Abstract
Objective
Stromal derived factor-1α/CXCL12 is a chemoattractant responsible for homing of progenitor cells to ischemic tissues. We aimed to investigate the association of plasma CXCL12 with long-term cardiovascular outcomes in patients with coronary artery disease (CAD).
Methods
785 patients aged: 63±12 undergoing coronary angiography were independently enrolled into discovery (N=186) and replication (N=599) cohorts. Baseline levels of plasma CXCL12 were measured using Quantikine CXCL12 ELISA assay (R&D systems). Patients were followed for cardiovascular death and/or myocardial infarction (MI) for a mean of 2.6 yrs. Cox proportional hazard was used to determine independent predictors of cardiovascular death/MI.
Results
The incidence of cardiovascular death/MI was 13% (N=99). High CXCL12 level based on best discriminatory threshold derived from the ROC analysis predicted risk of cardiovascular death/MI (HR=4.81, p=1 × 10−6) independent of traditional risk factors in the pooled cohort. Addition of CXCL12 to a baseline model was associated with a significant improvement in c-statistic (AUC: 0.67 to 0.73, p=0.03). Addition of CXCL12 was associated with correct risk reclassification of 40% of events and 10.5% of non-events. Similarly for the outcome of cardiovascular death, the addition of the CXCL12 to the baseline model was associated with correct reclassification of 20.7% of events and 9% of non-events. These results were replicated in two independent cohorts.
Conclusion
Plasma CXCL12 level is a strong independent predictor of adverse cardiovascular outcomes in patients with CAD and improves risk reclassification.
Keywords: Stromal Cell-Derived Factor1α, CXCL12, coronary artery disease, cardiovascular outcomes
Introduction
Stromal cell-derived factor-1α also known as CXCL12 is a chemokine that plays a key role in recruitment of stem cells and myocardial regeneration after myocardial infarction.1, 2 CXCL12 mediates homing of progenitor cells to areas of ischemic tissues.3 It is expressed on the surface of platelets and endothelial cells and is secreted in plasma after activation, facilitating mobilization, migration, and domiciliation of progenitor cells in ischemic tissues.4, 5 On the other hand, CXCL12 by activating several signaling pathways has been shown to induce an inflammatory response by activation of chemotaxis, cell migration, and secretion of several inflammatory biomarkers.6 Limited numbers of clinical studies have reported differences in CXCL12 levels in patients with a variety of clinical manifestations of coronary artery disease (CAD) and with varied exposure to traditional cardiovascular risk factors.7, 8 However, the data on prognostic role of CXCL12 level, a key modulator of circulating progenitor cells, in patients with CAD is limited.9 The goal of the present study was to investigate the prognostic role of plasma CXCL12 levels on long-term cardiovascular outcomes in patients with suspected or confirmed CAD, with the hypothesis that higher CXCL12 would be associated with higher incidence of adverse cardiovascular events.
Methods
Study population: 785 patients, aged 63±12 years, undergoing cardiac catheterization were enrolled independently into discovery (N=186) and replication (N=599) cohorts. The discovery cohort was established at the Atlanta Veterans Affairs and Emory University hospitals between years 2004 to 2006 and includes patients with stable CAD undergoing percutaneous coronary intervention and stenting. The replication cohort was a nested study within the Emory Cardiovascular Biobank with subjects enrolled between years 2008 to 2011. Demographics, medical, and behavioral characteristics as well as risk factor prevalence were documented as previously described.10 Subjects were classified as current or non- smokers. Acute MI at enrollment was defined using universal criteria for diagnosis.11 Subjects were noted to have hypertension or dyslipidemia if they had a documented history or they were on treatment. Subjects were excluded if they had a history of heart transplantation, immunosuppressant use, malignancy, or significant infections. The Institutional Review Board at Emory University approved both cohorts and all subjects provided written informed consent.
Follow-up data collection
Outcomes data were collected by independent personnel who were blinded to the study data. Record of death was obtained from the Social Security Death Index and/or via direct contact with subjects’ family members. Cause of death was adjudicated from medical records or direct contact. Follow-up was conducted at 1 and 5 years from the date of enrollment to identify cases of myocardial infarction. MI occurring within a month of enrollment was not included in the final analysis.
Identification of CAD and severity scoring
Coronary angiograms were scored for luminal narrowing based on the modified AHA/ACC classification of the coronary tree.12 Patients were classified as having non-obstructive (visible plaque resulting in <50% luminal stenosis) or obstructive CAD (plaque resulting in ≥50% stenosis.
Sample Collection
Fasting arterial blood samples for plasma were drawn at cardiac catheterization and stored at −80°C before analysis. CXCL12 levels were measured using the Human CXCL12 Quantikine ELISA kit (R&D systems). Discovery cohort samples were processed at the time of enrollment and replication cohort samples were processed after an average 3.2 years. The inter-assay precision for the CXCL12 assay varies between 8.2% to 13.4% for the different concentrations. In a batch of 20 samples from the discovery cohort that were re-analyzed with the assay kits used for the replication cohort, an insignificant 3.48% (p=0.56) difference in means of CXCL12 level was found. Mean CXCL12 was 1623±606 pg/ml in the discovery cohort compared to 2534±1011 pg/ml in the replication cohort.
Statistical Analysis
Continuous variables are presented as means ± SD and categorical variables as proportions (%). The student t-test and Chi-square analyses were performed when appropriate. Univariate predictors of the primary endpoint were identified using univariate Cox proportional hazard models. Multivariate Cox regression models were created adjusting for traditional cardiovascular risk factors as well as medication use, serum creatinine, LVEF, history of CABG, and presence of ≥50% stenosis in any major epicardial vessel. The best discriminatory cutoff for CXCL12 in association with outcomes was determined using the Youden’s index (Sensitivity – (1-Specificity)) from the Receiver Operating Characteristic (ROC) analysis to identify “high” and “low” CXCL12 levels in both cohorts separately. Both cohorts were then pooled to determine cases with “high” or “low” CXCL12 based on the same method of categorization. Discrimination analysis for prediction of the primary endpoint was calculated as the difference in C-statistic comparing baseline model with a model containing CXCL12 (high/low) variable in addition to the baseline variables. Using multivariate Cox models with the clinical covariates noted above, net reclassification improvement (NRI) and integrated discrimination improvement (IDI) metrics were calculated.13, 14 Annual event rates for each outcome measure were calculated by dividing the observed number of events by the observed event-specific number of person-years of follow-up. P values <0.05 from two-sided tests were considered statistically significant. Analyses were performed with SAS (Version 9.3; SAS Institute, NC, USA).
Results
Baseline characteristics of the 186 subjects in the discovery and 599 subjects in the replication cohorts are presented in Table 1. In the combined cohort, the mean age was 63±12 years, 76% were male, 40% had a history of diabetes mellitus, 76% had ≥50% stenosis in at least one epicardial vessel, and 7% had acute MI on presentation. Incidence rate of cardiovascular death and MI in the discovery cohort was 17% (n=32) and 11% (n=20), respectively, with a mean follow-up duration of 5.6 years. In the replication cohort the incidence of cardiovascular death and MI was 6% (n=36) and 3.4% (n=20) with a mean follow-up of 1.6 years. The incidence of the primary endpoint of cardiovascular death/MI in the pooled cohort was 13% (n=99) with a mean follow-up duration of 2.6 years.
Table 1.
Baseline Characteristics
| Discovery (N=186) |
Replication (N=599) |
Pooled (N=785) |
|
|---|---|---|---|
| Age, years | 61±10 | 63±12 | 63±12 |
| Male Gender (%) | 84 | 74 | 76 |
| African American Race (%) | 19 | 22 | 22 |
| Serum Creatinine (mg/dL) | 1.08±0.31 | 1.43±175 | 1.35±1.55 |
| Diabetes Mellitus (%) | 44 | 39 | 40 |
| Hypertension (%) | 83 | 89 | 88 |
| Dyslipidemia (%) | 89 | 78 | 81 |
| LVEF (%) | 53±9.2 | 53±13 | 53±12 |
| History of CABG (%) | 23 | 23 | 23 |
| Active Smoking (%) | 27 | 19 | 21 |
| Aspirin Use (%) | 97 | 60 | 69 |
| ACE-inhibitor/ARB use (%) | 71 | 52 | 57 |
| Beta blocker Use (%) | 75 | 66 | 68 |
| Statin Use (%) | 78 | 71 | 72 |
| Total Cholesterol (mg/dL) | 172±41 | 161±50 | 164±48 |
| LDL (mg/dL) | 94±33 | 92±43 | 92±41 |
| HDL (mg/dL) | 43±13 | 40±15 | 40±14 |
| Triglyceride (mg/dL) | 187±168 | 170±109 | 175±127 |
| Presence of CAD ≥ 50% (%) | 100 | 69 | 76 |
| Acute MI (%) | 0.0 | 9.0 | 7.0 |
| Management Strategy | |||
| Medical Management Only (%) | 0 | 61 | 47 |
| PCI (%) | 100 | 33 | 49 |
| CABG (%) | 0 | 6 | 4 |
| High CXCL12 (%) | 35 | 34 | 34 |
Data presented as Mean±SD. CAD: coronary artery disease, LVEF: left ventricular ejection fraction, CABG: Coronary artery bypass surgery, PCI: Percutaneous Coronary Intervention, LDL: low density lipoprotein, HDL: high density lipoprotein, MI: myocardial infarction, High CXCL12 based on based discriminatory cutoff for the composite cardiovascular death/MI outcome.
Association of CXCL12 levels and baseline cardiovascular risk factors
Univariate comparison of cardiovascular risk factors and high/low CXCL12 levels based on best discriminatory cutoff for cardiovascular death/MI outcome in the pooled cohort revealed that those with higher CXCL12 levels were more likely to have left ventricular systolic dysfunction with ejection fraction < 45% (24% vs. 15%, p=0.003) but less likely to have dyslipidemia (74% vs. 84%, p<0.001) and less likely to be on statins (65% vs. 76%, p=0.002) at the time of enrollment. Moreover, those with higher CXCL12 were more likely to have higher serum creatinine > 1.5 mg/dl (25% vs. 6%, p<0.001), Table 2. In a logistic regression model adjusting for gender, statin use, serum creatinine > 1.5 mg/dl, CAD ≥ 50% stenosis, and LVEF < 0.45, independent predictors of high CXCL12 levels were high serum creatinine > 1.5 mg/dl (OR=5.6, p<0.001), LVEF < 0.45 (OR=2.02, p=0.001), and statin use (OR=0.65, p=0.02).
Table 2.
Chi-Square comparison of distribution of risk factors between patients with high vs. low CXCL12 levels based on best discriminatory cutoff for cardiovascular death/MI outcome
| Risk Factors | Discovery Cohort | Replication Cohort | Pooled Cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low CXCL12 |
High CXCL12 |
P value | Low CXCL12 |
High CXCL12 |
P value | Low CXCL12 |
High CXCL12 |
P value | |
| Age ≥65, % | 32 | 41 | 0.22 | 45 | 48 | 0.45 | 42 | 46 | 0.22 |
| Male Gender, % | 85 | 81 | 0.54 | 75 | 70 | 0.11 | 78 | 72 | 0.09 |
| African American Race, % | 20.3 | 20.5 | 0.97 | 19 | 27 | 0.02 | 20 | 24 | 0.19 |
| Diabetes Mellitus, % | 44 | 43 | 0.84 | 37 | 42 | 0.26 | 39 | 42 | 0.37 |
| Hypertension, % | 82 | 89 | 0.20 | 88 | 89 | 0.75 | 87 | 89 | 0.35 |
| Dyslipidemia, % | 91 | 89 | 0.73 | 83 | 68 | <0.001 | 84 | 74 | <0.001 |
| Active smoking, % | 26 | 28 | 0.76 | 20 | 17 | 0.43 | 21 | 20 | 0.63 |
| LVEF <0.45, % | 14 | 20 | 0.35 | 15 | 25 | 0.002 | 15 | 24 | 0.003 |
| ≥50% stenosis in any major epicardial vessel, % |
98 | 99 | 0.67 | 73 | 60 | 0.001 | 79 | 69 | 0.002 |
| Serum Creatinine>1.5 | 7 | 14 | 0.12 | 6 | 30 | <0.001 | 6 | 25 | <0.001 |
| Statin Use, % | 80 | 74 | 0.36 | 75 | 63 | 0.002 | 76 | 65 | 0.002 |
| Acute MI, % | -- | -- | -- | 9.2 | 8.8 | 0.89 | 7 | 6.7 | 0.87 |
Clinical and demographic predictors of adverse cardiovascular outcomes
In the discovery cohort, 65 (35%) patients, and in the replication cohort, 269 (34%) patients had elevated CXCL12 levels. Univariate predictors of cardiovascular death/MI in the pooled cohort were serum creatinine (p=0.01), diabetes mellitus (p=0.03), hypertension (p=0.05), LVEF (p<0.001), history of CABG (p=0.03), and high CXCL12 (p=1 × 10−6).
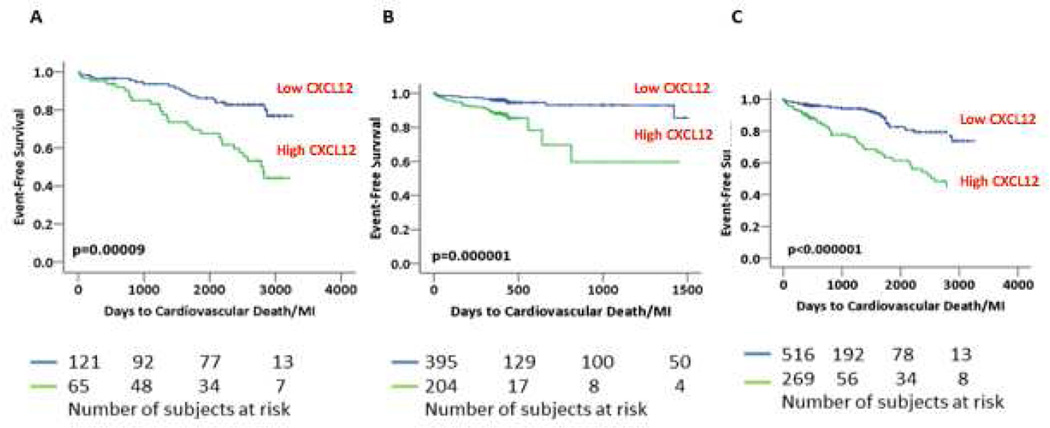
In multivariate models, adjusting for age, gender, diabetes, hypertension, active smoking, acute MI, serum creatinine, LVEF, history of CABG, statin use, aspirin use, presence of at least 50% stenosis in at least one major epicardial vessel, and LDL levels, high CXCL12 levels independently predicted risk of cardiovascular death/MI in both cohorts. Thus, in the discovery cohort, high CXCL12 level was associated with 6.24-fold increased risk of cardiovascular death/MI at follow-up (p=3.8 × 10−4). Similarly in the replication cohort, high CXCL12 levels predicted risk of cardiovascular death/MI (HR: 4.36, p=0.001), Table 3. In the pooled cohort, high CXCL12 was associated with an adjusted HR of 4.81 (p=1 × 10−6) for the primary endpoint, Figure 1. Other independent predictors of cardiovascular death/MI were left ventricular ejection fraction (LVEF, HR: 0.97, p=0.004), with diabetes (HR: 1.40, p=0.07) and hypertension (HR: 2.43, p=0.08) trending towards significance. Similarly, high CXCL12 was associated with increased risk of cardiovascular death (HR=5.70,p<10−6), all-cause death (HR=4.52, p=1 × 10−6), and all-cause death/MI (HR=3.02, p=1 × 10−6) independent of all aforementioned variables. Treatment strategy after cardiac catheterization was not a predictor of outcomes in either the discovery or the replication cohort. In the discovery cohort, CXCL12 level remained an independent predictor even after adjustment for serum C-reactive protein (CRP) levels (CRP levels were unavailable in the replication cohort). There was no correlation between CXCL12 and CRP levels (r=−0.04, p=0.51). When CXCL12 was analyzed as a continuous measure in relation to outcomes, similar findings were observed in the discovery and replication cohorts (data not shown).
Table 3.
Cox regression survival analysis of CXCL12 with adverse events
| ROC cut |
Discovery cohort Unadjusted HR (95% CI) |
Discovery cohort adjusted HR (95% CI) |
ROC cut |
Replication Cohort unadjusted HR (95% CI) |
Replication cohort adjusted HR (95% CI) |
Pooled cohort Unadjusted HR (95% CI) |
Pooled cohort adjusted HR (95% CI) |
|
|---|---|---|---|---|---|---|---|---|
| Outcome | pg/ml | High vs. Low CXCL12 |
High vs. Low CXCL12 |
pg/ml | High vs. Low CXCL12 | High vs. Low CXCL12 |
High vs. Low CXCL12 |
High vs. Low CXCL12 |
| Death | 1559 | 3.31 (1.56–6.98) | 4.63 (1.39–15.45) | 2679 | 4.92 (2.58–9.38) | 5.53 (2.54–12.03) | 3.66 (2.28–5.89) | 4.52 (2.48–8.24) |
| CV Death | 1734 | 3.14 (1.53–6.43) | 4.84 (1.52–15.35) | 2679 | 4.96 (2.42–10.14) | 6.10 (2.51–14.80) | 4.03 (2.44–6.66) | 5.70 (2.95–10.99) |
| Death/MI | 1661 | 3.18 (1.79–5.66) | 6.02 (2.47–14.67) | 2679 | 3.72 (2.08–6.63) | 3.44 (1.72–6.89) | 3.02 (2.06–4.44) | 3.75 (2.28–6.15) |
| CV Death/MI | 1734 | 3.01 (1.68–5.40) | 6.24 (2.61–14.91) | 2679 | 4.27 (2.30–7.91) | 4.36 (2.05–9.28) | 3.18 (2.13–4.76) | 4.81 (2.86–8.10) |
Models adjusted for age, gender, diabetes, hypertension, active smoking, acute MI on presentation, serum creatinine, LVEF, history of CABG, aspirin use, statin use, presence of at least 50% stenosis in one of major epicardial vessels, and LDL levels.
Figure 1.
Kaplan Meier Survival Analysis for high vs. low CXCL12 and cardiovascular death/MI. Panels A, B, and C represent survival curves in the discovery, replication, and pooled cohorts respectively. Numbers of subjects at risk are presented below each panel.
Sensitivity analysis to check for interaction between each of the aforementioned covariates and the predictive ability of CXCL12 with the cardiovascular death/MI outcome revealed that only history of diabetes had a significant interaction term with CXCL12 (p value for interaction=0.025). There was no significant interaction term between other covariates and CXCL12. When we further conducted a stratified analysis for presence of diabetes, we found that high CXCL12 was significantly predictive of future cardiovascular death/MI with greater risk in non-diabetics compared to diabetics (HR=10.79, p=0.000001 vs. HR=2.84, p=0.004 respectively).
Adding the binary CXCL12 variable to a baseline model containing the aforementioned covariates was associated with a significant improvement in C-statistic (AUC: 0.67 to 0.73, p=0.03). Detailed Cox regression analysis of each outcome measure is presented in Table 4. Further discrimination analysis revealed that the addition of the binary CXCL12 variable to the baseline model was associated with correct reclassification of 40% of events and 10.5% of non-events. Similarly for the outcome of cardiovascular death, the addition of the binary CXCL12 variable to the baseline model was associated with correct reclassification of 20.7% of events and 9% of non-events. Detailed discrimination analysis for other outcome measures are presented in Table 4.
Table 4.
Risk prediction metrics for CXCL12 high vs. low in the pooled cohort in relation to outcome.
| Pooled Cohort | C-statistic (95% CI) | Categorical NRI | Relative IDI (95% CI) |
|---|---|---|---|
| Cardiovascular death/MI | |||
| Baseline Model* | 0.67 (0.60–0.73) | - | - |
| Baseline Model + CXCL12 | 0.73 (0.67–0.78), p=0.03 | 0.23 | 0.22 (0.009–0.47) |
| Cardiovascular Death | |||
| Baseline Model* | 0.69 (0.62–0.77) | - | - |
| Baseline Model + CXCL12 | 0.75 (0.68–0.82), p=0.04 | 0.29 | 0.38 (0.079–0.64) |
| All-Cause Death/MI | |||
| Baseline Model* | 0.67 (0.61–0.73) | - | - |
| Baseline Model + CXCL12 | 0.72 (0.66–0.77), p=0.059 | 0.20 | 0.17 (0.005–0.34) |
| All-Cause Death | |||
| Baseline Model* | 0.69 (0.62–0.76) | - | - |
| Baseline Model + CXCL12 | 0.75 (0.68–0.81), p=0.04 | 0.30 | 0.31 (0.092–0.54) |
Baseline models contain: age, gender, diabetes, hypertension, acute MI on presentation, active smoking, statin use, LVEF, serum creatinine, and presence of CAD≥ 50%
Discussion
Herein we have described the prognostic implications of plasma CXCL12 levels in patients with CAD and have validated our results in an independent cohort. We have shown that plasma CXCL12 level is superior to traditional risk factors in predicting adverse cardiovascular outcomes and that it improves discrimination and risk classification. An elevated CXCL12 level was associated with a 5.7-fold increased risk of cardiovascular death and a 4.8-fold increased risk of cardiovascular death/MI in the pooled analysis after fully adjusting for all traditional risk factors. This corresponded to an average annual event rate of 7% for cardiovascular death and 10% for cardiovascular death/MI in those with high CXCL12 level compared to a 1% risk of cardiovascular death and 2% for cardiovascular death/MI in those with low CXCL12 levels, Figure 2.
Figure 2.
Annual risk of outcomes between groups with high vs. low baseline CXCL12 levels. Green bars represent those with high CXCL12 levels and the blue bars represent those with low CXCL12 levels.
CXCL12 is a cytokine of the CXC family involved in homing, mobilization, and differentiation of circulating progenitor cells in response to tissue ischemia.15–17 Binding of CXCL12 to its receptor CXCR4 activates several signaling pathways leading to a variety of biological responses including activation of Gi proteins which inhibits adenylyl cyclase and instead activates tyrosine kinase, phospholipase C-β (PLC-β)18, mitogen activated protein kinase (MAPK)19, and phosphoinbositide-3 kinase (PI3K)9 signaling pathways. Activation of these pathways contributes to chemotaxis, cell migration, and secretion of several matrix metalloproteinase (MMP’s) such as MMP-2 and MMP-9, which lead to migration of cells through the basement membrane.6 These pathways also stimulate secretion of vascular endothelial growth factor (VEGF), which induces angiogenesis.20
The pathophysiologic role of CXCL12 in relation to atherosclerosis has been a subject of controversy. While CXCL12 can promote regenerative/reparative angiogenesis and thus be potentially cardioprotective, it also promotes platelet aggregation by activation of the CXCR4 receptor.12, 13 CXCL12 is highly expressed in atherosclerotic plaques of human carotid arteries but not in normal vessels suggesting that CXCL12 may in fact be involved in formation of platelet-rich thrombus after plaque disruption, and CXCL12/CXCR4 inhibition may be a novel approach to treatment of atherosclerosis and acute coronary syndrome.21, 22 CXCL12 appears to be upregulated in platelet-rich thrombus as early as 30 minutes after vessel injury23 and enhanced platelet expression of CXCL12 in another study was associated with a 1.4-fold increased risk of having an acute coronary syndrome.8 Finally, CXCL12 expression is upregulated in the infarcted tissue and surrounding myocardium that also facilitates signaling and homing of progenitor cells.16, 17,5 Moreover, CXCL12 is secreted by hematopoietic stem cells and is involved in autocrine/paracrine regulation of their development and survival.24 In a rat model of ischemia/reperfusion injury, delivery of the CXCR4 gene vector was associated with an increase in infarct area, decrease in fractional shortening, as well as a simultaneous increase in both CXCL12 and tumor necrosis factor expression in myocardium.25 CXCR4 overexpression was also associated with increased influx of inflammatory cells and enhanced apoptosis of cardiomyocytes. In contrast to these findings, Damas and colleagues reported lower levels of plasma CXCL12 in 30 subjects with unstable angina compared to 30 subjects with stable CAD and the control group.26 They showed that CXCL12 might mediate anti-inflammatory and matrix stabilizing effects in unstable angina by inhibition of MMP-9, potentially promoting plaque stabilization.
Although CXCL12 has been extensively studied in animal models, the prognostic role of CXCL12 in humans, especially in those with CAD is not well defined. Fortunato and colleagues in a smaller study of 172 patients with acute MI and shorter follow-up found that high CXCL12 levels were associated with a 3.8-fold greater risk of adverse events including hospitalization.27 In the Framingham Offspring cohort of community participants, plasma CXCL12 levels were associated with incident heart failure, all-cause mortality, and cardiovascular mortality as observed in our high-risk cohort.28 Our study confirms the prognostic implications of high CXCL12 levels and extends these findings to patients with stable CAD. We have validated our findings in an independent cohort with relatively large number of patients and have shown that CXCL12 level not only is a predictor of adverse cardiovascular outcomes but also improves risk re-classification for this high-risk group of patients. We have also demonstrated similar findings with regards to outcomes of cardiovascular death, cardiovascular death/MI, all-cause death, and all-cause death/MI.
Consistent with previous reports, we also found that patients without left ventricular systolic dysfunction and those on statin therapy have lower CXCL12 levels.29,30, 31 We also found that chronic kidney disease and elevated serum creatinine levels were associated with higher plasma CXCL12 levels. Plasma CXCL12 levels were higher in our replication cohort compared to discovery cohort probably because of a higher prevalence of statin use and elevation of creatinine in the former group. When 20 discovery samples were re-analyzed with the replication cohort samples, we found no significant difference, confirming validity of the assay.
Interaction of CXCL12 with other CXCR4 ligands such as macrophage migration inhibitory factor (MIF) remains to be established. MIF is a cytokine involved in inflammatory pathogenesis of atherosclerosis and progression of atherosclerotic plaque.32, 33 Binding of MIF to CXCR4 leads to upregulation of adhesion molecules and chemokines, inflammatory cell recruitment, conversion of macrophages to foam cells, and regulation of smooth muscle cell proliferation and progression.32 Increased plasma MIF levels have also been shown to predict cardiovascular events in diabetics with CAD.34 The joint effect of increased MIF and CXCL12 on modulation of cardiovascular risk is currently unknown and requires further investigation.
Conclusion
Plasma CXCL12 level is a strong independent predictor of adverse cardiovascular outcomes in patients with CAD and helps to improve risk re-classification in these patients. Whether targeted therapies that modulate the CXCL12/CXCR4 pathway would reduce this risk requires further investigation. This association may not be generalizable to patients without CAD and patient with low-moderate risk of cardiovascular disease.
Highlights.
Increased levels of Plasma SDF-1 α is associated with increased risk of major cardiovascular outcomes including cardiovascular death, all-cause death, and cardiovascular death/MI in patients with stable coronary artery disease independent of traditional cardiovascular risk factors.
Plasma SDF-1 α level improves discrimination by a significant change in c-statistic and is associated with improved risk reclassification of cardiovascular death and cardiovascular death/MI when added to a baseline model of traditional cardiovascular risk factors.
These findings were first shown in a discovery cohort (N=186) and replicated in an independent cohort (N=599).
This study to our knowledge is the first study that examines the association of plasma SDF-1 α levels with major cardiovascular outcomes in patients with stable coronary artery disease.
Acknowledgments
We would like to thank the members of the Emory Biobank Team, the Emory Clinical Cardiovascular Research Institute (ECCRI), and the Atlanta Clinical and Translational Science Institute for recruitment of participants, compilation of data, and preparation of samples
Funding:
Funding for collection and management of samples was received from the Robert W. Woodruff Health Sciences Center Fund (Atlanta, GA), Emory Heart and Vascular Center (Atlanta, GA), Katz Family Foundation Preventive Cardiology Grant (Atlanta, GA) and NIH Grant UL1 RR025008 from the Clinical and Translational Science Award program NIH grant R01HL089650-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Nothing to disclose. No relationship with industry exists.
References
- 1.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 2.Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. Journal of molecular and cellular cardiology. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang G, Nakamura Y, Wang X, Hu Q, Suggs LJ, Zhang J. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit+ cell homing to the infarcted heart. Tissue engineering. 2007;13:2063–2071. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 4.Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, Bigalke B, Mueller I, Schumm M, Schaefer I, Seizer P, Kraemer BF, Siegel-Axel D, May AE, Lindemann S, Gawaz M. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117:206–215. doi: 10.1161/CIRCULATIONAHA.107.714691. [DOI] [PubMed] [Google Scholar]
- 5.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 6.Tang CH, Tan TW, Fu WM, Yang RS. Involvement of matrix metalloproteinase-9 in stromal cell-derived factor-1/CXCR4 pathway of lung cancer metastasis. Carcinogenesis. 2008;29:35–43. doi: 10.1093/carcin/bgm220. [DOI] [PubMed] [Google Scholar]
- 7.Stellos K, Ruf M, Sopova K, Kilias A, Rahmann A, Stamatelopoulos K, Jorbenadze R, Geisler T, Gawaz M, Bigalke B. Plasma levels of stromal cell-derived factor-1 in patients with coronary artery disease: effect of clinical presentation and cardiovascular risk factors. Atherosclerosis. 2011;219:913–916. doi: 10.1016/j.atherosclerosis.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Wurster T, Stellos K, Haap M, Seizer P, Geisler T, Otton J, Indermuehle A, Ishida M, Schuster A, Nagel E, Gawaz M, Bigalke B. Platelet expression of stromal-cell-derived factor-1 (SDF-1): an indicator for ACS? International journal of cardiology. 2013;164:111–115. doi: 10.1016/j.ijcard.2011.06.082. [DOI] [PubMed] [Google Scholar]
- 9.Vicente-Manzanares M, Rey M, Jones DR, Sancho D, Mellado M, Rodriguez-Frade JM, del Pozo MA, Yanez-Mo M, de Ana AM, Martinez AC, Merida I, Sanchez-Madrid F. Involvement of phosphatidylinositol 3-kinase in stromal cell-derived factor-1 alpha-induced lymphocyte polarization and chemotaxis. J Immunol. 1999;163:4001–4012. [PubMed] [Google Scholar]
- 10.Eapen DJ, Manocha P, Patel RS, Hammadah M, Veledar E, Wassel C, Nanjundappa RA, Sikora S, Malayter D, Wilson PW, Sperling L, Quyyumi AA, Epstein SE. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. Journal of the American College of Cardiology. 2013;62:329–337. doi: 10.1016/j.jacc.2013.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 12.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 13.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 14.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Annals of internal medicine. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 16.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 17.Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG, Rafii S. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Annals of the New York Academy of Sciences. 2001;938:36–45. doi: 10.1111/j.1749-6632.2001.tb03572.x. discussion 45-7. [DOI] [PubMed] [Google Scholar]
- 18.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochimica et biophysica acta. 2007;1768:952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. The Journal of biological chemistry. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 20.Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, Shim H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochemical and biophysical research communications. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei D, Wang G, Tang C, Qiu J, Zhao J, Gregersen H, Deng L. Upregulation of SDF-1 is associated with atherosclerosis lesions induced by LDL concentration polarization. Annals of biomedical engineering. 2012;40:1018–1027. doi: 10.1007/s10439-011-0486-z. [DOI] [PubMed] [Google Scholar]
- 22.Abi-Younes S, Sauty A, Mach F, Sukhova GK, Libby P, Luster AD. The stromal cell-derived factor-1 chemokine is a potent platelet agonist highly expressed in atherosclerotic plaques. Circulation research. 2000;86:131–138. doi: 10.1161/01.res.86.2.131. [DOI] [PubMed] [Google Scholar]
- 23.Massberg S, Konrad I, Schurzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, Schiemann M, Kennerknecht E, Sauer S, Schulz C, Kerstan S, Rudelius M, Seidl S, Sorge F, Langer H, Peluso M, Goyal P, Vestweber D, Emambokus NR, Busch DH, Frampton J, Gawaz M. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. The Journal of experimental medicine. 2006;203:1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. Journal of molecular histology. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Chemaly E, Liang L, Kho C, Lee A, Park J, Altman P, Schecter AD, Hajjar RJ, Tarzami ST. Effects of CXCR4 gene transfer on cardiac function after ischemia-reperfusion injury. The American journal of pathology. 2010;176:1705–1715. doi: 10.2353/ajpath.2010.090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damas JK, Waehre T, Yndestad A, Ueland T, Muller F, Eiken HG, Holm AM, Halvorsen B, Froland SS, Gullestad L, Aukrust P. Stromal cell-derived factor-1alpha in unstable angina: potential antiinflammatory and matrix-stabilizing effects. Circulation. 2002;106:36–42. doi: 10.1161/01.cir.0000020001.09990.90. [DOI] [PubMed] [Google Scholar]
- 27.Fortunato O, Spinetti G, Specchia C, Cangiano E, Valgimigli M, Madeddu P. Migratory activity of circulating progenitor cells and serum SDF-1alpha predict adverse events in patients with myocardial infarction. Cardiovascular research. 2013 doi: 10.1093/cvr/cvt153. [DOI] [PubMed] [Google Scholar]
- 28.Subramanian S, Liu C, Aviv A, Ho JE, Courchesne P, Muntendam P, Larson MG, Cheng S, Wang TJ, Mehta NN, Levy D. Stromal cell-derived factor 1 as a biomarker of heart failure and mortality risk. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2100–2105. doi: 10.1161/ATVBAHA.114.303579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stellos K, Bigalke B, Langer H, Geisler T, Schad A, Kogel A, Pfaff F, Stakos D, Seizer P, Muller I, Htun P, Lindemann S, Gawaz M. Expression of stromal-cell-derived factor-1 on circulating platelets is increased in patients with acute coronary syndrome and correlates with the number of CD34+ progenitor cells. European heart journal. 2009;30:584–593. doi: 10.1093/eurheartj/ehn566. [DOI] [PubMed] [Google Scholar]
- 30.Camnitz W, Burdick MD, Strieter RM, Mehrad B, Keeley EC. Dose-dependent Effect of Statin Therapy on Circulating CXCL12 Levels in Patients with Hyperlipidemia. Clinical and translational medicine. 2012;1:23. doi: 10.1186/2001-1326-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satoh K, Fukumoto Y, Nakano M, Sugimura K, Nawata J, Demachi J, Karibe A, Kagaya Y, Ishii N, Sugamura K, Shimokawa H. Statin ameliorates hypoxia-induced pulmonary hypertension associated with down-regulated stromal cell-derived factor-1. Cardiovascular research. 2009;81:226–234. doi: 10.1093/cvr/cvn244. [DOI] [PubMed] [Google Scholar]
- 32.Zernecke A, Bernhagen J, Weber C. Macrophage migration inhibitory factor in cardiovascular disease. Circulation. 2008;117:1594–1602. doi: 10.1161/CIRCULATIONAHA.107.729125. [DOI] [PubMed] [Google Scholar]
- 33.Burger-Kentischer A, Goebel H, Seiler R, Fraedrich G, Schaefer HE, Dimmeler S, Kleemann R, Bernhagen J, Ihling C. Expression of macrophage migration inhibitory factor in different stages of human atherosclerosis. Circulation. 2002;105:1561–1566. doi: 10.1161/01.cir.0000012942.49244.82. [DOI] [PubMed] [Google Scholar]
- 34.Makino A, Nakamura T, Hirano M, Kitta Y, Sano K, Kobayashi T, Fujioka D, Saito Y, Watanabe K, Watanabe Y, Kawabata K, Obata JE, Kugiyama K. High plasma levels of macrophage migration inhibitory factor are associated with adverse long-term outcome in patients with stable coronary artery disease and impaired glucose tolerance or type 2 diabetes mellitus. Atherosclerosis. 2010;213:573–578. doi: 10.1016/j.atherosclerosis.2010.09.004. [DOI] [PubMed] [Google Scholar]