Abstract
Sporadic or idiopathic Parkinson's disease (PD) is an age-related neurodegenerative disorder of unknown origin that ranks only second behind Alzheimer's disease (AD) in prevalence and its consequent social and economic burden. PD neuropathology is characterized by a selective loss of dopaminergic neurons in the substantia nigra pars compacta; however, more widespread involvement of other CNS structures and peripheral tissues now is widely documented. The onset of molecular and cellular neuropathology of PD likely occurs decades before the onset of the motor symptoms characteristic of PD. The hallmark symptoms of PD, resting tremors, rigidity and postural disabilities, are related to dopamine (DA) deficiency. Current therapies treat these symptoms by replacing or boosting existing DA. All current interventions have limited therapeutic benefit for disease progression because damage likely has progressed over an estimated period of ~5 to 15 years to a loss of 60%–80% of the nigral DA neurons, before symptoms emerge. There is no accepted definitive biomarker of PD. An urgent need exists to develop early diagnostic biomarkers for two reasons: (1) to intervene at the onset of disease and (2) to monitor the progress of therapeutic interventions that may slow or stop the course of the disease. In the context of disease development, one of the promises of personalized medicine is the ability to predict, on an individual basis, factors contributing to the susceptibility for the development of a given disease. Recent advances in our understanding of genetic factors underlying or contributing to PD offer the potential for monitoring susceptibility biomarkers that can be used to identify at-risk individuals and possibly prevent the onset of disease through treatment. Finally, the exposome concept is new in the biomarker discovery arena and it is suggested as a way to move forward in identifying biomarkers of neurological diseases. It is a two-stage scheme involving a first stage of exposome-wide association studies (EWAS) to profile omic features in serum to discover molecular biomarkers. The second stage involves application of this knowledge base in follow-up studies. This strategy is unique in that it promotes the use of data-driven (omic) strategies in interrogating diseased and healthy populations and encourages a movement away from using only reductionist strategies to discover biomarkers of exposure and disease. In this short review we will examine 1) advances in our understanding of the molecular mechanisms underlying PD that have led to candidate biomarkers for diagnosis and treatment efficacy and 2) new technologies on the horizon that will lead to novel approaches in biomarker development.
Keywords: Imaging, Fluid biomarkers, α-Synuclein, Transcranial sonography
1. Introduction
Parkinson's disease, which is the second most common neurodegenerative disorder after Alzheimer's disease, has a late onset and is diagnosed in about 1% of individuals over the age of 65. PD is an incurable and progressive disorder that gradually robs the individual of motor control. Traditionally, PD has been considered an idiopathic or sporadic disease characterized pathologically by the degeneration and loss of the dopaminergic neurons in the nigral striatal pathway and the presence of Lewy pathology [1]. The diagnosis of PD usually is clinical and an autopsy is considered necessary for disease confirmation. No validated diagnostic biomarker of PD is available. Unfortunately, the cardinal and defining motor symptoms (Parkinsonism) used in the clinical diagnosis of PD also occur in other disorders, but not all of these clinical “look-a-likes” have a neurodegenerative component. In idiopathic PD, these cardinal features, which are not apparent until a great deal of degeneration has already occurred, include body tremors, slowed movement, muscle rigidity, and an irregular posture, but more recently non-motor extra-nigral symptoms also have been recognized [1,2]. PD is not just a complex motor disorder but now is considered a systemic disease as its non-motor symptoms often precede the clinical motor signs. These include olfactory and autonomic dysfunction (e.g., constipation), as well as sleep and cognitive disturbances; their presence in most patients suggests that they could be used as prodromal/pre-clinical markers of PD [3–5]. The lack of motor symptoms during the early disease stage may be due to “neuronal reserve” or active compensatory mechanism(s) (e.g., collateral axonal sprouting from surviving DA neurons) [6]. Early in the disease the diagnostic error rate can be as high as 25% among practitioners with limited clinical experience in PD. This high level of misdiagnosis affirms the strong need for a diagnostic biomarker of PD. A suitable biomarker would allow treatment with putative neuroprotective agents to begin long before the significant and irreversible loss of neurons, and would enable the assessment of disease modification in individuals receiving treatments.
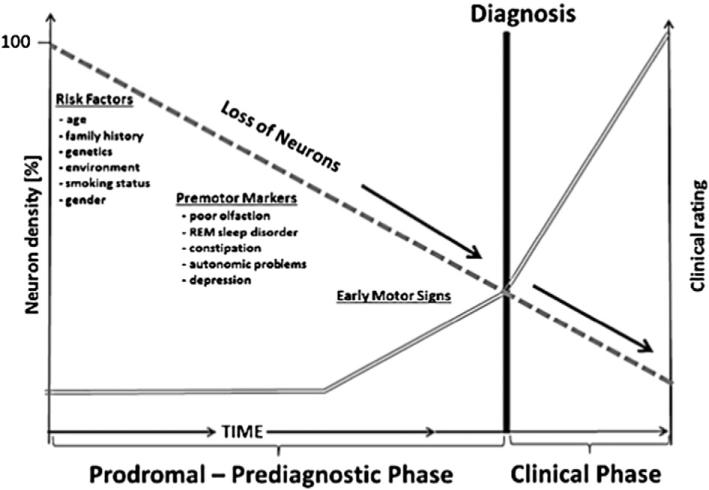
1.1. The need for several types of PD biomarkers
The Biomarkers Definitions Working Group [7] has defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention”. No presently available biomarker able can predict the onset of PD or constitutes a definite diagnostic test. Misdiagnosis often occurs early in the disease and an autopsy is needed to confirm diagnosis. The difficulties in diagnosing PD make the search for biomarkers difficult as identifying the diagnostic criteria for a disease is an important step in beginning to identify and validate biomarkers. Since the clinical diagnosis of PD usually occurs only after a substantial number of SN neurons have degenerated, there is a need for PD biomarkers that include (1) prodromal, preclinical or premotor stage biomarkers;(2) biomarkers of risk or susceptibility; and (3) motor stage biomarkers (Fig. 1) [8,9]. Biomarkers addressing these categories could be based on clinical, imaging, genetic, proteomic, or biochemical factors or various combinations of these [2]. A sole reliance on a clinical assessment to identify PD subjects impedes research progress and even seasoned clinicians make diagnostic errors early in the disease. This is seen in the ELLDOPA, CALM-PD and REAL-PET studies where imaging analyses found no evidence of striatal dopaminergic deficits in some subjects diagnosed as having early PD based on clinical signs [10]. Further, progression and treatment response are mostly determined using semi quantitative subjective clinical scales like the UPDRS that focus almost exclusively on the motor symptoms. The reliance on clinical diagnosis and the ensuing problems have instigated a call to conceptualize PD differently and change the criteria for the diagnosis and staging of PD by using, for example, a tier approach in research efforts. Even the use of pathologic findings as the final judge in diagnosis is being questioned. For example, pathology can be inconclusive in genetic forms of PD [11–14]. Additional criteria that could involve, for example, non-motor clinical signs like anosmia, would not replace the existing clinical criteria. Rather, they would supplement them to better stratify subjects in research efforts as well as in clinical trials involving treatment and neuroprotective agents. However, some of these schemes are criticized for their failure to include the core DA deficit of PD or address the issue of false positives [15].
Fig. 1.
Factors and premotor markers associated with loss of neurons (————) prior to onset of motor signs and clinical diagnosis (====). Adapted from [48].
Obvious problems are inherent in PD diagnosis and there is a great need for reliable and cost-effective biomarkers of PD. Adhering to the broad definition described above, a wide range of candidate measurements has been evaluated, including olfactory testing, tissue and fluid analysis, functional neuroimaging, and genetic susceptibility. Future diagnosis of PD is likely to involve multiple indices including clinical, laboratory, imaging and genetics [16–18]. The best and most useful biomarker(s) would be sensitive, reproducible, and technically feasible for most labs, inexpensive, noninvasive and most important, thoroughly validated [19].
1.2. Types of biomarkers and their purpose
Prodromal or premotor biomarkers would be diagnostic biomarkers and would identify PD before a significant degree of damage has occurred and at a stage when neuroprotective therapies could halt or slow neuronal loss. Risk biomarkers are needed to identify cohorts with a high probability of developing clinical PD. Premotor symptoms may reflect pathogenic processes in the development of PD, and understanding them may have etiological implications [20]. Motor stage biomarkers would serve to chart disease progression and aid in determining the efficacy of various therapies given in the period when motor symptoms are readily apparent due to the marked degeneration of SN neurons. An obvious goal would be the identification of prodromal biomarkers early in the disease. Although it is widely acknowledged that prodromal, motor stage and other biomarkers of PD are needed, there has been limited progress despite an intensive investment of time and effort. This failure could be a consequence of the strategies used to identify biomarkers of PD that involve an intensive characterization of specimens from individuals already diagnosed and that are in the late stages of PD. Some failures may be due to issues concerning the lack of strict sample collection under specific PD diagnostic criteria, resulting in poor sample quality as well as the inappropriate storage of what would have been high quality specimens [21,22]. The Parkinson's Progression Markers Initiative (PPMI), which involves 20 centers in the USA and Europe, aims to combat this problem by enrolling equal numbers of early stage (before medication) PD patients and matched controls [23]. All involved centers will adhere to standardized techniques for repeated biosampling (blood, CSF, urine), clinical assessments and imaging. Strict standards for sample archiving, storage and analysis also are part of the initiative. NINDS has followed suit with its Parkinson's Disease Biomarkers Program emphasizing cooperation and collaboration between consortium members, a sample repository and a Data Management Resource.
1.3. Strategies for biomarker discovery
A number of different strategies have been utilized in the search for PD biomarkers. Many efforts have relied on an extensive characterization of those diagnosed with PD. Such efforts have added to the current knowledge concerning the molecular neuropathology of PD and identified possible targets for neuroprotection and development of viable preclinical animal models of PD. They also have identified functional and imaging changes accompanying disease progression as well as the possible contribution of genetics. Biomarkers emanating from the characterization of the molecular neuropathology of PD would be considered pathogenesis-based (e.g., biomarkers based on mitochondrial dysfunction) (Fig. 2). A newer, less restricted approach, an exposome strategy, encourages a more expansive and less reductionist strategy with less of an emphasis on single candidate biomarkers [4,24]. Below we will provide a brief synopsis of the current molecular characterization of PD. Genetic, functional, fluid and tissue, as well as imaging biomarkers will be discussed. Finally, we will examine the conceptual and methodological barriers to the development of useful PD biomarkers and how newer discovery approaches may move the field forward.
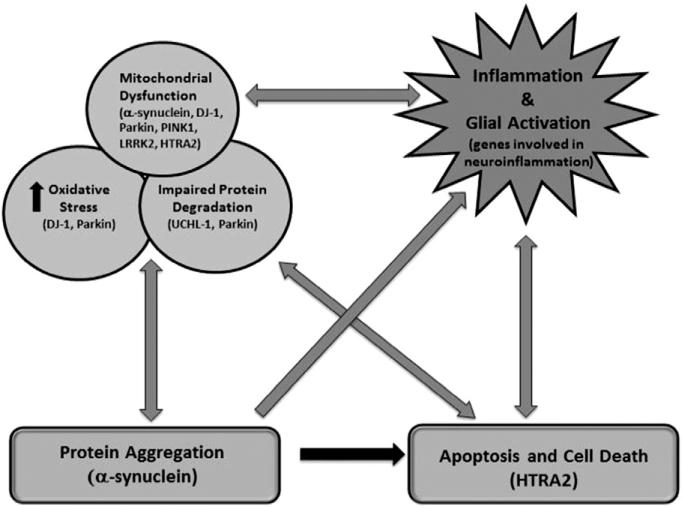
Fig. 2.
Proposed mechanisms and gene expression events underlying pathogenesis of PD. Adapted from [25].
1.4. Molecular neuropathology of PD
As already noted, PD is a disease characterized by the loss of pigmented DA neurons in the SN accompanied by the cardinal motor symptoms of bradykinesia, tremor at rest, rigidity, and postural instability. Considerable effort has been directed at using post-mortem tissue to investigate the molecular neuropathology accompanying these defining characteristics [25]. It is believed that determining and characterizing the molecular underpinnings of PD will lead to early biomarkers of PD as well as viable treatments for preventing or halting it. These investigational studies have focused on the pathological hallmarks of PD, namely (1) the degeneration and death of the melanin containing neurons of the SN, and (2) Lewy pathology — the presence of intra-cytoplasmic Lewy bodies with inclusions containing mainly α-synuclein and ubiquitin. Neuronal projections, called Lewy neurites, containing similar inclusions also are present.
Naturally, these investigations have focused on cell death mechanisms as the DA containing neurons of the SN die. The presence of nuclear TUNEL labeling and chromatin condensation in PD brains suggests that these SN neurons may suffer a programmed cell death (i.e., apoptosis). Further examination of PD brains identified changes in mitochondrial function, increased oxidative stress, lysosomal dysfunction, protein aggregation and impaired degradation, deposition of iron as well as inflammation and glial activation. All of these observations suggest target areas to be explored for potential biomarkers and development of useful animal models of PD [26].
Many failed PD neuroprotection clinical trials have been based on the putative mechanisms discussed above, such as those accounting for the neuronal loss (e.g., apoptosis, mitochrondrial dysfunction). These “cause-directed” trials involved antioxidants, trophic factors, and enhancers of mitochondrial function, agents to block excitotoxicity or apoptosis. To date no disease modifying or neuroprotective treatments have been identified. This failure likely is due to an incomplete understanding of the multiple pathogenic elements of PD, a lack of validated biomarkers of the stages of PD and its progression, a lack of target engagement by the test agent, due to an ineffective dose or failure to reach the site, or an incorrect assessment of clinical state due to concurrent administration of agents providing symptomatic relief. If multiple mechanisms are involved, then targeting one pathway is likely to be ineffective. It is also important to note that disease modification can occur without altering neuronal loss; a test agent may merely ameliorate symptoms giving the appearance of neuroprotection [27–29].
1.5. Genetic biomarkers
Although having a family member with PD is a risk factor, for many years genetics was not considered to have much of an influence on susceptibility to PD because of its late onset and the sporadic nature of the idiopathic form. Idiopathic PD now is considered to have a complex etiology involving multiple influences including lifestyle, genetics, and environment. Investigations involving associations between single nucleotide polymorphisms in many candidate genes (e.g. ones related to detoxification like CYPs or DA) and PD risk have shown no relationship or only weak ones [30–32]. To date, genome wide association surveys (GWAS) have identified 16 PD loci but these explain only a small percentage of the heritability. However, the discovery of rare, early onset familial forms engendered a belated recognition of the possible importance of genetics in that these forms can be caused by a variety of mutations in a single gene (Fig. 2). For example, point mutations, duplications, and triplication in the SNCA gene cause PD with high penetrance and interestingly some SNPs of this gene appear related to risk [8,33]. Accordingly, characterizing the monogenetic autosomal dominant forms of PD has identified genes and gene products (Fig. 2) that may be critical for determining the underlying cause(s) of the pathology in PD. These efforts have focused attention on mitochondrial dysfunction and mutations in mitochondrial genes (e.g., SNCA) and gene products (α-synuclein). Many of the gene products of the mutated genes in the autosomal dominant forms have been linked to oxidative stress, mitochondrial dysfunction and mishandling of impaired or aberrant forms of the gene products (e.g, oligometric α-synuclein) [19]. Further, these proteins because of their intimate association with disease pathophysiology (e.g., α-synuclein) have been identified as candidate biomarkers for PD.
1.6. Fluid and tissue biomarkers of PD — α-synuclein as an example
The identification and development of useful biomarkers for neurodegenerative diseases have been hindered by the limited access to diseased brain tissue until death and autopsy. Further hampering the efforts is the fact that most patients do not go to autopsy. As there is no barrier between CSF and the brain this fluid is considered to be ideal to interrogate for biomarkers of neurodegenerative diseases. However, CSF is not as easily accessed as other body fluids such as blood and urine, whose collection, unlike CSF, is considered almost non-invasive. Consequently efforts are intensifying in determining the potential benefits of using these convenient fluids to screen for PD biomarkers. Although a variety of candidates related to aspects of PD pathology have been evaluated for their suitability as fluid biomarkers (e.g., neurofilaments, neurotransmitters, urate, DJ-1), to date α-synuclein is probably one of the most investigated, for several reasons [4,34]. For example, Lewy pathology, a hallmark of PD, is found in the brain tissue of PD patients and consists of neurons and neurites containing inclusions of misfolded and aggregated α-synuclein. Also, α-synuclein is the protein product of the SNCA gene; its mutation causes one of the monogenetic forms of PD, further strengthening the rationale for investigating its potential as a PD biomarker [34]. α-synuclein in its native, aggregated and putative pathological (oligomeric, phosphorylated) forms has been found in a variety of tissues from both living and deceased PD patients including CSF, blood, urine, saliva, gastrointestinal tract, vagus nerve, sympathetic and stellate ganglia, cutaneous autonomic nerves, and submandibular gland. As some of these tissues are easily accessed in living patients (e.g., colon) and there is at present no tissue biopsy test for PD, they may be useful for developing such a test [19,35–39].
It should be noted that there are technical and methodological issues associated with the collection and storage of the fluids evaluated for biomarker content that may contribute to the high degree of variability in fluid biomarkers (e.g., location of the needle insert for CSF) observed between collection centers. For certain proteins like a-synuclein and DJ-1 the levels in blood are much higher than in CSF suggesting that blood contamination of CSF may be a serious issue [40,41]. A standardization of procedures would help to reduce or eliminate these problems.
1.7. Imaging and other functional biomarkers of PD
1.7.1. Imaging
Neuroimaging using single-photon emission tomography (SPECT), positron emission tomography (PET), magnetic resonance imaging (MRI) and transcranial sonography (TCS) can provide important information on brain structure and function in PD and serve as an adjunct to clinical assessment. As these approaches are non-invasive they can repeatedly assess the integrity of the DA system and provide anatomical profiles (e.g., asymmetry of uptake, etc), as well as information about the time frame of neuron loss. In some instances they are correlated with disease severity. All, with the exception of TCS, are expensive, limiting their usefulness in standard diagnostic situations [2,42–44]. MRI, functional MRI, SPECT, PET as well as transcranial sonography all have been used in efforts to differentiate PD from other movement disorders and can facilitate diagnostic accuracy. Transcranial sonography can detect an echo of greater density in midbrain with good accuracy in PD patients in both hospital-based and community settings. This midbrain hyperechogenicity may reflect increased iron content in the SN of PD patients even early in disease, although 10% of controls also show a heightened signal. Although, TCS is cost-effective and has shown promise as a possible imaging biomarker in PD, it is very dependent on operator skill, is not specific and requires an adequate temporal acoustic bone window for good imaging. Also, the size of the echogenic signal is not related to PD duration nor do longitudinal studies show an increased signal with disease progression. Thus, the usefulness of TCS may be limited to differential diagnosis in the early disease stage especially when combined with other prodromal signs like anosmia. Voxel-based morphometry techniques can reveal structural similarities and volume differences that aid in differentiating between PD and other motor disorders in the early stages of the diseases where misdiagnosis is more prevalent [45,46].
1.8. Functional/behavioral indices
Early non-motor symptoms of PD are believed to reflect degeneration in extra-nigral areas before the loss of DA nigral neurons and include disturbances in olfaction, sleep, visuo-spatial abilities, cognition including diminished executive function as well as changes in behavior [11]. Functional tests aimed at these symptoms may indicate PD risk, are noninvasive and may be cost effective, are usually easy to administer and include some evaluations which can be done at home and/or on-line by participants themselves. These include olfaction acuity tests (e.g., the University of Pennsylvania Smell Identification Test — the UPSIT), the REM sleep behavior disorder screening questionnaire, a keyboard tapping test, the bradykinesia akinesia incoordination test (BRAIN), and accelerometer based exams. These tests have been used in several studies (e.g., PREDICT-PD) and have shown promise for the eventual development of screening programs for PD risk that can be used in community settings or at a population level. The test scores can be used alone or along with other risk factors (e.g., age) to develop risk algorithms [47–50]. Although these approaches show promise at identifying risk their real utility will not be known until they have been validated. There is a need to know which if any of these pre-motor features will have a predictive time course, change in an orderly fashion, or predict the individuals who will develop PD. Many of these features are not specific to PD. There are also ethical considerations associated with both false positive and negative outcomes. Would the knowledge be beneficial considering there are no treatments to halt the neurodegeneration? Would benefits outweigh the needless worry caused by a false positive diagnosis (see [18,20,47] for a discussion of the issues and caveats)?
1.9. The future — new approaches in disease biomarker discovery
In many diseases, not just PD, the search for biomarkers has been driven by an extensive investigation and characterization of the disease itself as well as diseased tissue. In PD, the examination of post-mortem brain tissue has led to the identification of relevant molecular pathways and genes that have allowed for targeted therapies, development of animal models, and new drug delivery systems [8,26,51–53]. These targeted strategies have identified many biomarker candidates that are being actively evaluated in various tissue compartments for their potential as different types of PD biomarkers (e.g, α-synuclein) [19,25,34,38,54].
Newer approaches espouse casting a broader net and utilizing more global non-targeted strategies, such as omics (e.g., genomics, proteomics, metabolomics, etc.), for identifying multiple biomarkers in tissue from healthy and diseased individuals [4,16,24,55,56]. For example, a metabolomics evaluation of plasma generated a set of metabolites (i.e., a signature) able to differentiate PD patients from controls irrespective of medication status. Recent work using CSF and serum from deceased PD patients with a complete pathological assessment shows that the profiling of these cell-free peripheral fluids provides a true reflection of the cellular pathological changes in the diseased tissue [57]. These positive results suggest that the use of platforms that more easily identify groups of analytes that differ between disease and control subjects may advance the search for biomarkers of PD more quickly (however, see [41] for more of a tempered view of large-scale molecular profiling approaches). Despite the enthusiastic acceptance of the technological advances and the collection of multiple large data sets, these approaches have not yet produced any useful clinical biomarkers of PD. The promise of these approaches may yet come to fruition with a greater attention to standardization of clinical assessment and appropriate stratification of study subjects, as well as the standardized and appropriate collection and storage of biosamples.
Finally, future efforts should be directed towards the interrogation of existing data sets from large population based studies as well as the analysis of archived fluid and tissue samples from large prospective population-based cohort studies, where there has been an emphasis on clinical observations and repeated sample collection over a long period (e.g., Framingham Heart study; Honolulu Asian Aging Study, a continuation of the Honolulu Heart Program) [47,58]. Every effort should be made to use current bioinformatics and technological approaches (e.g., omics) to thoroughly evaluate these valuable commodities.
REFERENCES
- 1.Olanow CW, Obeso JA. The significance of defining preclinical or prodromal Parkinson's disease. 2012;27:666–9. doi: 10.1002/mds.25019. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S, Moon CS, Khogali A, Haidous A, Chabenne A, Ojo C, et al. Biomarkers in Parkinson's disease (recent update). Neurochem Int. 2013;63:201–29. doi: 10.1016/j.neuint.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Ferrer I, Lopez-Gonzalez I, Carmona M, Dalfo E, Pujol A, Martinez A. Neurochemistry and the non-motor aspects of PD. Neurobiol Dis. 2012;46:508–26. doi: 10.1016/j.nbd.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Mielke MM, Maetzler W. A ‘bird's eye’ view on the current status and potential benefits of blood biomarkers for Parkinson's disease. Biomark Med. 2014;8:225–7. doi: 10.2217/bmm.13.139. [DOI] [PubMed] [Google Scholar]
- 5.Truong DD, Bhidayasiri R, Wolters E. Management of non-motor symptoms in advanced Parkinson disease. J Neurol Sci. 2008;266:216–28. doi: 10.1016/j.jns.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Arkadir D, Bergman H, Fahn S. Redundant dopaminergic activity may enable compensatory axonal sprouting in Parkinson disease. Neurology. 2014;82:1093–8. doi: 10.1212/WNL.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 7.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 8.DeKosky ST, Marek K. Looking backward to move forward: early detection of neurodegenerative disorders. Science. 2003;302:830–4. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- 9.Scherzer C. Searching for biomarkers of Parkinson's disease. Biomark Med. 2009;3:113–4. doi: 10.2217/bmm.09.10. [DOI] [PubMed] [Google Scholar]
- 10.Fahn S, Parkinson Study Group Does levodopa slow or hasten the rate of progression of Parkinson's disease? J Neurol. 2005;252(Suppl. 4):IV37–42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- 11.Berg D, Lang AE, Postuma RB, Maetzler W, Deuschl G, Gasser T, et al. Chang the research criteria for the diagnosis of Parkinson's disease: obstacles and opportunities. Lancet Neurol. 2013;12:514–24. doi: 10.1016/S1474-4422(13)70047-4. [DOI] [PubMed] [Google Scholar]
- 12.Berg D, Postuma RB, Bloem B, Chan P, Dubois B, Gasser T, et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson's disease. Mov Disord. 2014;29:454–62. doi: 10.1002/mds.25844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhidayasiri R, Reichmann H. Different diagnostic criteria for Parkinson disease: what are the pitfalls? J Neural Transm. 2013;120:619–25. doi: 10.1007/s00702-013-1007-z. [DOI] [PubMed] [Google Scholar]
- 14.Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–66. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 15.Deutch AY. Parkinson's disease redefined. Lancet Neurol. 2013;12:422–3. doi: 10.1016/S1474-4422(13)70052-8. [DOI] [PubMed] [Google Scholar]
- 16.Saracchi E, Fermi S, Brighina L. Emerging candidate biomarkers for Parkinson's disease: a review. 2014;5:27–34. doi: 10.14366/AD.2014.050027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlossmacher MG, Mollenhauer B. Biomarker research in Parkinson's disease: objective measures needed for patient stratification in future cause-directed trials. Biomark Med. 2010;4:647–50. doi: 10.2217/bmm.10.93. [DOI] [PubMed] [Google Scholar]
- 18.Streffer JR, Grachev ID, Fitzer-Attas C, Gomez-Mancilla B, Boroojerdi B, Bronzova J, et al. Prerequisites to launch neuroprotective trials in Parkinson's disease: an industry perspective. Mov Disord. 2012;27:651–5. doi: 10.1002/mds.25017. [DOI] [PubMed] [Google Scholar]
- 19.Parnetti L, Castrioto A, Chiasserini D, Persicheti E, Tambasco N, El-Agnaf O, et al. Cerebrospinal fluid biomarkers in Parkinson disease. Nat Rev Neurol. 2013;9:131–40. doi: 10.1038/nrneurol.2013.10. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Burton EA, Ross GW, Huang X, Savica R, Abbott RD, et al. Research on the pre-motor symptoms of Parkinson's disease: clinical and etiological implications. Environ Health Perspect. 2013;121:1245–52. doi: 10.1289/ehp.1306967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammadi D. The Harvard Biomarker Study's big plan. Lancet Neurol. 2013;12:739–40. doi: 10.1016/S1474-4422(13)70155-8. [DOI] [PubMed] [Google Scholar]
- 22.Poste G. Bring on the biomarkers. Nature. 2011;469:156–7. doi: 10.1038/469156a. [DOI] [PubMed] [Google Scholar]
- 23.Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simjuni T, et al. The Paarkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011;95:629–35. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rappaport SM. Biomarkers intersect with the exposome. 2012;17:483–9. doi: 10.3109/1354750X.2012.691553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Dijk KD, Teunissen CE, Drukarch B, Jimenez CR, Groenewegen HJ, Berendse HW, et al. Diagnostic cerebrospinal fluid biomarkers for Parkinson's disease: a pathogenetically based approach. Neurobiol Dis. 2010;39:229–41. doi: 10.1016/j.nbd.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yadav S, Dixit A, Agrawal S, Singh A, Srivastava G, Singh AK, et al. Rodent models and contemporary molecular techniques: notable feats yet incomplete explanations of Parkinson's disease pathogenesis. Mol Neurobiol. 2012;46:495–512. doi: 10.1007/s12035-012-8291-8. [DOI] [PubMed] [Google Scholar]
- 27.AIDakheel A, Kalia LV, Lang AE. Pathogenesis-targeted, disease-modifying therapies in Parkinson disease. Neurotherapeutics. 2014;11:6–23. doi: 10.1007/s13311-013-0218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart RG, Pearce LA, Ravina BM, Yaltho TC, Marler JR. Neuroprotection trials in Parkinson's disease: systematic review. Mov Disord. 2009;24:647–54. doi: 10.1002/mds.22432. [DOI] [PubMed] [Google Scholar]
- 29.Lang AE, Melamed E, Poewe W, Rascol O. Trial designs used to study neuroprotective therapy in Parkinson's disease. Mov Disord. 2013;28:86–95. doi: 10.1002/mds.24997. [DOI] [PubMed] [Google Scholar]
- 30.Alonso-Navarro H, Jimenez-Jimenez FJ, Garcia-Martin E, Agundez JA, et al. Genomic and pharmacogenomic biomarkers of Parkinson's disease. Curr Drug Metab. 2014;15:129–81. doi: 10.2174/138920021502140327175404. [DOI] [PubMed] [Google Scholar]
- 31.Klein C, Westenberger A. Genetics of Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scholz SW, Mhyre T, Ressom H, Shah S, Federoff HJ. Genomics and bioinformatics of Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a009449. doi: 10.1101/cshperspect.a009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasser T. Genomic and proteomic biomarkers for Parkinson disease. Neurology. 2009;72(Suppl. 2):S27–31. doi: 10.1212/WNL.0b013e318198e054. [DOI] [PubMed] [Google Scholar]
- 34.Nyhlen J, Constantinescu R, Zetterberg H. Problems associated with fluid biomarkers for Parkinson's disease. Biomark Med. 2010;4:671–81. doi: 10.2217/bmm.10.84. [DOI] [PubMed] [Google Scholar]
- 35.Adler CH, Dugger BN, Hinni ML, Lott DG, Driver-Dunckley E, Hidalgo J. Submandibular gland needle biopsy for the diagnosis of Parkinson disease. Neurology. 2014;82:858–64. doi: 10.1212/WNL.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eller M, Williams DR. α-Synuclein in Parkinson disease and other neurodegenerative disorders. Clin Chem Lab Med. 2011;49:403–8. doi: 10.1515/CCLM.2011.077. [DOI] [PubMed] [Google Scholar]
- 37.Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ, et al. Multiple organ involvement by alpha-synuclein pathology in Lewy Body disorders. Mov Disord. 2013;00:1–9. doi: 10.1002/mds.25776. http://dx.doi.org/10.1002/mds.25776. [DOI] [PubMed] [Google Scholar]
- 38.Visanji NP, Marras C, Hazrati LN, Liu LN, Liu LW, Lang AE. Alimentary, my dear Watson? The challenges of enteric α-synuclein as a Parkinson's disease biomarker. Mov Disord. 2014;29:444–50. doi: 10.1002/mds.25789. [DOI] [PubMed] [Google Scholar]
- 39.Wang N, Gibbons CH, Lafo J, Freeman R. α-Synuclein in cutaneous autonomic nerves. Neurology. 2013;81:1604–10. doi: 10.1212/WNL.0b013e3182a9f449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.del Campo M, Mollenhauer B, Bertolotto A, Engelborghs S, Hampel H, Simonsen AH, et al. Recommendations to standarize preanalyticl confounding factors in Alzheimer's and Parkinson's disease cerebrospinal fluid biomarkers: an update. Biomark Med. 2012;6:419–30. doi: 10.2217/bmm.12.46. [DOI] [PubMed] [Google Scholar]
- 41.Mellick GD, Silburn PA, Sutherland GT, Siebert GA. Exploiting the potential of molecular profiling in Parkinson's disease: current practice and future probabilities. Expert Rev Mol Diagn. 2010;10:1035–50. doi: 10.1586/erm.10.86. [DOI] [PubMed] [Google Scholar]
- 42.Berg D, Godau J, Walter U. Transcranial sonography in movement disorders. Lancet Neurol. 2008;7:1044–55. doi: 10.1016/S1474-4422(08)70239-4. [DOI] [PubMed] [Google Scholar]
- 43.Gaig C, Tolosa E. When does Parkinson's disease begin? Mov Disord. 2009;24(Suppl. 2):S656–64. doi: 10.1002/mds.22672. [DOI] [PubMed] [Google Scholar]
- 44.Tuite PJ, Mangia S, Michaeli S. Magnetic resonance imaging (MRI) in Parkinson's disease. J Alzheimers Dis Res. 2013;S1:001. doi: 10.4172/2161-0460.S1-001. http://dx.doi.org/10.4172/2161-0460.S1-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poewe W, Mahlknecht P. Combined assessment of midbrain hyperechogenicity, hyposmia and motor asymmetry improves diagnostic accuracy in early Parkinson's disease. Expert Rev Neurother. 2012;12:911–4. doi: 10.1586/ern.12.75. [DOI] [PubMed] [Google Scholar]
- 46.Shao N, Yang J, Li J, Shang HF, Ross GW, Poewe W. Voxelwise meta-analysis of gray matter anomalies in progressive supranuclear palsy and Parkinson's disease using anatomic likelihood estimation. Front Hum Neurosci. 2014;8:63. doi: 10.3389/fnhum.2014.00063. http://dx.doi.org/10.3389/fnhum.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berg D, Marek K, Ross GW, Poewe W. Defining at-risk populations for Parkinson's disease: lessons from ongoing studies. Mov Disord. 2012;27:656–64. doi: 10.1002/mds.24985. [DOI] [PubMed] [Google Scholar]
- 48.Lerche S, Hobert M, Brockmann K, Wurster I, Gaenslen A, Hasmann S, et al. Mild parkinsonian signs in the elderly — is there an association with PD? Crossectional findings in 992 individuals. PLoS ONE. 2014;9:e92878. doi: 10.1371/journal.pone.0092878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liepelt-Scarfone I, Gauss K, Maetzler W, Muller K, Fruhmann Berger W, et al. Evaluation of progression markers in the premotor phase of Parkinson's disease: the progression markers in the premotor phase study. Neuroepidemiology. 2013;41:174–82. doi: 10.1159/000353560. [DOI] [PubMed] [Google Scholar]
- 50.Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Hardy J, et al. PREDICT-PD: identifying risk of Parkinson's disease in the community: methods and baseline results. J Neurol Neurosurg Psychiatry. 2013:1–7. doi: 10.1136/jnnp-2013-305420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garbayo E, Ansorena E, Blanco-Preito MJ. Drug development in Parkinson's disease: from emerging molecules to innovative drug delivery systems. Maturitas. 2013;76:272–8. doi: 10.1016/j.maturitas.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 52.Schneeberger A, Mandler M, Mattner F, Schmidt W. Vaccination for Parkinson's disease. Parkinsonism Relat Disord. 2012;18(Suppl. 1):S11–3. doi: 10.1016/S1353-8020(11)70006-2. [DOI] [PubMed] [Google Scholar]
- 53.Valera E, Masliah E. Immunotherapy for neurodegenerative diseases: focus on a-synucleinopathies. Pharmacol Ther. 2013;138:311–22. doi: 10.1016/j.pharmthera.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devic I, Hwang H, Edgar JS, Izutsu K, Presland R, Pan C, et al. Salivary a-synuclein and DJ-1: potential biomarkers for Parkinson's disease. Brain. 2011;134:e178. doi: 10.1093/brain/awr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bogdanov M, Matson WR, Wang L, Matson T, Saunders-Pullman R, Bressman SS, et al. Metabolomic profiling to develop blood biomarkers for Parkinson's disease. Brain. 2008;131:389–96. doi: 10.1093/brain/awm304. [DOI] [PubMed] [Google Scholar]
- 56.Rappaport SM, Barupal DK, Wishart D, Vineis P, Scalbert A. The blood exposome and its role in discovering causes of disease. Environ Health Perspect. 2014 doi: 10.1289/ehp.1308015. http://dx.doi.org/10.1289/ehp.1308015. [DOI] [PMC free article] [PubMed]
- 57.Burgos K, Malenica I, Metpally R, Courtright A, Rakela B, Beach T, et al. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer's and Parkinson's diseases correlate with disease status and features of pathology. PLoS ONE. 2014;9:e94839. doi: 10.1371/journal.pone.0094839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeKosky ST, Gandy S. Environmental exposures and the risk for Alzheimer disease. JAMA Neurol. 2014;71:273–5. doi: 10.1001/jamaneurol.2013.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]