Abstract
Background
Identifying effective intervention strategies to combat age-related decline in mobility and brain health is a priority. The primary aim of our study was to examine whether 12 months of aerobic training (AT) versus balance and toning (BAT) exercises moderates the relationship between change in mobility and change in basal ganglia volume in older adults.
Design
Secondary analysis of a randomized controlled trial.
Setting
Champaign-Urbana, Illinois.
Participants
Community-dwelling older adults (N = 101; mean age = 66.41 years)
Intervention
12-month exercise trial with two groups: AT and BAT.
Measurements
Mobility was assessed by the Timed Up and Go (TUG) test. Basal ganglia (putamen, caudate nucleus, pallidum) was segmented from T1-weighted MR images using FIRST. Measurements were obtained at baseline and trial completion. Hierarchical multiple regression was conducted to examine whether exercise mode moderates the relationship between change in mobility and change in basal ganglia volume over 12 months. Age, sex, and education were included as covariates.
Results
Exercise mode significantly moderated the relationship between change in mobility and change in left putamen volume. Specifically, for the AT group, volume of the left putamen did not change, regardless of change in mobility. Similarly, in the BAT group, those who improved their mobility most over 12 months had no change in left putamen volume; however, those who declined in mobility levels significantly decreased in left putamen volume.
Conclusion
Our primary finding that older adults who engage in 12 months of balance and tone training and improve mobility exhibit maintenance of brain volume in a key region responsible for motor control provides compelling evidence that such exercises can contribute to the promotion of functional independence and healthy aging.
Keywords: Basal ganglia, mobility, aging, exercise mode
INTRODUCTION
With the number of adults aged 65 years and older expected to triple worldwide by the year 2050 (1), understanding the factors that contribute to healthy and successful aging is an important public health priority. Both mobility and brain health (e.g., structure and function of the brain) deteriorate with age, and can negatively impact quality of life and functional independence for older adults. For example, changes in gait speed predicted mortality in an 8-year prospective study (2), with an increase of 0.1 metres per second in usual gait speed predicting a 58% reduction in relative risk of death. Similarly, greater reductions in brain volume over time are associated with a higher risk of impairment in instrumental activities of daily living (IADLs) (3). Thus, extending our knowledge about developmental changes in brain volume and mobility may provide insight into how we can effectively combat functional decline in our aging population.
Recent literature has highlighted the connection between mobility and structural integrity of the brain. In a population-based longitudinal study of older adults aged 60–86 years, decreased total white matter volume and greater white matter lesion progression were both significantly associated with reduced gait speed over 2.5 years (4). Further, in another population-based study of community-dwelling adults aged 65 and older that examined grey matter volumes in regions specifically relevant for motor control – such as the putamen and cerebellum – smaller volumes were significantly associated with poorer mobility, including slower gait speed and reduced balance (5). While such studies point towards a clear correlation between mobility and brain health, it is important to consider that these constructs likely have a bi-directional relationship – that is, deterioration in one can negatively impact the other, and vice versa. Therefore, identifying strategic interventions that improve both mobility and brain health is an important public health goal.
Exercise training is a promising intervention strategy that has multiple systemic benefits. In cross-sectional and intervention strategies alike, exercise has been found to improve both mobility (6) and brain health (7–11). However, different modes of exercise have differential physiological effects on the body. For example, aerobic training (AT) is aimed at improving cardiovascular health, and includes activities such as walking or running. A second type of exercise is balance and toning (BAT), which includes exercises aimed at improving muscle tone, flexibility, and balance. Given that BAT exercises can target lower extremity strength and balance, this form of exercise is likely better than AT for improving key components of mobility, such as postural stability. Indeed, Gothe and colleagues (6) previously reported that those who completed a 12-month BAT program exhibited greater improvements on the Timed Up and Go (TUG) – a measure of mobility in older adults – compared with those in an AT program. Given that different types of exercise have different physiological objectives, might they have different impacts on the relationship between changes in mobility and brain volume?
In this secondary analysis of a 12-month randomized controlled trial (RCT) (6, 12–14), our primary aim was to examine the association between change in mobility and change in brain volume in subcortical regions of the basal ganglia that are part of the motor circuit: the putamen, caudate nucleus, and pallidum. Our focus on the basal ganglia for this study was motivated by its role in locomotion and motor coordination (15). Further, we aimed to examine whether this relationship between mobility and basal ganglia volume is moderated by exercise type. Specifically, we examined the effects of AT versus BAT exercise programs on the relationship between mobility and basal ganglia volume. Given that BAT training includes mobility-relevant exercises, we hypothesized that a stronger relationship between changes in mobility and regional volume would be observed for this group compared with the AT group. Such findings would have the potential to inform future intervention strategies for older adults to improve multiple outcomes critical for maintaining an independent and active lifestyle.
METHODS
Participants
Older, community-dwelling adults were recruited to participate in a 12-month RCT examining the effects of exercise on cognition and brain health. Details of the study have been reported elsewhere (6, 13, 16). Briefly, participants were eligible if they: 1) were aged 60–80 years; 2) were right handed; 3) scored ≥ 51 on the modified Mini-Mental Status Exam (mMMSE) (17), a screening questionnaire for cognitive status; 4) scored < 3 on the Geriatric Depression Scale (GDS) (18); 5) had normal colour vision and corrected visual acuity of at least 20/40; 6) were physically inactive over the past six months (i.e., having been physically active for 30 minutes or more, no more than two times per week in the last six months). Ethics approval was obtained from the institutional review board at the University of Illinois at Urbana-Champaign, and all participants provided written informed consent.
Exercise intervention
This study was a 12-month RCT, with assessments at baseline, mid-point, and trial completion. Participants were randomized into one of two groups: AT group or BAT control group. Both programs consisted of three 40-minute group sessions per week and were led by trained exercise leaders.
In the AT program, participants started by walking for 10 minutes on an indoor track, and progressed their walking duration weekly by 5-minute increments until a duration of 40 minutes was achieved at week seven. Participants walked for 40 minutes per session for the remainder of the program. All walking sessions started and ended with 5 minutes of stretching to warm up and cool down. Exertion was monitored via heart rate monitors, and participants were encouraged to walk at 50–60% of their maximum heart rate reserve (HRR) for weeks one to seven, and to increase to 60–75% HRR for the remainder of the program.
The BAT program consisted of warm-up and cool-down stretches, four muscle toning exercises utilizing dumbbells or resistance bands, two balance exercises, a yoga sequence, and an exercise of their choice. New exercises were introduced every 3 weeks to maintain interest. They were encouraged to exercise at an intensity of 13–15 on the Borg RPE scale (Borg, 1985). This group served as to control for non-exercise based effects of participation in a program, such as socialization and study commitment.
Measures
Mobility
To assess mobility, participants completed a modified version of the valid and reliable Timed Up and Go (TUG) test (19), which required participants to stand up from a seated position, walk a distance of 8 feet at their usual pace, return to the chair, and sit back down. The shortest time of two trials was recorded in seconds. Change over time for TUG performance was calculated as baseline score minus trial completion score, with higher scores indicating improved mobility over the 12-month trial. The TUG is a useful measure of mobility given that it can differentiate between healthy older adults and those at risk of falling (20), and correlates with other measures of mobility (19).
Magnetic resonance imaging (MRI)
Participants completed MRI scanning on a 3T head-only Siemens Allegra MRI scanner. High resolution T1-weighted brain images using a 3D Magnetization Prepared Rapid Gradient Echo Imaging (MPRAGE) protocol with 144 contiguous axial slices, collected in ascending fashion parallel to the anterior and posterior commissures, echo time (TE) = 3.87 ms, repetition time (TR) = 1800 ms, field of view (FOV) = 256 mm, acquisition matrix 192 mm × 192 mm, slice thickness = 1.3 mm, and flip angle = 80.
Brain volume of left and right putamen, caudate (head and tail), and pallidum were calculated using the FMRIB Software Library’s (FSL) Integrated Registration and Segmentation Tool (FIRST) (21) a semi-automated model-based segmentation and registration tool within the Oxford Centre’s FSL software package v5.0. FIRST uses a point distribution model to extract the volume of segmented subcortical structures based on a priori manually segmented images of subcortical structures provided by the Center for Morphometric Analysis (CMA), Massachusetts General Hospital, Boston MA. First, the algorithm uses an affine transformation to register the T1-weighted image to the Montreal Neurological Institute (MNI) template. Next, the algorithm searches through linear combinations of shape modes of variation for the most probable shape instance given the observed intensities in the T1-weighted image. The algorithm then transfers the images back to native space. Finally, boundary correction is applied to classify whether the boundary voxels belong to the structure or not, using a threshold of p < 0.001 (z > 3.00), before the final volume estimation is extracted. The quality of the segmentations was checked manually by raters naiive to participant exercise group allocation. See (21) for further details on the FIRST algorithm.
Statistical analysis
Volumetric data was imported into SPSS (Version 21, Mac) for analysis. To control for baseline basal ganglia volume, percent change over time for subcortical brain volume was calculated as [(trial completion volume – baseline volume)/baseline volume] × 100; higher scores indicate increased volume over the 12-month trial. To examine the relationship between mobility, basal ganglia volume, and exercise mode, we conducted hierarchical multiple regression analyses separately for each brain region (left and right putamen, caudate, and pallidum). In the first step, age, sex, and education were included due to their relationship to brain processes and mobility. Next, two variables were included to predict percent change in basal ganglia brain volume: change in TUG performance and exercise mode (AT vs. BAT). Last, the interaction term between change in TUG performance and exercise mode was added to the regression model. Alpha was set at p ≤ 0.05.
RESULTS
Baseline demographic characteristics are presented in Table 1. A total of 179 participants were recruited and randomized for the RCT. Of those, 101 had usable MRI scans and mobility assessments at baseline and trial completion and were therefore included in our secondary analysis. The mean age of participants included in our secondary analysis was 66.41 (± 5.84) years, which was nearly identical to the mean age of all participants in the intervention (66.43 years).
Table 1.
Baseline Demographic Characteristics for Participants.
| Variablea | Aerobic training (n = 54) |
Balance and tone (n = 47) |
Total (N = 101) |
|---|---|---|---|
| Age, years | 67.37 (5.68) | 65.30 (5.89) | 66.41 (5.84) |
| Sex, % female | 74.1 | 61.7 | 68.3 |
| Education, no. (%) | |||
| Ninth grade or less | 1 (1.9) | 0 (0.0) | 1 (1.0) |
| High school graduate | 7 (13.0) | 7 (14.9) | 14 (13.9) |
| Some college or vocational school | 17 (31.5) | 11 (23.4) | 28 (27.7) |
| College graduate | 11 (20.4) | 10 (21.3) | 21 (20.8) |
| Master’s degree | 13 (24.1) | 12 (25.5) | 25 (24.8) |
| PhD or equivalent | 5 (9.3) | 7 (14.9) | 12 (11.9) |
| Modified Mini-Mental Status Examination, max. 100 | 54.92 (1.88) | 55.28 (1.70) | 55.09 (1.80) |
| Timed Up and Go, s | 5.62 (0.96) | 5.68 (1.01) | 5.65 (0.98) |
| Left putamen volume, mm3 | 4378.36 (575.62)* | 4624.53 (597.81) | 4492.91 (596.01) |
| Right putamen volume, mm3 | 4444.74 (505.05) | 4616.81 (608.85) | 4524.81 (559.60) |
| Left caudate volume, mm3 | 3110.28 (449.90) | 3103.53 (485.24) | 3107.14 (464.33) |
| Right caudate volume, mm3 | 3280.88 (470.40) | 3287.53 (443.81) | 3283.97 (455.95) |
| Left pallidum volume, mm3 | 1842.43 (334.25) | 1815.55 (333.94) | 1829.92 (332.71) |
| Right pallidum volume, mm3 | 1810.97 (349.39) | 1772.57 (264.54) | 1793.10 (311.86) |
Data presented as Mean (SD) unless otherwise indicated.
p ≤ 0.05
Change scores over the 12-month intervention for relevant variables are presented in Table 2. The effects of the exercise intervention on physical functioning (6), cognition (12), and brain structure (16) and function (12) have been reported elsewhere. Pertinent to our current study, there was a significant time by exercise group interaction for TUG performance, where participants in the BAT group improved their mobility significantly more than the AT group (6). For basal ganglia volume, the BAT group exhibited significant decreases in the left putamen over the 12 month intervention period compared with the AT group. This was evidenced by a significant time by exercise group interaction, covarying for age, sex, and education, F(1,96) = 5.27, p = 0.02, partial η2 = 0.05. There were no between-group differences for the other basal ganglia regions (i.e., right putamen, left or right caudate and pallidum), all p’s > 0.07.
Table 2.
Change Scores over 12 Months.
| Aerobic training (n = 54) Mean (SD) |
Balance and tone (n = 47) Mean (SD) |
Total (N = 101) Mean (SD) |
|
|---|---|---|---|
| Timed Up and Goa, s | 0.52 (0.81) | 0.68 (0.86) | 0.60 (0.83) |
| Left putamen volumeb, mm3 | 26.32 (241.89)** | −189.76 (507.33) | −74.23 (401.42) |
| Right putamen volumeb, mm3 | −120.01 (260.06)* | −276.20 (459.75) | −192.69 (373.10) |
| Left caudate volumeb, mm3 | −25.42 (177.82) | −49.57 (300.08) | −36.66 (241.51) |
| Right caudate volumeb, mm3 | −26.62 (189.46) | −70.91 (197.84) | −47.23 (193.71) |
| Left pallidum volumeb, mm3 | −33.38 (102.88) | −41.21 (314.71) | −37.02 (226.24) |
| Right pallidum volumeb, mm3 | −63.77 (206.12) | −84.72 (206.35) | −73.52 (205.46) |
Calculated as baseline minus trial completion.
Calculated as trial completion minus baseline.
p ≤ 0.05
p ≤ 0.01
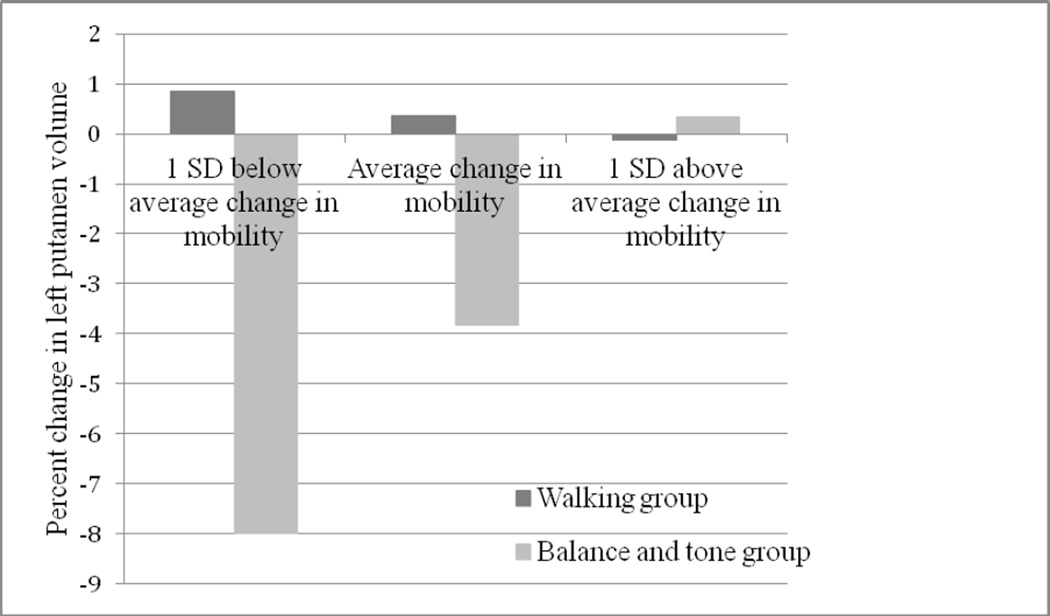
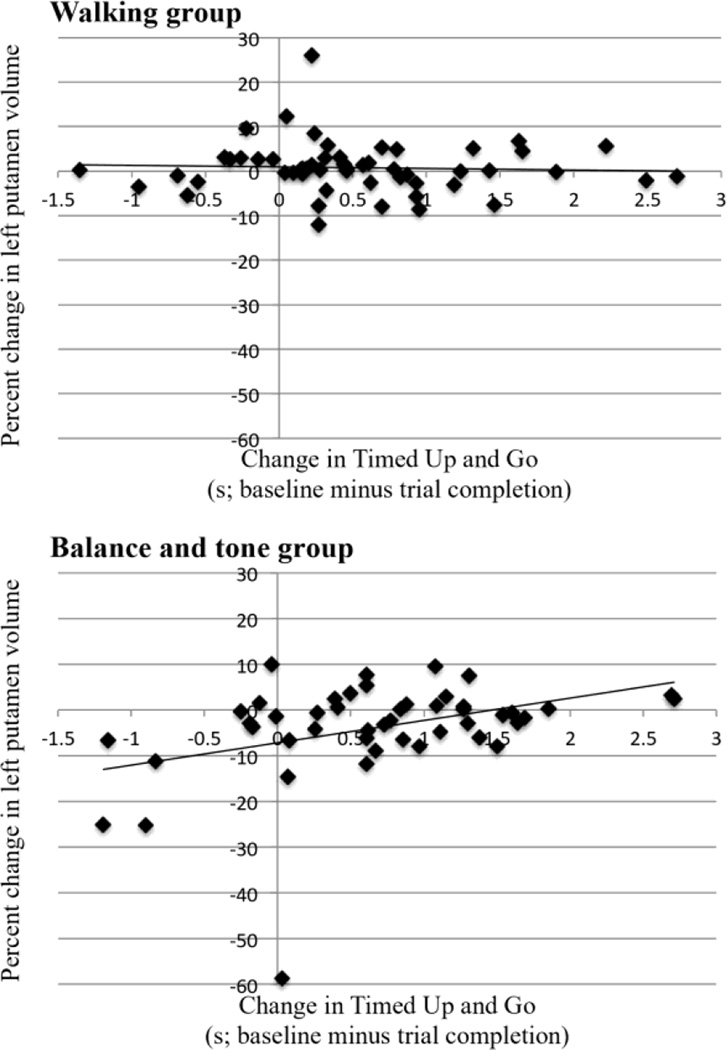
For our hierarchical regression (see Table 3), change in mobility and exercise mode accounted for a significant amount of variance in percent change in volume of the left putamen, R2 = 0.165, F(5,95) = 3.75, p = 0.0041. In addition, the interaction term between change in mobility and exercise mode accounted for a significantly larger amount of variance in predicting percent change in volume of the left putamen, ΔR2 = 0.07, ΔF(1,94) = 7.93, p = 0.006, b = 5.571, t(94) = 2.82, p = 0.006. Examining the data (Figures 1 and 2) shows that for the AT group, volume of the left putamen did not change, regardless of change in mobility. Similarly, in the BAT group, those who improved their mobility most over 12 months had little to no change in left putamen volume; however, those who declined in mobility levels had a significant decrease in left putamen volume. Exercise type did not significantly moderate the relationship between mobility and brain volume for any other basal ganglia regions (i.e., right putamen or bilateral caudate and pallidum), all p’s > 0.06.
Table 3.
Hierarchical Regression Model.
| Independent variable | Percent Δ Left putamen volumea | |||||
|---|---|---|---|---|---|---|
| r | R2 | R2 change |
Unstandardized B (Standard error) |
Standardized β |
p value | |
| Model 1 | 0.281 | 0.079 | 0.079 | |||
| Age | 0.232* | 0.314 (0.148) | 0.209 | 0.037 | ||
| Sex | 0.059 | 0.381 (1.864) | 0.020 | 0.838 | ||
| Education | −0.189 | −1.037 (0.671) | −0.155 | 0.125 | ||
| Model 2 | 0.406 | 0.165 | 0.086 | |||
| Age | 0.232* | 0.261 (0.145) | 0.173 | 0.074 | ||
| Sex | 0.059 | 0.495 (1.826) | 0.026 | 0.787 | ||
| Education | −0.189 | −0.818 (0.650) | −0.122 | 0.211 | ||
| Δ TUGb | 0.184 | 2.155 (1.007) | 0.205 | 0.035 | ||
| Exercise modec | −0.263** | −4.158 (1.691) | −0.237 | 0.016 | ||
| Model 3 | 0.479 | 0.230 | 0.065 | |||
| Age | 0.232* | 0.199 (0.141) | 0.132 | 0.162 | ||
| Sex | 0.059 | 1.122 (1.777) | 0.060 | 0.529 | ||
| Education | −0.189 | −1.081 (0.635) | −0.161 | 0.092 | ||
| Δ TUG | 0.184 | −0.579 (1.374) | −0.055 | 0.674 | ||
| Exercise mode | −0.263** | −7.502 (2.018) | −0.428 | < 0.001 | ||
| Δ TUG × Exercise mode | 0.146 | 5.571 (1.978) | 0.429 | 0.006 | ||
Calculated as [(trial completion – baseline)/baseline] × 100.
Calculated as baseline minus trial completion.
AT coded as reference group in model.
p ≤ 0.05
p ≤ 0.01
Figure 1.
Percent change in left putamen volume as a function of change in mobility and group (walking vs. balance and tone). Negative values of percent change in left putamen volume represent decreases in volume over time. Change in mobility represents average change in TUG scores + 1 SD. The association between change in volume of the left putamen and change in mobility is moderated by type of exercise, where change in left putamen volume remains stable across change in mobility in the walking group, whereas left putamen volume depends on change in mobility in the balance and tone group.
Figure 2.
Scatterplots displaying the relationship between percent change in left putamen volume and change in mobility over 12 months in the walking group (top) and balance and tone group (bottom).
DISCUSSION
In this secondary analysis of a randomized controlled trial of exercise training, we found that exercise mode moderated the relationship between change in mobility and change in left putamen volume. Specifically, in the balance and tone exercise group, decline in mobility function over 12 months was significantly associated with reduced subcortical brain volume in the left putamen – a brain region involved in motor control, including selection of movement (22). While previous work has established a link between mobility and brain health, our study is the first to examine this relationship in the context of longitudinal changes as a function of two distinct modes of exercise training. Hence, our results provide evidence for effective strategies to maintain mobility and putamen volume in older adults – two essential components for successful aging.
In our current study, 12 months of regular aerobic exercise resulted in maintenance of basal ganglia volume in older adults. This finding aligns with previous work by Erickson and colleagues (14) and ten Brinke and colleagues (23) who independently found that aerobic exercise significantly increased hippocampal volume in healthy older adults and those with mild cognitive impairment, respectively. Further, Chaddock et al. (24) found that in children, greater aerobic fitness was associated with greater volume of the basal ganglia. It has been well documented that the aging brain endures global volumetric loss (25, 26), including atrophy in the putamen (27). However, larger-than-normal declines in volume are associated with neurodegenerative disease (28); indeed, it is estimated that annual global brain atrophy is 5–10 times greater in patients with Alzheimer’s disease compared to healthy controls (29). More specifically, changes in putamen volume are associated with motor-related disorders, such as Parkinson’s disease (30). Thus, the maintenance of brain volume over time may help to evade cognitive- and motor-related impairment, including risk for Parkinson’s disease, in older age. Interestingly, the AT group exhibited preservation of putamen volume without any concomitant changes in mobility. Although it is beyond the scope of our current study, one possible mechanism through which AT may impact brain health is through physical fitness, such as improvement of Vo2 max. Future research is required to extend our understanding of the biological and physiological mechanisms that impact brain volume.
Furthermore, we found that those in the balance and tone group who improved their mobility the most after 12 months of training also showed the greatest preservation of left putamen volume. Therefore, our results suggest that even though balance and tone training may not directly provide the same neuro-protective benefits as aerobic training, it appears to buffer against brain volume loss through improvement in mobility. That is, participants in this group improved their mobility significantly more than the aerobic training group – which in turn supported the preservation of subcortical brain volume. While our longitudinal study does not provide insight into the mechanisms that may account for this result, we cautiously speculate that improving mobility in the balance and tone group may strengthen relevant brain regions, which is certainly viable given the role of the putamen in motor control. However, we also note that future studies to further examine the relationship between mobility, brain volume, and exercise type are warranted – including those that may elucidate how other brain regions, such as the cerebellum or motor cortex, may also be impacted by type of exercise.
The strengths of our study include a relatively large sample size with a long intervention period. However, we note the limitations of our study. First, our sample is comprised of highly educated older adults, which is not representative of the entire population; thus our results may not generalize to more heterogeneous samples. Second, we defined mobility in our study by TUG performance. Given that the TUG may not be sensitive to subtle changes in mobility, future studies encompassing diverse measures of mobility are essential before broad generalizations can be made. Related to this, the scope of our current study was limited to analysis of basal ganglia volume; future studies should investigate whether other subcortical volumes, such as the cerebellum, may also play a role in the relationship between exercise and mobility. We also acknowledge that our study did not include a no-contact control group. While this means that we are unable to examine changes in brain volume and mobility over one year in the absence of exercise, this does allow us to control for extraneous variables which may impact functional outcomes, such as participation in a study and socialization. Last, our moderation model only permits correlational inferences to be made. We recognize that the relationship between changes in mobility and brain health are likely bi-directional, such that each may impact one another. Further, this is a dynamic and complex relationship with multiple other relevant variables. As such, elucidating factors that may contribute to individual-level differences in mobility improvement in the balance and tone group above and beyond age, sex, and education would be an important next line of inquiry; indeed, our model accounted for a modest 23% of variance for predicting change in left putamen volume. Hence, future intervention studies to examine the intricacies of this model are warranted.
In conclusion, our study is the first to examine whether exercise mode moderates the relationship between change in mobility and change in basal ganglia brain volume. Our primary novel finding that improving mobility through balance and tone training may potentially buffer against brain volume loss in a key region responsible for motor control provides compelling evidence that balance and tone training can contribute to the promotion of functional independence and healthy aging among older men and women. To the extent that aerobic training may not be accessible to a significant portion of older adults, such as those with poor mobility, balance and tone training may provide a feasible alternative for this population. We note that such findings should also be considered in light of the federal guidelines for physical activity participation which recommend the accumulation of at least 30 minutes of aerobic activity on five or more days per week and participation in flexibility and strengthening activities on at least two days per week. Incorporating the combination of these activities in older adults’ lifestyles is likely to result in an array of psychological and physical health benefits, not the least being the potential preservation of brain volume.
ACKNOWLEDGMENTS
This study was funded by a grant from the National Institute on Aging (Grant # AG025667) and was registered at Clinical Trials.gov (NCT00438347). The authors extend appreciation to Ms. Susan Herrel for project coordination.
LSN is a Canadian Institute for Health Research (CIHR) Postdoctoral Fellow. AMW was supported by the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health under Award Number F31NS089111. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Sponsor’s Role: None
Footnotes
Examining our data for heteroskedasticity, there was one outlier for change in left putamen volume (z = −6.09). Inclusion/exclusion of this participant did not significantly change any results or interpretations; therefore, the participant has been included in our analyses.
No financial disclosures were reported by the authors of this paper.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author’s Contributions: Lindsay S. Nagamatsu and Edward McAuley: Study conception and design, analysis and interpretation of data, manuscript preparation; Andrea M. Weinstein, Kirk I. Erickson: Acquisition of data, data analysis, and critical review of manuscript; Jason Fanning and Elizabeth A. Awick: Acquisition of data and critical review of manuscript; Arthur F. Kramer: Study conception and design, analysis and interpretation of data, critical review of manuscript.
REFERENCES
- 1.United Nations. World Population Ageing 2013. In: Affairs PD, editor. Department of Economic and Social. New York: ST/ESA/SER.A/348; 2013. [Google Scholar]
- 2.Hardy SE, Perera S, Roumani YF, et al. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55:1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 3.Verlinden VJ, van der Geest JN, de Groot M, et al. Structural and microstructural brain changes predict impairment in daily functioning. Am J Med. 2014;127:1089–1096. doi: 10.1016/j.amjmed.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 4.Callisaya ML, Beare R, Phan TG, et al. Brain structural change and gait decline: A longitudinal population-based study. J Am Geriatr Soc. 2013;61:1074–1079. doi: 10.1111/jgs.12331. [DOI] [PubMed] [Google Scholar]
- 5.Rosano C, Aizenstein HJ, Studentski S, et al. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol: A-Biol. 2007;62:1048–1055. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- 6.Gothe N, Fanning J, Awick E, et al. Executive function processes predict mobility outcomes in older adults. J Am Geriatr Soc. 2014;62:285–290. doi: 10.1111/jgs.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 9.Liu-Ambrose T, Nagamatsu LS, Graf P, et al. Resistance training and executive functions: A 12-month randomized controlled trial. Arch Intern Med. 2010;170:170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu-Ambrose T, Nagamatsu LS, Voss MW, et al. Resistance training and functional plasticity of the aging brain: A 12-month randomized controlled trial. Neurobiol Aging. 2011;33:1690–1698. doi: 10.1016/j.neurobiolaging.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Nagamatsu LS, Handy TC, Hsu CL, et al. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med. 2012;172:666–668. doi: 10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voss MW, Prakash RS, Erickson KI, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAuley E, Mullen SP, Szabo AN, et al. Self-regulatory processes and exercise adherence in older adults: Executive function and self-efficacy effects. Am J Prev Med. 2011;41:284–290. doi: 10.1016/j.amepre.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takakusaki K, Tomita N, Yano M. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. J Neurol. 2008;255:19–29. doi: 10.1007/s00415-008-4004-7. [DOI] [PubMed] [Google Scholar]
- 16.Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern Y, Sano M, Paulsen J, et al. Modified mini-mental state examination: Validity and reliability. Neurology. 1987;37:179. [Google Scholar]
- 18.Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986. pp. 165–173. [Google Scholar]
- 19.Podsiadlo D, Richardson S. The timed "Up and Go": A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 20.DiFabio RP, Seay R. Use of the "fast evaluation of mobility, balance, and fear" in elderly community dwellers: Validity and reliability. Phys Ther. 1997;77:904–917. doi: 10.1093/ptj/77.9.904. [DOI] [PubMed] [Google Scholar]
- 21.Patenaude B, Smith SM, Kennedy DN, et al. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jueptner M, Weiller C. A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain. 1998;121:1437–1449. doi: 10.1093/brain/121.8.1437. [DOI] [PubMed] [Google Scholar]
- 23.ten Brinke LF, Bolandzadeh N, Nagamatsu LS, et al. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. Br J Sports Med. 2015;49:248–254. doi: 10.1136/bjsports-2013-093184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaddock L, Erickson KI, Prakash RS, et al. Basal ganglia volume is associated with aerobic fitness in preadolescent children. Dev Neurosci. 2010;32:249–256. doi: 10.1159/000316648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fotenos AF, Snyder AZ, Girton LE, et al. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- 26.Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: General trends, individual differences, and modifiers. Cerebr Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 27.Long X, Liao W, Jiang C, et al. Healthy aging: An automatic analysis of global and regional morphological alterations of human brain. Acad Radiol. 2012;19:785–793. doi: 10.1016/j.acra.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Kiuchi K, Kitamura S, Taoka T, et al. Gray and white matter changes in subjective cognitive impairment, amnestic mild cognitive impairment and Alzheimer's disease: A voxel-based analysis study. PLoS One. 2014;9:e104007. doi: 10.1371/journal.pone.0104007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox NC, Schott JM. Imaging cerebral atrophy: Normal ageing to Alzheimer's disease. Lancet. 2004;363:392–394. doi: 10.1016/S0140-6736(04)15441-X. [DOI] [PubMed] [Google Scholar]
- 30.Pitcher T, Melzer T, MacAskill M, et al. Reduced striatal volumes in Parkinson's disease: A magnetic resonance imaging study. Transl Neurodegener. 2012;1:17. doi: 10.1186/2047-9158-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]