Abstract
Introduction
The N-methyl-d-aspartate receptor-ionophore complex plays a key role in learning and memory and has efficacy in animals and humans with affective disorders. GLYX-13 is an N-methyl-d-aspartate receptor (NMDAR) glycine-site functional partial agonist and cognitive enhancer that also shows rapid antidepressant activity without psychotomimetic side effects.
Areas covered
The authors review the mechanism of action of GLYX-13 that was investigated in preclinical studies and evaluated in clinical studies. Specifically, the authors review its pharmacology, pharmacokinetics, and drug safety that were demonstrated in clinical studies.
Expert opinion
NMDAR full antagonists can produce rapid antidepressant effects in treatment-resistant subjects; however, they are often accompanied by psychotomimetic effects that make chronic use outside of a clinical trial inpatient setting problematic. GLYX-13 appears to exert its antidepressant effects in the frontal cortex via NMDAR-triggered synaptic plasticity. Understanding the mechanistic underpinning of GLYX-13’s antidepressant action should provide both novel insights into the role of the glutamatergic system in depression and identify new targets for therapeutic development.
Keywords: antidepressant, depression, glycine site, major depressive disorder, NMDA receptor, rapid-acting
1. Introduction
N-methyl-d-aspartate receptors (NMDARs) are part of the ionotropic glutamate receptor family that also includes AMPA and kainate receptors. NMDARs exist in multiple subtypes – at least 12 functionally distinct ones have been characterized to date, and are developmentally regulated and differentially distributed in various brain regions [1–7]. It is now well established that NMDARs play a critical role in learning and memory [8,9].
NMDAR-triggered intracellular calcium influx which, in turn, leads to synaptic membrane-associated AMPA receptor insertion/removal is thought to be an important pathway for the induction of both long-term potentiation (LTP) and long-term depression (LTD) of synaptic strength; forms of activity-dependent synaptic plasticity believed to be necessary for learning and memory formation [9–18]. Additionally, NMDAR activation has been suggested to be critical for the acquisition of hippocampal-dependent learning tasks such as trace eye blink conditioning (tEBC; [19,20]). Finally, mice with point mutations in the NMDAR glycine binding site that greatly reduce NMDAR function show severe deficits in learning in the Morris Water Maze (MWM), and learning is rescued by administration of the NMDAR glycine site agonist D-serine, now believed to be the in vivo modulator of the NMDAR glycine site [21–23].
The development of GLYX-13 began with the creation of monoclonal antibodies generated against freshly prepared developing rat dentate gyrus membrane preparations and screened for their ability to recognize neurons in unfixed frozen sections of adult rat hippocampi. These antibodies were further screened for their ability to bind to neuronal cell surface antigens using live, unfixed cultures of primary rat hippocampal neurons [24]. Antibodies that passed these two screens were then examined for their ability to modulate Schaffer collateral-CA1 LTP using rat hippocampal slice preparations [25,26]. Finally, an LTP-enhancing monoclonal antibody, B6B21, which was found to be an NMDAR glycine site partial agonist, and was also found to be a robust cognitive enhancer when injected intraventricularly in rabbits undergoing trace eyeblink conditioning [27].
Next, the heavy and light chains of B6B21 were cloned and sequenced as described previously [28] and peptides were synthesized based on the sequences identified in each of the hypervariable regions of the light chain. GLYX-13 was identified from peptide screening assays that involved the use of synaptic membranes prepared using rat hippocampal tissue [25]. These membrane preparations were monitored for the effect of peptides on [3H]MK-801 binding in the presence of 7-chlorokynurenic acid as previously reported [25,28].
GLYX-13 acts as an NMDAR functional glycine site modulator and cognitive enhancer. GLYX-13 simultaneously enhanced the magnitude of LTP of synaptic transmission, while reducing LTD. GLYX-13 reduced NMDAR-mediated synaptic currents in CA1 pyramidal neurons evoked by low-frequency Schaffer collateral stimulation, but enhanced NMDAR currents during high-frequency bursts of activity, and these actions were occluded by a saturating concentration of the glycine site agonist D-serine [29]. GLYX-13 (1 mg/kg, IV) also enhanced learning in both young adult and learning-impaired aged rats in MWM and alternating T-maze, and increased tEBC in both young and aging rats. Examination of the induction of LTP and LTD at Schaffer collateral-CA1 synapses in hippocampal slices found that aged rats showed marked, selective impairment in the magnitude of LTP evoked by a submaximal tetanus, and that GLYX-13 significantly enhanced the magnitude of LTP in slices from both young adult and aged rats without affecting LTD [30]. Finally, GLYX-13, when injected directly into the rat medial prefrontal cortex (MPFC), significantly increased positive emotional learning (PEL; [31,32]).
Thus, it appears reasonable that GLYX-13 directly modulates NMDAR in a glycine-like fashion and based on the effects of GLYX-13 on LTP/LTD, as well as enhancement of cognition in four different behavioral paradigms in young and learning-impaired aged rats, that GLYX-13 interacting with NMDARs triggers NMDAR-mediated synaptic plasticity. Since many of the physiological and molecular underpinnings of LTP and LTD have begun to be identified, this hypothesis will be readily testable.
GLYX-13 was derived from the amino acid sequence of one of the hypervariable regions of the light chain of a monoclonal antibody [33] which interacts with ionotropic NMDARs at the NMDAR glycine site [25]. GLYX-13 acts like an NMDAR glycine site functional partial agonist [29,33,34] to modulate NMDAR channel currents in rat hippocampal slices, with 25% of the activity of a glycine site full agonist. In animals, GLYX-13 exhibited antidepressant-like effects in several rat models [34]. In rats and dogs, GLYX-13 is rapidly cleared from plasma with a half-life of 10 min or less [35]. Toxicology studies in rats and dogs found GLYX-13 to be safe and well tolerated at single doses of 500 mg/kg IV and in 3-month studies at 300 mg/kg IV in rats and 200 mg/kg IV in dogs [35]. GLYX-13 caused no changes in the hERG assay at concentrations up to 2 mM, or cardiac or cardiovascular changes in conscious dogs at 500 mg/kg IV, or respiratory changes in rats at 415 mg/kg [35]. GLYX-13 did not increase locomotor activity at low doses in rats – a predictor of psychotomimetic effects in humans [34,35]. Further, GLYX-13 did not substitute for ketamine in ketamine-trained rats, elicit ketamine-like psychotomimetic effects in a prepulse inhibition assay, or cause conditioned place preference, all unlike ketamine [34].
2. Pharmacology
2.1 GLYX-13 is an NMDAR glycine site functional partial agonist
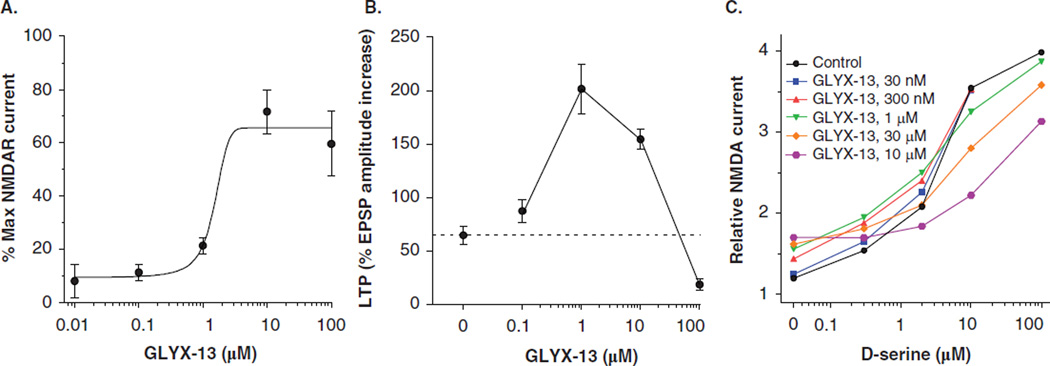
In oocytes expressing NMDAR, electrophysiological recordings demonstrate that GLYX-13 acts as a partial agonist at the glycine modulatory site. As shown in Figure 1A, GLYX-13, in the absence of exogenous glycine, can act as a co-agonist to evoke a response to NMDA using standard two-electrode whole-cell voltage clamp techniques [33,36–38]. As shown in Figure 1B, GLYX-13 enhanced the magnitude of LTP of Schaffer collateral–CA1 synaptic transmission at 1 – 10 µM and inhibited LTP at 100 µM [33]. In the absence of exogenous NMDAR glycine site agonist, GLYX-13 caused a concentration–dependent increase in pharmacologically isolated NMDAR current in rat hippocampal slices (Figure 1C). Maximum stimulation of current was approximately 20% of that elicited by the NMDAR glycine site full agonist D-serine. In the presence of increasing concentrations of GLYX-13 the concentration–response curve to D-serine shifted progressively to the right, consistent with a co-agonist site partial agonist. The affinity constant (Kp) for GLYX-13 activity was assessed using the method of Stephenson [39] and was found to be 1.3 µM.
Figure 1. GLYX-13 is an NMDAR functional glycine site partial agonist.
(A) The effects of GLYX-13 on NMDA currents in oocytes. Cells were injected with e1/z1 cRNA, voltage-clamped at −80 mV in the presence of 100 µM NMDA, varying concentrations of GLYX-13, and no exogenous glycine. Data are expressed as a percentage of the current elicited by 10 µM glycine in the same cell, usually in the same trial. Results from nine cells injected with e1/z1 cRNA; error bars indicate SEM. (B) The effects of GLYX-13 (0.1 – 100 µM) on LTP induced by a high-frequency stimulus train (3 × 100 Hz/500 ms) at Schaffer collateral–CA1 synapses. Each point represents Mean ± SEM of normalized field EPSP slope. (C) Pharmacologically isolated NMDAR currents were recorded from whole-cell patch clamp recordings of CA1 pyramidal neurons in slices. GLYX-13 by itself (left-most symbols) elicited increased NMDAR channel current to 20% the maximum current elicited by the full-agonist D-serine. GLYX-13 added in increasing concentrations shifted the dose response of D-serine to the right. Kp = 1.3 µM was calculated using the Stephenson method [39].
Data were adapted from [29] and [33] with permission of Elsevier.
2.2 GLYX-13 is a cognitive enhancer
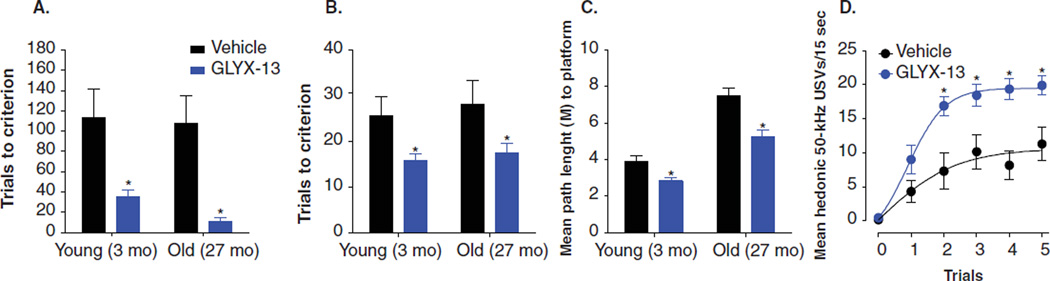
GLYX-13 (1 mg/kg IV) facilitates learning and memory in both young adult and learning impaired aged rats in hippocampal-dependent trace eyeblink conditioning (Figure 2A), food-motivated alternating T-maze (Figure 2B), and movable platform MWM (Figure 2C) [30]. GLYX-13 also facilitated positive emotional learning (PEL; Figure 2D) defined as increased rates of hedonic 50-kHz ultrasonic vocalizations in response to a conditioned stimulus that predicts heterospecific play that is acquired gradually across time [31]. PEL is modulated by NR2B-containing NMDAR in the MPFC, and is relevant to anhedonic and negative cognitive bias symptoms of depression [31]. It should be noted that because GLYX-13 acts as a cognitive enhancer, clinical trials have begun examining its effects on episodic and declarative memory in normal human volunteers and will be extended to depressed patients.
Figure 2. GLYX-13 is a cognitive enhancer.
(A– C) The effects of an optimal cognitive enhancing dose of GLYX-13 (1 mg/kg, IV, 15 min post-dosing) in young adult (3 months) or learning-impaired aged (27 months old) rats in hippocampal-dependent trace eyeblink conditioning (A), alternating T-maze (B), and Morris water maze (C) tests. (D) GLYX-13 (1 mg/kg, IV, 15 min post-dosing) in young adult (3 months) rats facilitated positive emotional learning as measured by rates of hedonic 50 kHz ultrasonic vocalizations in response to a conditioned stimulus that predicts heterospecific rough-and-tumble play.
Data are expressed as Mean ± SEM. *p < 0.05 Fisher’s PLSD post hoc test vs. vehicle.
2.3 GLYX-13 produces antidepressant-like effects in several animal models
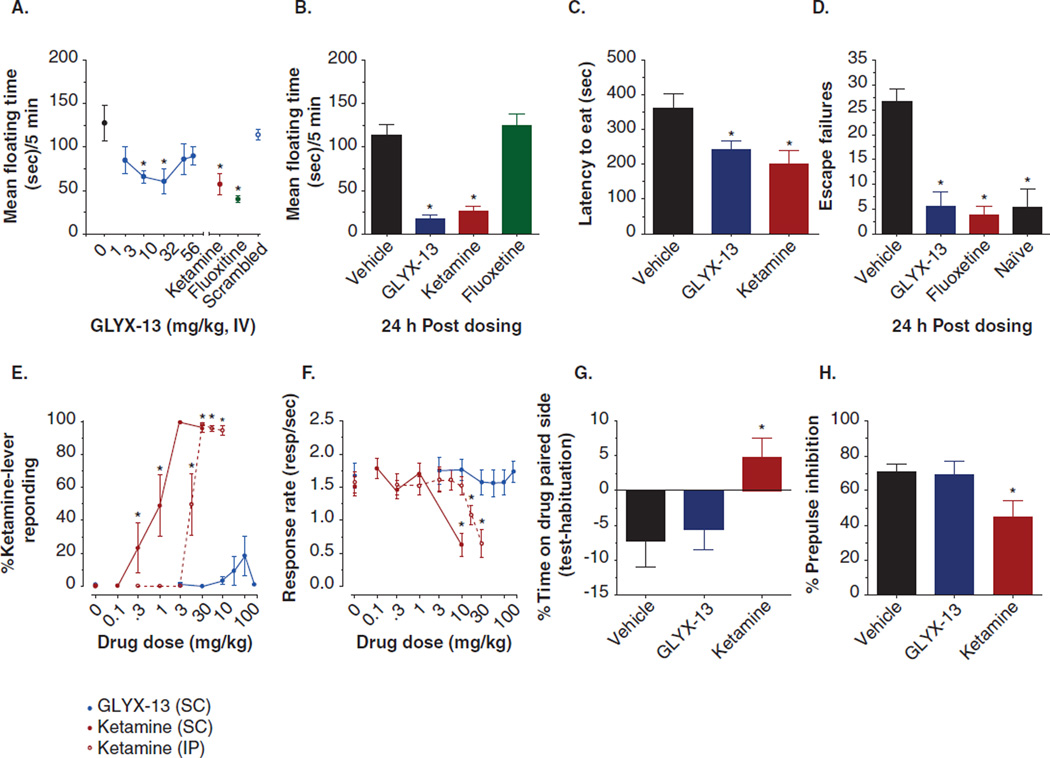
GLYX-13 produced rapid-acting (1 h; Figure 3A) and long-lasting (24 h; Figure 3B) antidepressant-like effects in the rat Porsolt test, rapid-acting (1 h) antidepressant effects in the novelty-induced hypophagia test (NIH; Figure 3C) and a long-lasting (24 h) antidepressant-like effect in the learned helplessness test (LH; Figure 3D) [34]. The version of the Porsolt test used has been shown to have high sensitivity and specificity for detecting the antidepressant effects of drugs [40], and the versions of the NIH and LH tests used require chronic administration of typical antidepressants such as fluoxetine to show efficacy but can detect the acute and long-lasting antidepressant-like effects of ketamine [40–43].
Figure 3. GLYX-13 has antidepressant-like properties without psychotomimetic side effects in rats.
(A – D) The antidepressant-like effects of an optimal antidepressant-like dose of GLYX-13 (3 mg/kg IV) in the rat Porsolt test measured at 1 h (A) or 24 h (B) post-dosing G, the novelty-induced hypophagia test 1 h post-dosing (C) or the learned helplessness test 24 h post dosing (D). GLYX-13 does not show ketamine-like discriminative stimulus effects (E) or sedative effects (F) in rats trained to discriminate 10 mg/kg ketamine from saline. (G – H) GLYX-13 (10 mg/kg IV) does not show ketamine-like rewarding effects in the conditioned place preference (G) or sensory-motor gating deficits in the pre-pulse inhibition (H) tests in rats.
Data are expressed as Mean ± SEM. *p < 0.05 Fisher’s PLSD post hoc test vs. vehicle.
Data adapted from [34] with permission of Nature Publishing Group.
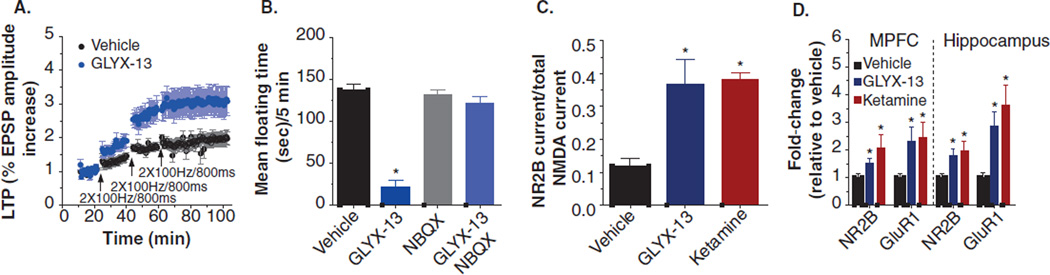
Therefore, it is suggested that long-lasting antidepressant effects of GLYX-13 are due to metaplastic enhancement in long-term activity-dependent synaptic plasticity, that is, a shift in the threshold of activation needed for inducing long-term changes in synaptic strength [34]. An important feature of metaplasticity is that such changes can last much longer than the triggering mechanism. NMDAR activation is a key component of both the induction of LTP and of meta-plastic regulation of the threshold for future LTP [8,44–50]. As shown in Figure 4A, a single dose of GLYX-13 facilitated the induction of Schaffer collateral LTP from 24 h to at least 1 week post-dosing, far outlasting the presence of the drug, and this effect did not habituate following repeated dosing (i.e., no signs of tachyphylaxis). The AMPA/kainate receptor antagonist, NBQX, blocked the maintenance of the antidepressant-like effects of GLYX-13 (Figure 4B). GLYX-13 increases synaptic currents gated by NR2B-containing NMDARs in hippocampal slices (Figure 4C), as well as significantly increasing cell-surface expression of NR2B and GluR1 protein levels in both the hippocampus and MPFC (Figure 4D) [34]. This suggests that the long-lasting antidepressant-like effects of GLYX-13 are due at least in part to metaplasticity mechanisms associated with NMDAR-triggered induction of LTP that results in a persistent shift in threshold for future LTP that underlies an antidepressant effect.
Figure 4. The antidepressant effects of GLYX-13 are due to NMDAR-triggered synaptic plasticity.
GLYX-13 (3 mg/kg IV, 24 h post-dosing): (A) enhances the magnitude of hippocampus ex vivo long-term potentiation, (B) antidepressant-like effect is blocked by the AMPA/kainate antagonist NBQX, (C) increases hippocampus NR2B current, and (D) increases cell surface expression of NR2B and GluR1 protein levels in the medial prefrontal cortex and hippocampus.
Data are expressed as Mean ± SEM. *p < 0.05 Fisher’s PLSD post hoc test vs. vehicle.
Data adapted from [34] with permission of Nature Publishing Group.
Ketamine is a robust antidepressant that is also an NMDAR modulator. Unlike GLYX-13, it is an open channel blocker. Ketamine also causes unacceptable dissociative side effects and is a substance of abuse. Mechanistically, ketamine works at least in part by modulation of the mTOR pathway which is coupled to the canonical Wnt receptor system and also involves the modulation of GSK-3β [41,51,52]. NMDAR channel blockers inhibit NMDARs and thus would inhibit synaptic plasticity processes associated with LTP. Ketamine has been shown to lead to enhanced synaptic glutamate release in the prefrontal cortex [53] suggesting NMDAR activation, and it has been postulated that ketamine may be a blockade of GABAergic interneurons projecting to prefrontal cortical synapses that disinhibit glutamate release, or perhaps blockade of presynaptic NMDARs that modulates glutamate release from excitatory terminals [54]. Interestingly, it has been shown that GSK-3β plays a key role in regulating one form of synaptic plasticity, LTD [55,56]. As such it may be that ketamine inhibits LTD at critical synapses and thereby indirectly increasing LTP. In contrast, GLYX-13 directly activates NMDARs, enhancing LTP and reducing LTD without the dissociative or ‘substance abusing’ side effects. Thus, ketamine, and perhaps all NMDAR channel blockers, appear to activate these receptors via an indirect mechanism that may be difficult to separate from dissociative side effects.
2.3. Nonclinical safety and toxicology
2.3.1 Safety
No adverse behavioral responses were observed in either male or female rats upon administration of GLYX-13 in single doses up to 500 mg/kg IV, the highest dose evaluated. Further, GLYX-13, up to 500 mg/kg IV, did not affect motor coordination in male or female rats. GLYX-13 did not induce hyperlocomotion used as a signal for psychotomimetic effects of NMDAR modulators in humans [57] at any dose evaluated.
GLYX-13 did not show any psychotomimetic side effects in rats [34]. GLYX-13 did not show any ketamine-like discriminative stimulus effects (Figure 3E) or sedative effects (Figure 3F) in rats trained to discriminate 10 mg/kg ketamine from saline. Using this drug discrimination protocol, NMDAR antagonists that have been shown to have psychotomimetic properties show ketamine-like discriminative stimulus effects, while NMDAR glycine site modulators do not show ketamine/PCP discriminative stimulus effects or psychotomimetic effects in humans [58,59]. In addition, GLYX-13 (10 mg/kg IV) did not produce a rewarding effect in the conditioned place-preference test (Figure 3G), relevant to the addictive side effects of NMDAR anatagonists such as ketamine or PCP [60], and did not suppress pre-pulse inhibition (Figure 3H) relevant to the sensory-motor gating deficits seen following ketamine or PCP that are associated with the positive symptoms of schizophrenia [61].
A single dose of GLYX-13, 500 mg/kg IV, 60 min following pentylenetetrazole administration, did not affect convulsant activity in rats. GLYX-13 did not affect current through the hERG channel at concentrations up to 5 mM. GLYX-13 at concentrations up to 1 mM, the highest dose evaluated, did not significantly alter any of the electrophysiological parameters measured in dog-isolated Purkinje fibers nor did GLYX-13 administered at single IV bolus doses up to 500 mg/kg affect heart rate, blood pressure, or any ECG parameter in conscious instrumented dogs, nor did GLYX-13 affect any respiratory parameter measured plethysmographically in conscious rats following a single IV bolus dose up to 415 mg/kg, the highest dose evaluated. In summary, GLYX-13 did not raise any safety concerns up to doses of 500 mg/kg.
2.3.2 Toxicology
In rats and dogs, single-dose IV toxicology studies were performed in both rats and dogs at doses up to 1000 mg/kg IV. No observational, body weight, clinical chemistry, hematology, urinalysis, or gross or microscopic pathological signals were observed. At 1000 mg/kg, transient respiratory depression and sedation were observed (about 10 min in duration), which in later studies have been determined to be due to a general anesthetic effect. Similarly, in 90-day twice-weekly IV administration repeated-dose studies, GLYX-13 was well tolerated at doses up to the highest evaluated, 300 mg/kg in rats and 200 mg/kg in dogs. GLYX-13 was inactive in the Ames mutagenesis, mouse lymphoma, and mouse micronucleus assays. GLYX-13 was completely compatible with human blood, serum, and plasma at concentrations up to 3 mg/ml in vitro.
In summary, GLYX-13 was examined in a series of toxicology studies including single dose and 3-month, repeated IV dose toxicity studies in rats and dogs, and genetic toxicity studies. These studies found that GLYX-13 is well tolerated in rats and dogs at doses up to 500 mg/kg IV in single doses and 200 – 300 mg/kg IV (highest doses evaluated) in twice-weekly dosing for 3 months. The maximum therapeutic effect in animals occurs at 10 mg/kg; thus, therapeutic index is 50-fold in single doses and at least 20 – 30-fold with chronic dosing.
3. Pharmacokinetics
3.1 Animals
GLYX-13 (30 – 300 mg/kg IV) exhibited a plasma half-life of 2–3 min in rats. Following 20 – 200 mg/kg IV administration in dogs, plasma half-life was 5–8 min, as would be expected for a tetrapeptide composed of natural amino acids. Following repeated, twice-weekly dosing in rats and dogs for 3 months, GLYX-13 retained its short half-life.
Thus, plasma half-life is considerably shorter than duration of action, which in the various pharmacologic studies was as long as 96 h to 2 weeks. Evidence suggests that prolonged (24 h post-dosing) antidepressant-like effects of both GLYX-13 and other NMDAR antagonists can be ascribed to the induction of NMDAR-dependent synaptic plasticity (Figure 4) [34].
3.2 Pharmacokinetics of GLYX-13 in normal human volunteers
Clearance was rapid in normal human volunteers following IV administration via the antecubital vein over the dose range 0.5 – 25 mg/kg. Plasma Cmax and AUC increased with dose (Table 1). Cmax increased supralinearly over the dose range 0.5 – 10 mg/kg and linearly over the dose range 10–25 mg/kg. AUC increased supralinearly over the dose range 0.5 – 25 mg/kg. Plasma half-life was short, approximately 10 min or less.
Table 1.
GLYX-13 plasma concentration and pharmacokinetic parameters in normal human volunteers.
| Dose (mg/kg) | Cmax (µg/ml) | AUC∞ (µg·min/ml) | t1/2 initial (min) | Vd(l/kg) | Cl (l/h/kg) |
|---|---|---|---|---|---|
| 1 IV | 5.2 ± 0.9 | 32 ± 4.2 | 4.7 ± 0.5 | 0.17 ± 0.03 | 2.02 ± 0.36 |
| 5 IV | 26 ± 11 | 288 ±115 | 7.4 ± 0.9 | 0.31 ±0.14 | 2.09 ± 0.98 |
| 10 IV | 100 ± 21 | 1162 ± 121 | 9.0 ± 0.6 | 0.12 ± 0.01 | 0.54 ± 0.05 |
| 20 IV | 182 ± 56 | 4030 ± 405 | 14.0 ± 2.1 | 0.10 ± 0.03 | 0.30 ± 0.03 |
Pharmacokinetics parameters are presented as Mean ± SEM, n = 4 subjects at each dose.
4. Human efficacy and safety
4.1 Antidepressant efficacy
To date, a Phase I safety and pharmacokinetics study (Section 3.2) and a single-dose Phase IIa safety and efficacy study have been completed. A repeated dose safety and efficacy study is ongoing. The Phase IIa study was a randomized, double-blind, multiple-dose level, placebo-controlled, single IV dose parallel safety and efficacy study of GLYX-13 in subjects with major depressive disorder (MDD) diagnosed using the Structured Clinical Interview for DSM-IV TR Axis I Disorders [62] who had experienced poor response (< 25% using the ATRQ) [63] to antidepressants during their current episode of MDD. The study included 116 subjects in 12 US centers that recruited subjects from April 2011 – July 2012.
Subjects must have undergone a 14-day washout period for all antidepressant drugs prior to randomization and could not receive any other antidepressant drug during the follow-up period. Subjects received a single IV dose of placebo (normal saline) or GLYX 13 at a dose level of 1, 5, 10, or 30 mg/kg. Depending upon dose level and mass, infusion occurred over 3–15 min. Subjects were confined to the study unit for at least 24 h following administration of study drug to allow for safety evaluations and efficacy assessments. Continuous ECG and pulse oximetry monitoring occurred from baseline through 4 h after dosing with additional assessments being made at intervals throughout the inpatient period and at follow-up visits.
A total of 116 subjects were included in the single IV dose efficacy trial. Of these, 33 subjects received placebo, and 25, 20, 17, and 21 subjects received GLYX-13 at dose levels of 1, 5, 10, and 30 mg/kg, respectively.
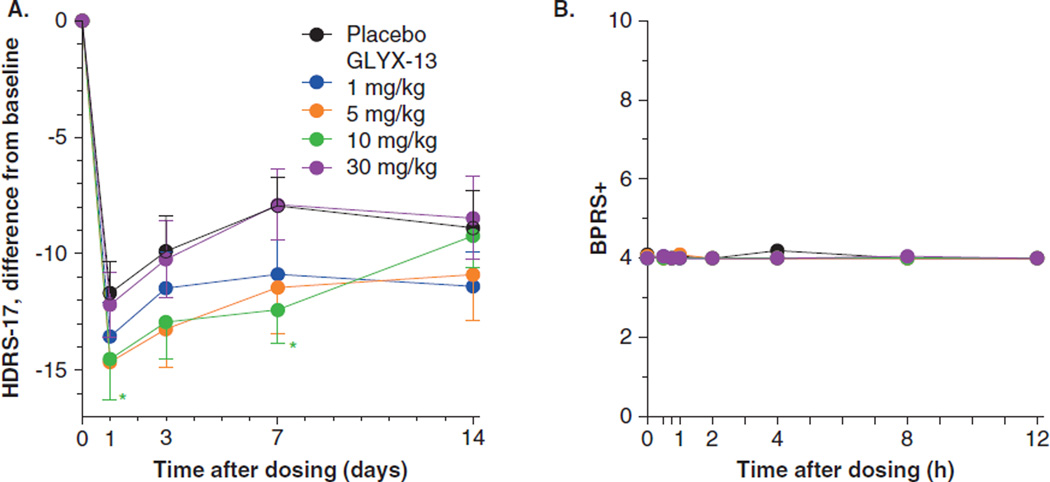
While the prolonged IV administration and the use of numerous assessment instruments resulted in a placebo response of 45% in reduction of HDRS-17, single IV administration of GLYX-13, at 1, 5, or 10 mg/kg resulted in rapid dose-dependent reduction in HDRS-17 score compared to placebo, apparent at the first assessment at the end of Day 1, and continuing through Days 3 and 7 (Figure 5A). At a dose of 1 mg/kg, GLYX-13 reduced HDRS-17 score numerically compared to placebo but the effect was not statistically significant. Dose levels of 5 and 10 mg/kg reduced HDRS-17 statistically significantly compared to placebo (analysis of covariance [ANCOVA] drug effect of 5 and 10 mg/kg, p < 0.05). The effect of GLYX-13 was statistically significant compared to placebo at Day 7 following administration but was not different from placebo at Day 14. Cohen’s d was 0.56, 0.41, 0.39 and 0.37, 0.36, 0.60 for 5 mg/kg at Days 1, 3, and 7 and 10 mg/kg at Days 1, 3, and 7, respectively with difference from placebo ranging from −2.8 and −2.5 HDRS-17 units (5 and 10 mg/kg, respectively) at Day 1 following dose administration to −3.1 to −4.3 HDRS-17 units (5 and 10 mg/kg, respectively) at Day 7 following dose administration. Reduction in HDRS-17 was most pronounced at the 5 and 10 mg/kg doses and antidepressant effect was lost when dose was increased to 30 mg/kg, similar to the dose response observed in animals (Figure 1A). Analysis of the data using a mixed effects model with repeated measures also revealed that GLYX-13 reduced HDRS-17 compared to placebo (ANCOVA drug effect at 5 and 10 mg/kg, p < 0.05) at the 5 and 10 mg/kg IV dose levels but the effect was lost when dose was increased to 30 mg/kg.
Figure 5. GLYX-13 reduces depression scores assessed as HDRS-17 score and does not cause psychotomimetic side effects assessed as BPRS+ scores.
(A) HDRS-17 scores are presented as difference from baseline. Mean ± SEM. Baseline scores were 26.1 (n = 33) placebo, and 26.1 (n = 25), 25.2 (n = 20), 25.1 (n = 17), and 24.6 (n = 21) for GLYX-13, 1, 5, 10, and 30 mg/kg, respectively. *p < 0.05 for 10 mg/kg compared to placebo at that time point. *p < 0.05 ANCOVA for 5 and 10 mg/kg compared to placebo. GLYX-13 reduces depressive symptoms assessed as Bech-6 scores. (B) GLYX-13 does not cause psychotomimetic side effects assessed as BPRS+ scores.
Mean + SEM, SEM were all 0.02 or less. Placebo, n = 33, GLYX-13 1 mg/kg, n = 25, 5 mg/kg, n = 20, 10 mg/kg, n = 17, 30 mg/kg, n = 21.
4.2 Human safety
No treatment-related serious adverse events occurred during the study. No differences were noted between treatment groups in the emergence of adverse events, ECG, oxygen saturation, laboratory or hematologic data, or vital signs. Few adverse events were reported by 5% or more of subjects and these were rated as mild or moderate. These included headache, somnolence, dizziness, dysgeusia, and fatigue. With the exception of dizziness, which was reported by approximately 10% of subjects who received GLYX-13 compared to no reports in subjects who received placebo, incidence of the other adverse events was similar in subjects who received GLYX-13 and placebo, and no dose response was apparent for GLYX-13. The assignment of dizziness as a specific side effect of GLYX-13 is unclear since the incidence of dizziness was not related to pharmacokinetics of GLYX-13, having been reported for only for a short period by any subject and occurring from 5 min to several hours after administration; however, 10% of subjects who received GLYX-13 in the Phase I study also reported dizziness. GLYX-13 did not cause any increase in suicidality over the course of the 14- or 28-day course of observation following dosing (Figure 5B) using the Columbia-Suicide Severity Rating Scale [64]. Importantly, unlike other NMDAR modulators studied in humans, GLYX-13 did not increase BPRS+ score (psychotomimetic effects) at any time following dosing.
5. Conclusion
These studies add further support to the hypothesis that GLYX-13 acts as a partial agonist at the glycine site of the NMDAR as assessed by evaluation of its effects on whole-cell NMDAR channel current in rat hippocampal CA1 pyramidal neurons [29,33,34]. GLYX-13 displayed approximately 20% of the activity of the glycine site full agonist D-serine. The dose–response relationship of GLYX-13 in the Porsolt assay was ‘U-shaped.’ At a dose of 1 mg/kg IV, floating time was reduced numerically compared to placebo and was statistically significantly different from placebo at 3 and 10 mg/kg. When the dose was further increased to 30 mg/kg, the effect of GLYX-13 was diminished.
In a double-blind, placebo-controlled, proof-of-concept clinical trial, a single IV dose of GLYX-13 to subjects with MDD who had not responded to an adequate trial with at least one antidepressant agent during their current depressive episode, improved depression score within hours without psychotomimetic effects. The improvement was sustained, lasting 7 days on average when assessed using HDRS-17 [65] score or 3 days when assessed using Bech-6 score [66].
The data suggested that a dose of 1 mg/kg IV would be minimally efficacious, a dose of 5 mg/kg would be at or above the ED50, and a dose of 10 mg/kg would be at the peak of the dose response. The magnitude of effect of GLYX-13 was robust, with Cohen’s d as great at 0.60, somewhat less than has been reported for ketamine. In this study, an appreciable immediate placebo effect, averaging approximately 45%, was observed. In spite of this, the effect of GLYX-13 on depression scores was readily apparent using the composite HDRS-17 score, the core symptom Bech-6 score, as well as several individual items that compose the HDRS-17 which all revealed a statistically greater reduction in depression score compared to placebo.
A large placebo effect was observed in this study. The reasons for this are likely several-fold. First, the study drugs are administered intravenously. Injection is associated with large placebo effects compared to oral placebo administration [67]. Second, infusion time is prolonged several minutes. This may allow a significant ‘therapeutic relationship’ to develop during drug administration. Further, many of the subjects in the trial expected a strong response based on the efficacy of ketamine in depressed patients, which has received wide media attention. Finally, in this study, subjects were administered 31 evaluation instruments during the 24-h in-patient period following administration which may have influenced expectation as well.
The duration of the antidepressant effect of GLYX-13 was 7 days using the HDRS-17 and is similar to the duration of the effect of ketamine and longer than CP-101,606 or AZD6765. In rats, GLYX-13 produces long-lasting efficacy in the Porsolt assay and induces LTP which lasts at least 24 h after a single dose [34]. Long-lasting neuroplastic changes such as LTP may subserve the long-lasting efficacy of GLYX-13.
GLYX-13 demonstrated a dose response in human subjects with depression similar to its dose response in the Porsolt assay in rats. That is, a dose of 1 mg/kg GLYX-13, while numerically superior to placebo, did not produce a statistically significant effect on depression scores, whereas 5 and 10 mg/kg produced robust effects. Similar to results in the Porsolt assay, when the dose of GLYX-13 was increased further to 30 mg/kg, its antidepressant effect was lost. The mechanism for this ‘U-shaped’ dose response is not clear at present. Nevertheless, U-shaped dose–response curves are common in pharmacology and have been studied extensively [68]. Two plausible explanations for the U-shaped dose–response curves observed with GLYX-13 include reports by Sheinin et al. [69] and Dravid et al. [70] showing that D-cycloserine, a glycine site partial agonist, interacts with each NMDAR subtype uniquely leading to quite different ion channel kinetic patterns ranging from partial agonist-driven channel opening to super-agonist-driven channel modulation. Thus, it is conceivable that, as the concentration of GLYX-13 is increased, different NMDAR channel opening kinetics at different receptor subtypes occur, leading to an overall decrease in receptor function. A second hypothesis is based on the observation that increasing the glycine coagonist concentration facilitates NMDAR endocytosis [71]. Thus, an increase in GLYX-13 concentration could eventually lead to increased removal of NMDARs from the cell surface with a concomitant decrease in activity leading directly to a reduction in therapeutic efficacy of GLYX-13.
GLYX-13 has rapid efficacy that lasts for approximately 1–2 weeks after a single injection. Thus, it is likely that GLYX-13 will be used in combination with traditional anti-depressants and will also need to be administered repeatedly over time as well. The current Phase II program is examining the effects of repeated dosing with GLYX-13. Preclinical studies have shown that repeated dosing with GLYX-13 produces neither tachyphylaxis in antidepressant models nor negative effects in a variety of learning and memory paradigms [29,34]. Moreover, it has also been shown in preclinical models that GLYX-13 did not interfere with the antidepressant-like effects of fluoxetine in the forced swim test using rats.
Of great interest is the observation in this study that GLYX-13 produced antidepressant effects without any evidence of psychotomimetic side effects. This is a therapeutically important observation in that NMDAR full antagonists such as ketamine produce psychotomimetic side effects at doses that reduce depression scores. Additional studies with repeated dosing of GLYX-13 are warranted by the results of this study. A 12-week repeated-dose study of GLYX-13 as add-on therapy is under way.
6. Expert opinion
Studies conducted by several investigators have demonstrated that NMDAR modulators produce rapid antidepressant effects, often within a few hours, in subjects with MDD who had not responded adequately to other antidepressant agents. Many investigators believe that the discovery of rapid antidepressant activity in these resistant subjects may lead to a paradigm shift in treatment of MDD aimed at rapid control with resulting decrease in the disruption in the lives of depressed patients and their families. Unfortunately, NMDAR full antagonists induce psychotomimetic side effects at dose levels that are efficacious in MDD. For example, ketamine, a potent NMDA channel blocker, has been demonstrated to reduce depression scores in subjects with MDD [72–74], with efficacy apparent within a few hours [72–76]. However, ketamine produces psychotomimetic side effects at doses that reduce depression scores. Similarly, CP-101,606, an NR2B subunit-selective NMDAR antagonist, reduced depression scores in an add-on study but was also associated with psychotomimetic side effects although these were reduced when the dose level of CP-101,606 was reduced [76]. Another NR2B-selective antagonist, MK-0657, also reduced secondary efficacy measures by Day 5 of oral administration in 5 subjects without causing psychotomimetic side effects [75].
Thus, it has been suggested that NMDAR modulators that do not completely block NMDAR activity may be effective antidepressant agents without producing psychotomimetic side effects [6]. D-cycloserine is an NMDAR glycine site partial agonist at NR2B-containing NMDARs, but it is a superagonist at NR2C-containing NMDARs [69,70]. The antidepressant activity of D-cycloserine has been studied in clinical trials, where it was found to not possess antidepressant activity [77], or, in a recent study at higher dose, it has been reported that 2 weeks of daily oral treatment resulted in decreased depression score without psychotomimetic side effects and antidepressant activity was maintained during a further 4-week period [78]. Memantine, a weak NMDAR channel blocker, was found not to have antidepressant activity in a clinical trial [79]. AZD6765, another weak NMDA channel blocker, demonstrated short-term reduction of depression scores, within 80 min of dosing but lasting < 24 h, without causing increased BPRS+ scores [80]. Tianeptine is a unique antidepressant and anxiolytic agent with serotonin reuptake inhibitory activity as well as activity at glutamate receptors [81]. Tianeptine alters phosphorylation of glutamate receptors [82] and augments neuroplasticity [83]. However, the clinical data regarding the effectiveness of tianeptine in the treatment of depression have been largely inconclusive.
GLYX-13 appears promising because it appears it seems to act rapidly with antidepressant effects that persist for approximately a week in humans and show no psychotomimetic side effects. Its partial agonist properties, like D-cycloserine, likely play a role in this lack of side effects. GLYX-13 is a cognitive enhancer that facilitates synaptic plasticity in frontal cortex and hippocampus by triggering NMDAR-mediated calcium influx leading to increased membrane insertion of AMPA receptors, one of the mechanisms shown previously to underlie the induction of some forms of both LTP and LTD of synaptic strength. Thus, GLYX-13 appears to operate as an antidepressant via mechanisms associated with upregulation of synaptic plasticity necessary for learning and memory. Understanding the mechanistic underpinning of GLYX-13’s antidepressant action should provide novel insights into the role of the glutamatergic system in depression, insights for clinical development, and help to identify additional new targets for therapeutic development.
Highlights.
GLYX-13 is a novel glutamatergic-based antidepressant in both animal studies and in humans based on clinical trial data presented.
GLYX-13, unlike NMDAR channel blockers such as ketamine, does not elicit psychotomimetic side effects.
GLYX-13 is a novel first-in-class antidepressant in that it appears to operate via NMDAR triggering setting off a cascade of biochemical and physiological processes akin to LTP-based synaptic plasticity.
The clinical trial results with GLYX-13 together with those previously reported for ketamine add strong support for the NMDAR as a target for the creation of new antidepressants.
Acknowledgments
The authors wish to thank Derek Small for his helpful discussions.
Declaration of interest
JR Moskal is the founder of Naurex, Inc. He has founders’ shares of stock in the company. JR Moskal also receives financial compensation as a consultant. PK Stanton, RA Kroes, JS Burgdorf, and JF Disterhoft have been consultants for Naurex, Inc. for the past 3 years, and have received financial compensation and stock. RM Burch is an employee of Naurex and has received financial compensation and stock. J David Leander is a paid consultant for Naurex, and also has stock in the company. Over the last 3 years he has received financial compensation and/or stock with the following companies: AgeneBio, Nektar, and CoLucid. JR Moskal is further supported by grants from The Ralph and Marian Falk Medical Research Trust (Chicago, IL) while JS Burgdorf is supported by NIH grant MH094835 and PK Stanton with NS044421.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1. Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14(6):383–400. doi: 10.1038/nrn3504. •• Most recent review of biochemical pharmacological and physiological properties of known NMDA receptor subtypes: the future of NMDA receptor drug discovery programs will evolve to address receptor subtype specific disease modulation.
- 2.Traynelis SF, Wollmuth LP, McBain CJ, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J Neurosci. 1994;14(5 Pt 2):3180–3194. doi: 10.1523/JNEUROSCI.14-05-03180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monyer H, Burnashev N, Laurie DJ, et al. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992;3(12):1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Akazawa C, Shigemoto R, Bessho Y, et al. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347(1):150–160. doi: 10.1002/cne.903470112. [DOI] [PubMed] [Google Scholar]
- 7.Sheng M, Cummings J, Roldan LA, et al. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368(6467):144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 8.Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9(5):387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 9. Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. • This review laid the groundwork for studying the two key mechanisms for the study of synaptic plasticity as it relates to learning and memory.
- 10.Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1998;273(33):20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- 11.Faas GC, Raghavachari S, Lisman JE, Mody I. Calmodulin as a direct detector of Ca2+ signals. Nat Neurosci. 2011;14(3):301–304. doi: 10.1038/nn.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313(5790):1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 13.Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48(2):289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 14.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284(5411):162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 15.Leonard AS, Lim IA, Hemsworth DE, et al. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 1999;96(6):3239–3244. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 17.Stanton PK. LTD. LTP, and the sliding threshold for long-term synaptic plasticity. Hippocampus. 1996;6(1):35–42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Rison RA, Stanton PK. Long-term potentiation and N-methyl-D-aspartate receptors: foundations of memory and neurologic disease? Neurosci Biobehav Rev. 1995;19(4):533–552. doi: 10.1016/0149-7634(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 19.Weiss C, Knuttinen MG, Power JM, et al. Trace eyeblink conditioning in the freely moving rat: optimizing the conditioning parameters. Behav Neurosci. 1999;113(5):1100–1105. doi: 10.1037//0735-7044.113.5.1100. [DOI] [PubMed] [Google Scholar]
- 20.Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav Brain Res. 1999;99(2):123–132. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 21.Ballard TM, Pauly-Evers M, Higgins GA, et al. Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug-resistant nonhabituating hyperactivity. J Neurosci. 2002;22(15):6713–6723. doi: 10.1523/JNEUROSCI.22-15-06713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kew JN, Koester A, Moreau JL, et al. Functional consequences of reduction in NMDA receptor glycine affinity in mice carrying targeted point mutations in the glycine binding site. J Neurosci. 2000;20(11):4037–4049. doi: 10.1523/JNEUROSCI.20-11-04037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mothet JP, Parent AT, Wolosker H, et al. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 2000;97(9):4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moskal JR, Schaffner AE. Monoclonal antibodies to the dentate gyrus: immunocytochemical characterization and flow cytometric analysis of hippocampal neurons bearing a unique cell-surface antigen. J Neurosci. 1986;6(7):2045–2053. doi: 10.1523/JNEUROSCI.06-07-02045.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haring R, Stanton PK, Scheideler MA. Moskal JR Glycine-like modulation of N-methyl-D-aspartate receptors by a monoclonal antibody that enhances long-term potentiation. J Neurochem. 1991;57(1):323–332. doi: 10.1111/j.1471-4159.1991.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 26.Stanton PK, Sarvey JM, Moskal JR. Inhibition of the production and maintenance of long-term potentiation in rat hippocampal slices by a monoclonal antibody. Proc Natl Acad Sci USA. 1987;84(6):1684–1688. doi: 10.1073/pnas.84.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson LT, Moskal JR, Disterhoft JF. Hippocampus-dependent learning facilitated by a monoclonal antibody or D-cycloserine. Nature. 1992;359(6396):638–41. doi: 10.1038/359638a0. [DOI] [PubMed] [Google Scholar]
- 28.Moskal JR, Yamamoto H, Colley PA. The use of antibody engineering to create novel drugs that target N-methyl-D-aspartate receptors. Curr Drug Targets. 2001;2(3):331–345. doi: 10.2174/1389450013348399. [DOI] [PubMed] [Google Scholar]
- 29.Zhang XL, Sullivan JA, Moskal JR, Stanton PK. A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus. Neuropharmacology. 2008;55(7):1238–1250. doi: 10.1016/j.neuropharm.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgdorf J, Zhang XL, Weiss C, et al. The N-methyl-D-aspartate receptor modulator GLYX-13 enhances learning and memory, in young adult and learning impaired aging rats. Neurobiol Aging. 2011;32(4):698–706. doi: 10.1016/j.neurobiolaging.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgdorf J, Kroes RA, Weiss C, et al. Positive emotional learning is regulated in the medial prefrontal cortex by GluN2B-containing NMDA receptors. Neuroscience. 2011;192:515–523. doi: 10.1016/j.neuroscience.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgdorf J, Panksepp J, Moskal JR. Frequency-modulated 50 kHz ultrasonic vocalizations: a tool for uncovering the molecular substrates of positive affect. Neurosci Biobehav Rev. 2011;35(9):1831–1836. doi: 10.1016/j.neubiorev.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Moskal JR, Kuo AG, Weiss C, et al. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacology. 2005;49(7):1077–1087. doi: 10.1016/j.neuropharm.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 34. Burgdorf J, Zhang X-I, Nicholson KL, et al. GLYX-13, an NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38(5):729–742. doi: 10.1038/npp.2012.246. • The long-lasting antidepressant properties of glutamatergic compounds may involve the mechanisms of metaplasticity.
- 35.Hanan EA, Godin CS, Bruening-Wright A, et al. In vitro and in vivo safety pharmacology assessment of GLYX-13. American College of Toxicology 33rd Annual Meeting; 2012. P710. [Google Scholar]
- 36.Leonard JP, Kelso SR. Apparent desensitization of NMDA responses in Xenopus oocytes involves calcium-dependent chloride current. Neuron. 1990;4(1):53–60. doi: 10.1016/0896-6273(90)90443-j. [DOI] [PubMed] [Google Scholar]
- 37.Meguro H, Mori H, Araki K, et al. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992;357(6373):70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- 38.Yamazaki M, Mori H, Araki K, et al. Cloning, expression and modulation of a mouse NMDA receptor subunit. FEBS Lett. 1992;300(1):39–45. doi: 10.1016/0014-5793(92)80160-i. [DOI] [PubMed] [Google Scholar]
- 39.Stephenson RP. A modification of receptor theory. Br Pharmacol Chemother. 1956;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23(5):238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 41. Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. •• First paper to discuss some of the molecular mechanisms underlying the antidepressant effects of MDA receptor channel Mockers.
- 42.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29(4–5):771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Valentine G, Dow A, Banasr M, et al. Differential effects of chronic antidepressant treatment on shuttle box escape deficits induced by uncontrollable stress. Psychopharmacology (Berl) 2008;200(4):585–596. doi: 10.1007/s00213-008-1239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang YY, Colino A, Selig DK, Malenka RC. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992;255(5045):730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- 45.Buschler A, Manahan-Vaughan D. Brief environmental enrichment elicits metaplasticity of hippocampal synaptic potentiation in vivo. Front Behav Neurosci. 2012;6:85. doi: 10.3389/fnbeh.2012.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper LN, Bear MF. The BCM theory of synapse modification at 30: interaction of theory with experiment. Nat Rev Neurosci. 2012;13(11):798–810. doi: 10.1038/nrn3353. [DOI] [PubMed] [Google Scholar]
- 47.Makino H, Malinow R. Compartmentalized versus global synaptic plasticity on dendrites controlled by experience. Neuron. 2011;72(6):1001–1011. doi: 10.1016/j.neuron.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redondo RL, Morris RG. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12(1):17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- 49.Disterhoft JF, Oh MM. Learning, aging and intrinsic neuronal plasticity. Trends Neurosci. 2006;29(10):587–599. doi: 10.1016/j.tins.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2(1):32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35(1):47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorrain DS, Baccei CS, Bristow LJ, et al. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117(3):697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- 54.Duman RS, Li N, Liu RJ, et al. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peineau S, Nicolas CS, Bortolotto ZA, et al. A systematic investigation of the protein kinases involved in NMDA receptor-dependent LTD: evidence for a role of GSK-3 but not other serine/threonine kinases. Mol Brain. 2009;2:22. doi: 10.1186/1756-6606-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradley CA, Peineau S, Taghibiglou C, et al. A pivotal role of GSK-3 in synaptic plasticity. Front Molecular Neurosci. 2012;5:13. doi: 10.3389/fnmol.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tricklebank MD, Singh L, Oles RJ, et al. The behavioural effects of MK-801: a comparison with antagonists acting non-competitively and competitively at the NMDA receptor. Eur J Pharmacol. 1989;167(1):127–135. doi: 10.1016/0014-2999(89)90754-1. [DOI] [PubMed] [Google Scholar]
- 58. Nicholson KL, Balster RL. The discriminative stimulus effects of N-methyl-D-aspartate glycine-site ligands in NMDA antagonist-trained rats. Psychopharmacology (Berl) 2009;203(2):441–451. doi: 10.1007/s00213-009-1469-8. • This paper evaluated the abuse potential of NMDA receptor glycine site modulators.
- 59.Nicholson KL, Balster RL. Evaluation of the phencyclidine-like discriminative stimulus effects of novel NMDA channel blockers in rats. Psychopharmacology (Berl) 2003;170(2):215–224. doi: 10.1007/s00213-003-1527-6. [DOI] [PubMed] [Google Scholar]
- 60.Li F, Fang Q, Liu Y, et al. Cannabinoid CB(1) receptor antagonist rimonabant attenuates reinstatement of ketamine conditioned place preference in rats. Eur J Pharmacol. 2008;589(1–3):122–126. doi: 10.1016/j.ejphar.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 61.de Bruin NM, Ellenbroek BA, Cools AR, et al. Differential effects of ketamine on gating of auditory evoked potentials and prepulse inhibition in rats. Psychopharmacology (Berl) 1999;142(1):9–17. doi: 10.1007/s002130050856. [DOI] [PubMed] [Google Scholar]
- 62.First MB, Spitzer RL, Gibbon M, Williams AR. Structured Clinical Interview of DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- 63.Chandler GM, Iosifescu DV, Pollack MH, et al. RESEARCH: validation of the massachusetts general hospital Antidepressant Treatment History Questionnaire (ATRQ) CNS Neurosci Ther. 2010;16(5):322–325. doi: 10.1111/j.1755-5949.2009.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamilton M. A rating scale for depression. J Neurol Neurosur Ps. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 67.de Craen AJ, Tijssen JG, de Gans J, Kleijnen J. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J Neurol. 2000;247(3):183–188. doi: 10.1007/s004150050560. [DOI] [PubMed] [Google Scholar]
- 68.Calabrese EJ, Baldwin LA. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001;22(6):285–291. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- 69.Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology. 2001;41(2):151–158. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- 70.Dravid SM, Burger PB, Prakash A, et al. Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors. J Neurosci. 2010;30(7):2741–2754. doi: 10.1523/JNEUROSCI.5390-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nong Y, Huang YQ, Ju W, et al. Glycine binding primes NMDA receptor internalization. Nature. 2003;422(6929):302–307. doi: 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- 72.aan het Rot M, Collins KA, Murrough JW, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 73.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 74. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. •• The first published account of rapid antidepressant effect of a NMDAR antagonist.
- 75.Ibrahim L, Diaz Granados N, Jolkovsky L, et al. A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012;32(4):551–557. doi: 10.1097/JCP.0b013e31825d70d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Preskorn SH, Baker B, Kolluri S, et al. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28(6):631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 77.Heresco-Levy U, Javitt DC, Gelfin Y, et al. Controlled trial of D-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. J Affect Disord. 2006;93(1–3):239–243. doi: 10.1016/j.jad.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Heresco-Levy U, Gelfin G, Bloch B, et al. A randomized add-on trial of high-dose D-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol. 2013;16(3):501–506. doi: 10.1017/S1461145712000910. [DOI] [PubMed] [Google Scholar]
- 79.Zarate CA, Jr, Singh JB, Quiroz JA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163(1):153–155. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- 80.Zarate CA, Jr, Mathews D, Ibrahim L, et al. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2012;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McEwen BS, Chattarji S, Diamond DM, et al. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry. 2010;15(3):237–249. doi: 10.1038/mp.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Svenningsson P, Bateup H, Qi H, et al. Involvement of AMPA receptor phosphorylation in antidepressant actions with special reference to tianeptine. Eur J Neurosci. 2007;26(12):3509–3517. doi: 10.1111/j.1460-9568.2007.05952.x. [DOI] [PubMed] [Google Scholar]
- 83.Qi H, Mailliet F, Spedding M, et al. Antidepressants reverse the attenuation of the neurotrophic MEK/MAPK cascade in frontal cortex by elevated platform stress; reversal of effects on LTP is associated with GluA1 phosphorylation. Neuropharmacology. 2009;56(1):37–46. doi: 10.1016/j.neuropharm.2008.06.068. [DOI] [PubMed] [Google Scholar]