Abstract
Background
In neonatal rats, early post-hypoxia-ischemia administration of the omega-3-fatty acid docosahexaenoic acid (DHA) improves sensorimotor function but does not attenuate brain damage.
Objective
To determine if DHA administration in addition to hypothermia, now standard care for neonatal asphyxial brain injury, attenuates post-hypoxia-ischemia damage and sensorimotor deficits.
Methods
Seven-day-old (P7) rats underwent right carotid ligation followed by 90 min 8% O2 exposure. Fifteen min later, pups received injections of DHA 2.5 mg/kg (complexed to 25% albumin) or equal volumes of albumin. After 1 h recovery, pups were cooled (3 h, 30°C). Sensorimotor and pathology outcomes were initially evaluated on P14. In subsequent experiments, sensorimotor function was evaluated on P14, 21, and 28; histopathology was assessed on P28.
Results
At P14, left forepaw function scores (normal: 20/20), were near normal in DHA+Hypothermia-treated animals (mean±SD: 19.7±0.7 DHA+Hypothermia vs. 12.7±3.5 Albumin+Hypothermia, p<0.0001) and brain damage was reduced (mean±SD right hemisphere damage (%) 38±17 DHA+Hypothermia vs. 56±15 Albumin+Hypothermia, p=0.003). Substantial improvements on 3 sensorimotor function measures and reduced brain damage were evident up to P28.
Conclusion
Unlike post-hypoxia-ischemia treatment with DHA alone, treatment with DHA plus hypothermia produced both sustained functional improvement and reduced brain damage after neonatal hypoxia-ischemia.
Introduction
Docosahexaenoic acid (DHA) is a dietary long-chain omega-3 polyunsaturated fatty acid with neuroprotective properties [1-7]. DHA modulates neuroinflammation, oxidative stress, and apoptosis [7-9]. We previously reported that DHA pre-treatment is neuroprotective in a neonatal rodent model of hypoxic-ischemic (HI) brain injury, elicited by right carotid artery ligation and timed exposure to 8% oxygen in 7 day old (P7) rats [4]; a single injection of DHA prior to lesioning improved sensorimotor function and reduced brain damage. Subsequently, we found that a single DHA injection after the end of hypoxia exposure improved the same neurologic function but did not reduce tissue injury [5]. Other investigators showed that chronic maternal dietary supplementation with DHA or fish oil (enriched in DHA) in the prenatal and lactation period also confers neuroprotection and reduces brain damage in their offspring in this model [6,7]. Since most perinatal asphyxia insults occur sporadically and unpredictably in term infants, we focused our pre-clinical investigations on optimization of the efficacy of DHA administered after acute HI injury.
Hypothermia decreases death or disability in neonates with HI brain injury [10]. Yet, 40-50% of infants treated with hypothermia have adverse outcomes and treatments to augment the neuroprotective efficacy of hypothermia are needed [11]. Potentially neuroprotective drugs, such as DHA, administered in conjunction with therapeutic hypothermia, could exert additive or synergistic neuroprotection or paradoxically counteract the benefits of cooling. To further evaluate the neuroprotective properties of DHA, it was thus essential to examine its efficacy in combination with hypothermia. We developed a protocol for delayed onset, relatively brief post injury cooling to evaluate drug-hypothermia interactions, either beneficial or harmful, in the neonatal rodent hypoxic-ischemic (HI) brain injury model [12-14]. The stepwise evaluation begins with a comparison of drug vs. vehicle and then comparison of adding hypothermia to drug and vehicle-treated groups. Based on our prior report [5], in this study, we selected a post-HI DHA dose that resulted in better sensorimotor function than in vehicle-treated controls without any benefit on tissue damage. We examined the impact of combining this DHA dose with brief hypothermia on function and neuropathology outcomes. We found sustained benefits of combination treatment with DHA and brief hypothermia.
Methods
DHA treatment
DHA (Sigma-Aldrich, St. Louis, MO) was complexed to human 25% albumin, as previously described (final concentration 0.5 mg/1 ml) [4]. The dose selected, 2.5 mg/kg, was more effective than either higher (5 mg/kg) or lower (1 mg/kg) doses in the post-HI treatment protocol reported previously [5].
Wistar rat pups were obtained in litters adjusted to equal sex distribution (Charles River Laboratories, Portage, MI) and were weaned on P21. Animals were treated in accordance with protocols approved by the University of Michigan Committee on the Use and Care of Animals.
Each of six independent experiments (n=12 animals/experiment) included equal numbers of litter-mate DHA-treated and albumin-treated pups; animals of both genders were allocated equally between groups in each experiment Isoflurane-anesthetized pups underwent right carotid artery ligation, as previously described [4,15]. Pups recovered (36.5°C, 90 min), and then were exposed to 8% oxygen (90 min). After 15 min recovery periods (in 37°C incubators), they received equal volume intra-peritoneal injections (0.05 ml/10 gm) of DHA or 25% albumin; they returned to incubators (45 min) until initiation of hypothermia. Pups were then placed in a circulating air incubator with cuffed portals (30°C, 3h) and were separated with partitions to prevent huddling [13,14]. Pups then returned to dams until P14 (3 experiments) or P28 (3 experiments). Seven sequential temperature measurements were obtained (YSI thermometer 43T with probe 554, Yellow Springs, OH) from baseline to the conclusion of hypothermia [14].
Sensorimotor Testing
In this HI model, a variety of sensorimotor tests provide useful outcome measures in neuroprotection studies [4,5,7,12,13,14,16,17]. We incorporated 3 quantifiable, objective tests.
1. Vibrissae-stimulated forepaw place testing
On P14, or weekly on P14, 21 and 28, animals underwent lateral vibrissae-stimulated forepaw place testing [4,5]. Vibrissae are stimulated unilaterally on a surface edge. As early as P14, the normal complete response is consistent immediate extension of the ipsilateral forepaw to contact the stimulus surface. Performance is impaired in the forepaw contralateral to an HI cerebral hemisphere lesion. The impaired forepaw either does not move or demonstrates a partial extension movement that is insufficient to reach the stimulus surface. Performance was videotaped and scored in 10 trials/forepaw by an observer unaware of treatment group (partial response score=1, complete response score=2) [4].
2. Grip Traction Test
On P21 and P28, forepaw grip strength (maximal force applied in grasping a pull bar) was measured using a Grip strength meter (3 measurements/ forepaw; Columbus Instruments, Columbus, OH). Normal animals have equal strength bilaterally, and grip strength increases between P21 and P28 [14]. Absolute values for grip strength and left/right forepaw grip strength ratios were calculated for each animal. After 90 min duration HI lesioning on P7, animals typically have about 40-50% reduction in contralateral grip strength 2-4 weeks later [14].
3. Vertical Cylinder Exploration
On P28, forepaw preference was evaluated. Normal animals initiate exploratory movements equally with both forepaws; animals that underwent HI lesioning on P7 typically display decreased initiation of weight-bearing contacts with the forepaw contralateral to brain injury [16]. Animals were videotaped in a vertical clear plastic cylinder (20×30 cm, 2 min); initial weight-bearing contacts of the right, left, or both forepaws with the cylinder wall were counted. Right forepaw preference scores were calculated with the formula 100*(Right-Left)/(Right+Left+Both) (normal=0) [18].
Histopathology
Animals were euthanized and brains removed and frozen on P14 or on P28. Regularly spaced coronal 20-micrometer sections, from the anterior genu to the posterior genu of the corpus callosum, were cresyl-violet stained. Using ImageJ software (US National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/), bilateral volumes were calculated from hemisphere and regional area measurements in at least 10 sections/brain; percent reductions in right hemisphere volumes, compared to left, were calculated {100*[left-right/left]} [14].
Statistics
Sample sizes (18/group) were selected based on power calculations with data from prior similar experiments. Differences in contralateral vibrissae-stimulated forepaw placing responses, forepaw grip strength and cylinder right forepaw preference score were compared using repeated measures ANOVA. Post-hoc comparisons of treatment group means were carried out using the Tukey Kramer single step multiple comparison procedure. A linear mixed models analysis of variance (ANOVA) was applied to evaluate differences in percent brain damage. We used litter as a random effect, with treatment, sex and brain region as fixed effects. Differences in body temperatures and weights were assessed by repeated measures ANOVA, factoring treatment and sex.
RESULTS
Survival/morbidity
There were no differences in mortality, mean temperatures (Table 1), or in weights or weight changes (not shown).
Table 1.
Sequential temperature measurementsa
| Time-points | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Experiment/Groupb | N | Pre-surgery | End of HI | 15 min post-HI | 60 min post-HI | 75 min post-HI | 90 min post-HI | 240 min post-HI |
| DHA+HT | 36 | 33.1 ±0.8 | 35.0 ±0.6 | 35.2 ±0.5 | 36.0 ±0.4 | 31.3 ±0.8 | 31.2 ±0.7 | 32.2 ±1.0 |
| ALB+HT | 36 | 33.3 ±0.8 | 34.8 ±0.7 | 35.3 ±0.6 | 36.1 ±0.3 | 31.3 ±0.8 | 31.1 ±0.6 | 32.2 ±1.1 |
HI: hypoxia-ischemia; ALB: 25%albumin; DHA: docosahexaenoic acid; HT: hypothermia.
: temperatures (°C) are expressed as mean ±SD.
: All animals underwent HI lesioning (see Methods); 15 min after the end of HI all received injections of DHA 2.5 mg/kg (in 25% albumin) or an equivalent volume of 25% albumin. All animals underwent HT (30°C incubator for 3h, beginning at 1 h after HI); time-point 4 corresponds with the beginning of HT and time-point 7 is at the end of HT. Data presented includes 3 litters with outcome to P14 and 3 litters with outcome to P28.
Initial (P14) Outcomes
Sensorimotor function
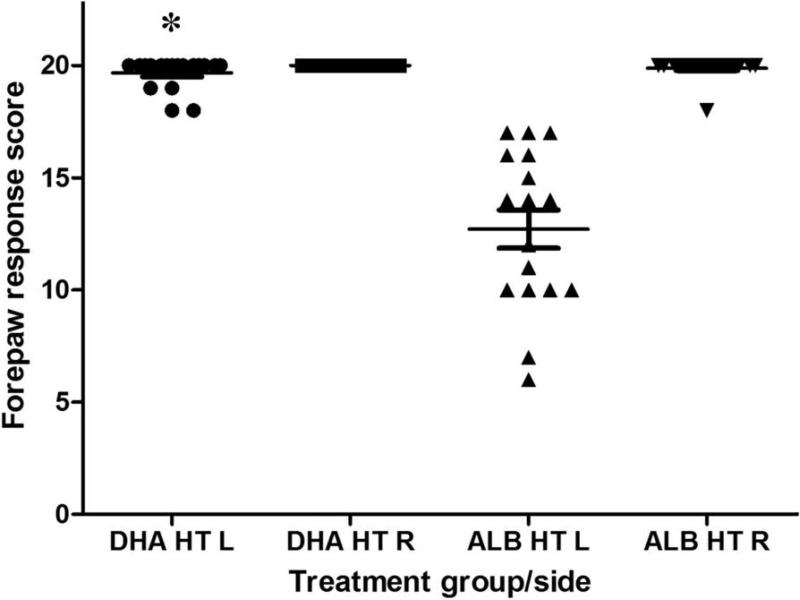
Right forepaw placing scores were normal in all DHA-treated and 16/17 albumin-treated animals (one scored 18/20). Contralateral (left) placing responses were near normal in the DHA+Hypothermia group, but significantly impaired in the Albumin+Hypothermia group (Figure 1) (total scores: 19.7/20±3.5 vs. 12.7/20±0.7, p< 0.0001, t test; complete responses: 9.7/10±0.6 vs. 5.9/10±1.8, p< 0.0001, t test; no gender effect).
Figure 1. Treatment with DHA and delayed-onset hypothermia attenuates P14 forepaw placing deficits.
P14 bilateral vibrissae-stimulated forepaw placing test scores (mean±SEM, normal score=20, see Methods) in animals that underwent right carotid ligation followed by 90 min 8% O2 exposure on P7, and received DHA, 2.5 mg/kg or 25% albumin, followed 1h later by hypothermia (3h, 30 °C). Right(R) forepaw scores were all normal; left forepaw placement was impaired in albumin-treated controls (n=17, ALB HT L) and but performance near-normal in the DHA-HT group (n=18, DHA HT L, *p<0.01 ANOVA, Tukey Kramer post-hoc test).
Histopathology
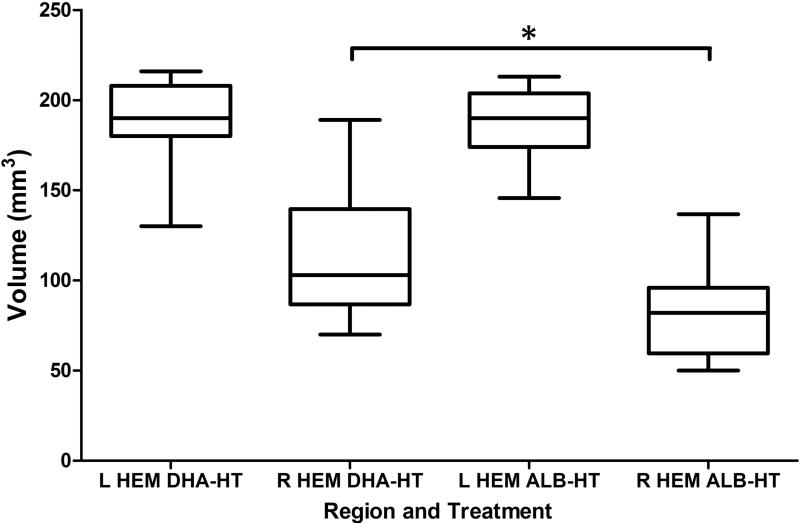
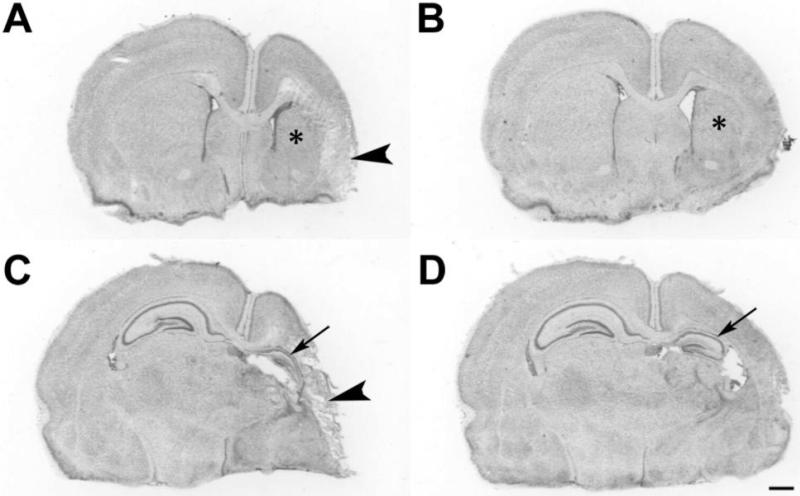
In controls, there was 55±15 % right hemisphere volume loss; damage was modestly attenuated in the DHA+Hypothermia group (38±17 %) (p=0.003, t-test) (Figure 2). Figure 3 (A, C) illustrates a representative control lesion with right striatal and hippocampal atrophy and cortical infarction; Figure 3 (panels B, D) illustrates representative pathology from the DHA+Hypothermia group with greater tissue preservation. Table 2 presents regional volume data. On P14, tissue damage was reduced in the DHA+Hypothermia group (ANOVA, p < 0.001) with region-specific treatment effects in cortex (p = 0.0004) and striatum (p = 0.0014), and no gender effects.
Figure 2. Treatment with DHA and hypothermia reduces P14 brain damage.
Bilateral cerebral hemisphere volumes on P14, calculated from cross sectional area measurements (see Methods), are compared in DHA+Hypothermia (DHA-HT, n=18) and Albumin+Hypothermia (ALB-HT, n=17) groups (Box: median and interquartile range; whiskers: minimum and maximum). Right hemisphere damage is reduced in the DHA-hypothermia treated group (*p< 0.05, t-test).
Figure 3. Neuropathology.
These cresyl violet stained coronal sections at the level of striatum (A,B) and hippocampus (C,D) illustrate representative histopathology in DHA+hypothermia (B,D) and albumin-hypothermia (A,C) treated animals on P14. After right carotid ligation+90 min 8% O2 exposure, P7 pups received DHA, 2.5 mg/kg (B,D) or 25% albumin vehicle, followed 1h later by hypothermia (3h, 30 °C). In the control (A,C) there is right cortical infarction (arrowheads), striatal atrophy and infarction (*) and hippocampal atrophy (arrow). In the DHA+Hypothermia brain (B,D), there is less right hemisphere, striatal (*) and hippocampal (arrow) atrophy (scale bar=1mm).
Table 2.
Effect of docosahexaenoic acid (DHA) and therapeutic hypothermia (HT) on regional brain damage severity a
| Cortex |
Striatum |
Hippocampus |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P14 Outcomes | ||||||||||
| Treatment | n | Left b | Right b | % Diffc | Left | Right | % Diff | Left | Right | % Diff |
| 25% Albumin +HT | 17 | 69 ± 16 | 25 ± 15 | 67 ± 11 | 23 ± 4 | 13 ± 4 | 46 ± 3 | 6 ± 3 | 2± 1 | 64 ± 1 |
| DHA 2.5 mg/kg+HT | 18 | 62 ± 16 | 42 ± 19* | 40 ± 13* | 23 ± 4 | 16 ± 5* | 32 ± 3* | 6 ± 3 | 2 ± 1 | 61 ± 1 |
| P28 Outcomes | ||||||||||
| Treatment | n | Leftb | Rightb | % Diffc | Left | Right | % Diff | Left | Right | % Diff |
| 25% Albumin +HT | 17 | 98 ± 7 | 29 ± 13 | 70 ± 13 | 34 ± 4 | 13 ± 3 | 62 ± 10 | 13 ± 3 | 2± 2 | 83 ± 13 |
| DHA 2.5 mg/kg+HT | 18 | 98 ± 8 | 41 ± 18* | 59 ± 17* | 34 ± 3 | 17 ± 5* | 50 ± 13* | 13 ± 3 | 4 ± 3* | 68 ±18* |
: All animals were lesioned on P7 and tissue damage was evaluated on P14 (A) or P28 (B) (see Methods).
: Left and right values represent regional volumes in each cerebral hemisphere (mm3, mean ± SD)
: Diff = difference; % Diff values (mean ± SD) represent regional % damage, calculated from regional volumes with the formula 100 * (Left-Right)/Left.
p <0.05 compared to Albumin+HT (ANOVA with Tukey Kramer post-hoc test)
Outcomes on P14-P28
Sensorimotor function
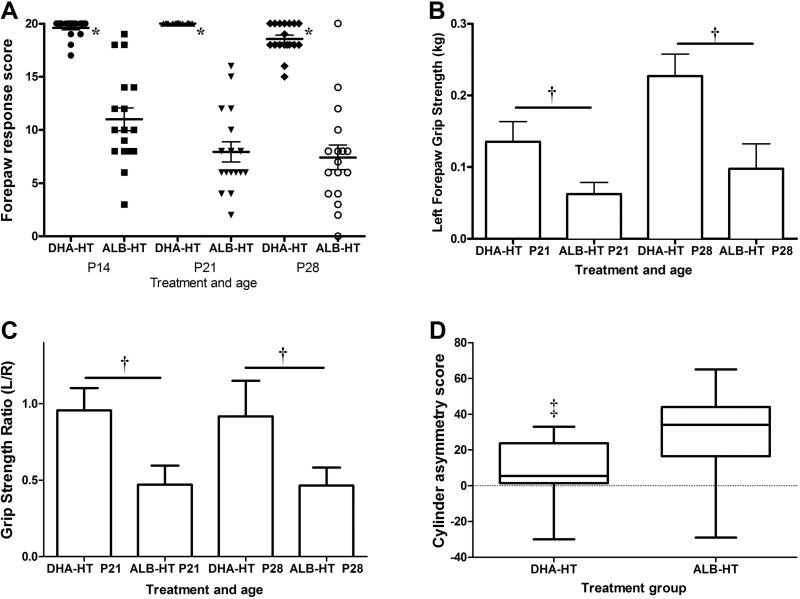
All right forepaw placing scores were normal. Left forepaw scores remained near normal in the DHA+Hypothermia group, and deficits persisted in the Albumin+Hypothermia group (p<0.0001 for treatment, p<0.01 for time, with no treatment*time interaction, repeated measures ANOVA, Figure 4A). Right forepaw grip strength increased from P21 to P28 (Figure 4B). In the DHA+Hypothermia group, left and right forepaw grip strength did not differ; in the Albumin+Hypothermia group, left forepaw strength remained at about 50% of right forepaw grip strength (Grip strength ratio, p<0.0001 for treatment, p=NS for time, repeated measures ANOVA, Figure 4C). In the cylinder test, at P28, there were asymmetries in both groups; asymmetric function was greater in Albumin+Hypothermia than DHA+ Hypothermia groups (p<0.005, t-test) (Figure 4D). There were no gender effects on any scores.
Figure 4. Performance on three measures of sensorimotor function.
Vibrissae-stimulated forepaw placing was evaluated on P14, P21, P28 (A), forepaw grip strength on P21 and P28 (B, C) and vertical cylinder forepaw contact preference on P28 (D) in DHA-hypothermia (DHA-HT) and control albumin-hypothermia (ALB-HT) groups. Left forepaw vibrissae-stimulated placing scores (mean±SEM) remained near normal in the DHA-HT group (n=18); impaired placing persisted in ALB-HT controls (n=17) [A, * p<0.0001 for treatment, p<0.01 for time, with no treatment*time interaction, repeated-measures ANOVA (RMANOVA)]. Right forepaw grip strength (mean ±SD) increased from P21 to P28 (B, p<0.0001, RMANOVA). In the DHA-HT group there was no right-left difference at P21 or P28 (C); in ALB-HT controls, left forepaw grip strength was reduced at both ages, compared to right (mean ±SD L/R ratio, C), and compared to left forepaw in the DHA+Hypothermia group (B, C † p<0.0001 ANOVA), and remained at about 50% of right forepaw strength at both ages (C, L/R grip strength ratio † p<0.0001 for treatment, RMANOVA). In the vertical cylinder test, on P28, both groups had positive scores, indicating right forepaw preference (Box: median and interquartile range; whiskers: minimum and maximum), but asymmetry was attenuated (i.e. lower score) in the DHA-HT group (‡ p<0.005 t-test).
Histopathology
Results were congruent with P14 outcomes (Figure 5). In the DHA+Hypothermia group there were region-specific effects in cortex, striatum and hippocampus (p<0.05 ANOVA; p<0.05, Tukey-Kramer post-hoc tests). There were no gender effects.
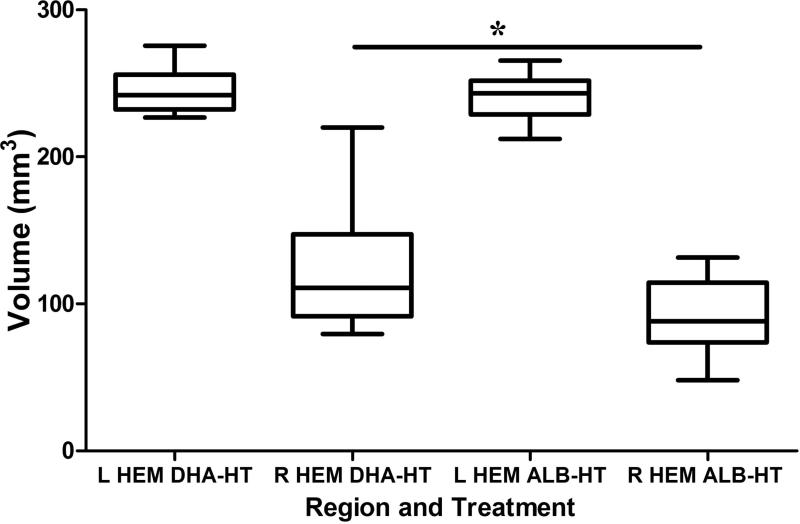
Figure 5. Sustained attenuation of brain damage by DHA+Hypothermia.
P28 bilateral cerebral hemisphere volumes were compared in DHA+Hypothermia (DHA-HT, n=18) and Albumin+Hypothermia (ALB-HT, n=17) groups (Box: median and interquartile range; whiskers: minimum and maximum). Right hemisphere damage is less severe in the DHA+Hypothermia group (*p<0.05).
DISCUSSION
Our results demonstrate that the combination of post-hypoxia-ischemia treatment with DHA and brief delayed hypothermia confers markedly improved sensorimotor function and modestly reduced tissue injury, compared to treatment with delayed hypothermia without DHA.
Brief post-HI hypothermia (started immediately after hypoxia) may delay progression of brain injury without conferring sustained neuroprotection [19]. Therefore, we replicated the P14 outcome experiments and confirmed that the beneficial effects of DHA+Hypothermia treatment were sustained. In addition, we incorporated three complementary sensorimotor tests to further characterize functional outcomes and to confirm persistence of improvements. Each testing method included objective, quantifiable measures. All three measures demonstrated substantially better function in the DHA+Hypothermia group; persistent asymmetries were most evident in the cylinder test. This task may be more sensitive to detection of subtle sensorimotor deficits.
Although this brief duration of hypothermia is not equivalent to the much longer duration of hypothermia that is currently applied clinically, our data provide important proof-of-principle evidence that the combination of DHA with hypothermia could represent a more effective post-asphyxia treatment than hypothermia alone. In an adult rat focal cerebral ischemia with reperfusion model, post-reperfusion treatment with a fatty acid mixture that included DHA aggravated injury, presumably by increasing the ischemia-induced oxidative burden [20]. Thus, in terms of translational potential for DHA in neonates, it is reassuring that we found no adverse interactions between DHA and hypothermia. The finding that DHA+hypothermia improved outcomes in a neonatal rodent model provide the impetus for more complex and costly experiments in larger neonatal animal models (e.g. piglets) in which prolonged hypothermia and physiologic monitoring are feasible. Future experiments could also clarify whether combination treatment conferred additive or synergistic neuroprotection.
The current study has several additional limitations. Although inclusion of a DHA-no hypothermia control group would have been ideal, we employed a pragmatic design that has been used to evaluate other novel therapeutic agents in combination with hypothermia [13,14]. We prioritized comparisons with litter-mate controls and evaluation of gender effects. DHA was complexed to albumin; although higher albumin doses may have neuroprotective properties, we previously showed that the albumin doses and administration route used in these experiments had no effects on outcomes in comparison with saline-injected controls [4].
Hypothermia could, hypothetically, either attenuate or amplify DHA neuroprotection. Hypothermia could result in increased accumulation of DHA-derived toxic isoprostanes and/or impaired conversion of DHA to its neuroprotective metabolite [8,9] however, we found no detrimental interactions. We speculate that in the injured neonatal brain hypothermia increased the neuroprotective efficacy of DHA by reducing non-enzymatic DHA peroxidation [21,22, 23] and/or by preserving DHA for enzymatic conversion via 15-lipoxygenase to the reported neuroprotective metabolite 10,17S-docosatriene (neuroprotectin D1) [9]. In an adult rat acute hypoxia model, hypothermia reduced lipid peroxidation [21]. We did not explore the underlying cellular and molecular mechanisms whereby DHA and hypothermia act in concert to attenuate brain injury and preserve sensorimotor function; this is a complex and challenging goal, and beyond the scope of this proof of principle study.
One of the intriguing findings at P28 was the near-normal sensorimotor function in the DHA+Hypothermia combination group, despite substantial forebrain tissue damage. This could suggest that DHA and hypothermia together promote plasticity mechanisms during recovery e.g. increased production of neurotrophins and/or stimulation of neurogenesis. In adult brain after global cerebral ischemia, post-resuscitation hypothermia augments brain-derived neurotrophic factor (BDNF) expression [24], and in normal adult rats a diet enriched in DHA increased BDNF expression in brain (the time course for this response was not examined) [25]. Similarly both interventions may also increase neurogenesis and could contribute to promotion of recovery, in part, through this mechanism [26,27].
Several drugs, including DHA, are attractive candidates for post-hypoxia-ischemia therapy in combination with hypothermia [11]. It will be challenging to decide which agents should move forward to clinical trial assessment. DHA readily crosses the blood-brain barrier, is designated Generally Regarded as Safe (GRAS) by the United States Food and Drug Administration [28], and can be administered parenterally. An investigational omega-3-enriched parenteral lipid emulsion is currently under study in neonates [29] and additional safety data is likely to emerge. The dose of DHA tested, 2.5 mg/kg, is within the feasible range for clinical applications with this emulsion.
Conclusions
Our findings provide the impetus for future studies to determine if findings with DHA+hypothermia can be replicated in large animal pre-clinical models of neonatal brain injury. The optimal dose(s) and routes of administration of DHA for post-ischemic neuroprotection also remain to be determined. Confirmatory results from additional pre-clinical studies could provide a compelling rationale for initiation of randomized controlled trials to test the effectiveness of DHA in combination with therapeutic hypothermia in neonates with moderate-to-severe hypoxic-ischemic encephalopathy.
Acknowledgments
ROLE OF THE FUNDING SOURCE:
The funding sources played no role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Statement of Financial Support: This work was supported through the NIH/NCRR CTSA grant UL1 RR024986, by NIH grant R21 AT006636 (to J.D.B. and F.S.S.) and by the GorgEffen Gift Fund of the Department of Pediatrics, The University of Michigan.
Footnotes
DISCLOSURE / CONFLICT OF INTEREST:
Drs. Berman, Liu, and Silverstein and Mr. Shangguan have no conflicts of interest to disclose. Dr. Mozurkewich is the principal investigator on an NIH-sponsored randomized controlled trial that has received donated fish oil capsules and placebo capsules from Nordic Naturals, Watsonville, CA. Dr. Barks received a donation of DHASCO oil from Martek Biosciences Inc., Columbia MD, for a different rodent research project.
REFERENCES
- 1.Belayev L, Marcheselli VL, Khoutorova L, Rodriguez de Turco EB, Busto R, Ginsberg MD, Bazan NG. Docosahexaenoic acid complexed to albumin elicits high-grade ischemic neuroprotection. Stroke. 2005;36:118–23. doi: 10.1161/01.STR.0000149620.74770.2e. [DOI] [PubMed] [Google Scholar]
- 2.Huang WL, King VR, Curran OE, Dyall SC, Ward RE, Lal N, Priestly JV, Michael-Titus AT. A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord injury. Brain. 2007;130:3004–19. doi: 10.1093/brain/awm223. [DOI] [PubMed] [Google Scholar]
- 3.Pan H, Kao T, Ou Y, Yang DY, Yen YJ, Wang CC, Chuang YH, Liao SL, Raung SL, Wu CW, Chiang AN, Chen CJ. Protective effect of docosahexaenoic acid against brain injury in ischemic rats. J Nutr Biochem. 2009;20:715–25. doi: 10.1016/j.jnutbio.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Berman DR, Mozurkewich E, Liu Y, Barks J. Docosahexaenoic acid pretreatment confers neuroprotection in a rat model of perinatal cerebral hypoxia-ischemia. Am J Obstet Gynecol. 2009;200:305, e1–6. doi: 10.1016/j.ajog.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berman D, Liu Y, Barks J, Mozurkewich E. Treatment with docosahexaenoic acid after hypoxia-ischemia improves forepaw placing in a rat model of perinatal hypoxia ischemia. Am J Obstet Gynecol. 2010;203:385.e1–5. doi: 10.1016/j.ajog.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suganuma H, Arai Y, Kitamura Y, Hayashi M, Okumura A, Shimizu T. Maternal docosahexaenoic acid-enriched diet prevents neonatal brain injury. Neuropathology. 2010;30:597–605. doi: 10.1111/j.1440-1789.2010.01114.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Hu X, Yang W, Gao Y, Chen J. Omega-3 polyunsaturated fatty acid supplementation confers long-term neuroprotection against neonatal hypoxic-ischemic brain injury through anti-inflammatory actions. Stroke. 2010;41:2341–7. doi: 10.1161/STROKEAHA.110.586081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcheselli V, Hong S, Lukiw W, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–17. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 9.Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations and Alzheimer's disease. J Lipid Res. 2009;50(Suppl):S400–5. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: Synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cilio MR, Ferriero DM. Synergistic neuroprotective therapies with hypothermia. Semin Fetal Neonat Med. 2010;15:293–8. doi: 10.1016/j.siny.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu YQ, Barks JD, Xu G, Silverstein FS. Topiramate extends the therapeutic window for hypothermia-mediated neuroprotection after stroke in neonatal rats. Stroke. 2004;35:1460–5. doi: 10.1161/01.STR.0000128029.50221.fa. [DOI] [PubMed] [Google Scholar]
- 13.Barks JD, Liu YQ, Shangguan Y, Silverstein FS. Phenobarbital augments hypothermic neuroprotection. Pediatr Res. 2010;67:532–7. doi: 10.1203/PDR.0b013e3181d4ff4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu YQ, Shangguan Y, Barks JDE, Silverstein FS. Bumetanide augments the neuroprotective efficacy of phenobarbital plus hypothermia in a neonatal hypoxia–ischemia model. Pediatr Res. 2012;71:559–65. doi: 10.1038/pr.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic ischemic brain damage in the rat. Ann Neurol. 1981;9:131–41. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 16.Grow JL, Liu YQ, Barks JDE. Can lateralizing sensorimotor deficits be identified after neonatal cerebral hypoxia-ischemia in rats? Dev Neurosci. 2003;25:394–402. doi: 10.1159/000075665. [DOI] [PubMed] [Google Scholar]
- 17.Pazaiti A, Soubasi V, Spandou E, Karkavelas G, Georgiou T, Karalis P. Guiba-Tziampiri. Evaluation of long-lasting sensorimotor consequences following neonatal hypoxic-ischemic brain injury in rats: The neuroprotective role of MgSO4. Neonatology. 2009;95:33–40. doi: 10.1159/000151753. [DOI] [PubMed] [Google Scholar]
- 18.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–87. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 19.Trescher WH, Ishiwa S, Johnston MV. Brief post-hypoxic-ischemic hypothermia markedly delays neonatal brain injury. Brain Devel. 1997;19:326–38. doi: 10.1016/s0387-7604(97)00027-2. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Pan HC, Yen YJ, Wang CC, Chuang YH, Chen SY, Lin SY, Liao SL, Raung SL, Wu CW, Chou MC, Chiang AN, Chen CJ. Detrimental effects of post-treatment with fatty acids on brain injury in ischemic rats. Neurotoxicology. 2007;28:1220–9. doi: 10.1016/j.neuro.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Alva N, Carbonell T, Palomeque J. Hypothermic protection in an acute hypoxia model in rats: Acid-base and oxidant/antioxidant profiles. Resuscitation. 2010;81:609–16. doi: 10.1016/j.resuscitation.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Roberts LJ, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, Dettbarn WD, Morrow JD. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273:13605–12. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 23.Signorini C, Ciccoli L, Leoncini S, Carloni S, Perrone S, Comporti M, Balduini M, Buonocore G. Free iron, total F-isoprostanes and total F-neuroprostanes in a model of neonatal hypoxic-ischemic encephalopathy: Neuroprotective effect of melatonin. J Pineal Res. 2009;46:148–54. doi: 10.1111/j.1600-079X.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- 24.D'Cruz BJ, Fertig KC, Filiano AJ, Hicks SD, DeFranco DB, Callaway CW. Hypothermic reperfusion after cardiac arrest augments brain-derived neurotrophic factor activation. J Cereb Blood Flow Metab. 2002;l22:843–51. doi: 10.1097/00004647-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–9. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He C, Qu X, Cui L, Wang J, Kang JX. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc Natl Acad Sci U S A. 2009;106:11370–5. doi: 10.1073/pnas.0904835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong M, Cheng GQ, Ma SM, Yang Y, Shao XM, Zhou WH. Post-ischemic hypothermia promotes generation of neural cells and reduces apoptosis by Bcl-2 in the striatum of neonatal rat brain. Neurochem Int. 2011;58:625–33. doi: 10.1016/j.neuint.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Hubbard WK, United States Food and Drug Administration Letter responding to health claim petition dated November 3, 2003 (Martek petition): Omega-3 fatty acids and reduced risk of coronary heart disease (docket no. 2003Q-0401) Date of issue September 8, 2004. http://www.fda.gov/Food/LabelingNutrition/LabelClaims/QualifiedHealthClaims/ucm072932.htm 30 September, 2011.
- 29.Diamond IR, Sterescu A, Pencharz PB, Kim JH, Wales PW. Changing the paradigm: Omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48:209–15. doi: 10.1097/MPG.0b013e318182c8f6. [DOI] [PubMed] [Google Scholar]