Abstract
A significant proportion of children and adolescents with chronic pain endorse elevated pain-related fear. Pain-related fear is associated with high levels of disability, depressive symptoms, and school impairment. Due to faulty nerve signaling, individuals with neuropathic pain and CRPS may be more prone to develop pain-related fear as they avoid use of and neglect the affected body area(s), resulting in exacerbated symptoms, muscle atrophy, maintenance of pain signaling, and ongoing pain-related disability. Not surprisingly, effective treatments for elevated pain-related fears involve exposure to previously avoided activities to down-regulate incorrect pain signaling. In the context of intensive interdisciplinary pain treatment of youth with neuropathic pain, decreasing pain-related fear is associated with improved physical and psychological functioning, while high initial pain-related fear is a risk factor for less treatment responsiveness. An innovative approach to targeting pain-related fear as well as evidence of a neural response to treatment involving decoupling of the amygdala with key fear circuits in youth with CRPS suggest breakthroughs in our ability to ameliorate these issues.
Keywords: chronic pain, youth, pain-related anxiety, brain imaging, amygdala
Chronic pain in childhood is a significant public health concern with median prevalence rates of 11 to 38% [25], with 3 to 5% of children suffering from significant pain-related disability [12; 22], costing society $19.5 billion annually in the US alone [18]. Although no published prevalence rates for neuropathic pain in childhood exist, approximately one quarter of patients who present to our tertiary care pain clinic are diagnosed with neuropathic pain (complex regional pain syndrome [CRPS] and non-CRPS), ranging from 23% (total sample=697;[47]) to 26% (total sample=321;[57]). Based on these estimates, it is expected that approximately 3–9% of children suffer from chronic neuropathic pain. Beyond the personal suffering and persistent physical and economic consequences for families, chronic pain in childhood can predispose the development of adult chronic pain[69]. Identifying targets for intervention is a challenge as the chronic pain experience involves physiological, psychological, and social factors contributing to pain-related outcomes [14]. The current review will focus on one particularly salient influence on pain outcomes, fear[13; 51; 74; 75].
The evolution of pain-related fear
Humans are programmed to experience fear, and in most cases, fear is adaptive. Pain (an unconditioned noxious stimulus; US) triggers our fear response and alerts our flight-or-fight system to act (an unconditioned response; UR). This is where pain and fear can go awry. After experiencing a painful stimulus or injury, a previously neutral experience, such as movement or even anticipation of movement (a conditioned stimulus; CS) can elicit fear (a conditioned response; CR), even in the absence of pain. Although adaptive in the short-term to promote healing, individuals who continue to perceive movement as threatening after the painful stimuli are no longer present or the initial injury has healed (conditioned fear) can experience a number of psychological and physical sequelae, including hypervigilance, muscular reactivity, escape/avoidance, and guarding behaviors that maintain or exacerbate pain and promote pain-related disability [65]. Unfortunately, pain-related fear learning can develop after only a few repetitions, generalizes quickly, and can be maintained through pain anticipation, converting to an operant process of reinforcement [67].
Pain-related fear and associated behavioral avoidance has been identified as a key factor influencing the development and persistence of pain-related disability across patients with low back[21; 60; 61], neuropathic[8; 52], upper extremity[10], abdominal[39], fibromyalgia[7; 29], and headache pain[5; 41; 54], irrespective of pain intensity. The Fear Avoidance (FA) Model of Chronic Pain [67] is a theoretical framework put forth to explain the impact of pain-related fear. According to the model, individuals who develop fear of pain, injury, and/or physical activity engage in guarding or avoidance behaviors that maintain or exacerbate pain[31]. Continued avoidance and physical inactivity leads to disuse, disability, and depression[24; 65]. Conversely, individuals who confront their pain experience and progressively resume physical activities, thereby testing and correcting pain expectations, subsequently experience a recovery in their pain symptoms[33]. Although some debate exists regarding the cyclical nature of the model [73], evidence for its core tenets is robust [77].
Pain-related fear in youth
Studies that have examined pain-related fear in children have supported its link to adverse outcomes. In a small pilot study of youth with chronic pain, pain-related fear accounted for 40% of the variance in pain-related disability [35] with a second small study finding that pain-related fear predicted child physical activity limitations beyond the influence of pain level and depressive symptoms [76]. When examined within a larger tertiary care pain clinic sample, higher levels of pain-related fear were associated with more healthcare utilization and functional disability [56]. In the context of acute post-surgical pain, pain-related fear was associated with pain unpleasantness and functional disability two weeks after major surgery [45].
Most studies have focused on the influence of pain-related fear on disability-related outcomes [77], rather than the temporal influence of fear on subsequent pain levels. The current evidence suggests that the concurrent relation between pain-related fear and pain intensity in children and adolescents is modest (Table 1). Although more research is needed, it appears that the magnitude of the association is larger among post-surgical patients (in an acute pain context) and among patients after completing intensive pain rehabilitative treatment.
Table 1.
Association between pain and pain-related fear in children
| Authors | Sample Size | Zero-order correlation: pain & pain-related fear | Pain-related fear | Pain rating | |
|---|---|---|---|---|---|
| Martin et al., 2007 | Chronic pain | n=21 | −0.04 | CPASS | Current |
| Wilson et al., 2011 | Chronic pain | n=42 | 0.22 | FABQ | Usual |
| Simons et al., 2011 | Chronic pain | n=299 | 0.24 | FOPQ | Average |
| Huguet et al., 2011 | School children | n=225 | 0.301 | PPFS | Highest (FPS-R) |
| Chronic pain | n=159 | 0.15 | PPFS | Highest (FPS-R) | |
| Page et al., 2011 | Post-surgical | n=83 | 0.43a | CPASS | Current |
| n=69 | 0.36b | CPASS | Current | ||
| Simons et al., 2012 | Intensive pain rehabilitation | n=134 | 0.15aa | FOPQ | Average |
| n=122 | 0.30bb | FOPQ | Average | ||
| n=110 | 0.43cc | FOPQ | Average | ||
| Carpino et al., 2014 | Headache | N=195 | 0.22 | FOPQ | Average |
Note. CPASS=Child Pain Anxiety Symptom Scale; FABQ=Fear Avoidance Beliefs Questionnaire; FOPQ=Fear of Pain Questionnaire; FPS-R=Faces Pain Rating Scale-Revised; PPFS=Pediatric Pain Fear Scale.
Correlation was corrected for gender.
48–72 hours post-surgery,
2-weeks post-surgery,
admission,
discharge,
1-month follow-up.
Applying the Fear Avoidance (FA) Model of Chronic Pain to the pediatric context has yielded promising results (Simons & Kaczynski, 2012; Figure 1a–b). Key elements of the model for the individual patient (pain, pain catastrophizing, fear of pain, avoidance of activities, disability/depression) have been thoroughly examined to evaluate separate models for children and adolescents. The predictive model for functional disability was robust and consistent with the theorized adult model (catastrophizing -> fear -> avoidance -> disability; Figure 1a), whereas for the outcome of depression, pain-related distress (pain catastrophizing, fear of pain) had a direct influence on depressive symptoms (Figure 1b). Developmental differences emerged with fear of pain more influential on avoidance behavior among adolescents, suggesting that targeting anxiety-related pain cognitions (“I walk around in constant fear of hurting”) may potentially yield greater gains in returning to previously avoided activities than in younger pain patients.
Figure 1. Model with Functional Disability and Depression as Concurrent Outcome.
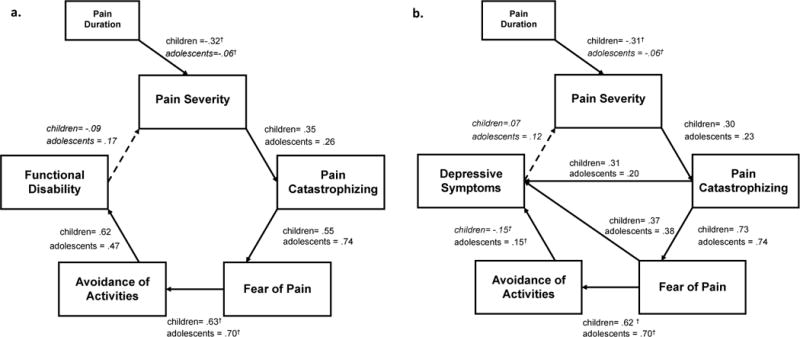
For functional disability (a) the FAM was robustly upheld. For depressive symptoms (b), additional direct pathways for pain catastrophizing and fear of pain were necessary for acceptable model fit. Solid lines are significant at p < .05. Error variances were not included in the figure for simplicity and interpretability. Italicized regression coefficients are non-significant pathways. †denotes values that significantly differ from one another within a specific pathway.
Beyond individual factors, researchers have called for modifications to the FA model for pediatric application to incorporate contextual influences (Asumundson et al., 2012; [16]). Fear can be acquired, reinforced, or resistant to extinction through social transmission[43] with parent emotional responses serving as key guides to a child’s learning about safety and danger and influencing subsequent behavior[30; 38]. New neurobiological evidence in a preclinical model supports parental transmission of fear behaviorally[11]. Growing evidence in the psychosocial literature supports parent pain catastrophizing and protective behavior in prioritizing pain control[4], higher functional disability[34; 70], and school dysfunction[32] in children with pain. This is coupled with recent evidence supporting the influential role of parents on the formation of negative pain memories[40].
As a result, the interpersonal fear avoidance model (IFAM) of pain was recently developed [16] and tested (Simons et al., 2015; Figure 2a-b). The IFAM expands upon the FA Model to include parent cognitive-affective and behavioral factors that have either been proven to impact child outcomes or are theorized to do so. Within the IFAM (Figure 2a), when parents interpret a child’s pain expression through the lens of their own catastrophic appraisals and pain-related fears, they are more likely to engage in maladaptive avoidant or protective parenting behaviors. These parent behaviors influence whether the child avoids or limits activities, potentially leading to greater functional disability. In addition, parents may suffer emotionally and experience interference of their own life goals as a result of their child’s pain, getting caught up in a cycle of avoidance and activity restriction. To examine parent pain-related fear and avoidance, we developed and validated the Parent Fear of Pain Questionnaire (PFOPQ)[58] in a sample of 321 parents of chronic pain patients and tested the Interpersonal Fear Avoidance Model in a separate sample of 163 patients[58]. Parent protective and avoidance behavior contributed directly and indirectly to child avoidance. Additionally, parent fear and catastrophizing contributed indirectly to child avoidance through parent behavior and child fear and catastrophizing, in turn, influencing child functional disability levels (Figure 2b).
Figure 2. Interpersonal Fear Avoidance Model of Pain (IFAM).
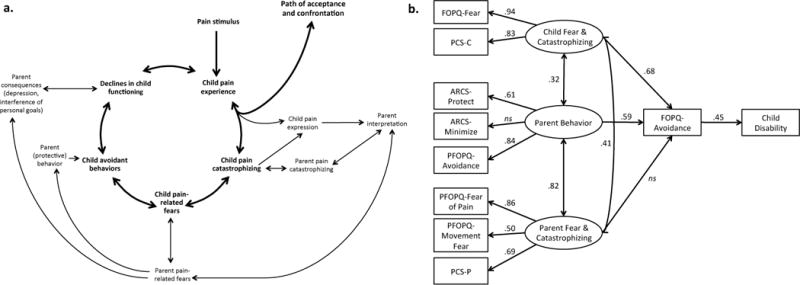
a. In the IFAM model, a child or adolescent develops pain, often in response to an event; although, it can also begin spontaneously. In the context of the child’s pain experience, (s)he either responds with progressive confrontation and acceptance of pain in his/her life for now or continues to perceive the presence of pain as threatening. This expression of threat and fear is observed and interpreted by the parent and reciprocally interacts with a parent’s own catastrophic thinking about his/her child’s pain. This, in turn, leads to hypervigilance and persistent fear in the child and parent. In the context of heightened fear, the child avoids activities that (s)he perceives as potentially harmful to his/her with parent protective behavior providing further encouragement for avoidance behavior. Ultimately, this leads to declines in child functioning and limitations in the life of the parent/caregiver. b. Child fear and catastrophizing and parent behavior had a direct and indirect influence on child avoidance behavior, while parent fear and catastrophizing indirectly influenced child avoidance. Overall, the model accounted for 20% of the variance in functional disability, X2(30) = 46.95, CFI = .97, RSMEA = .06 (CI=.02–.09). Child avoidance is measured with the Avoidance of Activities subscale of the Fear of Pain Questionnaire, Child report (FOPQ). Child disability is measured with the Functional Disability Inventory (FDI). Parent Avoidance is measured with the Parent Avoidance subscale of the Parent Fear of Pain Questionnaire (PFOPQ). PCS-C: Pain Catastrophizing Scale, Child report; ARCS: Adult Responses to Child’s Symptoms; PCS-P: Pain Catastrophizing Scale, Parent report.
Treatment of pain-related fear
Reversing the impact of fear learning is complex and difficult. Extinction, or learning of an inhibitory response, of acquired fear is resistant to automatic generalization, requiring massed rehearsal in a variety of contexts during stressful and non-stressful circumstances to prevent renewal [44; 48]. Graded in-vivo exposure (GEXP), a cognitive-behavioral therapy developed by Vlaeyen and colleagues [66] targets fear of pain and disability through exposing patients to activities previously avoided due to fear of pain or re-injury [9; 10]. A pain psychologist and physical therapist jointly deliver GEXP in an outpatient setting. This intervention has been identified as more effective than wait-list controls in improving disability and reducing pain-related fear [1].
To date, no studies have explicitly targeted pain-related fear through GEXP in children. One study with adolescents who participated in an exposure and acceptance-based treatment program reported decreases in pain-related fear [71], and when examining mediators of change, the investigators identified pain impairment beliefs (psychological flexibility/pain acceptance) and pain reactivity (worry and emotional reactivity to pain) as significant mediators of improvements in pain interference and depressive symptoms [72]. Although not a primary target of the exposure and acceptance-based program, the investigators also observed a significant decrease in pain intensity that was maintained across follow-up periods and was not observed in the multidisciplinary treatment group [71]. Within the context of an intensive day hospital pain rehabilitation program that involves psychological, physical, and occupational therapy, a decrease in pain-related fear was associated with improvements in functional disability and depressive symptoms at the end of treatment and at two month follow-up among patients with neuropathic pain [52]. In this investigation pain intensity was observed to significantly decrease across time, and interestingly as detailed in Table 1, the concurrent association between fear of pain and pain intensity actually increased in magnitude from 0.15 at admission to 0.43 at 1-month follow-up. The increasing resonance between pain intensity and fear of pain deserves further inquiry. Of note, high levels of pain-related fear at admission predicted less reduction in functional disability and depression at discharge, suggesting that high levels of pain-related fear may be a risk factor in relation to treatment outcomes. These findings underscore the need to specifically target pain-related fear in children to potentially avert sustained pain-related disability.
We recently initiated the first pilot implementation of GEXP for youth with chronic pain. In this intervention, the primary goal for the patient is returning to valued activities of daily life and restoring daily functioning, including returning to school. Sessions are conducted with a cognitive-behavioral (CB) therapist, physical therapist (PT), the child, and a parent (as developmentally appropriate). The treatment manual for children and adolescents with chronic pain was adapted from the adult published treatment manual[68]. Briefly, the initial phase of treatment focuses on psychoeducation of the Interpersonal Fear Avoidance Model, values-based goal setting, and fear hierarchy development through the use of the English version (Simons et al., unpublished) of Photographs of Daily Activities (PHODA)-Youth, a diagnostic tool to determine the perceived harmfulness of different activities[64]. In the second phase of treatment, graded exposure begins. The CB therapist and PT jointly lead some of the exposure sessions. During other sessions, the PT leads the exposures while the CB therapist meets individually with the parent. The final phase of treatment focuses on relapse prevention and future goal setting. Results of this initial trial are forthcoming with GEXP expanding treatment options for children struggling with persistent pain, fear, and disability.
Brain circuitry of pain-related fear
Beyond the phenotypic presentation of pain-related fear and its treatment, brain imaging can provide insight into the underlying neural pathways of fear of pain and fear conditioning in humans, work previously limited to animal models (Figure 3). Evoked brain response to fear conditioning is observed in the amygdala[3; 28], insula[17; 63], and anterior cingulate[26], with the magnitude of the amygdala response predictive of conditioned response strength[28; 46]. Brain structure correlates of conditioned fear have associated greater posterior insula thickness with a larger conditioned response, while greater amygdala volume has been associated with less fear acquisition[19].
Figure 3. Neural Pathways of Fear Learning.
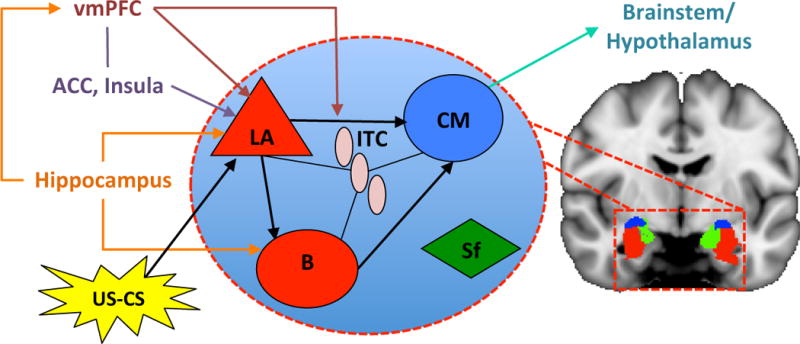
In fear conditioning, an emotionally neutral conditioned stimulus (CS; e.g., movement) is presented with an aversive unconditioned stimulus (US; pain). The CS and US converge at the lateral nucleus (LA). The LA then connects with the centromedial nucleus (CM), controlling the expression of the conditioned fear responses (CR) in the brain stem and hypothalamus. Projections from the hippocampus to the basal nucleus (B) of the amygdala process contextual information during conditioning, while the insula and anterior cingulate (ACC) influence threat encoding. During extinction learning, inhibitory connections between the ventromedial prefrontal cortex (vmPFC) and intercalcated (ITC) cell masses are established. During extinction recall, these connections inhibit fear expression through projections to the CM. Inhibitory connections between the vmPFC and LA may also regulate fear expression through the CM. Contextual modulation of extinction expression is mediated by projections from the hippocampus to the vmPFC and/or LA. Adapted from[20; 53].
Brain regions critical to the acquisition of extinction learning (the target of GEXP) include the amygdala, ventromedial prefrontal cortex (vmPFC), and hippocampus. Recent pre-clinical evidence suggests that successful extinction silences basal amygdala excitatory neurons previously active during fear conditioning[62] and decreases excitatory tranismssion of mPFC projections to the basolateral nucleus of the amygdala[6]. The vmPFC is necessary for the retention and recall of extinction, wherein damage to this area results in preservative behavior[59]. fMRI studies of extinction learning in humans report a decrease in amygdala activation[15; 27; 28; 46] while BOLD signals in the vmPFC increase during extinction learning and extinction retrieval[46]. This association is extended to measures of brain structure with increased thickness in the vmPFC associated with extinction retention[19]. Lastly, fMRI studies that examine context-dependent recall of extinction point to the function of the hippocampus[23; 37] and vmPFC[49]. Overall, extinction learning in humans seems to depend on the integrated functioning of a neural circuit that includes the amygdala, the vmPFC, and the hippocampus.
Beyond evaluation of fear learning/extinction pathways, few studies have examined neural correlates of pain-related fear, particularly among patients with chronic pain. In a study conducted with healthy adults, the authors found a link between higher fear of pain levels and greater activation in the medial PFC, orbitofrontal cortex, and cingulate in response to noxious stimuli [42]. In a more recent study of healthy adults, higher back pain-related fear avoidance beliefs were associated with greater amygdala activity and greater synchronicity in activity between the amygdala and pregenual anterior cingulate cortex [36]. In the only study in adults with chronic pain examining the influence of pain-related fear on evoked brain activity, participants viewed aversive movements, and no differences were noted among high and low fear back pain patients and healthy controls in brain responses to stimuli [2]; although, this may be explained by the method used to elicit fear [50].
Our group has recently reported the first data for youth with chronic pain that suggests that fear circuits are altered [55]. In this study, patients with Complex Regional Pain Syndrome (CRPS) who presented for intensive interdisciplinary pain rehabilitation were scanned prior to the start of treatment and at discharge from the program. Within the sample, 70% of patients reported elevated or clinically elevated pain-related fear at treatment admission. When examining resting state functional connectivity of the amygdala to the rest of the brain, higher pain-related fear scores were associated with stronger functional connectivity between the left amygdala and insula, hippocampus, prefrontal cortex, anterior cingulate, middle temporal gyrus, brain stem and cerebellum, after controlling for pain level (Figure 4a). After intensive psychophysical treatment, persistently higher fear scores were associated with stronger functional connectivity between the left amygdala and hippocampus, prefrontal cortex, anterior cingulate, and middle temporal gyrus (Figure 4b). Together, these results link key fear brain circuits with the pain-related fear phenotype; although, further research is needed to evaluate the degree to which these alterations are due to heightened fear learning and ineffective extinction.
Figure 4. Connectivity strength by levels of pain-related fear in patients (Left Amygdala).
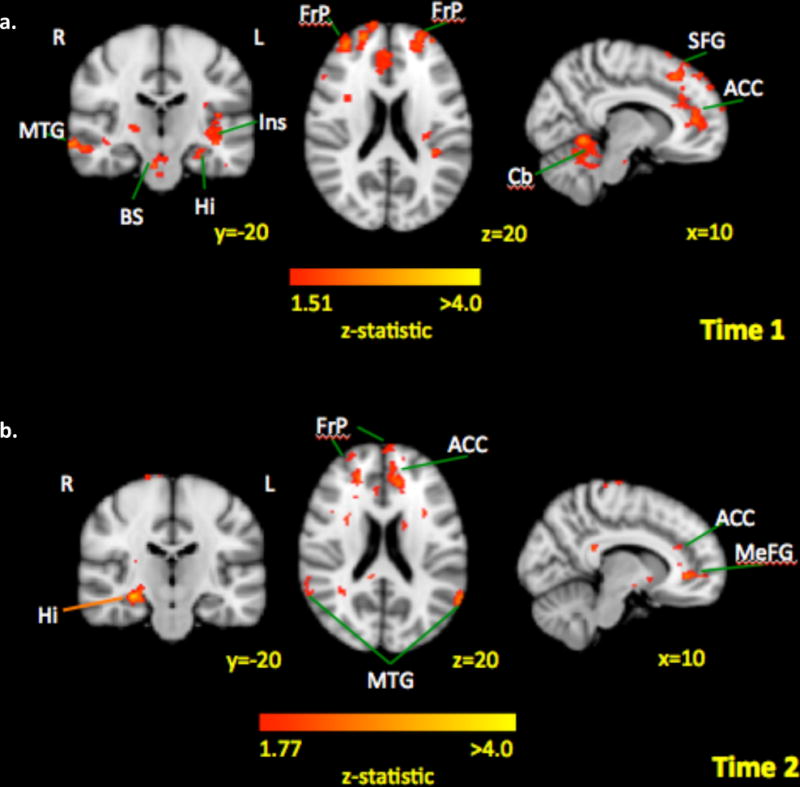
Across time, areas associated with fear circuitry were consistently associated with higher pain-related fear scores. Key: MTG: middle temporal gyrus; BS: brain stem; Hi: hippocampus; Ins: insula; FrP: frontal pole; ACC: anterior cingulate cortex; SFG: superior frontal gyrus; Cb: cerebellum; MeFG: medial frontal gyrus.
Beyond alterations in fear circuitry, we found that brain response to intensive pain rehabilitative treatment can be tied to reductions in pain-related fear[55]. Paired analysis of amygdala connectivity changes within patients was examined (Figure 5). Decreased functional coupling between the left amygdala and prefrontal cortex, supplementary motor area, anterior cingulate cortex, and insula after treatment was associated with decreases in pain-related fear. Changes in pain did not correlate with changes in amygdala connectivity. These results suggest that improvements in function and fear are closely linked to decoupling of the amygdala with key fear circuits in youth with CRPS. It is yet unknown if these changes reflect greater neural plasticity in children compared to adults, and future research that includes both pediatric and adult patients is necessary to answer this lingering question.
Figure 5. Treatment Response from Time 1 to Time 2: Decrease in functional connectivity and pain-related fear.
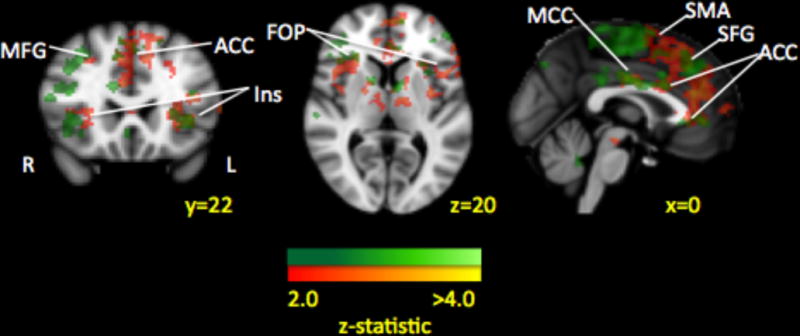
Paired analysis of amygdala connectivity changes within patients is depicted in green while connectivity changes that correlated with changes in pain-related fear are displayed in red. Many of the amygdala connectivity decreases were correlated with decreases in pain-related fear after treatment, suggesting that changes in intrinsic brain functional connectivity can be linked to symptom improvement. Key: MFG: middle frontal gyrus; Ins: insula; SMA: supplementary motor area; ACC: anterior cingulate cortex; MCC: middle cingulate cortex; FOP: frontal opercular cortex.
Conclusions
There is strong support for the detrimental impact of fear of pain and associated avoidance and disability on children suffering with chronic pain. Additionally, emerging evidence highlights the additive impact of parent variables, including fear and avoidance, on the child chronic pain experience. Fortunately, initial findings suggest that exposure-based treatments and intensive interdisciplinary pain programs result in improvements in pain-related fear. Additionally, innovative imaging studies that examine fear-related brain circuitry coincide with the phenotypic presentation of pain-related fear in the clinic. Moreover, we have captured changes in fear circuits that correlate with improvements in pain-related fear, suggesting brain plasticity with recovery. Further work is necessary to determine if these changes are unique to the pediatric CRPS patient. As we look forward, it will be essential to integrate our accumulating knowledge of basic learning mechanisms to our understanding and treatment of pain-related fear in youth. Tenets and perhaps even whole theories will need to be refined or redefined to keep pace with the rapidly evolving field.
Acknowledgments
This study were supported by an NIH grant (K23 HD067202) awarded to LS and the Sara Page Mayo Endowment for Pediatric Pain Research and Treatment, and the Department of Anesthesiology, Perioperative and Pain Medicine at Boston Children’s Hospital.
Footnotes
Disclosures: Dr. Simons reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Bailey KM, Carleton RN, Vlaeyen JW, Asmundson GJ. Treatments Addressing Pain-Related Fear and Anxiety in Patients with Chronic Musculoskeletal Pain: A Preliminary Review. Cognitive behaviour therapy. 2009:1. doi: 10.1080/16506070902980711. [DOI] [PubMed] [Google Scholar]
- 2.Barke A, Baudewig J, Schmidt-Samoa C, Dechent P, Kroner-Herwig B. Neural correlates of fear of movement in high and low fear-avoidant chronic low back pain patients: an event-related fMRI study. Pain. 2012;153(3):540–552. doi: 10.1016/j.pain.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20(5):947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 4.Caes L, Vervoort T, Eccleston C, Goubert L. Parents who catastrophize about their child’s pain prioritize attempts to control pain. Pain. 2012;153(8):1695–1701. doi: 10.1016/j.pain.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Carpino E, Segal S, Logan D, Lebel A, Simons LE. The interplay of pain-related self-efficacy and fear on functional outcomes among youth with headache. J Pain. 2014;15(5):527–534. doi: 10.1016/j.jpain.2014.01.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho JH, Deisseroth K, Bolshakov VY. Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron. 2013;80(6):1491–1507. doi: 10.1016/j.neuron.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Gier M, Peters ML, Vlaeyen JW. Fear of pain, physical performance, and attentional processes in patients with fibromyalgia. Pain. 2003;104(1–2):121–130. doi: 10.1016/s0304-3959(02)00487-6. [DOI] [PubMed] [Google Scholar]
- 8.de Jong JR, Vlaeyen JW, de Gelder JM, Patijn J. Pain-related fear, perceived harmfulness of activities, and functional limitations in complex regional pain syndrome type I. The journal of pain: official journal of the American Pain Society. 2011;12(12):1209–1218. doi: 10.1016/j.jpain.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 9.de Jong JR, Vlaeyen JW, Onghena P, Cuypers C, den Hollander M, Ruijgrok J. Reduction of pain-related fear in complex regional pain syndrome type I: the application of graded exposure in vivo. Pain. 2005;116(3):264–275. doi: 10.1016/j.pain.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 10.de Jong JR, Vlaeyen JW, van Eijsden M, Loo C, Onghena P. Reduction of pain-related fear and increased function and participation in work-related upper extremity pain (WRUEP): effects of exposure in vivo. Pain. 2012;153(10):2109–2118. doi: 10.1016/j.pain.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Debiec J, Sullivan RM. Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(33):12222–12227. doi: 10.1073/pnas.1316740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn KM, Jordan KP, Mancl L, Drangsholt MT, Le Resche L. Trajectories of pain in adolescents: a prospective cohort study. Pain. 2011;152(1):66–73. doi: 10.1016/j.pain.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flor H. New developments in the understanding and management of persistent pain. Current opinion in psychiatry. 2012;25(2):109–113. doi: 10.1097/YCO.0b013e3283503510. [DOI] [PubMed] [Google Scholar]
- 14.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133(4):581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 15.Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nature neuroscience. 2004;7(10):1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- 16.Goubert L, Simons LE. Cognitive styles and processes in pediatric pain. In: McGrath P, Stevens B, Walker S, Zempsky W, editors. Oxford Textbook of Pediatric Pain. Oxford University Press; 2014. pp. 95–101. [Google Scholar]
- 17.Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR. Neural reactivity tracks fear generalization gradients. Biological psychology. 2013;92(1):2–8. doi: 10.1016/j.biopsycho.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Groenewald CB, Essner BS, Wright D, Fesinmeyer MD, Palermo TM. The economic costs of chronic pain among a cohort of treatment-seeking adolescents in the United States. The journal of pain: official journal of the American Pain Society. 2014;15(9):925–933. doi: 10.1016/j.jpain.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartley CA, Fischl B, Phelps EA. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cerebral cortex. 2011;21(9):1954–1962. doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35(1):136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasenbring MI, Hallner D, Klasen B, Streitlein-Bohme I, Willburger R, Rusche H. Pain-related avoidance versus endurance in primary care patients with subacute back pain: psychological characteristics and outcome at a 6-month follow-up. Pain. 2012;153(1):211–217. doi: 10.1016/j.pain.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Huguet A, Miro J. The severity of chronic pediatric pain: an epidemiological study. The journal of pain: official journal of the American Pain Society. 2008;9(3):226–236. doi: 10.1016/j.jpain.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(37):9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashikar-Zuck S, Vaught MH, Goldschneider KR, Graham TB, Miller JC. Depression, coping, and functional disability in juvenile primary fibromyalgia syndrome. The journal of pain: official journal of the American Pain Society. 2002;3(5):412–419. doi: 10.1054/jpai.2002.126786. [DOI] [PubMed] [Google Scholar]
- 25.King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152(12):2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(1):218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognitive, affective & behavioral neuroscience. 2004;4(3):317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- 28.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20(5):937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 29.Lambin DI, Thibault P, Simmonds M, Lariviere C, Sullivan MJ. Repetition-induced activity-related summation of pain in patients with fibromyalgia. Pain. 2011;152(6):1424–1430. doi: 10.1016/j.pain.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 30.Lebowitz ER, Shic F, Campbell D, MacLeod J, Silverman WK. Avoidance moderates the association between mothers’ and children’s fears: findings from a novel motion-tracking behavioral assessment. Depression and anxiety. 2015;32(2):91–98. doi: 10.1002/da.22333. [DOI] [PubMed] [Google Scholar]
- 31.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. Journal of behavioral medicine. 2007;30(1):77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 32.Logan DE, Simons LE, Carpino EA. Too sick for school? Parent influences on school functioning among children with chronic pain. Pain. 2012;153(2):437–443. doi: 10.1016/j.pain.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohnberg JA. A review of outcome studies on cognitive-behavioral therapy for reducing fear-avoidance beliefs among individuals with chronic pain. Journal of Clinical Psychology in Medical Settings. 2007;14(2):113–122. [Google Scholar]
- 34.Lynch-Jordan AM, Kashikar-Zuck S, Szabova A, Goldschneider KR. The interplay of parent and adolescent catastrophizing and its impact on adolescents’ pain, functioning, and pain behavior. The Clinical journal of pain. 2013;29(8):681–688. doi: 10.1097/AJP.0b013e3182757720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin AL, McGrath PA, Brown SC, Katz J. Anxiety sensitivity, fear of pain and pain-related disability in children and adolescents with chronic pain. Pain Res Manag. 2007;12(4):267–272. doi: 10.1155/2007/897395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meier ML, Stampfli P, Vrana A, Humphreys BK, Seifritz E, Hotz-Boendermaker S. Fear avoidance beliefs in back pain-free subjects are reflected by amygdala-cingulate responses. Frontiers in human neuroscience. 2015;9:424. doi: 10.3389/fnhum.2015.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Muris P, Steerneman P, Merckelbach H, Meesters C. The role of parental fearfulness and modeling in children’s fear. Behaviour research and therapy. 1996;34(3):265–268. doi: 10.1016/0005-7967(95)00067-4. [DOI] [PubMed] [Google Scholar]
- 39.Naliboff BD, Waters AM, Labus JS, Kilpatrick L, Craske MG, Chang L, Negoro H, Ibrahimovic H, Mayer EA, Ornitz E. Increased acoustic startle responses in IBS patients during abdominal and nonabdominal threat. Psychosomatic medicine. 2008;70(8):920–927. doi: 10.1097/PSY.0b013e318186d858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noel M, Rabbitts JA, Tai GG, Palermo TM. Remembering Pain after Surgery: A Longitudinal Examination of the Role of Pain Catastrophizing in Children’s and Parents’ Recall. Pain. 2015 doi: 10.1097/j.pain.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norton PJ, Asmundson GJ. Anxiety sensitivity, fear, and avoidance behavior in headache pain. Pain. 2004;111(1–2):218–223. doi: 10.1016/j.pain.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Ochsner KN, Ludlow DH, Knierim K, Hanelin J, Ramachandran T, Glover GC, Mackey SC. Neural correlates of individual differences in pain-related fear and anxiety. Pain. 2006;120(1–2):69–77. doi: 10.1016/j.pain.2005.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsson A, Phelps EA. Social learning of fear. Nature neuroscience. 2007;10(9):1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- 44.Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neuroscience and biobehavioral reviews. 2012;36(7):1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Page MG, Campbell F, Isaac L, Stinson J, Martin-Pichora AL, Katz J. Reliability and validity of the Child Pain Anxiety Symptoms Scale (CPASS) in a clinical sample of children and adolescents with acute postsurgical pain. Pain. 2011;152(9):1958–1965. doi: 10.1016/j.pain.2011.02.053. [DOI] [PubMed] [Google Scholar]
- 46.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 47.Pielech M, Ryan M, Logan D, Kaczynski K, White MT, Simons LE. Pain catastrophizing in children with chronic pain and their parents: proposed clinical reference points and reexamination of the Pain Catastrophizing Scale measure. Pain. 2014;155(11):2360–2367. doi: 10.1016/j.pain.2014.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS neuroscience & therapeutics. 2011;17(4):227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salomons TV, Davis KD. Fear avoidance and neuroimaging: falsification or just failure to confirm? Pain. 2012;153(3):511–512. doi: 10.1016/j.pain.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 51.Simons LE, Elman I, Borsook D. Psychological processing in chronic pain: a neural systems approach. Neuroscience and biobehavioral reviews. 2014;39:61–78. doi: 10.1016/j.neubiorev.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simons LE, Kaczynski KJ. The Fear Avoidance model of chronic pain: examination for pediatric application. J Pain. 2012;13(9):827–835. doi: 10.1016/j.jpain.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: evidence from neuroimaging. Human brain mapping. 2014;35(2):527–538. doi: 10.1002/hbm.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simons LE, Pielech M, Cappucci S, Lebel A. Fear of pain in pediatric headache. Cephalalgia: an international journal of headache. 2015;35(1):36–44. doi: 10.1177/0333102414534084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simons LE, Pielech M, Erpelding N, Linnman C, Moulton E, Sava S, Lebel A, Serrano P, Sethna N, Berde C, Becerra L, Borsook D. The responsive amygdala: treatment-induced alterations in functional connectivity in pediatric complex regional pain syndrome. Pain. 2014;155(9):1727–1742. doi: 10.1016/j.pain.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simons LE, Sieberg CB, Carpino E, Logan D, Berde C. The Fear of Pain Questionnaire (FOPQ): assessment of pain-related fear among children and adolescents with chronic pain. J Pain. 2011;12(6):677–686. doi: 10.1016/j.jpain.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Simons LE, Smith A, Ibagon C, Coakley R, Logan DE, Schechter N, Borsook D, Hill JC. Pediatric Pain Screening Tool: rapid identification of risk in youth with pain complaints. Pain. 2015;156(8):1511–1518. doi: 10.1097/j.pain.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simons LE, Smith A, Kaczynski K, Basch M. Living in Fear of Your Child’s Pain: The Parent Fear of Pain Questionnaire. Pain. 2015 doi: 10.1097/j.pain.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biological psychiatry. 2006;60(4):329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Thibodeau MA, Fetzner MG, Carleton RN, Kachur SS, Asmundson GJ. Fear of injury predicts self-reported and behavioral impairment in patients with chronic low back pain. The journal of pain: official journal of the American Pain Society. 2013;14(2):172–181. doi: 10.1016/j.jpain.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Trost Z, France CR, Thomas JS. Examination of the photograph series of daily activities (PHODA) scale in chronic low back pain patients with high and low kinesiophobia. Pain. 2009;141(3):276–282. doi: 10.1016/j.pain.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 62.Trouche S, Sasaki JM, Tu T, Reijmers LG. Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron. 2013;80(4):1054–1065. doi: 10.1016/j.neuron.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Well S, Visser RM, Scholte HS, Kindt M. Neural substrates of individual differences in human fear learning: evidence from concurrent fMRI, fear-potentiated startle, and US-expectancy data. Cognitive, affective & behavioral neuroscience. 2012;12(3):499–512. doi: 10.3758/s13415-012-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verbunt JA, Nijhuis A, Vikstrom M, Stevens A, Haga N, de Jong J, Goossens M. The psychometric characteristics of an assessment instrument for perceived harmfulness in adolescents with musculoskeletal pain (PHODA-youth) European journal of pain. 2014 doi: 10.1002/ejp.592. [DOI] [PubMed] [Google Scholar]
- 65.Verbunt JA, Seelen HA, Vlaeyen JW, van der Heijden GJ, Knottnerus JA. Fear of injury and physical deconditioning in patients with chronic low back pain. Archives of physical medicine and rehabilitation. 2003;84(8):1227–1232. doi: 10.1016/s0003-9993(03)00132-1. [DOI] [PubMed] [Google Scholar]
- 66.Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. The treatment of fear of movement/(re)injury in chronic low back pain: further evidence on the effectiveness of exposure in vivo. The Clinical journal of pain. 2002;18(4):251–261. doi: 10.1097/00002508-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153(6):1144–1147. doi: 10.1016/j.pain.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 68.Vlaeyen JW, Morley S, Linton S, Boersma K, De Jong J. Pain-related fear: Exposure-based treatment of chronic pain. Seattle: IASP Press; 2012. [Google Scholar]
- 69.Walker LS, Dengler-Crish CM, Rippel S, Bruehl S. Functional abdominal pain in childhood and adolescence increases risk for chronic pain in adulthood. Pain. 2010;150(3):568–572. doi: 10.1016/j.pain.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Welkom JS, Hwang WT, Guite JW. Adolescent pain catastrophizing mediates the relationship between protective parental responses to pain and disability over time. Journal of pediatric psychology. 2013;38(5):541–550. doi: 10.1093/jpepsy/jst011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wicksell RK, Melin L, Lekander M, Olsson GL. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain–a randomized controlled trial. Pain. 2009;141(3):248–257. doi: 10.1016/j.pain.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 72.Wicksell RK, Olsson GL, Hayes SC. Mediators of change in acceptance and commitment therapy for pediatric chronic pain. Pain. 2011;152(12):2792–2801. doi: 10.1016/j.pain.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Wideman TH, Asmundson GG, Smeets RJ, Zautra AJ, Simmonds MJ, Sullivan MJ, Haythornthwaite JA, Edwards RR. Rethinking the fear avoidance model: toward a multidimensional framework of pain-related disability. Pain. 2013;154(11):2262–2265. doi: 10.1016/j.pain.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. NeuroImage. 2009;47(3):987–994. doi: 10.1016/j.neuroimage.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 75.Wiech K, Tracey I. Pain, decisions, and actions: a motivational perspective. Frontiers in neuroscience. 2013;7:46. doi: 10.3389/fnins.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson AC, Lewandowski AS, Palermo TM. Fear-avoidance beliefs and parental responses to pain in adolescents with chronic pain. Pain Res Manag. 2011;16(3):178–182. doi: 10.1155/2011/296298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zale EL, Lange KL, Fields SA, Ditre JW. The relation between pain-related fear and disability: a meta-analysis. J Pain. 2013;14(10):1019–1030. doi: 10.1016/j.jpain.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


