Abstract
OBJECTIVES
To characterize frailty in cognitively normal older adults at baseline and to investigate the relationship of frailty with mortality.
DESIGN
A population-based, prospective, cohort study; the Mayo Clinic Study of Aging.
SETTING
Olmsted County, Minnesota.
PARTICIPANTS
Cognitively normal older persons aged 70 years and older (n = 2,356).
MEASUREMENTS
Frailty was assessed at baseline using a 36-item Frailty Index. Four frailty subgroups were identified: Frailty Index ≤0.10 (fit); 0.10<Frailty Index ≤0.20 (at risk); 0.20<Frailty Index≤0.30 (frail) and Frailty Index >0.30 (frailest). All participants underwent comprehensive clinical and cognitive assessments. The association of frailty with mortality was assessed using Cox proportional hazards models.
RESULTS
The mean age (standard deviation) was 78.8 (±5.2) years, 50.2% were male, and the median (interquartile range) Frailty Index was 0.17 (0.11–0.22). Frailty increased with age and was more common in older men. Over a median follow-up of 6.5 years (range 7 days to 8.9 years), 500 of the 2,356 participants died, including 292 men. Compared to fit participants, the frailest participants had the greatest risk of death across the whole cohort (hazard ratio, HR= 3.91; 95% confidence interval, CI = 2.69–5.68). The association was stronger in women (HR= 5.26, 95% CI = 2.88–9.61) than men (HR= 3.15, 95% CI = 1.98–5.02).
CONCLUSION
Baseline frailty was common, especially in older men, and increased with age across our cohort. Frailty was associated with a significantly increased risk of death, particularly in women. These gender differences should be considered when designing a geriatric care plan.
Keywords: frailty, Frailty Index, mortality, aging, sex-differences
Frailty has been increasingly recognized as an important geriatric syndrome. In particular, an ever increasing evidence base shows that frailty is associated with adverse outcomes including longer hospital stays, falls, institutionalization and death.1–4 Reaching a decision regarding a formal consensus definition of frailty has been arduous. However, a consensus committee recently agreed that frailty should be defined as “a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function that increases an individual’s vulnerability for developing increased dependency and/or death”.5 The committee also recommended that all adults over 70 years should be screened for frailty.
Throughout the literature, frailty rates vary enormously. A recent systematic review reported prevalence rates in community dwelling adults of 4–59%.6 Inherent in this variability are the many different approaches to measuring frailty, mainly revolving around the physical phenotype2 and the Frailty Index.7 In general, higher frailty rates with aging and in women tend to be consistent among studies,2, 8 although the distinction with regard to sex has in some cases been less clear.9 Unlike the variability in prevalence rates of frailty with different frailty measures, recent evidence suggests that the association of frailty with mortality remains consistent.10
Although several studies have described the association of frailty with increased mortality, only a few have focused on the sex differences in frailty and mortality.11–15 In line with the recommendation to readily identify frailty in older populations,5 here we sought to conduct a population-based study to identify frailty using a Frailty Index and to investigate the association of frailty with mortality among cognitively normal participants in the Mayo Clinic Study of Aging (MCSA). We were also interested in characterizing any sex-differences in the frailty profile and frailty-mortality relationship.
METHODS
Study Population
Details of the design and conduct of the MCSA have been reported elsewhere.16 Briefly, it is an ongoing population-based study of normal aging and mild cognitive impairment (MCI). We used the medical records-linkage system of the Rochester Epidemiology Project to enumerate all Olmsted County, Minnesota residents aged 70 to 89 years on October 1, 2004 (n = 9953).17 From this sampling frame, we used an age- (70–79, 80–89) and sex-stratified sampling scheme to randomly select participants for recruitment.16 The study protocol was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center. Written informed consent was obtained from all participants. Starting in 2008, we enumerated the population again and sampled additional subjects for enrollment using the same protocols. We have repeated this process every year to maintain the sample size of the population.
Clinical and Cognitive Evaluation
Participants underwent 3 assessments at each evaluation, an interview with a nurse or a study coordinator, a physician evaluation, and extensive cognitive testing by a psychometrist supervised by a neuropsychologist. The interview included a self-assessment of mood,18 memory, and demographic information.16 The Clinical Dementia Rating scale,19 the Functional Activities Questionnaire,20 and the Neuropsychiatric Inventory Questionnaire were administered to an informant. Cognitive testing included tests of memory, executive function, language, and visuospatial skills domains. A final diagnosis about cognition was reached by a consensus panel. Participants were considered cognitively normal if they had scores within the normative range and did not meet criteria for MCI21 or dementia.22 Participants were followed every 15 months.
Assessment of Frailty
We used a standardized procedure to create a Frailty Index from the variables collected in the MCSA.7 The given set of variables covered a range of medical conditions (n=12), health symptoms or signs (n=9), functional impairments (n=8) and psychological variables (n=7). They incorporated self-reported data, informant information, medical record data and physician measures. In total, we included 36 variables (Appendix e1). Most of these frailty deficits had binary response options, coded as 1= present, 0= absent. Any variables with an intermediate response, namely psychological variables, had responses of 0.5 for ‘sometimes’ or ‘possible’, and a score of 1 for ‘all of the time’. Body Mass Index (BMI) was categorized as 1 if < 18 or ≥ 30 kg/m2, 0.5 if between 25 and 30, and 0 otherwise. If there was a missing variable, the index for that individual was calculated from the remaining variables present. For example an individual with 7 deficits who had data for 35 of the 36 variables in the index would have a Frailty Index of 0.2 (7/35).
Although the Frailty Index was not initially designed to be described by subgroups as with the phenotypic description, we also described our cohort by subgroups using previously described cut-points generating 4 groups: Frailty Index ≤0.10 (fit); 0.10<Frailty Index ≤0.20 (at risk); 0.20<Frailty Index≤0.30 (frail) and Frailty Index >0.30 (frailest).23 This study was restricted to all cognitively normal participants sampled from 2004 through 2009, to allow sufficient follow-up time.
Statistical Analysis
Participants were assessed a Frailty Index and assigned a frailty subgroup using cut-points as described above.23 Baseline characteristics for subgroups were compared using chi square tests for categorical data and Kruskal Wallis test for continuous data. The median (25th and 75th percentile) Frailty Indices were computed by age groups at enrollment: 70–74; 75–79; 80–84 and 85–91 years. Duration of follow-up was computed from the baseline visit to the date of death or date of last follow-up. The association of frailty (as the continuous number of frailty deficits and by frailty subgroups) with mortality was assessed using a Cox proportional hazards model adjusted for age (as the time variable), sex and years of education. We also used Cox models to examine interactions of frailty with sex, and to perform stratified analyses by sex. Robustness of the Frailty Index cut-points used was tested by dividing the cohort into quartiles, and performing the same analyses described above using quartiles as cut-points. No significant differences in the results were detected between the cut-points described above and quartile cut-points. For convenience, results are presented using the Armstrong et al cut-points23. We used Kaplan-Meier survival curves for visual display of data. Statistical analyses were performed using SAS System, version 9.3 software (SAS Institute, Cary, NC).
RESULTS
Baseline Sample
A total of 2356 participants were cognitively normal at their initial assessment and were included in this study. Of these, 2139 (90.8%) had data available for all variables required to compute the Frailty Index, 132 (5.6%) had less than 3 missing variables, and the remaining 85 (3.6%) had between 3 and 8 missing variables. The median age of the sample was 78.5 years (interquartile-range, IQR=74.1 – 82.8), 50.2% were men, and 1358 (57.6%) had more than 12 years of education. Table 1 presents the characteristics or participants at baseline. Frail and frailest participants were more likely to be older, male and to have fewer years of education.
Table 1.
Baseline Characteristics of Participants by Frailty Index Subgroups.
| Baseline Characteristic |
Total (N=2356) |
0<FI≤0.1 (N=494) |
0.1<FI≤0.2 (N=1048) |
0.2<FI≤0.3 (N=576) |
FI>0.3 (N=238) |
P valuea |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| Mean (SD) | 78.8 (5.2) | 76.7 (4.6) | 78.4 (5.0) | 80.2 (5.1) | 81.8 (5.1) | <0.001 |
| Median (IQR) | 78.5 [74.1, 82.8] | 75.3 [72.7, 80.2] | 77.8 [73.9, 82.3] | 80.8 [76.1, 83.8] | 82.3 [78.6, 85.9] | |
| Men, N (%) | 1182 (50.2%) | 212 (42.9%) | 519 (49.5%) | 314 (54.5%) | 137 (57.6%) | <0.001 |
| Education, y | ||||||
| Mean (SD) | 13.9 (2.9) | 14.4 (2.8) | 13.9 (2.9) | 13.9 (3.0) | 13.4 (3.0) | <0.001 |
| Median (IQR) | 13 [12, 16] | 14 [12, 16] | 13 [12, 16] | 13 [12, 16] | 13 [12, 15] |
FI= frailty index; SD= standard deviation; IQR= interquartile range; Y = years.
P-values shown are comparing the four groups (each pairwise comparison was significant for age, sex, and education, p<0.02).
Frailty Distribution
The most common frailty deficits were hypertension (76.1%), taking 5 or more medications (73.6%), cancer (55.3%) and ischemic heart disease (44.0%). Medical co-morbidities, cardiovascular diseases and diabetes in particular, were more frequent in men (Appendix e1).
The median (IQR) Frailty Index for the entire cohort was 0.17 (0.11–0.22), corresponding to 6 out of 36 possible deficits. The Frailty Index ranged from 0 to a maximum of 0.57 (20.5 deficits). Overall, 10.1% of our cohort had a Frailty Index >0.3 and were described as the ‘frailest’ group. The median Frailty Index increased with age for both sexes. Only 4% of those in the youngest age group (70–74) had a Frailty Index >0.3 compared with 22.6% of those aged 85 and older. The Frailty Index was consistently higher in men than in women across all age groups and was highest in the oldest men (Table 2). In the oldest age group 29.7% of men had a Frailty Index of ≥0.30 compared with 18.6% of females in that age category.
Table 2.
Frailty Index Values and Number of Frailty Deficits by Age and Gender
| Variable | Age 70–74 | Age 75–79 | Age 80–84 | Age 85–91 | Total Cohort |
||||
|---|---|---|---|---|---|---|---|---|---|
| Women N=336 |
Men N=385 |
Women N=294 |
Men N=312 |
Women N=350 |
Men N=374 |
Women N=194 |
Men N=111 |
N=2356 | |
| Frailty Index Median [IQR] | 0.13 [0.08, 0.18] | 0.15 [0.10, 0.19] | 0.15 [0.10, 0.19] | 0.17 [0.13, 0.24] | 0.18 [0.13, 0.24] | 0.19 [0.14, 0.26] | 0.22 [0.15, 0.28] | 0.24 [0.17, 0.33] | 0.17 [0.11, 0.22] |
| Frailty Deficits Median [IQR] | 4.5 [3.0, 6.5] | 5.5 [3.5, 7.0] | 5.5 [3.5, 7.0] | 6.0 [4.5, 8.5] | 6.5 [4.5, 8.5] | 7.0 [5.0, 9.5] | 8.0 [5.5, 10.0] | 8.5 [6.0, 12.0] | 6.0 [4.0, 8.0] |
IQR= interquartile range.
Frailty and Mortality
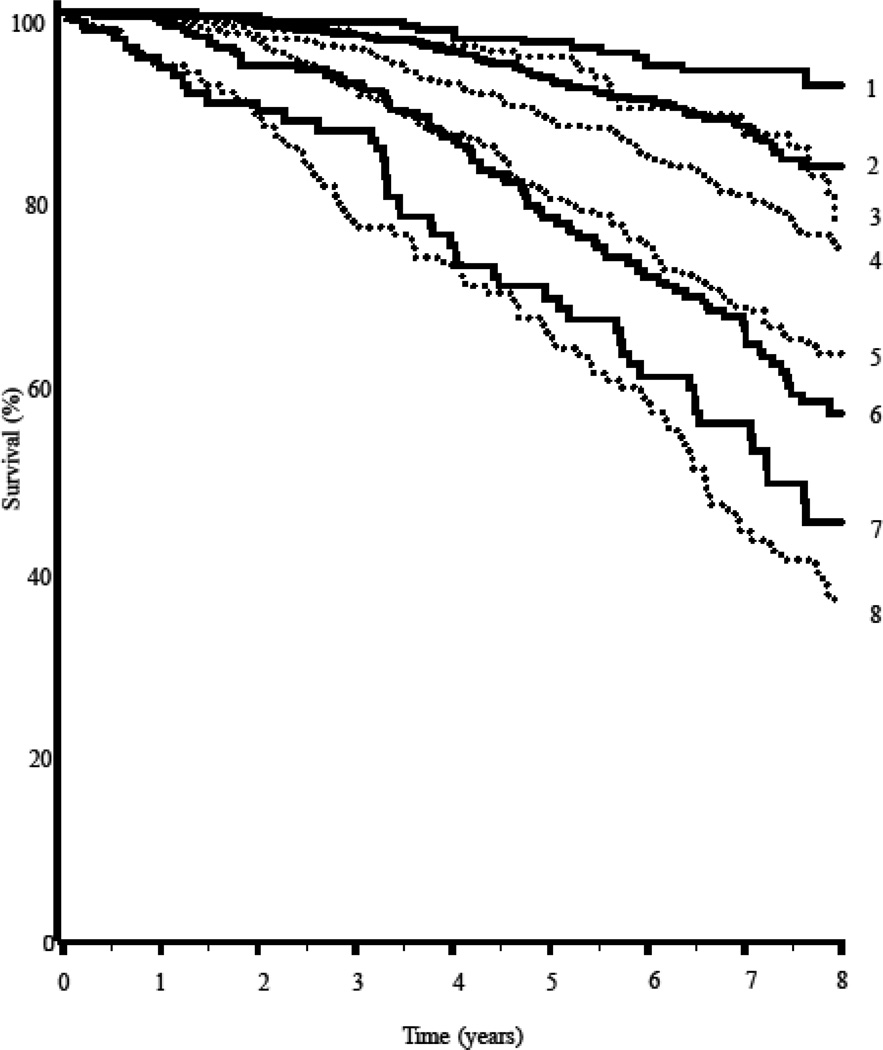
Over a median 6.5 years (range 7 days to 8.9 years) of follow-up, 500 of 2355 participants with survival data died (only 1 with missing data; 292 deaths were in men and 208 in women). In the whole cohort, number of frailty deficits predicted mortality; for each unit increase in number of frailty deficits the hazard ratio (HR) for mortality increased 1.12 (95% confidence interval, CI=1.10 – 1.15; Table 3). Participants who were ‘frailest’ had the greatest risk of death (HR = 3.91; 95% CI=2.69– 5.68, p<0.001) compared to those who were fit. In the multivariable models, gender was significantly associated with mortality, but education was not. There was a significant interaction of frailty with sex (p for interaction was 0.037 for frailty as a continuous measure and 0.063 for the frailty subgroups). In analyses stratified by sex, frailty was more strongly associated with mortality in women (HR = 5.26; 95% CI=2.88– 9.61, p<0.001 for the frailest vs. fit) than in men (HR = 3.15; 95% CI=1.98– 5.02, p<0.001 for the frailest vs. fit; Table 3). Figure 1 displays survival curves according to baseline frailty and sex.
Table 3.
Risk for Mortality According to Frailty Status at Baseline for all participants and stratified by sex
| All participants | Stratified analyses by Sex | ||||||
|---|---|---|---|---|---|---|---|
| N=2355, 500 events | Women n=1173, 208 events | Men n=1182, 292 events | Interaction | ||||
| HR (95% CI)a | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | p-valueb | |
| Frailty Indexc | 1.12 (1.10, 1.15) | <0.001 | 1.16 (1.12, 1.20) | <0.001 | 1.10 (1.07, 1.13) | <0.001 | 0.037 |
| Frailty Subgroup | <0.001c | <0.001c | <0.001c | 0.063c | |||
| Fit | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| At Risk | 1.47 (1.03, 2.10) | 0.035 | 1.76 (0.98, 3.16) | 0.057 | 1.27 (0.81, 1.99) | 0.30 | |
| Frail | 2.65 (1.86, 3.78) | <0.001 | 4.18 (2.36, 7.37) | <0.001 | 1.87 (1.19, 2.94) | 0.007 | |
| Frailest | 3.91 (2.69, 5.68) | <0.001 | 5.26 (2.88, 9.61) | <0.001 | 3.15 (1.98, 5.02) | <0.001 | |
All models are adjusted for age (as the time variable), education (continuous), and sex where applicable.
Interaction p values for frailty* sex derived from Cox proportional hazards models.
Estimates or risk per 1 point increase in the continuous Frailty Index (0–36).
P-value for trend.
Figure 1.
Survival curve showing the percentage of survivors according to their baseline frailty levels and sex; 1 = females Frailty Index ≤0.10 (fit); 2 = females 0.10<Frailty Index to ≤0.20 (at risk); 3 = males Frailty Index ≤0.10 (fit); 4 = males 0.10<Frailty Index to ≤0.20 (at risk); 5 = males 0.20<Frailty Index ≤0.30 (frail); 6 = females 0.20<Frailty Index ≤0.30 (frail); 7 = females FI >0.30 (frailest); 8 = males FI >0.30 (frailest).
DISCUSSION
This study sought to determine the baseline frailty status and mortality outcomes of cognitively normal older men and women in our population based study. We found that frailty is common and as expected, Frailty Index values increased with increasing age. Surprisingly, we note that median Frailty Index values are higher in men in our cohort across all age ranges. Frailty is associated with higher mortality and the effect of frailty on mortality was strongest in the frailest women, although mortality overall was higher in men over the study period.
Our study adds to the literature base of population-based studies of increased mortality associated with frailty. In the few studies that specifically looked at sex differences in the effects of frailty on mortality, the results were varied. Some noted a stronger association of frailty with mortality in older women13, 14 whereas Berges et al11 and Shi et al15 noted the opposite. Similar to the wide variation in frailty prevalence rates, these differences may well be explained by differences in frailty instruments used, including variations of the Fried physical frailty measure,11, 14 a 35-item Frailty Index15 and a composite score of 9 items used by Puts et al to measure frailty.13 Also, since these studies were conducted across different populations of community dwelling older people worldwide from Beijing,15 Finland,14 the Netherlands13 and the Hispanic populations in the Southwestern United States,11 the effects of physiologic (such as aging related sarcopenia) and societal factors (such as social supports) that have been proposed to influence the frailty process may be highly variable2.
Previous studies of various population-based samples have generally noted higher Frailty Index values in older women.3, 15, 24 Numerous hypotheses have been proposed as to the physiological mechanisms driving this.2 A few studies have shown that this sex difference has not always been so evident, particularly in persons age 80 years and older25 or less than 60 years old.26 One earlier study, which used the phenotypic definition of frailty, did not identify any gender difference in frailty rates.9 Hence, our finding of higher median Frailty Index values in older men across all age groups does deviate somewhat from the extant literature and may in part be due to restriction of the cohort to participants who were cognitively normal at baseline. Thus, a direct comparison with other studies is made more difficult. Dementia is more prevalent in older women, and higher frailty rates are likely associated with dementia;27 therefore restriction of the study to cognitively normal persons may have resulted in an underestimation of the frailty frequencies. Inclusion of persons with cognitive impairment at baseline may have generated a higher frequency of frailty that could have influenced the gender differences observed. Our focus on cognitively normal persons who were frail at baseline was due to the implications for future cognitive outcomes and trajectories as delineated later. A second and very plausible explanation for greater frailty in men could be due to the higher prevalence of medical co-morbidities, namely cardiovascular disease and diabetes in our cohort of older men. A recent study of multimorbidity in Olmsted County residents showed that vascular co-morbidities in particular, as well as several other chronic medical conditions, were more prevalent in older men than in older women.28 The higher prevalence of co-morbidities may be driving the higher frequency of frailty observed among men in our cohort compared to other populations as any of these co-morbidities could instigate or exacerbate the frailty process. This explanation is further supported by our finding that while the effect of frailty on risk of death was greater in women, more men died in our cohort, suggesting their risk of death was driven by other factors, such as cardiovascular disease. Another potential explanation for the gender difference found is that although we incorporated as broad a range of health deficits as possible from the data collected in our study, our index does not cover the broader range of disabilities assessed in a comprehensive geriatric assessment. As they age, older women accumulate more physical disabilities than their male counterparts29 so our Frailty Index may underestimate this difference. Indeed, for the activities of daily living (ADL) that were assessed, more women had difficulties than men; the actual numbers, however, were small due to the fact that these participants were all still cognitively intact.
Our study does have some additional methodological limitations. Firstly, the data were collected originally for purposes other than investigation of frailty and individual deficits were not weighted. However, we created the index from as many variables as possible in our database that would cover a range of systems, meet the required criteria and also on the basis that it is not the type of variables but rather the number present that is important.7 It should be noted that some deficits, namely the psychological variables and ADL deficits had a low frequency in the selected cohort, thus contributing little to the Index overall. However, we chose to retain them to maintain consistency within the Index for its future application to cognitively impaired participants in our cohort among whom these variables become more prevalent. Also, our use of the Frailty Index cut-points may not be relevant to our population; evaluating age-specific reference ranges may be more appropriate for our future studies where we have greater numbers of participants across the age ranges that would allow for greater precision.30 However, when we conducted further analyses using quartiles of the Frailty Index in our population, the results were similar. Finally, study participants were primarily of Northern European descent so the findings may have limited generalizability to persons not represented in our cohort. However, they generate hypotheses to be explored in other ethnicities.
The strengths of our study lie in the study design. The MCSA comprises of a large population-based sample with each participant undergoing a comprehensive assessment with self-report, objective and informant data, a consensus diagnosis and longitudinal follow up.
In conclusion, we found that the baseline Frailty Index values increased with age and were associated with an increase in mortality. Surprisingly, we noted that baseline median Frailty Index values were higher in men across all age ranges. However, the effect of frailty on mortality was stronger in women. This research contributes to the literature by providing further understanding of the sex-differences in the frailty syndrome in our population which can help clinicians developing geriatric care plans for frail older people and researchers involved in intervention studies on frailty.
Supplementary Material
ACKNOWLEDGMENTS
Dr. Roberts-research support from the National Institutes of Health (NIH).
Dr. Petersen-research support -NIH; scientific advisory boards -Pfizer, Inc., Janssen Alzheimer Immunotherapy, Elan Pharmaceuticals, GE Healthcare; CME lecture for Novartis, Inc.; royalties Mild Cognitive Impairment (Oxford University Press, 2003).
Sponsor's Role: The funding sources had no role in the design, methods, subject recruitment, data collections, analysis and preparation of this paper.
The research was funded by National Institute of Health grants (U01 AG006786, K01 AG028573 and K01 MH068351) and by the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program.
Footnotes
Conflict of Interest: The authors declare no competing interests.
Author Contributions: Dr. Bartley: study concept or design, analysis or interpretation of data, initial drafting/revising the manuscript. Dr. Geda and Dr. Roberts: drafting/ revising the manuscript, study concept or design, acquisition of data, obtaining funding. T.J.H. Christianson and Dr. Pankratz: analysis or interpretation of data, statistical analysis, drafting/revising the manuscript, obtaining funding. Dr. Petersen: study concept or design, drafting/ revising the manuscript, obtaining funding.
REFERENCES
- 1.Evans SJ, Sayers M, Mitnitski A, et al. The risk of adverse outcomes in hospitalized older patients in relation to a frailty index based on a comprehensive geriatric assessment. Age Ageing. 2014;43:127–132. doi: 10.1093/ageing/aft156. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 4.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 5.Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: A call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: A systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 7.Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004;59:M627–M632. doi: 10.1093/gerona/59.6.m627. [DOI] [PubMed] [Google Scholar]
- 9.Strawbridge WJ, Shema SJ, Balfour JL, et al. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci. 1998;53:S9–S16. doi: 10.1093/geronb/53b.1.s9. [DOI] [PubMed] [Google Scholar]
- 10.Ravindrarajah R, Lee DM, Pye SR, et al. The ability of three different models of frailty to predict all-cause mortality: Results from the European Male Aging Study (EMAS) Arch Gerontol Geriatr. 2013;57:360–368. doi: 10.1016/j.archger.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Berges IM, Graham JE, Ostir GV, et al. Sex differences in mortality among older frail Mexican Americans. J Womens Health (Larchmt) 2009;18:1647–1651. doi: 10.1089/jwh.2008.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 13.Puts MT, Lips P, Deeg DJ. Sex differences in the risk of frailty for mortality independent of disability and chronic diseases. J Am Geriatr Soc. 2005;53:40–47. doi: 10.1111/j.1532-5415.2005.53008.x. [DOI] [PubMed] [Google Scholar]
- 14.Kulmala J, Nykanen I, Hartikainen S. Frailty as a predictor of all-cause mortality in older men and women. Geriatr Gerontol Int. 2014;14:899–905. doi: 10.1111/ggi.12190. [DOI] [PubMed] [Google Scholar]
- 15.Shi J, Yang Z, Song X, et al. Sex differences in the limit to deficit accumulation in late middle-aged and older Chinese people: Results from the Beijing Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2014;69:702–709. doi: 10.1093/gerona/glt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: Design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: The Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II (BDI-II) San Antonio: Psychology Corporation; 2001. [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: Ten years later. Arch Neurol. 2009;66:1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diagnostic and Statstical Manual of Mental Disorders (DSM-IV) 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 23.Armstrong JJ, Andrew MK, Mitnitski A, et al. Social vulnerability and survival across levels of frailty in the Honolulu-Asia Aging Study. Age Ageing. 2015 Mar 10; doi: 10.1093/ageing/afv016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133:456–466. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Gonzalez JJ, Garcia-Pena C, Franco-Marina F, et al. A frailty index to predict the mortality risk in a population of senior Mexican adults. BMC Geriatr. 2009;9:47. doi: 10.1186/1471-2318-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saum KU, Dieffenbach AK, Muller H, et al. Frailty prevalence and 10-year survival in community-dwelling older adults: Results from the ESTHER cohort study. Eur J Epidemiol. 2014;29:171–179. doi: 10.1007/s10654-014-9891-6. [DOI] [PubMed] [Google Scholar]
- 27.Buchman AS, Boyle PA, Wilson RS, et al. Frailty is associated with incident Alzheimer's disease and cognitive decline in the elderly. Psychosom Med. 2007;69:483–489. doi: 10.1097/psy.0b013e318068de1d. [DOI] [PubMed] [Google Scholar]
- 28.Rocca WA, Boyd CM, Grossardt BR, et al. Prevalence of multimorbidity in a geographically defined american population: Patterns by age, sex, and race/ethnicity. Mayo Clin Proc. 2014;89:1336–1349. doi: 10.1016/j.mayocp.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu CJ, Wray LA. Physical disability trajectories in older Americans with and without diabetes: The role of age, gender, race or ethnicity, and education. Gerontologist. 2011;51:51–63. doi: 10.1093/geront/gnq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero-Ortuno R. An alternative method for Frailty Index cut-off points to define frailty categories. Eur Geriatr Med. 2013;4:299–303. doi: 10.1016/j.eurger.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.