Abstract
The NaKCl cotransporter NKCC1 facilitates intraneuronal chloride accumulation in the developing brain. Bumetanide, a clinically available diuretic, inhibits this chloride transporter, and augments the antiepileptic effects of phenobarbital in neonatal rodents. In a neonatal cerebral hypoxia-ischemia (HI) model, elicited by right carotid ligation, followed by 90 min 8% O2 exposure in 7-day-old(P7) rats, phenobarbital(PB) increases the neuroprotective efficacy of hypothermia. We evaluated whether bumetanide influenced the neuroprotective efficacy of combination treatment with PB and hypothermia(HT). P7 rats underwent HI lesioning; 15 min later, all received PB (30 mg/kg). 10 min later, half received bumetanide (10 mg/kg, PB-HT+BUM) and half received saline (PB-HT+SAL). One hour after HI, all were cooled (30°C, 3h). Contralateral forepaw sensorimotor function and brain damage were evaluated 1 to 4 weeks later. Forepaw functional measures were close to normal in the PB-HT+BUM group, while deficits persisted in PB-HT+SAL controls; there were corresponding reductions in right cerebral hemisphere damage (at P35, % damage: PB-HT+BUM, 21±16 versus 38±20 in controls). These results provide evidence that NKCC1 inhibition amplifies phenobarbital bioactivity in the immature brain, and suggest that co-administration of phenobarbital and bumetanide may represent a clinically feasible therapy to augment the neuroprotective efficacy of therapeutic hypothermia in asphyxiated neonates.
Keywords: Cerebral hypoxia-ischemia, Bumetanide, Phenobarbital, Hypothermia, Anticonvulsant drug
Introduction
In neonates with hypoxic-ischemic encephalopathy, therapeutic hypothermia (initiated within the first 6 hours of life) is associated with reductions in death and neurological impairment at 18 months (1). However, over 40% of treated infants have poor neurodevelopmental outcomes, and there is an urgent need to identify interventions that can effectively supplement hypothermia.
In experimental models of neonatal hypoxic-ischemic (HI) brain injury, a broad range of therapeutic agents including phenobarbital augment hypothermic neuroprotection (2, 3). In a well-characterized model of neonatal HI brain injury, elicited by unilateral carotid artery ligation, followed by 90 min 8% oxygen exposure, in 7 day old (P7) rats, early post-HI treatment with phenobarbital improved the neuroprotective efficacy of delayed onset brief moderate hypothermia. This combination therapy resulted in sustained improvements in sensorimotor function and a greater than 50% reduction in brain damage, in comparison with saline-injected hypothermia-treated controls (3).
Phenobarbital, a gamma-amino-butyric acid (GABA) agonist, is the antiepileptic drug used most frequently to treat neonatal seizures, although its efficacy is limited (4). Recent studies have provided important insights about the mechanisms underlying its limited anticonvulsant efficacy in neonates and have suggested pharmacological strategies to overcome them (5–7). In mature neurons, gamma-amino-butyric acid (GABA) triggers membrane hyperpolarization and neuronal inhibition due to the passive influx of chloride down its electrochemical gradient. In neonatal cortex (rodent and human), the developmentally regulated chloride transporter NKCC1 is expressed, and it facilitates intraneuronal chloride accumulation. Since immature neurons have high chloride concentrations, GABA triggers chloride efflux and membrane depolarization. Moreover, NKCC1 expression may be up-regulated both by neonatal hypoxia-ischemia (8) and by seizures (7). Bumetanide, a clinically available loop diuretic, inhibits NKCC1 and augments the antiepileptic effects of phenobarbital in a neonatal rat seizure model (6). A pilot clinical study of bumetanide as add-on treatment after phenobarbital administration for newborn seizures is underway (Clinical trials.gov, NCT00830531).
This study evaluated the impact of bumetanide on the neuroprotective efficacy of combination therapy with phenobarbital and hypothermia in the neonatal HI model. We found that bumetanide improved the neuroprotective efficacy of treatment with phenobarbital and hypothermia, and that hypothermia was essential to achieve optimal benefit from combination drug therapy.
Methods
Surgery
Seven-day-old (P7) Sprague-Dawley rats (11–12/experiment, gender-balanced; Charles River, Portage, MI), anesthetized with isofluorane, underwent right common carotid artery ligation; 90 min later, they were exposed to 8% oxygen (balance nitrogen) for 90 min (3, 9). This lesioning procedure typically results in 30–40% tissue loss of the right cerebral hemisphere, when compared with the weight or volume of the left cerebral hemisphere, one-five weeks later. Strengths of the model include relative simplicity and reproducibility, low mortality, and ability to integrate functional and pathological outcome measures. Weaknesses include intra- and inter-experiment variation in severity of damage, the limited behavioral repertoire of rodents, and inability to replicate important elements of neonatal intensive care unit practice. All procedures were approved by the University of Michigan Committee on Use and Care of Animals, and efforts were made to minimize the numbers of animals used.
Temperature management
In P7 rats, ambient temperature determines body and brain temperature (10). During hypoxia, animals remained in acrylic containers that were partially submerged in a water-bath (36.5°C, 90 min). Then they were placed in recovery incubators (36.5°C, 60 min). One hour later they were moved to circulating air incubators set at 30°C (“hypothermia”; 3h); the incubator was partitioned to prevent huddling. This delayed cooling intervention, alone, confers no benefit on sensorimotor or histology outcomes (9).
In experiments in which the impact of hypothermia was evaluated, controls remained in the recovery incubators (36.5°C, 3h; described in the protocol as “normothermia”). All animals were then returned to the dams. Rectal temperatures were measured intermittently (YSI thermometer 43T with probe 554; Yellow Springs, OH) before surgery, at the end of hypoxia, 15 min later, 60 min later (just before hypothermia), 15 and 30 min after cooling began, and at the end of hypothermia; in two experiments, temperatures were also measured 60 min after they were returned to the dams.
Drugs
Phenobarbital (PB), 40 mg/kg, together with delayed onset hypothermia, attenuates brain damage in this model (3). The dose was reduced to 30 mg/kg in this study; this dose is commonly used to treat neonatal seizures (4). Animals received intra-peritoneal injections of phenobarbital (30 mg/kg) at 15 min after the end of hypoxia, and a second injection (of bumetanide or saline) 10 min later. Since bumetanide has a very short half-life in rats, a high dose (10 mg/kg) was selected (11), and preliminary experiments (not shown) demonstrated that this dose of bumetanide, itself, in combination with hypothermia (3h) had no effects on survival or severity of brain damage. In this study we re-evaluated bumetanide plus hypothermia, and also evaluated a lower dose (2.5 mg/kg) in combination with PB and hypothermia. Drugs, purchased from Sigma Chemicals, St. Louis MO., were dissolved in saline.
Outcome measures
All experiments evaluated survival and weight gain. In initial experiments, brain damage was evaluated one week after lesioning. Subsequently, one combination treatment protocol was replicated and sensorimotor function and brain damage were evaluated up to four weeks later.
Brain damage
To quantify damage severity, coronal 20 micron brain sections were stained with cresyl violet; bilateral cross-sectional areas of striatum, neocortex, hippocampus, and cerebral hemisphere were measured on regularly spaced sections from the level of the anterior genu to the posterior genu of the corpus callosum, captured, and analyzed in ImageJ (http://rsbweb.nih.gov/ij/) using the dot-grid method. Bilateral volumes were estimated by multiplying the sum of areas by the distance between sections.
In experiments that evaluated P35 outcomes, neuropathology was also scored (range: 0–4) in 7 brain regions, by an observer unaware of treatment group assignment (FSS), as previously described (3).
Sensorimotor testing
Bilateral vibrissae-stimulated forepaw placement was tested (3); the number of successful placements in10 trials/side was recorded. In lesioned animals, contralateral deficits in forepaw placement are detectable at P14. One point was assigned for full forepaw extension; quality and speed of movement were not scored. Animals were tested at weekly intervals from P14–P35.
Forepaw grip strength (maximal force applied) was measured using a Grip strength meter (Columbus Instruments, Columbus, OH) on P21, P28, and P35. Three measurements were obtained for each forepaw; mean values/side and ratios of left/right forepaw strength were calculated.
Study design
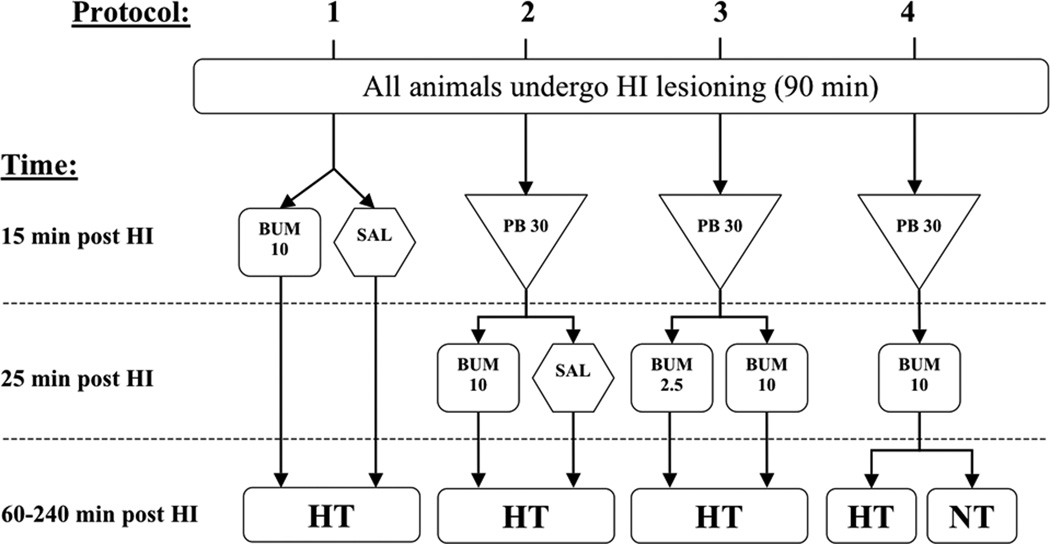
All animals underwent the same lesioning procedure; each experiment included equal numbers of animals of each gender in treatment and control groups. Initial studies (Protocols 1–4)(see Figure 1) evaluated brain damage on P14; then, the protocol to evaluate the impact of addition of bumetanide to combination treatment with phenobarbital and hypothermia was replicated, and sensorimotor and pathology outcomes were evaluated up to P35 (Protocol 5).
Figure 1. Summary of four treatment protocols.
This graphic outlines all components of the four treatment protocols studied. In all experiments, all animals (P7) underwent the same hypoxic-ischemic (HI) lesioning procedure (see Methods), and all treatments were administered after the end of hypoxia exposure [injections at 15 or 25 min later, and hypothermia (HT), beginning at 60 min later]. Only one phenobarbital (PB) dosage was included (30 mg/kg; PB 30). Two bumetanide (BUM) doses were studied, 10 mg/kg (BUM 10) and 2.5 mg/kg (BUM 2.5). Additional abbreviations: SAL = physiological saline; NT = normothermia.
Protocol 1 evaluated the impact of bumetanide in combination with delayed onset brief moderate hypothermia. Half received i.p. injections of bumetanide (10 mg/kg) and ½ received saline injections and all animals underwent the same hypothermia treatment (n=17–18/group).
Protocol 2 evaluated the impact of bumetanide on the neuroprotective efficacy of combined treatment with phenobarbital and hypothermia. All animals received phenobarbital; ½ received bumetanide (10 mg/kg) and ½ received saline injections; all underwent hypothermia (n=24/group).
Protocol 3 compared the two doses of bumetanide, 2.5 mg/kg and 10 mg/kg, on the neuroprotective efficacy of combined treatment with phenobarbital and hypothermia. All animals received phenobarbital; ½ then received bumetanide (10 mg/kg) and ½ received bumetanide (2.5 mg/kg) injections; and all underwent hypothermia (n=11/group).
Protocol 4 evaluated whether hypothermia contributed to the neuroprotection conferred by combination treatment with phenobarbital and bumetanide. All animals received phenobarbital and bumetanide; ½ underwent hypothermia and ½ remained in a “normothermia” (36.5°C) incubator for 3h (n=24/group).
Protocol 5 replicated Protocol 2 (all animals received phenobarbital; ½ received bumetanide and ½ received saline injections; all underwent hypothermia), and included sensorimotor testing up to P35 and pathology assessment (n=18/group).
Data analysis
Serial temperature measurements for each protocol were compared with repeated measure ANOVA. Percent (%) right cerebral hemisphere brain damage values on P14 and P35 were calculated from bilateral volume measurements with the formula [100 * (left − right)/left], and group values were compared with non-parametric Mann-Whitney tests. Serial vibrissae scores, and bilateral grip strength measurements were compared with ANOVA; post-hoc Bonferroni multiple comparison tests were applied to compare values at each age. Regional volumes and regional % Damage values on P35 were compared by 2 way ANOVA and post-hoc t-tests. Pathology scores on P35 were compared with Mann-Whitney tests. Linear regression modeling was applied to evaluate the relationship between pathology scores and right cerebral hemisphere % Damage.
Results
Physiological measures
Among animals allocated to Protocols 1–4, 152/154 survived until P14; in Protocol 5, 35/36 animals survived until P35. In the first day after lesioning bumetanide-treated animals gained less weight than controls (0.01±1 vs. 0.85± 0.9 grams), but weights did not differ between bumetanide and saline-treated animals at P14 or P35.
Table 1 summarizes sequential temperature measurements for all protocols. In the first two sets of experiments that evaluated combination treatment with bumetanide and hypothermia (Protocols 1 and 2), during hypothermia mean body temperatures were slightly lower in bumetanide-treated than in saline control groups (range: −0.3 to −0.9°C, p < 0.05, repeated-measures ANOVA). In the third set of experiments that evaluated combination treatment with two doses of bumetanide (Protocol 3), temperatures did not differ. In the fourth set of experiments (Protocol 4), the only variable was post-HI temperature management, and mean temperatures during this intervention differed substantially (range: −4.4 to −6°C, p < 0.001, ANOVA) between groups. Of note, when Protocol 2 was replicated to assess late outcomes (Protocol 5), there were no significant treatment-related temperature differences. In two Protocol 5 experiments, measurements were also obtained 60 min after the end of cooling, when pups were recovering with their dams, and their temperatures did not differ.
Table 1.
Sequential temperature measurementsa
| Time-points | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Protocol/Groupb | N | Pre- surgery |
End of HI | 15 min post-HI |
60 min post-HI |
75 min post-HI |
90 min post-HI |
240 min post-HI |
| Protocol 1 * | ||||||||
| BUM10+HT | 17 | 33.0 ±1.3 | 35.1 ±0.5 | 35.6 ±0.5 | 35.1 ±0.7 | 29.7 ±1.1 | 29.1 ±1 | 31.1 ±1.1 |
| SAL+HT | 18 | 33.0 ±1.4 | 35.0 ±0.7 | 35.6 ±0.4 | 35.7±0.5 | 30.4 ±0.7 | 30.0± 0.7 | 31.4 ±0.9 |
| Protocol 2 * | ||||||||
| PB+BUM10+HT | 24 | 33.6 ±1.1 | 35.2 ±0.6 | 35.3 ±0.5 | 35.3 ±0.5 | 30.8 ±0.8 | 30.4 ±0.8 | 30.6 ±0.7 |
| PB+SAL+HT | 24 | 33.7 ±1.0 | 35.2 ±0.9 | 35.3 ±0.6 | 35.6 ±0.5 | 31.1 ±0.6 | 30.9 ±0.6 | 31.1 ±0.8 |
| Protocol 3† | ||||||||
| PB+BUM10+HT | 11 | 33.5 ±0.6 | 34.8 ±0.6 | 35.3 ±0.6 | 34.7 ±0.8 | 30.2 ±1.0 | 30.0 ±0.6 | 30.6 ±0.6 |
| PB+BUM2.5+HT | 11 | 33.6 ±0.9 | 34.9 ±0.7 | 35.1 ±0.5 | 35.0 ±0.7 | 30.8 ±0.8 | 30.4 ±0.6 | 31.1 ±1.1 |
| Protocol 4 ** | ||||||||
| PB+BUM10+HT | 24 | 33.6 ±0.6 | 35.5 ±0.7 | 35.3 ±0.6 | 34.7 ±0.9 | 29.8 ±0.5 | 29.3 ±0.6 | 29.3 ±1.0 |
| PB+BUM10+NT | 24 | 33.5 ±0.7 | 35.5 ±0.6 | 35.3 ±0.6 | 34.8 ±0.8 | 34.2 ±0.8 | 34.1 ±1.0 | 35.3 ±0.4 |
| Protocol 5 † | ||||||||
| PB+BUM10+HT | 18 | 35.5 ±0.5 | 34.9 ±0.7 | 35.1 ±0.6 | 35.3 ±0.6 | 30.3 ±0.9 | 29.6 ±0.7 | 29.4 ±0.8 |
| PB+SAL+HT | 17 | 34.5 ±0.6 | 35.0 ±0.7 | 35.4 ±0.4 | 35.5 ±0.6 | 30.5 ±0.8 | 30.0 ±0.8 | 29.8 ±1.0 |
HI: hypoxia-ischemia; SAL: saline; BUM: bumetanide; PB: phenobarbital; HT: hypothermia; NT: normothermia.
: temperatures (°C) are expressed as mean ±SD.
: All animals underwent HI lesioning (see Methods); 15 min after the end of HI all received injections; when indicated, a second injection was administered 10 min later. In Protocols 2–5, all animals received PB, 30 mg/kg. Except in Protocol 4, which included an NT control group, all animals underwent HT (30°C, for 3h, beginning at 1h after HI); time-point 4 corresponds with the beginning of HT and time-point 7 is at the end of HT.
: p<0.05;
:p<0.0001,
: p=NS, repeated measures ANOVA.
Brain damage on P14
Figure 2 summarizes pathology outcomes from Protocols 1–4 on P14. Right cerebral hemisphere damage did not differ between animals that received injections of bumetanide (10 mg/kg) or saline and then underwent the brief hypothermia treatment (Protocol 1, Fig. 2.A); in both groups, the mean % right cerebral hemisphere damage was 34%, which was within the range expected after 90 min HI. Combination treatment with phenobarbital (30 mg/kg), bumetanide (10 mg/kg), and hypothermia resulted in attenuation of right cerebral hemisphere damage (18±16%, compared with 27±17% in animals that received saline instead of bumetanide, p<0.04, Mann-Whitney test) (Protocol 2, Fig.2.B). In the next group of experiments (Protocol 3, Fig. 2.C), damage was again attenuated by combination treatment with phenobarbital, bumetanide (10 mg/kg) and hypothermia; in contrast, treatment effects were lost with a lower dose of bumetanide (2.5 mg/kg) (p<0.01, Mann-Whitney). To determine if hypothermia contributed to neuroprotection conferred by the combination of phenobarbital and bumetanide, in Protocol 4 all animals were treated with phenobarbital and bumetanide and half underwent hypothermia (N=24/group; Fig. 2.D). Right cerebral hemisphere damage was lower in the hypothermia-treated group (15±17% vs. 27±20%, p < 0.04, Mann-Whitney). Note that results of the three groups of experiments that included a treatment arm that combined phenobarbital, bumetanide (10 mg/kg), and hypothermia, right cerebral hemisphere damage was similar (18±16%, 14±9%, and 15±17%).
Figure 2. Comparison of right cerebral hemisphere brain damage on P14.
All animals were lesioned on P7 (see Methods and Figure 1) and brain damage was measured on P14. % Damage was calculated from bilateral cerebral hemisphere measurements with the formula: 100*(Left-Right/Left); group data were compared with non-parametric Mann-Whitney tests. Results are presented as box and whisker plots (boxes extend from the 25th to 75th percentiles, and whiskers from 10th to 90th percentiles); horizontal bars, within each box, represent group medians. Panel A illustrates similar outcomes in bumetanide (BUM) and saline (SAL)-treated animals that also underwent hypothermia (HT). Panel B illustrates that in animals that received phenobarbital (PB), addition of BUM, compared with SAL, prior to hypothermia (HT), the severity of right cerebral hemisphere damage was reduced (*: p<0.04). Panel C compares the effects of two doses of BUM (2.5 mg/kg and 10 mg/kg), in groups that both were treated with PB+HT and illustrates that only the higher dose of BUM conferred neuroprotection (†: p <0.01). Panel D compares outcomes in animals that all received the same drug treatments (PB 30 mg/kg and BUM 10 mg/kg), followed either by HT or normothermia (see Methods). Brain damage was lower in the HT group (*: p < 0.04).
Sensorimotor function and pathology on P35
To determine if neuroprotection conferred by combination treatment was sustained, Protocol 2 was replicated. Animals were lesioned on P7, all received phenobarbital (30 mg/kg); ½ received bumetanide and ½ received saline injections, and all underwent hypothermia. Sensorimotor function was re-evaluated weekly up to P35, and then brain damage was assessed (n=17–18/group).
Figure 3.A summarizes results of left vibrissae-stimulated forepaw placement scores, assessed weekly. Right forepaw placement scores were all consistently normal (10/10) (not shown). In animals treated with phenobarbital, bumetanide, and hypothermia, mean left forepaw scores ranged from 9.5–10/10; in controls, mean scores remained at 5.3–5.5/10 from P14–P28, and improved modestly at P35 to 7.2/10. Performance differed markedly between groups at each age (p<0.001, ANOVA; p<0.01, Bonferroni post-hoc t-tests comparing scores at each age).
Figure 3. Measures of contralateral forepaw sensorimotor function.
Results of two measures of sensorimotor function in animals that were lesioned on P7, treated with phenobarbital (PB, 30 mg/kg), and bumetanide (BUM) or saline (SAL), and underwent the same hypothermia (HT) treatment. Sensorimotor function was re-evaluated weekly up to P35 (n=17–18/group).
Panel A. summarizes results of left (“Contralateral”) forepaw placement scores (10 trials; normal=10/10). In animals treated with PB+BUM+HT, mean scores ranged from 9.5–10/10. In SAL-controls, there were persistent deficits (*: p < 0.001, ANOVA; †:p<0.01, Bonferroni post-hoc t-tests comparing scores at each age). All right forepaw scores were normal (not shown).
Panel B. summarizes bilateral grip strength measurements. Grip strength increased in both groups; right forepaw grip strength did not differ between groups. In the controls, left forepaw strength was reduced compared to BUM-treated group at each age (* p<0.001, ANOVA; †: p<0.01; ‡: p<0.001, Bonferroni post-hoc tests comparing left-forepaw values at each age).
Figure 3.B summarizes results of sequential bilateral grip strength measurements. Grip strength increased in both groups from P21 to P35. Right forepaw grip strength was the same in both groups. In the saline-treated controls, left forepaw strength was reduced compared to the bumetanide-treated group at each age (ANOVA, p<0.001). Bilateral (left/right) forepaw grip strength ratios remained close to 1 in the bumetanide-treated group (0.94±0.13, 0.97±0.18, and 0.97±0.18 at P21, P28 and P35 respectively); in the controls, with persistent left forepaw deficits, corresponding ratios were 0.6±0.12, 0.6±0.14 and 0.53±0.1.
Figure 4 presents illustrative P35 histopathology; 4A–C demonstrate a severe lesion with marked right hemisphere atrophy and cortical infarction (from an animal in the PB-HT+SAL group), and D-F demonstrate a milder lesion with right striatal and hippocampal atrophy (from an animal in the PB-HT+BUM group).
Figure 4. Histopathology.
These cresyl-violet stained coronal brain sections illustrate features of histopathology on P35 at corresponding anatomic levels at mid-striatum (A, D) and hippocampus (B–C, E–F). In A–C (from an animal treated with PB-HT+SAL), there is marked right hemisphere atrophy and cortical infarction (arrowheads). In D–F (from an animal treated with PB-HT+BUM), there is right hemisphere atrophy but no infarction. Scale bar: 1 mm
Table 2 compares regional volume measurements and %Damage severity on P35. Left cerebral hemisphere volumes did not differ; right cerebral hemisphere volumes were larger in the bumetanide-treated group and mean % Damage was lower (21±16 vs. 39± 20%, p<0.01, Mann-Whitney test). Trends were similar across brain regions (ANOVA; p<0.0001). Figure 5.A compares the pathology scores in both groups. Although there is a broad range of scores in both groups, median scores are markedly lower in the PB-HT+BUM than in PB-HT+SAL groups (p<0.001, Mann-Whitney test). Figure 5.B evaluates the relationship between pathology scores and right cerebral hemisphere % Damage in all brain samples, and demonstrates that there is a close correlation between both measures of brain injury (r2=0.88; p<0.0001).
Table 2.
Bumetanide augments neuroprotection: Regional volumes and % Damage
| Regional volumes (mm3)* | % Damage** | ||
|---|---|---|---|
| Left | Right | ||
| Cerebral Hemisphere | |||
| PB-HT+BUM | 538±52 | 426±98 * | 21±16 * |
| PB-HT+SAL | 506±50 | 312±112 | 39±20 |
| Cortex | |||
| PB-HT+BUM | 220±20 | 174±50 † | 21±21 † |
| PB-HT+SAL | 206±18 | 116±64 | 43±27 |
| Striatum | |||
| PB-HT+BUM | 76±8 | 56±16 † | 28±8 † |
| PB-HT+SAL | 68±16 | 40±14 | 39±17 |
| Dorsal Hippocampus | |||
| PB-HT+BUM | 28±4 | 24±6† | 16±14† |
| PB-HT+SAL | 26±8 | 16±6 | 36±24 |
| Other regions | |||
| PB-HT+BUM | 212±24 | 174±2† | 19±13† |
| PB-HT+SAL | 194±48 | 132±44 | 29±15 |
PB: phenobarbital; HT: hypothermia; BUM: bumetanide; SAL: physiological saline Animals were lesioned on P7 (N=17–18/group); all received PB, 30 mg/kg; 1/2 received BUM, 10 mg/kg (PB-HT+BUM), and 1/2 received SAL injections (PB-HT+SAL); all underwent HT (3h). Tissue damage was assessed on P35 (see Methods). Regional % Damage was calculated from bilateral regional volume measurements with the formula: 100*(Left-Right/Left). Regional volumes and %Damage values were compared with ANOVA and post-hoc t-tests.
: p<0.01 Mann-Whitney, compared to controls.
: p<0.0001, ANOVA
: p<0.0001, 2-way ANOVA factoring treatment and region; p<0.05 post-hoc t-test for PB-HT+BUM treatment effect within each region.
Figure 5. Brain injury measures on P35.
A. Comparison of histopathology scores on P35 in animals that were lesioned on P7, treated with phenobarbital (PB, 30 mg/kg), and bumetanide (BUM) or saline (SAL), and underwent the same hypothermia (HT) treatment (n=17–18/group). Horizontal bars are median scores; boxes represent 25th and 75th percentiles, and whiskers are minimum and maximum values (*: p=0.001, Mann Whitney test). B. Illustrates the strong relationship between pathology scores and % right cerebral hemisphere damage values and includes data from both groups (open squares are values from PB-HT+BUM group and closed triangles are from PB-HT+SAL group; r2=0.88, p<0.0001)
In summary, in bumetanide-phenobarbital-hypothermia treated animals, sensorimotor function was preserved, and mean right cerebral hemisphere damage was less than 20%. There were no gender differences in treatment effects.
Discussion
Combined treatment with phenobarbital, bumetanide, and brief moderate hypothermia resulted in sustained improvements in sensorimotor function and reduced brain damage in this neonatal hypoxic-ischemic brain injury model. Although we did not perform a detailed dose-response study, the results demonstrated that the beneficial effects of bumetanide were dose-dependent. Of note, in the phenobarbital-saline-hypothermia control group, the phenobarbital dose administered to both groups was 25% lower dose than the dose that was administered in the prior study in which phenobarbital, in combination with hypothermia, substantially reduced brain damage (3).
Bumetanide enhances the anticonvulsant efficacy of phenobarbital in neonatal rodent brain (6), and the timing of drug administration in our protocols suggests that treatment may have limited post-ischemic seizures. We could not directly evaluate seizure activity because of the technical challenges inherent in performing and analyzing electroencephalograms in P7 rats. The issue of whether or not neonatal seizures amplify ischemic brain injury has been controversial and difficult to address both experimentally and clinically (12–14). In the fetal asphyxia sheep model which provided a foundation for clinical trials of neonatal hypothermia, hypothermia lost its protective efficacy if initiation of cooling was delayed until after seizure onset (15).
In the first group of experiments that evaluated combination treatment with PB, bumetanide, and hypothermia (Protocol 2), during hypothermia mean body temperatures were slightly lower (range: −0.3 to 0.9°C) in the bumetanide than in saline control group; temperatures were the same 90 min after the end of cooling. When the protocol was replicated to assess late outcomes (Protocol 5), there were no temperature differences between groups, and degree of hypothermia could not account for sustained neuroprotection.
NKCC1 activation may contribute to ischemic brain injury and bumetanide can attenuate ischemic injury by diverse mechanisms, including effects on cerebral vessels, astrocytes and neurons (8, 16, 17). Although bumetanide may have modest intrinsic neuroprotective properties, we found no attenuation of injury in animals treated with bumetanide in combination with hypothermia.
One of the important considerations in evaluating the translational potential of these results is whether these treatments are safe. Although both drugs have been used for many years to treat neonates, there is experimental evidence that both phenobarbital and bumetanide may have adverse effects on brain development. In rodents, several antiepileptic drugs, including phenobarbital, and anesthetics (i.e. agents that suppress synaptic activity) cause dose-dependent apoptotic neurodegeneration in the developing brain (18). The relevance of these findings to human neonates remains uncertain. Of interest in the context of this study, hypothermia may suppress this mechanism of drug-induced neuronal damage (19). There is also recent evidence that chronic bumetanide administration during the pre- and early post-natal period can have adverse effects on brain development and disrupt cortical excitatory synapse formation in mice (20). The authors of this study raised concerns regarding risks of treatment of neonates with bumetanide. Yet, it must be emphasized that deleterious effects were noted only with chronic fetal and early post-natal NKCC1 inhibition. Whether these findings are relevant to acute bumetanide treatment of human infants or in the setting of heightened NKCC1 expression, as occurs with hypoxia-ischemia, are uncertain. Moreover, the potential risks of chronic phenobarbital and/or bumetanide therapy are likely to be substantially outweighed, if brief treatment confers neuroprotection in the setting of neonatal hypoxic-ischemic brain injury.
Our findings raise many questions. We could not assess whether treatment effects were mediated by prevention of seizures. Nor did we evaluate the comparative safety and efficacy of the combination of phenobarbital and bumetanide with either higher doses of phenobarbital alone, or with other anticonvulsants such as topiramate. Nonetheless, our results provide support for the hypothesis that drug therapy could augment hypothermic neuroprotection in neonates with hypoxic-ischemic encephalopathy. It could be feasible to administer drugs either intra-partum in a high risk situation, or in the early postnatal period, after resuscitation, and prior to, or concurrently with initiation of hypothermia.
Implementation of combination therapy neonatal neuroprotection clinical trials will be complex and expensive. Yet, both infants who have poor outcomes with hypothermia treatments, and also infants who are ultimately classified as having “good” outcomes, but who may have the potential for better long-term function, could benefit. Our data provide evidence that rational combination therapies, such as phenobarbital plus bumetanide plus hypothermia, have potential to improve outcomes after neonatal hypoxic-ischemic brain injury.
Acknowledgements
HD 060348 (FS) and The GorgEffen Gift Fund (JB).
Abbreviations
- PB
phenobarbital
- BUM
bumetanide
- HT
hypothermia
- SAL
saline
- HI
hypoxia-ischemia
- P
day of life
References
- 1.Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cilio MR, Ferriero DM. Synergistic neuroprotective therapies with hypothermia. Semin Fetal Neonatal Med. 2010;15:293–298. doi: 10.1016/j.siny.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barks JD, Liu YQ, Shangguan Y, Silverstein FS. Phenobarbital augments hypothermic neuroprotection. Pediatr Res. 2010;67:532–537. doi: 10.1203/PDR.0b013e3181d4ff4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverstein FS, Jensen FE. Neurological progress: neonatal seizures. Ann Neurol. 2007;62:112–120. doi: 10.1002/ana.21167. [DOI] [PubMed] [Google Scholar]
- 5.Dzhala VI, Talos DM, Sdrulla DA, et al. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 6.Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizures model. Ann Neurol. 2008;63:222–235. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]
- 7.Dzhala VI, Kuchibhotla KV, Glykys JC, et al. Progressive NKCC1-dependent neuronal chloride accumulation during neonatal seizures. J Neurosci. 2010;30:11745–11761. doi: 10.1523/JNEUROSCI.1769-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai Y, Tang J, Zhang JH. Role of Cl− in cerebral vascular tone and expression of Na+-K+-2Cl− co-transporter after neonatal hypoxia-ischemia. Can J Physiol Pharmacol. 2005;83:767–773. doi: 10.1139/y05-076. [DOI] [PubMed] [Google Scholar]
- 9.Liu YQ, Barks J, Xu G, Silverstein FS. Topiramate extends the therapeutic window for hypothermia-mediated neuroprotection after stroke in neonatal rats. Stroke. 2004;35:1460–1465. doi: 10.1161/01.STR.0000128029.50221.fa. [DOI] [PubMed] [Google Scholar]
- 10.Yager J, Towfighi J, Vannucci RC. Influence of mild hypothermia on hypoxic-ischemic brain damage in the immature rat. Pediatr Res. 1993;34:525–529. doi: 10.1203/00006450-199310000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Brandt C, Nozadze M, Heuchert N, Rattka M, Loscher W. Disease-modifying effects of phenobarbital and the NKCC1 Inhibitor bumetanide in the pilocarpine model of temporal lobe epilepsy. J Neurosci. 2010;30:8602–8612. doi: 10.1523/JNEUROSCI.0633-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverstein FS. Do seizures contribute to neonatal hypoxic-ischemic brain injury? J Pediatr. 2009;155:305–306. doi: 10.1016/j.jpeds.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wirrell EC, Armstrong EA, Osman LD, Yager JY. Prolonged seizures exacerbate perinatal hypoxic-ischemic brain damage. Pediatr Res. 2001;50:445–454. doi: 10.1203/00006450-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Yager JY, Armstrong EA, Jaharus C, Saucier DM, Wirrell EC. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011:48–57. doi: 10.1016/j.brainres.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 15.Gunn AJ, Bennet L, Gunning MI, Gluckman PD, Gunn TR. Cerebral hypothermia is not neuroprotective when started after postischemic seizures in fetal sheep. Pediatr Res. 1999;46:274–280. doi: 10.1203/00006450-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Yan Y, Dempsey RJ, Flemmer A, Forbush B, Sun D. 2003 Inhibition of NaKCl cotransporter during focal cerebral ischemia decreases edema and neuronal damage. Brain Res. 2003;961:22–31. doi: 10.1016/s0006-8993(02)03832-5. [DOI] [PubMed] [Google Scholar]
- 17.Pond BB, Berglund K, Kuner T, Feng G, Augustine GJ, Schwartz-Bloom RD. The chloride transporter Na-K-Cl cotransporter isoform-1 contributes to intracellular chloride increases after in vitro ischemia. J Neurosci. 2006;26:1396–1406. doi: 10.1523/JNEUROSCI.1421-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann N Y Acad Sci. 2003;993:103–114. doi: 10.1111/j.1749-6632.2003.tb07517.x. [DOI] [PubMed] [Google Scholar]
- 19.Creeley CE, Olney JW. The young: neuroapoptosis induced by anesthetics and what to do about it. Anesth Analg. 2010;110:442–448. doi: 10.1213/ANE.0b013e3181c6b9ca. [DOI] [PubMed] [Google Scholar]
- 20.Wang DD, Kriegstein AR. Blocking early GABA depolarization with bumetanide results in permanent alterations in cortical circuits and sensorimotor gating deficits. Cereb Cortex. 2011;21:574–587. doi: 10.1093/cercor/bhq124. [DOI] [PMC free article] [PubMed] [Google Scholar]