Abstract
Neuroplasticity may be defined as the ability of the central nervous system (CNS) to respond to changes in the internal and external environment and it is well established that some stimuli have the ability to facilitate or impair neuroplasticity depending on the pre-existing milieu. A classic example of a stimulus that can both facilitate and impair neuroplasticity is stress. Indeed, the ability of CNS to respond to acute stress is often dependent upon the prior stress history of the individual. While responses to acute stress are often viewed as adaptive in nature, stress reactivity in subjects with prior chronic stress experiences are often linked to neuropsychiatric disorders, including major depressive disorder, post-traumatic stress disorder (PTSD) and anxiety. In rodent studies, chronic stress exposure produces structural and functional alterations in the hippocampus and medial prefrontal cortex that are consistent across different types of stress paradigms. Conversely, the amygdala appears to exhibit differential structural and functional responses to stress that are dependent on a variety of factors, including the type of stressor performed and the duration of the stress paradigm. This is most evident in output measures including morphological analysis of amygdala neurons, measurement of glutamatergic tone in amygdalar subdivisions and the analysis of amygdala-centric behaviors. Accordingly, this review will provide an overview of the effects of stress on the structural and functional plasticity of the rodent amygdala, especially in relation to the differential effects of repeated or chronic stress paradigms on dendritic architecture, neurochemistry of the glutamatergic system and behavior.
Keywords: Basolateral amygdala, Central amygdala, Glutamate, Major depressive disorder, Post-traumatic stress disorder, Anxiety
Highlights
-
•
Repeated exposure to stress elicits heterogeneous changes in the rodent amygdala.
-
•
Stress outcomes are dependent on stress duration and stress type in the amygdala.
-
•
Repeated stress exposure may induce atrophy or hypertrophy of amygdala neurons.
-
•
Acute versus repeated stress differentially impacts glutamate efflux in the amygdala.
-
•
Different stress paradigms differentially affect amygdalar-centric behaviors.
1. Introduction
Neuroplasticity may be defined as the ability of the central nervous system (CNS) to respond to changes in the external and internal milieu. Neuroplasticity can be evaluated by a variety of functional and morphological endpoint measures ranging from molecular/cellular indices to changes in synaptic transmission to neurochemical alterations to changes in dendritic architecture and spine density. Ultimately, all these neuroplasticity alterations impact behavior and cognition. Review of the literature clearly indicates that some factors such as exercise facilitate and promote neuroplasticity (Voss et al., 2013) while other factors impair neuroplasticity, as is seen in age-related cognitive decline (Small et al., 2011). Interestingly, acute stress-mediated activation of the hypothalamic–pituitary–adrenal (HPA) axis may both promote and impair neuroplasticity depending upon the conditions and prior experience of the individual. In this regard, glucocorticoids (GCs) may promote neuroplasticity and thereby facilitate the formation of strong emotional memories (Conrad, 2005, Roozendaal et al., 2009). Conversely, exposure to repeated or chronic stressful stimuli often results in maladaptive responses that impair neuroplasticity and ultimately lead to behavioral and cognitive deficits. For these reasons exposure to chronic stress has been implicated in the pathophysiology of neuropsychiatric disorders such as post-traumatic stress disorder (PTSD), major depressive disorder (MDD) and anxiety (Diamond et al., 2004, McEwen, 2008). These differential effects of acute versus chronic stress are most evident in stress-responsive regions like the medial prefrontal cortex (mPFC), hippocampus and amygdala, regions that have been identified as critical epicenters for neuropsychiatric disorders. Closer examination of these studies suggests that different stress paradigms generally produce similar effects on neuroplasticity endpoint measures in the hippocampus and mPFC. However, the amygdala appears to exhibit differential responses to stress that are dependent on the type of stressor performed and the duration of the stress paradigm. This includes analysis of differential effects of stress on amygdalar neuroanatomy, neurochemistry and amygdalar-dependent behaviors. In view of these observations, the aim of the current review is to provide a comprehensive analysis of the differential effects of stress on structural and functional plasticity of the rodent amygdala with a focus on glutamatergic systems, and provide some potential implications for neuropsychiatric disease states in humans.
2. Stress-dependent effects on amygdalar morphology
2.1. Brief overview of amygdalar anatomy
The amygdala consists of multiple nuclei, each with unique cytoarchitectonic, chemoarchitectonic and connectional characteristics. Although an exhaustive description of the functions and connections of each of these nuclei is beyond the scope of this review, in broad terms the amygdalar nuclei can be grouped into two major macrostructures. First is the corticobasolateral nuclei, whose cell types resemble those of the cerebral cortex, with glutamatergic, calcium-calmodulin dependent (CaM) kinase-positive principal cells that bear morphological resemblance to cortical pyramidal neurons (McDonald, 1996). The activity of these neurons is coordinated, synchronized, and otherwise modulated by a variety of different inhibitory, GABAergic-interneurons, which can be classified according to their expression of various calcium-binding proteins as well as by morphological and connectional features. The second major amygdalar macrostructure is the centromedial complex, whose cell types resemble those of the striatopallidal region. Our studies on the anatomical and neurochemical correlates of stress in the amygdala have focused on the basolateral complex (BLA) and central nucleus (CeA). In addition to serving as representative nuclei of the corticobasolateral and centromedial macrostructures, respectively, the BLA and CeA form the major input and output systems of the amygdala and thus are likely to be sites of stress-related changes in amygdalar function. For example, the BLA receives sensory information from various cortical and thalamic regions, and in turn sends projections to other amygdalar nuclei, as well as to several extrinsic structures such as the hippocampus, prefrontal cortex, basal forebrain, hypothalamus, and brainstem (Jolkkonen et al., 2002; McDonald and Culberson, 1986, McDonald et al., 1999 Pikkarainen and Pitkanen, 2001, Sarter and Markowitsch, 1984). This vast connectivity allows the BLA to play a critical role in pairing conditioned stimuli (CS) and unconditioned stimuli (US) during learned fear (Sigurdsson et al., 2007), as well as mediate and influence multiple behavioral responses. The CeA, on the other hand, receives information from multiple extrinsic sources such as the thalamus, hypothalamus, midbrain, brainstem, ventral tegmental area, insular cortex, dorsal raphe, entorhinal cortex and perirhinal cortex (McDonald et al., 1999; McDonald, 1998, Sun and Cassell, 1993, Sun et al., 1994). While many of these projections are reciprocal, a significant proportion of CeA efferents (particularly those from its medial subdivision) target hypothalamic and brainstem centers (Cassell et al., 1999; LeDoux et al., 1988, Veening et al., 1984). The CeA also shares robust interconnections with the principal extra-amygdalar component of the “extended amygdala” (Cassell et al., 1999), the bed nucleus of the stria terminalis, which is also heaviliy implicated in behavioral and endocrine responses to threats or other stressors (Fox et al., 2015, Walker et al., 2009). Collectively, this pattern of connectivity allows the amygdala to play a central role in coordinating autonomic, endocrine and behavioral responses to conditioned and unconditioned sensory cues with emotional valence. Thus, the BLA receives polymodal sensory information from the cortex and thalamus and conveys this information directly or indirectly (e.g. via the intercalated nuclei) to the CeA, which mediates amygdalar influence over autonomic, endocrine and behavioral functions via projections to the hypothalamus and brainstem.
2.2. Stress paradigms and amygdalar morphology
Because of the central role of the amygdala in stress responses (Herman and Cullinan, 1997, Van de Kar and Blair, 1999), this region may contribute directly to some of the specific behavioral deficits induced by stress, and may also be the site of initiation of changes in other brain regions, including the hippocampus and mPFC. In other words, the amygdala region may play a central role in integrating the well-established connection between stress and psychopathology. Stress influences the structure and function of the amygdala and clinical investigations suggest that neuroanatomical changes in the amygdala precede those that are observed in the hippocampus of patients with mood disorders, in particular depressive illness patients (McEwen, 2003). Pre-clinical studies also indicate that stress can alter the structural and functional plasticity of the rodent amygdala. In this regard, seminal observations by Chattarji and coworkers determined that a 10 day immobilization stress paradigm elicited hypertrophy of pyramidal neurons in the rat BLA. Specifically, chronic immobilization stress (CIS) increased total dendritic length and number of branch points of BLA pyramidal neurons when compared to non-stressed control rats (Vyas et al., 2002). These results represent an interesting and important departure from prior neuroanatomical analyses, which have demonstrated that exposure to repeated stress elicits atrophy of principal neurons in the rat hippocampus (Conrad et al., 1999; Watanabe et al., 1992, Magarinos et al., 1997, McLaughlin et al., 2007) and mPFC (Cook and Wellman, 2004; Holmes and Wellman, 2009, Radley et al., 2004, Liston et al., 2006). Similarly, while stress-induced dendritic atrophy is a reversible process in the hippocampus (Luine et al., 1994) and mPFC (Radley et al., 2005), subsequent studies from the Chattarji group determined dendritic hypertrophy elicited by CIS may represent a more permanent morphological alteration (Vyas et al., 2004). CIS also increases spine density in BLA pyramidal neurons, an effect not observed immediately following a single acute immobilization stress session. However, increases in spine density in the absence of dendritic changes were observed 10 days following exposure to a single immobilization stress session (Mitra et al., 2005a). Another important observation from these studies was that unlike the morphological changes induced by CIS, chronic unpredictable stress (CUS) did not affect the morphology of BLA pyramidal neurons but did induce dendritic atrophy of bipolar neurons in the rat BLA (Vyas et al., 2002). As will be detailed below, these initial observations suggested that morphological changes in the rat BLA may be stressor and cell-type specific. More importantly, the outcomes from different stress models provide important insight into the heterogeneous findings from clinical studies that have examined amygdalar volumes in patients with major depressive illness Box 1.
Box 1. Stress paradigms and amygdalar neuroplasticity.
A variety of different paradigms have been used to investigate the effects of acute, repeated and chronic stress exposure on neuroplasticity in the central nervous system. A comprehensive review of all the different types of stress paradigms is beyond the focus of this review; instead we will briefly describe the paradigms that have examined the effects of stress on functional and morphological plasticity in the rodent amygdala. For example, chronic immobilization stress (CIS) is normally performed by completely immobilizing rodents for 2 h per day for 10 days; see (Vyas et al., 2002). Chronic unpredictable stress (CUS) involves daily exposure to a single different stressor such as food/water deprivation, immobilization stress, cold stress, swim stress, cage movement, etc. The sequence of the stressors is randomized and rodents are subjected to these stressors for ‘chronic’ periods ranging from 10 days to several weeks; see (Vyas et al., 2002, Pego et al., 2008). Restraint stress (RS) involves the use of flexible wire mesh restrainers (rats) or conical tubes (mice) that allow enough space to compensate for slight movements of the animal within the restraining apparatus. Rodents may be exposed to restraint stress for differing lengths of time during a daily exposure (2 h–6 h daily). In the context of this review, we refer to repeated restraint stress (RRS) as a 10 day paradigm [see (Piroli et al., 2013 Reznikov et al., 2008, Reznikov et al., 2009, Grillo et al., 2015)] and to chronic restraint stress (CRS) as a 21 day paradigm [see (Hill et al., 2013 Eiland et al., 2012, Reagan et al., 2007)]. Social isolation stress involves housing animals individually compared to control rodents that are group housed; see (Wang et al., 2012, Tsai et al., 2014). Single-prolonged stress (Liberzon et al., 1997) involves exposure to several different stressors (restraint stress, swim stress and exposure to ether) in rapid succession, which is often followed by a recovery period; see (Cui et al., 2008). Social instability stress (SIS) involves daily switching of cage mates, often performed in combination with other stressors such as restraint; see (Tsai et al., 2014). Please note that we have not discussed other types of social stress paradigms, since we believe that this would be beyond the scope of the current review. Platform stress involves brief exposure (30 min) on an elevated platform in a brightly lit arena; see (Maroun et al., 2013). Beyond stress paradigms, several studies have more selectively examined the effects of acute and chronic corticosterone exposure; see (Kim et al., 2014 Mitra and Sapolsky, 2008, Morales-Medina et al., 2009, Pego et al., 2008). As described in this review, the results from these studies demonstrate that the effects of stress on the functional morphology of the rodent amygdala appear to be dependent on the type of stress paradigm performed, as well as the duration of the stress; see text for details.
2.3. Heterogeneity of stress-induced morphological changes in the rodent amygdala
The studies described above from Chattarji and coworkers served as the genesis for subsequent studies that examined the effects of stress paradigms of different duration and type. Many of these studies determined that repeated stress paradigms elicited hypertrophy of BLA pyramidal neurons. For example, similar to the 10-day paradigm, an immobilization stress paradigm spanning 21 days also produced dendritic hypertrophy of BLA pyramidal neurons, as well as increases in spine density that were accompanied by anxiety-like behaviors (Vyas et al., 2006). Similar to observations in rats, 21 days of restraint stress (Hill et al., 2013) or 10 days of immobilization stress (Qin et al., 2011) resulted in an increase in total dendritic length and increased spine density of BLA pyramidal neurons in wild-type mice, morphological alterations that were associated with anxiety-like behaviors (Qin et al., 2011). More discrete analysis of dendritic morphology within subregions of the BLA determined that intermittent restraint stress induced dendritic hypertrophy in the basal nucleus, but did not impact the dendritic length of pyramidal neurons in the lateral nucleus (Padival et al., 2013). Interestingly, this stress paradigm increased spine density in both subregions of the BLA. Prolonged isolation stress beginning on postnatal day (PND) 28 also increased the complexity of BLA pyramidal neurons without affecting total dendritic length (Wang et al., 2012). In addition to these repeated and chronic stress paradigms, analysis of dendritic morphology following exposure to a single prolonged stress paradigm followed by a 7 day recovery period identified increases in total dendritic length and branch points of BLA pyramidal neurons in the absence of changes in these parameters in the CeA (Cui et al., 2008). These results supported previous findings demonstrating that stress did not impact CeA dendritic morphology (Vyas et al., 2003). Collectively, the results from these studies indicate that a number of different stress paradigms elicits dendritic hypertrophy and increases in spine density of BLA pyramidal neurons that are accompanied by anxiety-like behaviors (see Table 1).
Table 1.
Effects of different stress paradigms on neuronal morphology in the rodent amygdala.
| Paradigm | Duration | Species | Sex | Dendritic morphology | Reference |
|---|---|---|---|---|---|
| CIS | 10 days | Adult Wistar rats | Male | Hypertrophy of pyramidal neurons | Vyas et al., 2002 |
| CUS | 10 days | Adult Wistar rats | Male | Hypertrophy of bipolar neurons | Vyas et al., 2002 |
| CIS | 21 days | Adult Wistar rats | Male | Hypertrophy of pyramidal neurons | Vyas et al., 2006 |
| CIS | 10 days | Adult Wistar rats | Male | Hypertrophy of pyramidal neurons; inhibited by tianeptine | Pillai et al., 2012 |
| Restraint | 21 days | Adult c57/Bl6 mice | Male | Hypertrophy of pyramidal neurons | Hill et al., 2013 |
| CIS | 10 days | Adult c57/Bl6 mice | Male | Hypertrophy of pyramidal neurons | Qin et al., 2011 |
| Restraint | 21 days | Adult SD rats | Male | Hypertrophy of pyramidal neurons; inhibited by lithium | Johnson et al., 2009 |
| Restraint | intermittent | Adult SD rats | Male | Hypertrophy of pyramidal neurons | Padival et al., 2013 |
| SPS + recovery | 1 day | Adult SD rats | Male | Hypertrophy of pyramidal neurons | Cui et al., 2008 |
| Isolation stress | 8 weeks | PDN 28 Wistar rats | Male | Increased complexity of BLA pyramidal neurons; no change in dendritic length | Wang et al., 2012 |
| Cort Rx | 1 day; 10 day delay | Adult Wistar rats | Male | Hypertrophy of pyramidal neurons | Mitra and Sapolsky, 2008 |
| Cort Rx | 1 day; 10 day delay | Adult SD rats | Male | Hypertrophy of pyramidal neurons | Kim et al., 2014 |
| SIS | 5 weeks | Adult SD rats | Male | Hypertrophy of pyramidal neurons | Tsai et al., 2014 |
| Restraint stress | 21 days | Juvenile SD rats | Male & female | Hypertrophy of pyramidal neurons | Eiland et al., 2012 |
| Restraint stress | 21 days | Adult SD rats | Male & female | Hypertrophy of pyramidal neurons | Eiland et al., 2012 |
| CIS | 21 days | GIN mice | Male | Atrophy of BLA interneurons | Gilabert-Juan et al., 2011 |
| Platform stress | 1 day | Adult SD rats | Male | Atrophy of BLA pyramidal neurons | Maroun, 2013 |
| SIS | 5 weeks | Adolescent SD rats | Male | Atrophy of BLA pyramidal neurons | Tsai et al., 2014 |
| Restraint | 10 days | Adult SD rats | Male | Atrophy of BLA pyramidal neurons; inhibited by agomelatine | Grillo et al., 2015 |
| CUS | 10 days | Adult Wistar rats | Male | No change in BLA morphology | Vyas et al., 2002 |
| CUS | 28 days | Adult Wistar rats | Male | No change in BLA morphology | Pego et al., 2008 |
| Cort Rx | 28 days | Adult Wistar rats | Male | No change in BLA morphology | Pego et al., 2008 |
| Cort Rx | 21 days | Adult SD rats | Male | No change in BLA morphology | Morales-Medina et al., 2009 |
| Cort Rx | 10 days | Adult Wistar rats | Male | No change in BLA morphology | Mitra and Sapolsky, 2008 |
| Maternal deprivation | 24 h | Adult Wistar rats | Male & female | No change in BLA morphology | Krugers et al., 2012 |
| Predator stress | 10 min | Adult Long-Evans rats | Male | No change in BLA morphology | Adamec et al., 2012 |
Abbreviations. CIS: chronic immobilization stress. CUS: Chronic unpredictable stress. SPS: single prolonged stress. CORT Rx: corticosterone administration. SIS: social instability stress. SD: Sprague Dawley. PND: post-natal day.
While these studies above reported that stress paradigms elicit dendritic hypertrophy of BLA neurons, other studies have yielded different stress-induced morphological consequences. For example, adult mice exposed to a chronic restraint stress (CRS) paradigm exhibited dendritic atrophy of interneurons in the lateral and basolateral amygdala (Gilabert-Juan et al., 2011). Recent studies from our group indicate that repeated restraint stress (i.e. 10 days; RRS) also produces atrophy of BLA pyramidal neurons (Grillo et al., 2015), while Wellman and coworkers reported that a single exposure to platform stress induced dendritic retraction (decreased branch points and total dendritic length) of BLA pyramidal neurons (Maroun et al., 2013). Such results suggest that while stress more consistently elicits dendritic atrophy in the hippocampus and mPFC irrespective of the duration or type of stress paradigm that is employed, the morphological consequences to stress in the rodent amygdala appear to be more heterogeneous; see Fig. 1.
Fig. 1.
Stress as a one-armed bandit: differential effects of stress paradigms on amygdalar morphology. While stress paradigms consistently elicit atrophy of neurons in the rodent hippocampus and medial prefrontal cortex (blue highlighted regions in rat brain), stress-induced morphological changes are more heterogeneous in the amygdala (red highlighted region). The factors that impact morphological parameters include the type of stressor performed (US: unpredictable stress; RS: restraint stress; IS: immobilization stress), as well as time (i.e. duration) of the stress paradigm (acute versus chronic versus repeated). As described, the outcome on dendritic morphology depends on the combination of stressor, time, age and sex of the rodent. See text for details.
It is interesting to note that although anxiety-like behaviors were observed in rats exposed to CUS, this stress paradigm did not modulate total dendritic length or spine density of BLA pyramidal neurons (Pego et al., 2008). As will be described below, these results suggest that stress-induced behavioral outcomes (i.e. anxiety-like behaviors) may not always reflect stress-induced morphological parameters in the rodent BLA. In support of this hypothesis, rats selected for a ‘well-adapted’ phenotype in response to predator stress exhibited dendritic atrophy (total dendritic length and dendritic extent) when compared to ‘mal-adapted’ rats and handled controls (Mitra et al., 2009). In addition, some studies suggest that stress-induced morphological changes are reversible. In this regard, increases in spine density and hypertrophy of BLA pyramidal neurons induced by fear (cue) conditioning were reversed with extinction training (Heinrichs et al., 2013). Such results suggest that like the heterogeneity of stress-induced morphological changes, stress associated with different behavioral paradigms influences morphological plasticity in the rodent amygdala.
2.4. Age-dependent effects of stress on morphological parameters
Studies that have examined the effects of stress on BLA morphology at different developmental time points have also yielded disparate findings. For example, while maternal deprivation stress did not affect BLA pyramidal cell morphology in male and female rats (Krugers et al., 2012), juvenile male and female rats exhibited dendritic hypertrophy and increased anxiety-like behaviors in response to a chronic restraint stress paradigm (Eiland et al., 2012). A more recent study that examined the effects of chronic stress in juvenile and adult rats provides some insight into these disparate findings. In this regard, juvenile rats exposed to 5 weeks of instability stress (daily immobilization stress combined with changing of cage mates) elicited atrophy of BLA pyramidal neurons, decreases in spine density and impairments in fear-potentiated startle. Conversely, this same instability stress paradigm elicited dendritic hypertrophy, increases in spine density and enhanced fear-potentiated startle in adult male rats (Tsai et al., 2014). These results suggest that in addition to the factors described above (i.e. type of stress, duration of stress, cell-type examined), that age may also be an important factor that influences stress-induced morphological changes in the rodent amygdala.
2.5. Stress-induced morphological changes: role of corticosterone
While not entirely recapitulating the experience of stress, administration of stress levels of corticosterone (CORT) provides an approach to more selectively examine the role of CORT in stress-induced morphological changes in the rodent brain. Unlike the CORT-induced morphological changes observed in hippocampus (Woolley et al., 1990) and mPFC (Wellman, 2001), chronic exposure to stress levels of CORT does not modulate morphological parameters in BLA pyramidal neurons, including total dendritic length, spine density, neuronal density or amygdalar volume (Pego et al., 2008, Morales-Medina et al., 2009). However, studies by Mitra and Sapolsky determined that a single exposure to stress levels of CORT elicited dendritic hypertrophy when morphological analyses were preformed 12 days later (Mitra and Sapolsky, 2008). More recent studies replicated these findings and also demonstrated that acute CORT-induced hypertrophy of BLA pyramidal neurons was no longer evident 20 days post CORT treatment (Kim et al., 2014). Collectively, these studies once again demonstrate that the timing and/or duration of CORT exposure are important factors that determine/influence stress-induced morphological changes in the rodent BLA. Another important observation from these studies is that under certain conditions morphological changes in the rodent BLA are reversible. Such results suggest that like the heterogeneity of stress-induced morphological changes, experimental conditions and endocrine status may also influence whether stress-induced morphological plasticity is a reversible process in the rodent amygdala.
2.6. The neuropharmacology of stress-induced morphological changes in the rodent BLA
In spite of these potential stress-specific responses, one consistent observation is that pharmacological interventions used in the treatment of mood disorders effectively inhibit stress-induced morphological changes in the rat amygdala. In this regard, Young and coworkers reported that the mood-stabilizer lithium effectively prevented hypertrophy of BLA pyramidal neurons elicited by CRS (Johnson et al., 2009). More recently, Chattarji and coworkers reported that the antidepressant tianeptine also prevented CIS-induced dendritic hypertrophy of BLA pyramidal neurons (Pillai et al., 2012). This same study also reported that tianeptine modulated NMDA receptor-mediated currents in the lateral amygdala, thereby providing additional evidence to support the hypothesis that the mechanism of action of tianeptine involves normalization of glutamatergic tone in the rat amygdala (Piroli et al., 2013; Reznikov et al., 2007, Vouimba et al., 2004, Vouimba et al., 2006, McEwen et al., 2010). More recently, we reported that the antidepressant agomelatine, a melatonergic receptor agonist and 5HT2C receptor antagonist, effectively prevents repeated restraint stress-induced dendritic atrophy of BLA pyramidal neurons (Grillo et al., 2015).
Taken together these studies suggest that different types of stressors can induce various changes in morphological characteristics of BLA neurons that are reversible, and differ from those seen in other brain areas such as the hippocampus and prefrontal cortex. A challenge for the field is to determine how (or if) these changes in different brain areas are linked and if it is a change in the cortical-hippocampal-amygdalar circuit that underlies the behavioral shifts described below. Understanding the stress-related endocrine and neurotransmitter changes that drive the morphological changes in BLA, and how they differ from the mediators in hippocampus and prefrontal cortex, will require systematic and parametric studies. The neural mechanisms that induce stress-related anatomical changes to revert to a normal state remain to be elucidated, although studies with various antidepressant agents continue to offer some intriguing clues. Such studies also implicate a critical role for the glutamatergic system in mediating stress-induced changes in the amygdala.
3. Glutamatergic correlates of stress in the amygdala
Glutamate is the primary fast-acting excitatory neurotransmitter in the mammalian brain, including the amygdalar complex. Thus, glutamate, along with its fast-acting inhibitory amino acid counterpart, GABA, mediates the basics of synaptic neurotransmission on which the numerous amygdalar neuromodulatory systems—including dopamine, serotonin, norepinephrine and numerous neuropeptides—exert their influence. Principal cells of the BLA are glutamatergic, bear morphological resemblance to cortical pyramidal neurons, and are immunohistochemically detectable by labeling for the enzyme CaM kinase (McDonald et al., 2002). BLA neurons also serve as a significant source of neuronal glutamate in other amygdalar nuclei, such as the CeA (Pitkanen et al., 1997), and extra-amygdalar nuclei that receive BLA inputs. Additional presumptive sources of neuronal glutamate in the amygdalar complex include afferents originating from the cortex, including prefrontal cortex and sensory areas, the thalamus and hypothalamus (LeDoux et al., 1990 McDonald et al., 1999, Turner and Herkenham, 1991). Stress has been shown to impact virtually all of these regions, and it is likely that stress-related increases in amygdalar glutamate reflect a summed neurochemical correlate of activity in these stress-responsive circuits. Finally, as in other brain regions, a substantial fraction of extracellular amygdalar glutamate is likely derived from non-vesicular and glial sources (Timmerman and Westerink, 1997). Nonetheless, even glutamate derived from non-exocytotically-mediated release may affect neurotransmission and alter behavioral correlates of glutamatergic function (Baker et al., 2003, Baker et al., 2002, Moran et al., 2003) and changes in extracellular glutamatergic tone are likely to play a role in the morphological, synaptic, and neuroendocrine changes associated with stress in the amygdala and other limbic regions (Gabr et al., 1995, Herman et al., 2003). Thus, regardless of the source, glutamatergic neurotransmission in the amygdala likely represents an important mediator of stress effects on this area and, in turn, may play a key role in facilitating amygdalar activation of the HPA axis (Gabr et al., 1995). The important role played by glutamate in both acute rapid neurotransmission, as well as processes related to long-term synaptic plasticity, suggests that the dynamics of extracellular glutamate may yield key insights into the mechanisms underlying the effects of stress on amygdalar function. We and others have investigated the role of amygdalar glutamate in stress responses using microdialysis and other approaches amenable to in vivo analysis in awake animals.
3.1. Acute stress effects on BLA glutamate
Several lines of evidence indicate that acute stress increases release of glutamate within the BLA. Using in vivo microdialysis in awake rats, we have demonstrated that a one-hour restraint stress in previously stress-naïve animals produces robust increases in BLA glutamate levels (Reagan et al., 2012, Reznikov et al., 2007). Several features of the BLA glutamate response to acute stress are worth noting: First, the response is rapid—peaking within the first 15 min stress collection—but transient, returning to baseline levels by the second 15 min interval of the restraint period. Secondly, while basal extracellular glutamate measured by microdialysis in the BLA appears largely axon depolarization-independent (Timmerman and Westerink, 1997), stress-elicited increases in glutamate are completely abolished by the voltage-gated sodium channel blocker, tetrodotoxin (TTX). Finally, of therapeutic significance, acute stress-elicited glutamate release in the BLA is prevented by acute pretreatment with some (e.g., tianeptine and agomelatine) but not all (e.g., fluoxetine) antidepressant drugs (Reagan et al., 2012, Reznikov et al., 2007). Indeed, acute fluoxetine increases glutamate efflux in the BLA in the pre-stress period and prolongs the duration of the stress-elicited glutamate release, which may provide a potential mechanism through which initiation of selective serotonin reuptake inhibitor (SSRI) treatment elicits anxiety in depressive illness patients (Goldstein and Goodnick, 1998).
The effects of acute stress on BLA glutamate do not appear to be specific to restraint stress as BLA glutamate is also increased by other acute stressors, including chemical-induced somatic pain [intraplantar formalin injection; (Deyama et al., 2007)] and visceral distress produced by systemic lithium chloride (Miranda et al., 2002). Hegoburu et al. (Hegoburu et al., 2009) used rapid-sampling in vivo microdialysis to demonstrate that BLA glutamate is elevated transiently following an initial pairing of odor with footshock, but not with subsequent pairings, consistent with the idea that extracellular BLA glutamate is an important neurotransmitter correlate of unconditioned or early-stage acquisition of conditioned stress responses. In addition, Singewald and colleagues (Singewald et al., 2000), using the push–pull cannula technique, found that acute noise stress significantly increased glutamate release in the BLA of spontaneously hypertensive rats. Interestingly, this effect, which was TTX-sensitive, was not observed in Wistar-Kyoto rats even though the two strains did not differ in basal or veratridine-elicited glutamate release, suggesting a true species difference in stress sensitivity. Recent work in mouse models further supports the idea that glutamatergic neurotransmission not only reflects strain differences in stress responsiveness, but may actually mediate these differences (Mozhui et al., 2010).
3.2. Acute stress effects on CeA glutamate
Although fewer studies have investigated the effects of acute stress alone on glutamate in the CeA, we have shown that the initial response in this region roughly parallels that seen in the BLA. That is, rats experiencing their first episode of acute stress exhibit a rapid, significant and relatively short-lasting increase in CeA glutamate which is TTX-sensitive and prevented by the antidepressants tianeptine or agomelatine (Reagan et al., 2012, Reznikov et al., 2007).
While there have been few investigations on the acute effects of other (non-restraint) stressors on CeA glutamate, there is evidence that glutamate in this region is modulated by several neuromodulatory peptides which, themselves, are considered to play important roles in stress responses. For example, both corticotropin-releasing factor (CRF) (Beckerman et al., 2013) and endocannabinoids (Ramikie et al., 2014) have been hypothesized to regulate CeA glutamatergic transmission in the context of stress responses. Skorzewska and colleagues showed that i.c.v. CRF administration in animals exposed to novelty stress produced a robust increase (∼200% relative to baseline) in CeA glutamate levels (Skorzewska et al., 2009). Bosch and colleagues (Bosch et al., 2007) showed an important role for oxytocin in differential regulation of CeA glutamate in rat dams selectively bred for high or low anxiety, whereas Ebner et al. (Ebner et al., 2005) demonstrated the importance of oxytocin in regulating swim stress-induced release of glutamate in the CeA. Finally, intravenous administration of the hypothalamic neuropeptide orexin A, which innervates the central amygdala and plays a crucial role in panic and stress responses (Johnson et al., 2012; Kuwaki, 2011, Berridge et al., 2010, Schmitt et al., 2012), has been shown by microdialysis to increase amygdala glutamate in a calcium-dependent manner (John et al., 2003).
One caveat with using in vivo microdialysis to measure extracellular glutamate responses in the CeA is the inability to differentiate the medial and lateral compartments of this nucleus in rodents due to the small size of these subnuclei and the relatively large diameter (approximately 400 μm) of conventional microdialysis probes. This is potentially important given the different patterns of connectivity—and putatively different functional roles in stress responses—of these divisions of the CeA (Pitkanen et al., 1997, Ciocchi et al., 2010). Nonetheless, the relatively few studies using static (e.g. c-Fos expression) or dynamic (e.g. electrophysiological recordings) approaches that allow for more discrete targeting fail to suggest qualitatively opposite responses of these regions to acute stress or other aversive stimuli. For example, c-Fos expression in both lateral and medial divisions of the CeA is elevated by renewal of conditioned fear or active threat responding (Knapska and Maren, 2009, Martinez et al., 2013). Thus, it is likely that the ability of acute stress to increase extracellular glutamate in the CeA as a whole holds significant implications for behavioral and autonomic correlates of stress responses mediated by this nucleus.
3.3. Chronic stress effects on amygdalar glutamate
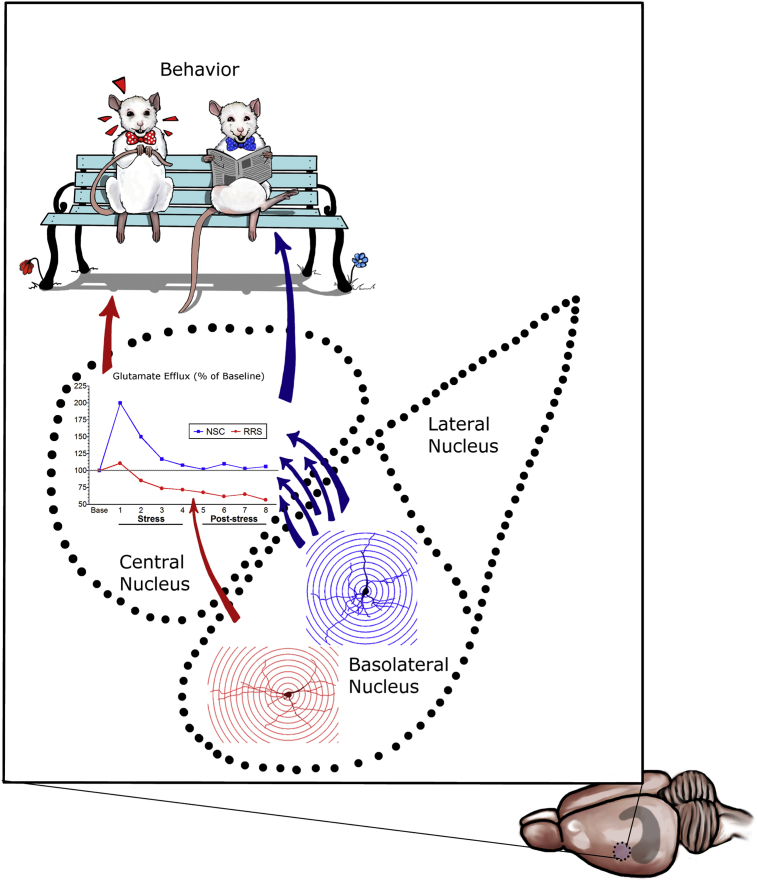
Studies utilizing in vivo neurochemical and behavioral approaches suggest that stress effects on extracellular glutamate in the amygdala are not just a neurotransmitter epiphenomenon, but may actually mediate functional consequences of chronic stress. As mentioned above, Mozhui and colleagues demonstrated an association between differences in trait- and stress-induced anxiety responses and measures of amygdalar glutamatergic neurotransmission in different strains of mice. Using in vivo microdialysis with elevated K+ to increase extracellular glutamate in rat amygdala (nucleus not specified), Fujikawa and colleagues showed that prolonged elevations in glutamate (∼300% relative to baseline) increased markers of neuronal damage (Fujikawa et al., 1996). Consistent with the hypothesis that chronic elevations in extracellular glutamate in BLA can have deleterious effects, our own work suggests that repeated restraint-induced decreases in CeA glutamate may be the consequence of RRS-elicited atrophy in BLA pyramidal neurons (Grillo et al., 2015), which represent a significant source of the neurotransmitter pool of glutamate in the CeA (Pitkanen et al., 1997). Thus, rats subjected to 10 days of RRS continued to show increased glutamate in the BLA in response to a subsequent acute stress challenge, although the magnitude of this increase tended to be smaller than in previously stress-naïve animals. However, these same animals showed marked reductions in extracellular glutamate, relative to animals experiencing their first episode of restraint stress, in the CeA. Importantly, chronic treatment with the antidepressant drug agomelatine normalized both the enhanced glutamate response in the BLA and the decrease in extracellular glutamate seen with chronic stress in the CeA. These studies demonstrate a qualitatively different response in glutamate homeostasis in the CeA and BLA of repeatedly-stressed rats and – combined with our morphological and c-Fos expression data (Reznikov et al., 2008) - suggest that repeated stress-induced decreases in extracellular glutamate in the CeA may result from dendritic atrophy and decreased activation of BLA inputs to this region (See Fig. 2). Indeed, repeated prenatal stress has been shown to result in reductions in hippocampal glutamate release in rats examined as adults, and this effect was prevented by chronic antidepressant treatment with either agomelatine or fluoxetine (Marrocco et al., 2014).
Fig. 2.
Neurochemical and behavioral correlates of repeated restraint stress (RRS)-induced atrophy of pyramidal neurons in the rat basolateral amygdala. Unlike the effects of immobilization stress (Vyas et al., 2002), our previous studies indicate that RRS elicits atrophy of pyramidal neurons in the rat BLA when compared to non-stressed control rats [Control BLA pyramidal neuron shown in blue; BLA pyramidal neuron from RRS rat shown in red; (Grillo et al., 2015)]. Since BLA pyramidal neurons extend their axons and axon collaterals to the central nucleus of the amygdala, a potential neurochemical consequence of RRS-induced atrophy of glutamatergic BLA neurons would be a decrease in glutamatergic tone in the central nucleus. In support of this hypothesis, unlike acute stress in a non-stress control (NSC) rat that elicits an increase in glutamate efflux in the central nucleus (Reznikov et al., 2007), rats with a prior RRS history exhibit decreases in glutamate efflux in the central nucleus in response to an acute stressor [(Grillo et al., 2015, Piroli et al., 2013) Blue arrows: BLA glutamatergic projections to central nucleus in NSC rat; red arrow: reduced BLA glutamatergic projections to central nucleus in RRS rat]. Ultimately, these neuroanatomical and neurochemical changes contribute to stress-induced changes in amygdalar-centric behaviors, as depicted by the ‘anxious’ rat wearing the red bowtie. See text for details.
An additional mechanism which may underlie the effects of repeated stress on amygdalar glutamate includes alterations in glial and/or neuronal reuptake and vesicular transport. This hypothesis stems, in part, from clinical data showing alterations in in vivo measures of glutamate levels (Auer et al., 2000; Sanacora et al., 2003, Sanacora et al., 2004), as well as post-mortem decreases in glial cell density (Ongur et al., 1998, Rajkowska and Miguel-Hidalgo, 2007) in some limbic brain regions. In our preclinical RRS model, repeated stress is associated with both blunted initial glutamate release in response to an acute stressor as well as dramatic reductions in extracellular glutamate levels in the CeA during both the stress and post-stress periods (Piroli et al., 2013). These changes in extracellular glutamate were also associated with dramatic reductions in expression of the vesicular glutamate transporter-2 (vGlut2). Importantly, both the neurochemical and vesicular protein deficits in markers of amygdalar glutamatergic neurotransmission in this study were prevented with daily administration of the antidepressant drug tianeptine (Piroli et al., 2013). Thus, restoration of capacity for glutamate release via increased vGlut2 expression may represent one mechanism by which some antidepressants restore glutamatergic neurotransmission and associated neuroplasticity in the amygdalar complex.
In summary, as shown in Table 2, the available data suggest that acute stress increases the neurotransmitter pool of extracellular glutamate in both central and basolateral divisions of the amygdala, but that these regions exhibit fundamentally different responses to repeated stress. Combined with morphological and neuronal activation studies, these data suggest the importance of glutamatergic neurotransmission in modulating the intra-amygdalar circuitry that contributes to behavioral and autonomic stress responses. These data further suggest the importance of amygdalar glutamate dynamics as a potential therapeutic neurochemical correlate of antidepressant treatment in animal models of stress. In the future, it will be interesting to directly examine the role of BLA inputs in repeated stress-induced changes in CeA neurochemistry, as well as to investigate how this phenomenon might be associated with a variety of other chronic stress paradigms. A challenge for the field will be to understand the relative role of glutamate release from glutamatergic inputs to specific amygdala regions, versus changes in extracellular glutamate levels induced by altered glial and/or neuronal reuptake or vesicular transport. Since these sources of extracellular glutamate flux will have distinct time courses, this could have interesting and complex effects on tripartite synapses. Stress related changes from different sources of glutamate might also be associated with specific types of stress and are likely to differ between acute and chronic stress paradigms.
Table 2.
Effects of acute and repeated stressors on glutamate neurochemistry in the rat amygdala.
| Paradigm | Region | Glutamate neurochemistry | Reference |
|---|---|---|---|
| Acute stress effects in BLA | |||
| Acute stress in naive rats | BLA | Increased above baseline; inhibited by tianeptine | Reznikov et al., 2007 |
| Acute stress in naive rats | BLA | Increased above baseline; inhibited by agomelatine | Reagan et al., 2012 |
| Chemical-induced somatic pain | BLA | Increased above baseline | Deyama et al., 2007 |
| Visceral distress | BLA | Increased above baseline | Miranda et al., 2002 |
| Acute odor + foot shock in naive rats | BLA | Increased above baseline only on initial presentation | Hegoburu et al., 2009 |
| Acute noise stress | BLA | Increased above baseline | Singewald et al., 2000 |
| Acute stress effects in CeA | |||
| Acute stress in naive rats | CeA | Increased above baseline; inhibited by tianpetine | Reznikov et al., 2007 |
| Acute stress in naive rats | CeA | Increased above baseline; inhibited by agomelatine | Reagan et al., 2012 |
| CRF + novelty stress | CeA | Increased above baseline | Skorzewska et al., 2009 |
| Oxytocin in high- and low-anxiety dams | CeA | Increased basal in high-anxiety; oxytocin antagonist increased in low-anxiety | Bosch et al., 2007 |
| Oxytocin regulation of swim stress | CeA | Swim stress increased above baseline; potentiated by oxytocin antagonist | Ebner et al., 2005 |
| Orexin A (hypocretin-1) | CeA | Increased above baseline | John et al., 2003 |
| Effects of repeated stress | |||
| Repeated restraint stress + acute stress | BLA | No changed vis-à-vis baseline | Piroli et al., 2013 |
| Repeated restraint stress + acute stress | BLA | No change vis-à-vis baseline | Grillo et al., 2015 |
| Repeated restraint stress + acute stress | CeA | Decrease below baseline; inhibited by tianeptine | Piroli et al., 2013 |
| Repeated restraint stress + acute stress | CeA | Decrease below baseline; inhibited by agomelatine | Grillo et al., 2015 |
Abbreviations. BLA: basolateral nucleus of the amygdala. CeA: central nucleus of the amygdala. CRF: corticotrophin releasing factor.
4. Stress and amygdala-centric behaviors
Various stress paradigms have been used to examine anxiety and depression-like behaviors in rodent models, since many of these acute or chronic stress models are thought to model the ability of stress to precipitate psychiatric conditions such as PTSD or depression. This section will focus on the effects of stress on unconditioned and conditioned anxiety-related behaviors that have been widely tested with numerous stress paradigms and are dependent (at least in part) on amygdalar processes, including responses to novelty and acoustic startle responses, as well conditioned fear or extinction. Like anatomical and neurochemical endpoints, the nature and length of the stressor, duration post-stress, strain and species of the rodents, and specific behavioral assay conditions all affect the changes observed in unconditioned and conditioned behaviors following acute or chronic stress. Of note, while some stressors produce effects on these behavioral endpoints that appear reversible and dissipate with recovery periods, other behavioral changes after stress (particularly more traumatic acute stressors) appear to require a sensitization period for the stress effects to emerge.
4.1. Responses to novelty in the open field test
Rodent responses to novelty have been tested in a variety of paradigms including the open field test (OF), holeboard test, elevated plus maze (EPM), and light:dark box (LDB). Enhanced levels of anxiety in these tests include decreased time in the center portion of an open field, time or relative (percent) time in the open arms of the EPM, or time in the light portion of the LDB. Additional behaviors, such as rearing, grooming, approaches, and risk assessment behaviors, as well as activity measures, have been assessed during these tasks in some studies as well.
Overall, stress effects in the open field (OF) are variable, most likely related to the various factors noted above. For example, RRS has been shown to decrease center time in the OF, but is generally without effects in general locomotion, although the RRS in one of these studies was done in adolescent (21 day old) rats (Chiba et al., 2012, Negron-Oyarzo et al., 2014). In contrast, the number of line crossings was reduced after 21 days of restraint stress performed after the animals had been previously exposed to a conditioned cue (Conrad et al., 1999). Although acute immobilization stress decreased ambulation in the OF 24 h after the stress session, this effect was prevented by chronic immobilization stress (CIS) for 9 days prior to the OF test suggesting protection or habituation with repeated stress exposure (Pastor-Ciurana et al., 2014). This habituation postulate is supported by the reduction in struggling behaviors during CIS protocols (Pastor-Ciurana et al., 2014, Wood et al., 2008). In the same Pastor-Ciurana et al. (2014) study, CUS for 9 days also produced a decrease in OF ambulation, although other studies have failed to show changes in OF locomotion (distance) or center time after longer CUS protocols [28 days; (Ventura-Silva et al., 2013)]. This could be related to the duration of stress, or the inclusion of footshock in these CUS protocols. Indeed, 5 day paradigms of repeated footshock decreased locomotion in the OF for up to 10 days after the end of the stress (Pijlman and van Ree, 2002; Pijlman et al., 2002, Pijlman et al., 2003b). Chronic mild stress paradigms have also been shown to decrease central OF time (Hu et al., 2010). Predator-related stress (cat exposure) did not change locomotion in a related holeboard task (Adamec et al., 2012, Adamec et al., 1999a, Adamec et al., 1999b, Adamec et al., 2005b, Blundell et al., 2005, Mitra et al., 2009), although one study demonstrated a decrease in center time following cat exposure (Adamec et al., 2012).
4.2. Elevated plus maze and light:dark box
In general, studies more consistently demonstrate a decrease in open arm time in the EPM following both CIS (Dagnino-Subiabre et al., 2005; Qin et al., 2011, Vyas et al., 2002, Vyas and Chattarji, 2004) and RRS (Chiba et al., 2012; Negron-Oyarzo et al., 2014, Zhang and Rosenkranz, 2013) paradigms. These protocols can decrease open arm time in both adolescent and adult rodents. Predator-induced stress (5–10 min cat exposure) also depresses open arm activity in the EPM, but this is following a seven to fourteen day sensitization period subsequent to this single traumatic stress (Adamec et al., 2012, Adamec et al., 1999a, Adamec et al., 1999b, Adamec et al., 2005b, Blundell et al., 2005, Mitra et al., 2009). This predator stress also induces a decrease in risk assessment behaviors in the EPM test (Adamec et al., 2005a, Adamec et al., 2012, Adamec et al., 1999a, Adamec et al., 1999b, Mitra et al., 2009). In contrast, despite the similarity of these tests, the effects on entries into the light compartment of the LDB were inconsistent between studies following predator exposure, perhaps due to the time point tested after cat exposure (Adamec et al., 2005b, Blundell et al., 2005). Mixed stressor paradigms including predator exposure, often viewed as models of PTSD, also decrease open arm time in the EPM. This includes a single prolonged stress (SPS) paradigm consisting of restraint, a forced swim and ether anesthesia followed by a 7 day (or longer) sensitization period [see review by (Yamamoto et al., 2009)] or a combination of predator (cat) exposure and immobilization followed by social instability for 21 days (Zoladz et al., 2008). The latter report suggested that the individual stressors in these paradigms were insufficient to induce changes in EPM behavior. The changes in EPM behaviors after CUS paradigms are more variable, with some investigators seeing decreases in open arm time following CUS (Bondi et al., 2008; Pego et al., 2008, Ventura-Silva et al., 2013), while others see no difference in EPM behaviors with this type of stress (Mitra et al., 2005b, Vyas et al., 2002).
Importantly, the effects of stress in these two tasks appear to be dependent upon both the sex and strain of the rodents. Unlike the changes observed in male rats, Mitra et al. reported that females fail to show shifts in EPM behaviors after either CIS or CUS (Mitra et al., 2005b). This suggests that there might be dramatic sex differences in responses to all these stress paradigms that predominantly use adult male subjects. In addition Mozhui et al. (2010), demonstrated that the changes in EPM and LDB in mice after 10 days of restraint in a plastic cone are dependent upon strain, although the changes after stress are somewhat confounded by baseline differences in these measures in the different mouse strains examined (Mozhui et al., 2010). In this regard, DBA/2J mice show decreased open arm time in the EPM and decreased time in the light compartment of the LDB after RRS, but C57BL/6J mice show an increase in these measures after stress (Masneuf et al., 2014, Mozhui et al., 2010). The increased time in the light compartment of the LDB in C57BL/6J mice after RRS was suggested to be a shift into a more active coping style (Masneuf et al., 2014).
4.3. Acoustic startle responses
Acoustic startle reflexes represent a conserved defensive reflex in response to a sudden, threatening stimulus. Since shifts in startle responses are seen in psychiatric disorders, including PTSD, many studies have examined stress-related changes in acoustic startle responses in rodent models. In general, different forms of repeated or traumatic stress paradigms sensitize the acoustic startle response, which is generally seen as an enhanced startle amplitude or delay in habituation of the acoustic startle response [for additional references see (Stam, 2007)]. Stressors that induce sensitized startle responses include repeated exposure to footshock (Davis and Walker, 2013; Walker and Davis, 1997, Gewirtz et al., 1998), a single prolonged stress paradigm (Yamamoto et al., 2009), predator stress (Adamec et al., 2012, Blundell et al., 2005), predator stress coupled with immobilization and social instability (Zoladz et al., 2008), and CUS (Pego et al., 2008, Ventura-Silva et al., 2013). Although there are reports that baseline startle responses are decreased by repeated daily footshocks for five days (Pijlman et al., 2003a), this difference may be due to the time of startle testing following stress. Davis and Walker (2013) have reported that this shock-induced sensitization diminishes over time, so the decreased basal startle might have been due to a long (10 day) delay after stress prior to behavioral testing (Pijlman et al., 2003b). There are also some reports that predator stress does not enhance baseline startle responses (Mackenzie et al., 2010). Interestingly, repeated footshock stress has been shown to increase prepulse inhibition of acoustic startle [PPI; (Pijlman et al., 2003a)], while neither psychological stress (observing other rats receiving footshock) or CUS altered PPI or fear potentiated startle responses (Pijlman et al., 2003a). Acoustic startle responses are also enhanced by testing in anxiogenic high light conditions (Davis and Walker, 2013, Walker and Davis, 1997).
4.4. Fear conditioning and extinction
In addition to stress- or fear-potentiated startle, studies have demonstrated stress effects on conditioning of other responses, including context or cue-conditioned freezing plus the extinction of this response during repeated cue presentation. There is considerable interest in assessing changes in fear conditioned responses, particularly extinction, given the relevance of such responses to disorders like PTSD. Several different stress paradigms either have been shown to enhance contextual- or cue-conditioned fear responses (primarily freezing) or reduce extinction of cue-conditioned fear. However, there are inconsistencies in the findings likely due to numerous factors including the stress paradigm, time after stress, and specifics of the cue conditioning and extinction protocols. Both RRS and CIS have been shown to enhance cue-conditioned freezing or contextual conditioning (Conrad et al., 1999; Farrell et al., 2013, Wood et al., 2008; Zhang and Rosenkranz, 2013, Farrell et al., 2010), although there are also reports that do not see changes in these responses (Baran et al., 2009, Miracle et al., 2006). Other stressors have also been seen to enhance context or cue conditioned freezing, such as prior exposure to multiple footshocks in another context (Rau et al., 2005) or a single prolonged stress exposure (Yamamoto et al., 2009). Further, conditioning of additional responses beyond freezing or startle is enhanced with other stressors such as footshock [inhibitory avoidance, (Roozendaal et al., 2002)] or predator exposure [locomotion in a conditioned context, (Mackenzie et al., 2010)].
Perhaps more uniformly, many stress paradigms demonstrate deficits in the recall after extinction, including RRS (Baran et al., 2009; Farrell et al., 2013, Zhang and Rosenkranz, 2013; Miracle et al., 2006, Farrell et al., 2010), single prolonged stress (Yamamoto et al., 2009), or an acute exposure to an elevated platform stress (Maroun et al., 2013), but not a single restraint stress (Zhang and Rosenkranz, 2013). Interestingly, these deficits in extinction recall display dependence on both age and sex (Zhang and Rosenkranz, 2013; Baran et al., 2009, Farrell et al., 2013). The ability of stress to produce deficits in extinction recall implicate effects of stress on prefrontal–amygdalar circuits underlying extinction learning, as PFC inputs to the amygdala play a crucial role in this phenomenon (Amano et al., 2010, Maroun, 2013). Indeed, the effects of chronic stress on the prefrontal cortex, which include dendritic atrophy, excitatory synapse loss, elevated extracellular glutamate and impaired plasticity of projections to the amygdala, are consistent with a powerful regulatory role of this structure in both BLA and CeA stress responses (Berretta, 2005; Maroun, 2006, Moghaddam, 2002, Radley and Morrison, 2005). Since increases in spine density and hypertrophy of BLA pyramidal neurons induced by cue conditioning are reversed with extinction training (Heinrichs et al., 2013), this might suggest stress modulates the ability of extinction training to induce morphological shifts in BLA neurons. Such results suggest that, like the heterogeneity of stress-induced morphological changes, behaviorally induced stress may also influence morphological plasticity in the rodent amygdala.
4.5. Amygdala glutamatergic processes mediating stress-induced behavioral changes
Several studies have implicated the glutamatergic system in the BLA in mediating the influences of stress on conditioned and unconditioned anxiety-related behaviors, although different glutamatergic receptors may influence different behavioral endpoints in these tasks. For example, NMDA NR2A (Grin2a) knockout mice fail to show the normal increase in time in the light compartment of the LDB after repeated stress observed in wildtype C57BL/6J mice, while mice lacking the AMPA receptor subunit (Gria1 null mutants) showed typical stress responses in this test (Mozhui et al., 2010). Further, injection of a kainate receptor agonist (APTA) into the BLA blocked the effect of RRS in C57BL/6J mice (Masneuf et al., 2014). Systemic injections of NMDA antagonists (MK801, AP7, CPP) administered before predator stress (cat exposure) also dose dependently attenuated the reduced open arm time and risk assessment behaviors in the EPM, and enhanced startle responses, seen 7–9 days later in vehicle-treated groups (Adamec et al., 1999a, Adamec et al., 1999b, Blundell et al., 2005). d-cycloserine has also been shown to attenuate the stress-induced deficits in extinction recall (Yamamoto et al., 2009) implicating NMDA-mediated process in that endpoint as well. In addition, bilateral injections of MK801 into the BLA, or unilateral injections into the left BLA, blocked predator induced decreases in risk assessment but not open arm time in the EPM (Adamec et al., 1999b). NMDA antagonists in the right amygdala have greater effects on increases in acoustic startle responses induced by predator stress, suggesting that there is lateralized control of these glutamatergic processes over stress effects. This intriguing study suggests that glutamatergic processes associated with stress in amygdala may exert very specific influences on distinct behavioral endpoints, even during the same task, and that there is a lateralized control of these glutamatergic processes over stress effects.
In contrast to unconditioned stress effects in novelty based tasks, sensitized startle responses appear to be dependent on BLA glutamatergic processes mediated via AMPA receptors. Studies by Davis and colleagues demonstrated that glutamatergic processes in the amygdala are important for fear-potentiated startle responses (Walker et al., 2002, Walker and Davis, 2000), and that microinjections of the AMPA antagonist NBQX into the BLA before the startle test blocked both shock (fear) potentiated startle and light enhanced startle responses (Davis and Walker, 2013, Walker and Davis, 1997). Studies also demonstrate that AMPA antagonists or lesions of the CeA block fear-potentiated startle, while AMPA antagonists or lesions on the bed nucleus of the stria terminalis (BNST) attenuate light-enhanced startle responses (Davis and Walker, 2013; Walker and Davis, 1997, Ventura-Silva et al., 2013). Overall, these studies implicate BLA glutamatergic processes in mediating stress effects in both conditioned and unconditioned anxiety-related responses, although there are distinct roles for NMDA, AMPA and kainate receptors in modulating specific behavioral endpoints. Moreover, these glutamatergic mechanisms in response to stress may be lateralized, which is not unlike the hemispheric changes seen in imaging studies of patients with conditions such as PTSD or other anxiety disorders. Lateralized effects could also explain the inconsistency between studies examining stress-related responses, morphological endpoints and the glutamatergic system. Thus, although it is clear that stressors create potentially long-lasting changes in behavior that involve glutamatergic mechanisms, it remains unknown if specific glutamatergic receptors mediate distinct behavioral endpoints and if these mechanisms differ between types of stress. In addition, since some types of stressors, such as predator stress, require a sensitization period before the behavioral shifts emerge, understanding how acute glutamatergic changes in the BLA drive long-term modifications in behavior represents a challenging scientific question, since it might be dependent on type of stress, timing, and sex or strain of the subjects. For example, it appears that chronic stress depresses glutamate release but ultimately sensitizes many amygdala-related behavioral endpoints. Finally, understanding how morphological changes in the BLA underlie behavioral shifts might require a more careful assessment of the lateralized effects of stress in this region.
5. Antidepressant drugs, stress and the amygdala
If glutamatergic neurotransmission is adversely affected by stress, drugs that restore appropriate glutamatergic tone may prevent or reverse the morphological changes in the CNS and would thereby be helpful in treating mood and anxiety disorders. Indeed, clinical research has shown alterations in levels, clearance and metabolism of glutamate in mood and anxiety disorders. Interestingly, these changes are consistent with volumetric changes in brain areas enriched in glutamate neurons (Gorman and Docherty, 2010). For these reasons, there is a growing interest in the development of drugs that directly target the glutamatergic system. The main advantages of this pharmacological approach are the rapid onset of the therapeutic effects, the avoidance of the typical side effects of monoaminergic antidepressants, as well as providing an alternative strategy in treatment-resistant patients. In this regard, it is well described that a single administration of non-specific NMDAR antagonists, such as ketamine, produces rapid antidepressant effects in clinical and preclinical settings (Berman et al., 2000; Autry et al., 2011, Sanacora et al., 2008). However, the mechanisms by which NMDAR blockers produce antidepressant effects remain to be fully elucidated. Ketamine increases the expression of synaptic proteins and the number of excitatory spines in PFC, suggesting that behavioral effects may be a consequence of changes in synaptic plasticity and synaptogenesis (Li et al., 2010, Li et al., 2011). More recently, Liu et al. showed that ketamine restores the strength of apical mPFC inputs from BLA, leading to the hypothesis that ketamine would ameliorate the disproportional negative influence of the amygdala in chronic stress and major depression (Liu et al., 2015). Taken together these recent studies suggest that ketamine exerts its rapid and prolonged antidepressant effect acting on the inputs that reach to the mPFC from BLA rather than acting directly on the BLA. There is a great interest in developing specific compounds that act on NMDAR subunits to avoid the adverse effects of ketamine. In this regard, Ro25-6981, a GluN2B selective antagonist, elicits anti-depressant-like behavior, although these effects were not sustained as long as those of ketamine (Maeng et al., 2008). Additional studies showed that GluN2B antagonist microinfusion into the mPFC, but not the BLA, reversed stress-related behavioral changes (Kiselycznyk et al., 2015). Beyond ionotropic glutamate receptors, the metabotropic glutamate receptor subtype 7 (mGlu7) has been identified as a key regulator of brain emotion circuits; specific blocking of mGlu7 has anti-stress, antidepressant and anxiolytic-like effects in rodent behavior (Gee et al., 2014). More critically, these pharmacological studies support the notion that glutamatergic projections between PFC and amygdala are important mediators of stress-induced behavioral changes and suggest that these glutamatergic projections provide a novel target for reversing the effects of chronic stress.
Monoaminergic-based antidepressants also interfere with the glutamate system, modifying the protein expression and the phosphorylation of different glutamate receptor subunits [for more details, see (Musazzi et al., 2013)]. Although the anxiolytic mechanism of the specific serotonin reuptake inhibitors (SSIRs) is unclear, these drugs are widely used for the treatment of anxiety disorders. Local administration of citalopram into the BLA decreases freezing behavior induced by conditioned fear stress, and serotonin levels are simultaneously increased, suggesting that increases of extracellular serotonin levels in the BLA may be related to the anxiolytic effects of SSRIs (Kitaichi et al., 2014). Chronic unpredicted mild stress (CUMS) produces considerable remodeling of type 1 synapses accompanied by changed expression of several synapse associated proteins in BLA. Chronic administration of another SSRI (escitalopram) decreased the length of the active zone of synapses that was increased by CUMS. In addition, escitalopram was able to restore spinophilin levels that were decreased in BLA after CUMS (Li et al., 2015).
Taken together, while the existing literature shows an important body of reports that studied the effects of antidepressants upon amygdala-dependent behavior, fewer reports have focused on the electrophysiology that underlies these behaviors and even fewer manuscripts investigated the structural synaptic plasticity of the amygdala after antidepressant treatment (Johnson et al., 2009 Grillo et al., 2015, Pillai et al., 2012). More systematic studies including behavior, electrophysiology, molecular mechanism and morphological analysis, will help to elucidate the drug mechanisms and assist in linking the remodeling of the glutamatergic neurons with the neurochemistry and the behavior. Understanding the mechanism of action will provide a pharmacological basis for the use of the antidepressant drugs, and subunit selective glutamatergic compounds, to treat amygdala–dependent behavior disorders. Going beyond empirical use will provide a more effective and appropriate use of the drugs used to treat the variety of disorders organized under the general term of “anxiety disorders”.
6. Conclusions
In recent years it has become clear that the amygdala represents a site of substantial neuroplasticity in the context of stress responses. The nature of these neuroplastic changes appears to differ from those observed in other stress-sensitive brain regions such as the PFC and hippocampus. The identification and further mechanistic characterization of the factors that drive amygdalar neuroplasticity will assuredly contribute to our understanding of the role of this brain structure in multiple stress- and anxiety-related disorders. Importantly, it may also lead to the development of novel tools from the fields of neuroimaging, neurogenetics and neuropharmacology that will aid the discovery of novel predictive biomarkers, prevention strategies, and drug-based treatments for these disorders. The full realization of these possibilities hinges on several unanswered questions going forward. For example, regarding the morphological alterations seen in the amygdala as a whole or in selected amygdalar neuronal populations—what is the precise time course of these morphological changes? How does this time course, and the accompanying variations in amygdalar nuclei or neuronal subpopulations, differ from other stress-responsive brains regions or across different stress paradigms? To what extent are these changes reversible? Or, for that matter, which of these changes are adaptive as opposed to facilitating cognitive, behavioral and affective dysfunction? The answers to such questions will require the field to adopt strategies to conduct parametric studies that include analysis of different brain areas with attention to potential hemispheric differences, different types of stressors, and different behavioral endpoints in conditioned and unconditioned tests. Most critically such studies will need to include a time course of changes both after a single stress and at time points during and after chronic stress exposures. A potentially very exciting and understudied area is the role of sex and sex hormones in these responses. The few studies that have examined sex differences have not shown differences in morphological changes, but demonstrate significant sex effects on behavioral endpoints and possible glutamatergic mechanisms. This might be due to fundamental differences between the sexes in baseline responses in glutamate tone and/or behavioral responses in these tests, so such studies will be a challenging prospect as the field moves forward.
The neurotransmitter correlates of stress-elicited neuroplasticity in the amygdala may also represent new opportunities for mechanistic descriptions of these changes as well as drug-based therapeutic targets. Glutamate-based therapeutics have received much interest in the past decade in the context of several neuropsychiatric disorders, and it appears this neurotransmitter system plays a crucial role in stress effects on the amygdala. Characterizing the role of glutamate—either as a secondary mediator of responses to drugs that act on other neurotransmitter systems, or as a primary target of drugs that directly target glutamate receptors or other molecular aspects of glutamatergic transmission—will be important for understanding the mechanism of existing drugs and the development of novel therapeutics. Understanding the nature of the relationship between stress-related glutamatergic neurotransmission and morphological alterations in the amygdala will also be necessary to develop new treatments that are informed by both of these levels of analysis. Finally, a better understanding of how preclinical stress paradigms model and recapitulate the amgydalar neuroplastic changes seen in human anxiety- and affective disorders will aid the translational impact of animal-based models.
Acknowledgments
Supported by the Department of Veterans Affairs (I21 BX002085 and IO1 BX001804: LPR; I01 BX001374: MAW) and the University of South Carolina Research Foundation. Funding agencies had no involvement in collection, analysis or interpretation of the data presented in this manuscript. The authors would like to thank Victoria Macht for the design and preparation of Fig. 1, Fig. 2.
References
- Adamec R., Blundell J., Burton P. Role of NMDA receptors in the lateralized potentiation of amygdala afferent and efferent neural transmission produced by predator stress. Physiol. Behav. 2005;86:75–91. doi: 10.1016/j.physbeh.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Adamec R., Hebert M., Blundell J., Mervis R.F. Dendritic morphology of amygdala and hippocampal neurons in more and less predator stress responsive rats and more and less spontaneously anxious handled controls. Behav. Brain Res. 2012;226:133–146. doi: 10.1016/j.bbr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamec R.E., Blundell J., Burton P. Neural circuit changes mediating lasting brain and behavioral response to predator stress. Neurosci. Biobehav. Rev. 2005;29:1225–1241. doi: 10.1016/j.neubiorev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Adamec R.E., Burton P., Shallow T., Budgell J. NMDA receptors mediate lasting increases in anxiety-like behavior produced by the stress of predator exposure–implications for anxiety associated with posttraumatic stress disorder. Physiol. Behav. 1999;65:723–737. doi: 10.1016/s0031-9384(98)00226-1. [DOI] [PubMed] [Google Scholar]
- Adamec R.E., Burton P., Shallow T., Budgell J. Unilateral block of NMDA receptors in the amygdala prevents predator stress-induced lasting increases in anxiety-like behavior and unconditioned startle–effective hemisphere depends on the behavior. Physiol. Behav. 1999;65:739–751. doi: 10.1016/s0031-9384(98)00225-x. [DOI] [PubMed] [Google Scholar]
- Amano T., Unal C.T., Pare D. Synaptic correlates of fear extinction in the amygdala. Nat. Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer D.P., Putz B., Kraft E., Lipinski B., Schill J., Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol. Psychiatr. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- Autry A.E., Adachi M., Nosyreva E., Na E.S., Los M.F., Cheng P.F., Kavalali E.T., Monteggia L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D.A., McFarland K., Lake R.W., Shen H., Tang X.C., Toda S., Kalivas P.W. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat. Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker D.A., Xi Z.X., Shen H., Swanson C.J., Kalivas P.W. The origin and neuronal function of in vivo nonsynaptic glutamate. J. Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran S.E., Armstrong C.E., Niren D.C., Hanna J.J., Conrad C.D. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol. Learn. Mem. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerman M.A., Van Kempen T.A., Justice N.J., Milner T.A., Glass M.J. Corticotropin-releasing factor in the mouse central nucleus of the amygdala: ultrastructural distribution in NMDA-NR1 receptor subunit expressing neurons as well as projection neurons to the bed nucleus of the stria terminalis. Exp. Neurol. 2013;239:120–132. doi: 10.1016/j.expneurol.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatr. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Berretta S. Cortico-amygdala circuits: role in the conditioned stress response. Stress. 2005;8:221–232. doi: 10.1080/10253890500489395. [DOI] [PubMed] [Google Scholar]
- Berridge C.W., Espana R.A., Vittoz N.M. Hypocretin/orexin in arousal and stress. Brain Res. 2010;1314:91–102. doi: 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J., Adamec R., Burton P. Role of NMDA receptors in the syndrome of behavioral changes produced by predator stress. Physiol. Behav. 2005;86:233–243. doi: 10.1016/j.physbeh.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Bondi C.O., Rodriguez G., Gould G.G., Frazer A., Morilak D.A. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Bosch O.J., Sartori S.B., Singewald N., Neumann I.D. Extracellular amino acid levels in the paraventricular nucleus and the central amygdala in high- and low-anxiety dams rats during maternal aggression: regulation by oxytocin. Stress. 2007;10:261–270. doi: 10.1080/10253890701223197. [DOI] [PubMed] [Google Scholar]
- Cassell M.D., Freedman L.J., Shi C. The intrinsic organization of the central extended amygdala. Ann. N. Y. Acad. Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Chiba S., Numakawa T., Ninomiya M., Richards M.C., Wakabayashi C., Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39:112–119. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Ciocchi S., Herry C., Grenier F., Wolff S.B., Letzkus J.J., Vlachos I., Ehrlich I., Sprengel R., Deisseroth K., Stadler M.B., Muller C., Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Conrad C.D. The relationship between acute glucocorticoid levels and hippocampal function depends upon task aversivenes and memory processing stage. Nonlinearity. Biol. Toxicol. Med. 2005;3:57–78. doi: 10.2201/nonlin.003.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C.D., LeDoux J.E., Magarinos A.M., McEwen B.S. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cook S.C., Wellman C.L. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Cui H., Sakamoto H., Higashi S., Kawata M. Effects of single-prolonged stress on neurons and their afferent inputs in the amygdala. Neuroscience. 2008;152:703–712. doi: 10.1016/j.neuroscience.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Dagnino-Subiabre A., Terreros G., Carmona-Fontaine C., Zepeda R., Orellana J.A., Diaz-Veliz G., Mora S., Aboitiz F. Chronic stress impairs acoustic conditioning more than visual conditioning in rats: morphological and behavioural evidence. Neuroscience. 2005;135:1067–1074. doi: 10.1016/j.neuroscience.2005.07.032. [DOI] [PubMed] [Google Scholar]
- Davis M., Walker D.L. Role of bed nucleus of the stria terminalis and amygdala AMPA receptors in the development and expression of context conditioning and sensitization of startle by prior shock. Brain Struct. Funct. 2013;219:1969–1982. doi: 10.1007/s00429-013-0616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyama S., Yamamoto J., Machida T., Tanimoto S., Nakagawa T., Kaneko S., Satoh M., Minami M. Inhibition of glutamatergic transmission by morphine in the basolateral amygdaloid nucleus reduces pain-induced aversion. Neurosci. Res. 2007;59:199–204. doi: 10.1016/j.neures.2007.06.1473. [DOI] [PubMed] [Google Scholar]
- Diamond D.M., Campbell A., Park C.R., Vouimba R.M. Preclinical research on stress, memory, and the brain in the development of pharmacotherapy for depression. Eur. Neuropsychopharmacol. 2004;14(Suppl. 5):S491–S495. doi: 10.1016/j.euroneuro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ebner K., Bosch O.J., Kromer S.A., Singewald N., Neumann I.D. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology. 2005;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- Eiland L., Ramroop J., Hill M.N., Manley J., McEwen B.S. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M.R., Sayed J.A., Underwood A.R., Wellman C.L. Lesion of infralimbic cortex occludes stress effects on retrieval of extinction but not fear conditioning. Neurobiol. Learn. Mem. 2010;94:240–246. doi: 10.1016/j.nlm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Farrell M.R., Sengelaub D.R., Wellman C.L. Sex differences and chronic stress effects on the neural circuitry underlying fear conditioning and extinction. Physiol. Behav. 2013;122:208–215. doi: 10.1016/j.physbeh.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A.S., Oler J.A., Tromp D.P., Fudge J.L., Kalin N.H. Extending the amygdala in theories of threat processing. Trends Neurosci. 2015;38:319–329. doi: 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa D.G., Kim J.S., Daniels A.H., Alcaraz A.F., Sohn T.B. In vivo elevation of extracellular potassium in the rat amygdala increases extracellular glutamate and aspartate and damages neurons. Neuroscience. 1996;74:695–706. doi: 10.1016/0306-4522(96)00171-6. [DOI] [PubMed] [Google Scholar]
- Gabr R.W., Birkle D.L., Azzaro A.J. Stimulation of the amygdala by glutamate facilitates corticotropin-releasing factor release from the median eminence and activation of the hypothalamic-pituitary-adrenal axis in stressed rats. Neuroendocrinology. 1995;62:333–339. doi: 10.1159/000127022. [DOI] [PubMed] [Google Scholar]
- Gee C.E., Peterlik D., Neuhauser C., Bouhelal R., Kaupmann K., Laue G., Uschold-Schmidt N., Feuerbach D., Zimmermann K., Ofner S., Cryan J.F., van der P.H., Fendt M., Vranesic I., Glatthar R., Flor P.J. Blocking metabotropic glutamate receptor subtype 7 (mGlu7) via the venus flytrap domain (VFTD) inhibits amygdala plasticity, stress, and anxiety-related behavior. J. Biol. Chem. 2014;289:10975–10987. doi: 10.1074/jbc.M113.542654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz J.C., McNish K.A., Davis M. Lesions of the bed nucleus of the stria terminalis block sensitization of the acoustic startle reflex produced by repeated stress, but not fear-potentiated startle. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1998;22:625–648. doi: 10.1016/s0278-5846(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Gilabert-Juan J., Castillo-Gomez E., Perez-Rando M., Molto M.D., Nacher J. Chronic stress induces changes in the structure of interneurons and in the expression of molecules related to neuronal structural plasticity and inhibitory neurotransmission in the amygdala of adult mice. Exp. Neurol. 2011;232:33–40. doi: 10.1016/j.expneurol.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Goldstein B.J., Goodnick P.J. Selective serotonin reuptake inhibitors in the treatment of affective disorders–III. Tolerability, safety and pharmacoeconomics. J. Psychopharmacol. 1998;12:S55–S87. doi: 10.1177/0269881198012003041. [DOI] [PubMed] [Google Scholar]
- Gorman J.M., Docherty J.P. A hypothesized role for dendritic remodeling in the etiology of mood and anxiety disorders. J. Neuropsychiatr. Clin. Neurosci. 2010;22:256–264. doi: 10.1176/jnp.2010.22.3.256. [DOI] [PubMed] [Google Scholar]
- Grillo C.A., Risher M., Macht V.A., Bumgardner A.L., Hang A., Gabriel C., Mocaer E., Piroli G.G., Fadel J.R., Reagan L.P. Repeated restraint stress-induced atrophy of glutamatergic pyramidal neurons and decreases in glutamatergic efflux in the rat amygdala are prevented by the antidepressant agomelatine. Neuroscience. 2015;284:430–443. doi: 10.1016/j.neuroscience.2014.09.047. [DOI] [PubMed] [Google Scholar]
- Hegoburu C., Sevelinges Y., Thevenet M., Gervais R., Parrot S., Mouly A.M. Differential dynamics of amino acid release in the amygdala and olfactory cortex during odor fear acquisition as revealed with simultaneous high temporal resolution microdialysis. Learn. Mem. 2009;16:687–697. doi: 10.1101/lm.1584209. [DOI] [PubMed] [Google Scholar]
- Heinrichs S.C., Leite-Morris K.A., Guy M.D., Goldberg L.R., Young A.J., Kaplan G.B. Dendritic structural plasticity in the basolateral amygdala after fear conditioning and its extinction in mice. Behav. Brain Res. 2013;248:80–84. doi: 10.1016/j.bbr.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Cullinan W.E. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Renda A., Bodie B. Norepinephrine-gamma-aminobutyric acid (GABA) interaction in limbic stress circuits: effects of reboxetine on GABAergic neurons. Biol. Psychiatr. 2003;53:166–174. doi: 10.1016/s0006-3223(02)01449-x. [DOI] [PubMed] [Google Scholar]
- Hill M.N., Kumar S.A., Filipski S.B., Iverson M., Stuhr K.L., Keith J.M., Cravatt B.F., Hillard C.J., Chattarji S., McEwen B.S. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol. Psychiatr. 2013;18:1125–1135. doi: 10.1038/mp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A., Wellman C.L. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci. Biobehav. Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Su L., Xu Y.Q., Zhang H., Wang L.W. Behavioral and [F-18] fluorodeoxyglucose micro positron emission tomography imaging study in a rat chronic mild stress model of depression. Neuroscience. 2010;169:171–181. doi: 10.1016/j.neuroscience.2010.04.057. [DOI] [PubMed] [Google Scholar]
- John J., Wu M.F., Kodama T., Siegel J.M. Intravenously administered hypocretin-1 alters brain amino acid release: an in vivo microdialysis study in rats. J. Physiol. 2003;548:557–562. doi: 10.1113/jphysiol.2002.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P.L., Molosh A., Fitz S.D., Truitt W.A., Shekhar A. Orexin, stress, and anxiety/panic states. Prog. Brain Res. 2012;198:133–161. doi: 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.A., Wang J.F., Sun X., McEwen B.S., Chattarji S., Young L.T. Lithium treatment prevents stress-induced dendritic remodeling in the rodent amygdala. Neuroscience. 2009;163:34–39. doi: 10.1016/j.neuroscience.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Jolkkonen E., Miettinen R., Pikkarainen M., Pitkanen A. Projections from the amygdaloid complex to the magnocellular cholinergic basal forebrain in rat. Neuroscience. 2002;111:133–149. doi: 10.1016/s0306-4522(01)00578-4. [DOI] [PubMed] [Google Scholar]
- Kim H., Yi J.H., Choi K., Hong S., Shin K.S., Kang S.J. Regional differences in acute corticosterone-induced dendritic remodeling in the rat brain and their behavioral consequences. BMC. Neurosci. 2014;15:65. doi: 10.1186/1471-2202-15-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselycznyk C., Jury N.J., Halladay L.R., Nakazawa K., Mishina M., Sprengel R., Grant S.G., Svenningsson P., Holmes A. NMDA receptor subunits and associated signaling molecules mediating antidepressant-related effects of NMDA-GluN2B antagonism. Behav. Brain Res. 2015;287:89–95. doi: 10.1016/j.bbr.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaichi Y., Inoue T., Nakagawa S., Omiya Y., Song N., An Y., Chen C., Kusumi I., Koyama T. Local infusion of citalopram into the basolateral amygdala decreased conditioned fear of rats through increasing extracellular serotonin levels. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;54:216–222. doi: 10.1016/j.pnpbp.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Knapska E., Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn. Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugers H.J., Oomen C.A., Gumbs M., Li M., Velzing E.H., Joels M., Lucassen P.J. Maternal deprivation and dendritic complexity in the basolateral amygdala. Neuropharm. 2012;62:534–537. doi: 10.1016/j.neuropharm.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Kuwaki T. Orexin links emotional stress to autonomic functions. Auton. Neurosci. 2011;161:20–27. doi: 10.1016/j.autneu.2010.08.004. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E., Farb C., Ruggiero D.A. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J. Neurosci. 1990;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E., Iwata J., Cicchetti P., Reis D.J. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Lee B., Liu R.J., Banasr M., Dwyer J.M., Iwata M., Li X.Y., Aghajanian G., Duman R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Liu R.J., Dwyer J.M., Banasr M., Lee B., Son H., Li X.Y., Aghajanian G., Duman R.S. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatr. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.L., Yuan Y.G., Xu H., Wu D., Gong W.G., Geng L.Y., Wu F.F., Tang H., Xu L., Zhang Z.J. Changed synaptic plasticity in neural circuits of depressive-like and escitalopram-treated rats. Int. J. Neuropsychopharmacol. 2015 doi: 10.1093/ijnp/pyv046. (epub May 2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I., Krstov M., Young E.A. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22:443–453. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- Liston C., Miller M.M., Goldwater D.S., Radley J.J., Rocher A.B., Hof P.R., Morrison J.H., McEwen B.S. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.J., Ota K.T., Dutheil S., Duman R.S., Aghajanian G.K. Ketamine strengthens CRF-activated amygdala inputs to basal dendrites in mPFC layer V pyramidal cells in the prelimbic but not infralimbic subregion, a key suppressor of stress responses. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.70. (epub March 2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V., Villegas M., Martinez C., McEwen B.S. Repeated stress causes reversible impairments of spatial memory impairments. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Mackenzie L., Nalivaiko E., Beig M.I., Day T.A., Walker F.R. Ability of predator odour exposure to elicit conditioned versus sensitised post traumatic stress disorder-like behaviours, and forebrain deltaFosB expression, in rats. Neuroscience. 2010;169:733–742. doi: 10.1016/j.neuroscience.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Maeng S., Zarate C.A., Jr., Du J., Schloesser R.J., McCammon J., Chen G., Manji H.K. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatr. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Magarinos A.M., Verdugo J.M., McEwen B.S. Chronic stress alters synaptic terminal structure in hippocampus. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M. Stress reverses plasticity in the pathway projecting from the ventromedial prefrontal cortex to the basolateral amygdala. Eur. J. Neurosci. 2006;24:2917–2922. doi: 10.1111/j.1460-9568.2006.05169.x. [DOI] [PubMed] [Google Scholar]
- Maroun M. Medial prefrontal cortex: multiple roles in fear and extinction. Neuroscientist. 2013;19:370–383. doi: 10.1177/1073858412464527. [DOI] [PubMed] [Google Scholar]
- Maroun M., Ioannides P.J., Bergman K.L., Kavushansky A., Holmes A., Wellman C.L. Fear extinction deficits following acute stress associate with increased spine density and dendritic retraction in basolateral amygdala neurons. Eur. J. Neurosci. 2013;38:2611–2620. doi: 10.1111/ejn.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco J., Reynaert M.L., Gatta E., Gabriel C., Mocaer E., Di Prisco S., Merega E., Pittaluga A., Nicoletti F., Maccari S., Morley-Fletcher S., Mairesse J. The effects of antidepressant treatment in prenatally stressed rats support the glutamatergic hypothesis of stress-related disorders. J. Neurosci. 2014;34:2015–2024. doi: 10.1523/JNEUROSCI.4131-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R.C., Gupta N., Lazaro-Munoz G., Sears R.M., Kim S., Moscarello J.M., LeDoux J.E., Cain C.K. Active vs. reactive threat responding is associated with differential c-Fos expression in specific regions of amygdala and prefrontal cortex. Learn. Mem. 2013;20:446–452. doi: 10.1101/lm.031047.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masneuf S., Lowery-Gionta E., Colacicco G., Pleil K.E., Li C., Crowley N., Flynn S., Holmes A., Kash T. Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior. Neuropharm. 2014;85:190–197. doi: 10.1016/j.neuropharm.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A.J. Glutamate and aspartate immunoreactive neurons of the rat basolateral amygdala: colocalization of excitatory amino acids and projections to the limbic circuit. J. Comp. Neurol. 1996;365:367–379. doi: 10.1002/(SICI)1096-9861(19960212)365:3<367::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- McDonald A.J. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald A.J., Culberson J.L. Efferent projections of the basolateral amygdala in the opossum, Didelphis virginiana. Brain Res. Bull. 1986;17:335–350. doi: 10.1016/0361-9230(86)90238-8. [DOI] [PubMed] [Google Scholar]
- McDonald A.J., Muller J.F., Mascagni F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J. Comp. Neurol. 2002;446:199–218. doi: 10.1002/cne.10204. [DOI] [PubMed] [Google Scholar]
- McDonald A.J., Shammah-Lagnado S.J., Shi C., Davis M. Cortical afferents to the extended amygdala. Ann. N. Y. Acad. Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Mood disorders and allostatic load. Biol. Psychiatr. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Chattarji S., Diamond D.M., Jay T.M., Reagan L.P., Svenningsson P., Fuchs E. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol. Psychiatr. 2010;15:237–249. doi: 10.1038/mp.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.J., Gomez J.L., Baran S.E., Conrad C.D. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle A.D., Brace M.F., Huyck K.D., Singler S.A., Wellman C.L. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol. Learn. Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Miranda M.I., Ferreira G., Ramirez-Lugo L., Bermudez-Rattoni F. Glutamatergic activity in the amygdala signals visceral input during taste memory formation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11417–11422. doi: 10.1073/pnas.182200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Adamec R., Sapolsky R. Resilience against predator stress and dendritic morphology of amygdala neurons. Behav. Brain Res. 2009;205:535–543. doi: 10.1016/j.bbr.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Jadhav S., McEwen B.S., Vyas A., Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Sapolsky R.M. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Vyas A., Chatterjee G., Chattarji S. Chronic-stress induced modulation of different states of anxiety-like behavior in female rats. Neurosci. Lett. 2005;383:278–283. doi: 10.1016/j.neulet.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol. Psychiatr. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Morales-Medina J.C., Sanchez F., Flores G., Dumont Y., Quirion R. Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat. J. Chem. Neuroanat. 2009;38:266–272. doi: 10.1016/j.jchemneu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Moran M.M., Melendez R., Baker D., Kalivas P.W., Seamans J.K. Cystine/glutamate antiporter regulation of vesicular glutamate release. Ann. N. Y. Acad. Sci. 2003;1003:445–447. doi: 10.1196/annals.1300.048. [DOI] [PubMed] [Google Scholar]
- Mozhui K., Karlsson R.M., Kash T.L., Ihne J., Norcross M., Patel S., Farrell M.R., Hill E.E., Graybeal C., Martin K.P., Camp M., Fitzgerald P.J., Ciobanu D.C., Sprengel R., Mishina M., Wellman C.L., Winder D.G., Williams R.W., Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J. Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L., Treccani G., Mallei A., Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol. Psychiatr. 2013;73:1180–1188. doi: 10.1016/j.biopsych.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Negron-Oyarzo I., Perez M.A., Terreros G., Munoz P., Dagnino-Subiabre A. Effects of chronic stress in adolescence on learned fear, anxiety, and synaptic transmission in the rat prelimbic cortex. Behav. Brain Res. 2014;259:342–353. doi: 10.1016/j.bbr.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Ongur D., Drevets W.C., Price J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padival M.A., Blume S.R., Rosenkranz J.A. Repeated restraint stress exerts different impact on structure of neurons in the lateral and basal nuclei of the amygdala. Neuroscience. 2013;246:230–242. doi: 10.1016/j.neuroscience.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Ciurana J., Rabasa C., Ortega-Sanchez J.A., Sanchis-Olle M., Gabriel-Salazar M., Ginesta M., Belda X., Daviu N., Nadal R., Armario A. Prior exposure to repeated immobilization or chronic unpredictable stress protects from some negative sequels of an acute immobilization. Behav. Brain Res. 2014;265:155–162. doi: 10.1016/j.bbr.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Pego J.M., Morgado P., Pinto L.G., Cerqueira J.J., Almeida O.F., Sousa N. Dissociation of the morphological correlates of stress-induced anxiety and fear. Eur. J. Neurosci. 2008;27:1503–1516. doi: 10.1111/j.1460-9568.2008.06112.x. [DOI] [PubMed] [Google Scholar]
- Pijlman F.T., Herremans A.H., van de K.J., Kruse C.G., van Ree J.M. Behavioural changes after different stress paradigms: prepulse inhibition increased after physical, but not emotional stress. Eur. Neuropsychopharmacol. 2003;13:369–380. doi: 10.1016/s0924-977x(03)00040-3. [DOI] [PubMed] [Google Scholar]
- Pijlman F.T., van Ree J.M. Physical but not emotional stress induces a delay in behavioural coping responses in rats. Behav. Brain Res. 2002;136:365–373. doi: 10.1016/s0166-4328(02)00128-6. [DOI] [PubMed] [Google Scholar]
- Pijlman F.T., Wolterink G., van Ree J.M. Cueing unavoidable physical but not emotional stress increases long-term behavioural effects in rats. Behav. Brain Res. 2002;134:393–401. doi: 10.1016/s0166-4328(02)00053-0. [DOI] [PubMed] [Google Scholar]
- Pijlman F.T., Wolterink G., van Ree J.M. Physical and emotional stress have differential effects on preference for saccharine and open field behaviour in rats. Behav. Brain Res. 2003;139:131–138. doi: 10.1016/s0166-4328(02)00124-9. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M., Pitkanen A. Projections from the lateral, basal and accessory basal nuclei of the amygdala to the perirhinal and postrhinal cortices in rat. Cereb. Cortex. 2001;11:1064–1082. doi: 10.1093/cercor/11.11.1064. [DOI] [PubMed] [Google Scholar]
- Pillai A.G., Anilkumar S., Chattarji S. The same antidepressant elicits contrasting patterns of synaptic changes in the amygdala vs hippocampus. Neuropsychopharmacology. 2012;37:2702–2711. doi: 10.1038/npp.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piroli G.G., Reznikov L.R., Grillo C.A., Hagar J.M., Fadel J.R., Reagan L.P. Tianeptine modulates amygdalar glutamate neurochemistry and synaptic proteins in rats subjected to repeated stress. Exp. Neurol. 2013;241:184–193. doi: 10.1016/j.expneurol.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Pitkanen A., Savander V., LeDoux J.E. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Qin M., Xia Z., Huang T., Smith C.B. Effects of chronic immobilization stress on anxiety-like behavior and basolateral amygdala morphology in Fmr1 knockout mice. Neuroscience. 2011;194:282–290. doi: 10.1016/j.neuroscience.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Morrison J.H. Repeated stress and structural plasticity in the brain. Ageing Res. Rev. 2005;4:271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Radley J.J., Rocher A.B., Janssen W.G., Hof P.R., McEwen B.S., Morrison J.H. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp. Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley J.J., Sisti H.M., Hao J., Rocher A.B., McCall T., Hof P.R., McEwen B.S., Morrison J.H. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rajkowska G., Miguel-Hidalgo J.J. Gliogenesis and glial pathology in depression. CNS. Neurol. Disord. Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramikie T.S., Nyilas R., Bluett R.J., Gamble-George J.C., Hartley N.D., Mackie K., Watanabe M., Katona I., Patel S. Multiple mechanistically distinct modes of endocannabinoid mobilization at central amygdala glutamatergic synapses. Neuron. 2014;81:1111–1125. doi: 10.1016/j.neuron.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V., DeCola J.P., Fanselow M.S. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Reagan L.P., Hendry R.M., Reznikov L.R., Piroli G.G., Wood G.E., McEwen B.S., Grillo C.A. Tianeptine increases brain-derived neurotrophic factor expression in the rat amygdala. Eur. J. Pharmacol. 2007;565:68–75. doi: 10.1016/j.ejphar.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Reagan L.P., Reznikov L.R., Evans A.N., Gabriel C., Mocaer E., Fadel J.R. The antidepressant agomelatine inhibits stress-mediated changes in amino acid efflux in the rat hippocampus and amygdala. Brain Res. 2012;1466:91–98. doi: 10.1016/j.brainres.2012.05.039. [DOI] [PubMed] [Google Scholar]
- Reznikov L.R., Grillo C.A., Piroli G.G., Pasumarthi R.K., Reagan L.P., Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur. J. Neurosci. 2007;25:3109–3114. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- Reznikov L.R., Reagan L.P., Fadel J.R. Activation of phenotypically distinct neuronal subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress. J. Comp. Neurol. 2008;508:458–472. doi: 10.1002/cne.21687. [DOI] [PubMed] [Google Scholar]
- Reznikov L.R., Reagan L.P., Fadel J.R. Effects of acute and repeated restraint stress on GABA efflux in the rat basolateral and central amygdala. Brain Res. 2009;1256:61–68. doi: 10.1016/j.brainres.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Brunson K.L., Holloway B.L., McGaugh J.L., Baram T.Z. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B., McEwen B.S., Chattarji S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Sanacora G., Gueorguieva R., Epperson C.N., Wu Y.T., Appel M., Rothman D.L., Krystal J.H., Mason G.F. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatr. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Sanacora G., Rothman D.L., Mason G., Krystal J.H. Clinical studies implementing glutamate neurotransmission in mood disorders. Ann. N. Y. Acad. Sci. 2003;1003:292–308. doi: 10.1196/annals.1300.018. [DOI] [PubMed] [Google Scholar]
- Sanacora G., Zarate C.A., Krystal J.H., Manji H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M., Markowitsch H.J. Collateral innervation of the medial and lateral prefrontal cortex by amygdaloid, thalamic, and brain-stem neurons. J. Comp. Neurol. 1984;224:445–460. doi: 10.1002/cne.902240312. [DOI] [PubMed] [Google Scholar]
- Schmitt O., Usunoff K.G., Lazarov N.E., Itzev D.E., Eipert P., Rolfs A., Wree A. Orexinergic innervation of the extended amygdala and basal ganglia in the rat. Brain Struct. Funct. 2012;217:233–256. doi: 10.1007/s00429-011-0343-8. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T., Doyere V., Cain C.K., LeDoux J.E. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharm. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Singewald N., Kouvelas D., Mostafa A., Sinner C., Philippu A. Release of glutamate and GABA in the amygdala of conscious rats by acute stress and baroreceptor activation: differences between SHR and WKY rats. Brain Res. 2000;864:138–141. doi: 10.1016/s0006-8993(00)02172-7. [DOI] [PubMed] [Google Scholar]
- Skorzewska A., Bidzinski A., Hamed A., Lehner M., Turzynska D., Sobolewska A., Szyndler J., Maciejak P., Wislowska-Stanek A., Plaznik A. The effect of CRF and alpha-helical CRF((9-41)) on rat fear responses and amino acids release in the central nucleus of the amygdala. Neuropharm. 2009;57:148–156. doi: 10.1016/j.neuropharm.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Small S.A., Schobel S.A., Buxton R.B., Witter M.P., Barnes C.A. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam R. PTSD and stress sensitisation: a tale of brain and body part 2: animal models. Neurosci. Biobehav. Rev. 2007;31:558–584. doi: 10.1016/j.neubiorev.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Sun N., Cassell M.D. Intrinsic GABAergic neurons in the rat central extended amygdala. J. Comp. Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Sun N., Yi H., Cassell M.D. Evidence for a GABAergic interface between cortical afferents and brainstem projection neurons in the rat central extended amygdala. J. Comp. Neurol. 1994;340:43–64. doi: 10.1002/cne.903400105. [DOI] [PubMed] [Google Scholar]
- Timmerman W., Westerink B.H. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Tsai S.F., Huang T.Y., Chang C.Y., Hsu Y.C., Chen S.J., Yu L., Kuo Y.M., Jen C.J. Social instability stress differentially affects amygdalar neuron adaptations and memory performance in adolescent and adult rats. Front. Behav. Neurosci. 2014;8:27. doi: 10.3389/fnbeh.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B.H., Herkenham M. Thalamoamygdaloid projections in the rat: a test of the amygdala's role in sensory processing. J. Comp. Neurol. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- Van de Kar L.D., Blair M.L. Forebrain pathways mediating stress-induced hormone secretion. Front. Neuroendocrinol. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- Veening J.G., Swanson L.W., Sawchenko P.E. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Ventura-Silva A.P., Melo A., Ferreira A.C., Carvalho M.M., Campos F.L., Sousa N., Pego J.M. Excitotoxic lesions in the central nucleus of the amygdala attenuate stress-induced anxiety behavior. Front. Behav. Neurosci. 2013;7:32. doi: 10.3389/fnbeh.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W., Vivar C., Kramer A.F., van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouimba R.M., Munoz C., Diamond D.M. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress. 2006;9:29–40. doi: 10.1080/10253890600610973. [DOI] [PubMed] [Google Scholar]
- Vouimba R.M., Yaniv D., Diamond D., Richter-Levin G. Effects of inescapable stress on LTP in the amygdala versus the dentate gyrus of freely behaving rats. Eur. J. Neurosci. 2004;19:1887–1894. doi: 10.1111/j.1460-9568.2004.03294.x. [DOI] [PubMed] [Google Scholar]
- Vyas A., Bernal S., Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vyas A., Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav. Neurosci. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- Vyas A., Jadhav S., Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A., Mitra R., Shankaranarayana Rao B.S., Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A., Pillai A.G., Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Walker D.L., Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J. Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.L., Davis M. Involvement of NMDA receptors within the amygdala in short- versus long-term memory for fear conditioning as assessed with fear-potentiated startle. Behav. Neurosci. 2000;114:1019–1033. [PubMed] [Google Scholar]
- Walker D.L., Miles L.A., Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.L., Ressler K.J., Lu K.T., Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J. Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.C., Ho U.C., Ko M.C., Liao C.C., Lee L.J. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct. Funct. 2012;217:337–351. doi: 10.1007/s00429-011-0355-4. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Gould E., McEwen B.S. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Wellman C.L. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wood G.E., Norris E.H., Waters E., Stoldt J.T., McEwen B.S. Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav. Neurosci. 2008;122:282–292. doi: 10.1037/0735-7044.122.2.282. [DOI] [PubMed] [Google Scholar]
- Woolley C.S., Gould E., McEwen B.S. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Morinobu S., Takei S., Fuchikami M., Matsuki A., Yamawaki S., Liberzon I. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress. Anxiety. 2009;26:1110–1117. doi: 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]
- Zhang W., Rosenkranz J.A. Repeated restraint stress enhances cue-elicited conditioned freezing and impairs acquisition of extinction in an age-dependent manner. Behav. Brain Res. 2013;248:12–24. doi: 10.1016/j.bbr.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz P.R., Conrad C.D., Fleshner M., Diamond D.M. Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress. 2008;11:259–281. doi: 10.1080/10253890701768613. [DOI] [PMC free article] [PubMed] [Google Scholar]