Abstract
The development of PTSD after military deployment is influenced by a combination of biopsychosocial risk and resilience factors. In particular, physiological factors may mark risk for symptom progression or resiliency. Research in civilian populations suggests elevated catecholamines after trauma are associated with PTSD months following the trauma. However, less is known regarding physiological markers of PTSD resilience among post-deployment service members (SM). We therefore assessed whether catecholamines obtained shortly after deployment were associated with combat-related PTSD symptoms three months later. Eighty-seven SMs completed the Clinician-Administered PTSD Scale for DSM-IV and blood draws within two months after return from deployment to Iraq or Afghanistan (“Time 1” or “T1”) and three months later (“Time 2” or “T2”). Linear regression analyses demonstrated that lower norepinephrine at T1 was associated with lower PTSD symptoms at T2. In particular, T1 norepinephrine was positively associated with T2 symptom intensity and avoidance symptoms. The present findings represent a biologically-informed method of assessing PTSD resilience after deployment, which may aid clinicians in providing tailored treatments for those in the greatest need. Further research is needed to validate these findings and incorporate physiological measures within an assessment battery.
Keywords: Resilience, Biological markers, Military, PTSD, Catecholamines, Deployment
1. Introduction
Military service members (SM) deployed to wars in Iraq and Afghanistan are more likely to experience posttraumatic stress disorder (PTSD) symptoms related to both warzone and homefront experiences (Smith et al., 2008, Vasterling et al., 2010) than those who did not deploy. Post-deployment PTSD symptoms are associated with reduced quality of life and functional status in SMs (Schnurr et al., 2009, Tsai et al., 2012), even for those whose symptoms fall short of meeting full diagnostic criteria for PTSD (Cukor et al., 2010, Grubaugh et al., 2005, Magruder et al., 2004). The current literature implies a variable risk for PTSD symptom development, attributable to a number of risk (e.g. number of deployments, traumatic brain injury, combat exposure, genetics) and resilience (e.g. unit support, post-deployment social support) factors (Hoge et al., 2008, Pietrzak et al., 2010, Reger et al., 2009, Seal et al., 2009, Skelton et al., 2012). However, less attention has been given to physiological factors that may signal risk for symptom progression, or conversely, the potential for resilience, after deployment, particularly with respect to subthreshold PTSD.
The catecholamines dopamine (DA), epinephrine (EPI), and norepinephrine (NE) are monoamines that are produced both within the central nervous system, where they act as neurotransmitters that are released to facilitate adaptive responses to acute stressors (Cahill and Alkire, 2003, Southwick et al., 2002), as well as in the sympathoadrenal system where they regulate physiologic responses to stressors. While beneficial in acute stress, repeated activation in chronic stress leads to dysregulation of catecholamine release, and increases the risk of PTSD; whereas adaptive catecholamine responses may signal resilience (Krystal and Neumeister, 2009). Precipitating events and psychological responses may dictate physiological reactions, but physiological reactions may also lead to psychological effects. Basal catecholamine levels have been associated with PTSD in a community sample (Young and Breslau, 2004), and there is some evidence that neuroendocrine measures (e.g. cortisol and NE) may be linked with trauma-related symptoms in adults within two months after trauma (Delahanty et al., 2000). Findings also indicate that catecholamine elevations can persist longer than 6 months given psychopathology. For example, combat veterans with chronic PTSD have higher baseline cerebral spinal fluid NE concentrations under unstressed conditions than their counterparts without PTSD (Geracioti et al., 2001). Furthermore, recent work suggests that variants of the gene coding for catechol-O-methyl transferase, an enzyme involved in catecholamine metabolism, are associated with PTSD (Kolassa et al., 2010, Norrholm et al., 2013).
Building upon these studies, we hypothesized that basal catecholamine levels obtained shortly after return from deployment could further improve our ability to predict resilience against combat-related PTSD symptoms in the subsequent months. We therefore compared catecholamine levels obtained within two months after return from deployment (T1) with PTSD symptoms three months later (T2). We expected that lower levels of DA, EPI, and NE at T1 would be associated with less severe PTSD symptoms at T2. Given that psychological states can induce changes in catecholamine systems, we also examined the reverse relationship, anticipating that lower T1 PTSD symptoms would be associated with lower catecholamine levels at T2.
2. Methods
2.1. Participants and procedure
SMs (N = 87) were recruited either through institutional review board-approved advertisements, or via direct contact immediately upon their return to the U.S. during postdeployment demobilization procedures at Fort Dix, NJ. Their current duty stations spanned the United States. They completed a T1 assessment within two months of return from deployment to Iraq or Afghanistan and then again three months later (T2). Inclusion criteria included the ability to complete informed consent, a structured interview, and a morning blood draw. Participants screening positive for probable PTSD (PTSD Checklist score ≥ 50), major depression (Patient Health Questionnaire-9 score ≥ 10), or post-concussive syndrome at baseline were excluded. Further exclusionary criteria included having a history of head injury resulting in a loss of consciousness for 60 minutes or more, a current Glasgow Coma Scale of less than 14 points, active psychotic symptoms, or active suicidal or homicidal ideation. Participants on alpha blockers or calcium channel blockers were excluded if they were unable to hold these medications for a 24-hour period preceding brain imaging scans. Combat-related PTSD symptoms were assessed by a trained, PhD-level psychologist using the Clinician-Administered PTSD Scale for DSM-IV (CAPS, Blake et al., 1995, Weathers et al., 2001), with traumatic events including improvised explosive devices, firefight, vehicular accidents, and other combat-related traumas. The frequency and intensity of each symptom cluster (e.g. re-experiencing, avoidance, hyperarousal) were combined to create a total score.
Blood draws occurred prior to 0900. Plasma samples were isolated from whole blood in sodium heparin preserved collection tubes with centrifugation for 10 minutes at room temperature. Samples were stored at −70 °C and run in a single batch. A commercially available Enzyme Immunoassay measured plasma catecholamine (EPI, NE, DA) levels, using Alpco Diagnostics (Salem, NH) Tri-Cat assay (17-TCTHUE03-RES).
2.2. Data analysis
First, step-wise linear regressions assessed whether T1 catecholamine levels were associated with T2 CAPS scores. Age, gender, and T1 CAPS score served as control variables. Catecholamine levels (DA, EPI, and NE) were entered as independent predictors. Both controls and predictors were entered in a step-wise fashion. The corresponding T2 CAPS score was analyzed as the dependent variable. T2 analyses modeled CAPS frequency and intensity, and CAPS symptom clusters, separately. Additional analyses were conducted to determine whether the reverse relationship was supported, such that T1 CAPS scores were associated with catecholamine levels at T2 when controlling for T1 catecholamine levels.
3. Results
Reflecting the overall deployed population, study participants were predominantly male (85%), single (53%), Caucasian (73%), and employed (71%). On average, participants were 30.0 (SD = 8.0) years old, had served 9.4 (5.9) years in the military, and had been deployed 1.7 (SD = 1.0) times since 2001. Total CAPS scores decreased from T1 to T2. In particular, symptom frequency and re-experiencing symptoms showed significant decreases between time points. Catecholamine levels and CAPS scores are shown in Table 1. There were no significant correlations between T1 catecholamine levels and T1 CAPS scores. The correlations between T1 variables, as well the correlations among T1 and T2 variables, are shown in Table 2.
Table 1.
Total, intensity, frequency, and cluster CAPS scores across time points.
| T1 mean (SD) | T2 mean (SD) | t (p) | |
|---|---|---|---|
| Dopamine | 28.64 (16.05) | 28.67 (16.03) | .26 (.79) |
| Epinephrine | 51.20 (25.57) | 61.01 (36.43) | 1.44 (.16) |
| Norepinephrine | 274.77 (173.50) | 309.67 (215.32) | 1.18 (.25) |
| Total CAPS | 19.6 (12.8) | 15.4 (15.1) | 2.32 (.02) |
| CAPS – intensity | 7.7 (5.4) | 6.1 (6.04) | 1.99 (.05) |
| CAPS – frequency | 12.0 (7.6) | 9.1 (9.0) | 2.56 (.01) |
| CAPS – re-experiencing | 4.3 (4.3) | 2.02 (3.7) | 3.65 (.001) |
| CAPS – avoidance | 4.9 (4.94) | 4.4 (6.5) | .62 (.54) |
| CAPS – hyperarousal | 10.5 (6.4) | 8.9 (7.5) | 1.65 (.11) |
Note: CAPS: Clinician Administered PTSD Scale.
Table 2.
Correlations among catecholamine levels and PTSD symptoms, with correlations among T1 variables shown below the diagonal and correlations between T1 with T2 variables shown above the diagonal.
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Dopamine | .19 | −.02 | .02 | −.02 | .04 | −.04 | .07 | −.004 | |
| 2. Epinephrine | .001 | .05 | .24 | .26 | .22 | .07 | .29* | .20 | |
| 3. Norepinephrine | .08 | .24* | .13 | .18 | .10 | −.06 | .19 | .13 | |
| 4. Total CAPS | −.001 | .07 | −.18 | .46** | .52** | .32* | .32* | .59** | |
| 5. CAPS – intensity | .04 | .10 | −.17 | .98** | .49** | .27** | .31* | .56** | |
| 6. CAPS – frequency | .04 | .07 | −.19 | .98** | .93** | .25 | .08 | .40** | |
| 7. CAPS – re-exper. | −.24* | −.04 | −.12 | .80** | .78** | .79** | .44** | .37** | |
| 8. CAPS – avoidance | .26* | .09 | −.16 | .74** | .74** | .71** | .38** | .59** | |
| 9. CAPS – hyperarousal | −.04 | .10 | −.15 | .89** | .85** | .89** | .63** | .45** |
Note: CAPS = Clinician Administered PTSD Scale, re-exper. = re-experiencing.
**p < .01, *p < .05.
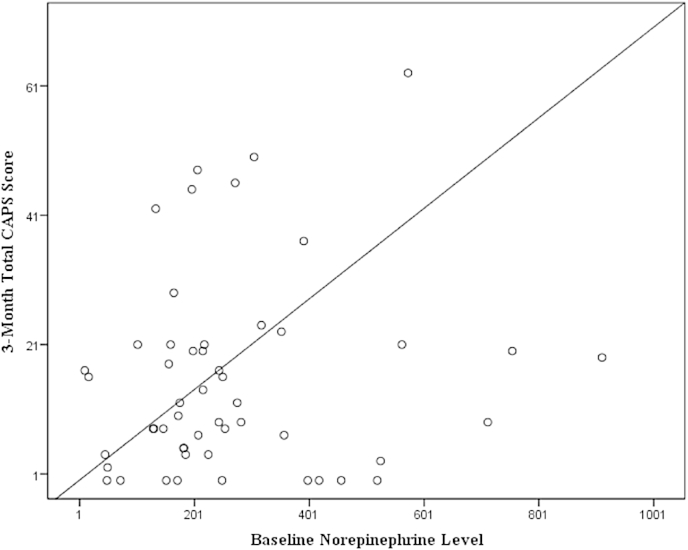
In order to examine the relationship between T1 catecholamines and PTSD symptomatology at T2, we completed step-wise linear regressions, while adjusting for T1 PTSD symptomatology. When controlling for T1 CAPS scores, step-wise linear regressions demonstrated that T1 NE was predictive of PTSD symptomatology at T2, in as much as T1 NE (β = .28, SE = .01, p = .03) was positively associated with T2 total CAPS scores (see Fig. 1). T2 step-wise linear regressions examined whether this association was due to symptom frequency or intensity. T1 NE levels (β = .32, SE = .004, p = .02) were associated with T2 CAPS intensity but not CAPS frequency. For these regressions, age, gender, and other catecholamines were not included in the final models as they were not significantly associated with T2 CAPS scores.
Fig. 1.
Relationship between T1 norepinephrine levels and T2 total CAPS score when controlling for T1 total CAPS score.
Next, step-wise linear regressions examined whether T1 catecholamine levels were associated with particular CAPS symptom clusters. T1 catecholamines were not associated with re-experiencing or hyperarousal at T2. However, T1 NE was positively associated with T2 avoidance scores (β = .38, SE = .004, p = .01). In particular, NE was positively associated with T2 avoidance frequency (β = .36, SE = .002, p = .006) and intensity (β = .38, SE = .002, p = .007). As noted in the previous step-wise regressions, age, gender, and other catecholamines were not retained in any of the final models.
Lastly, we completed T2 step-wise linear regressions to determine whether the reverse relationship was supported, such that T1 CAPS scores were associated with catecholamine levels at T2. In each of these analyses, the CAPS total score, frequency score, and intensity score were not associated with T2 catecholamine levels, when controlling for T1 catecholamine levels. When examining CAPS symptom clusters separately, T1 avoidance was associated with T2 DA levels (β = .40, SE = .63, p = .04).
4. Discussion
We found that lower blood NE levels obtained within two months after return from deployment were associated with lower combat-related PTSD symptoms three months later. In particular, T1 NE was positively correlated with overall symptom intensity and avoidance symptoms at T2. Avoidance is most strongly associated with reduced functioning among SM deployed to Iraq and Afghanistan (Rona et al., 2009, Sayers et al., 2009), therefore identifying a biomarker of resilience may be especially important when developing treatments focused on promoting recovery after deployment.
Elevated NE activity has been associated with both PTSD (Pietrzak et al., 2013), and with enhancement of memory consolidation in emotionally arousing situations in those with PTSD (Southwick et al., 2002). Increased NE can also result in heightened arousal and nightmares in PTSD patients, and prazosin, a post-synaptic α1-adrenergic receptor antagonist, has been shown to be a successful therapy for this element of combat-related PTSD (Raskind et al., 2013). Other studies have found mixed results when assessing psychopharmacological NE interventions (Steckler and Risbrough, 2012). For example, propranolol, a β-adrenergic antagonists that blocks post-synaptic NE receptors, has not been shown to reduce PTSD symptoms or the likelihood of a PTSD diagnosis when administered acutely after traumatic events (Hoge et al., 2012, Stein et al., 2007) Furthermore, clonidine, a α2-adrenergic receptor agonist, has been shown to reduce hyperarousal symptoms among individuals who have comorbid PTSD and borderline personality disorder (Ziegenhorn et al., 2009); whereas guanfacine, an α2-adrenergic receptor agonist, has been shown to have no effect on chronic PTSD among veterans (Neylan et al., 2006). The difference between the two studies' findings may be due to participant characteristics, comorbidities, sample sizes, or the medications themselves, despite having similar functioning. Our results are consistent with previous findings showing that NE and cortisol levels, measured acutely after trauma, are predictive of subsequent PTSD symptoms (Delahanty et al., 2000). Interestingly, a study involving treatment for complicated grief also found that individuals with lower basal NE levels had the lowest post-therapy complicated grief symptoms, even when controlling for T1 symptoms (O'Connor et al., 2013).
We found evidence that T1 PTSD symptoms are associated with subsequent catecholamine levels three months later. Lower T1 avoidance scores were associated with reductions in DA at T2. Dopaminergic systems have been associated with avoidance learning (Frank and Hutchison, 2009), whereas administration of a dopamine agonist (pramipexole) has been shown to diminish reinforcement-related behaviors in healthy participants (Pizzagalli et al., 2008). In the present findings, successful avoidance of trauma-related stimuli may enable avoidance learning. Across time, the act of avoidance may become physiologically reinforcing, even if an immediate perceived threat is not present. Historically, animal studies demonstrate the role of NE in avoidance. In the amygdala, NE increases during unconditioned stress (Heinsbroek et al., 1990, Tanaka et al., 2000), which in turn is associated with enhanced avoidance learning retention and avoidance behaviors in rats (Gold and van Buskirk, 1978, Tanaka et al., 2000). Taken together, the present findings demonstrate a bidirectional relationship between symptomology and physiology, an improved understanding of which may help identify individuals who experience sub-threshold combat-related PTSD symptoms in the months following return from deployment.
The present study possesses a number of strengths including the use of longitudinal data, analyses assessing for bi-directional relationships between variables, and exploration of individual symptom clusters, which may facilitate the development of novel therapies (Norrholm and Jovanovic, 2010). Exploration of the biological underpinnings of symptom severity has been highly advocated by the National Institutes of Health (Insel et al., 2010). The current study provides markers of resilience that are independent of DSM diagnostic criteria. Moreover, our timeline focused on the acute post-deployment period, a window of time which can set the course for future combat-related PTSD symptoms, and provides the potential for early intervention.
There are also some limitations to be acknowledged. The time course of combat-related PTSD symptoms may follow a variety of trajectories which may not be captured by only assessing two points in time. Second, we cannot rule out common precursors or mediating factors that may serve to link PTSD symptoms and catecholamine function and future studies would benefit from the addition of a non-deployed control group to determine the unique contributions of combat-related trauma in the relationship between symptoms and catecholamine function. Third, although our first time point is shortly after deployment, the deployment experience is highly variable, and some may have experienced significant traumas early in their deployment, so that nearly a year may have already passed by this point for some individuals. Fourth, we did not exclude individuals taking psychotropic medications that may affect catecholamine levels, future research would benefit from expanding and stratifying individuals by medication. Finally, in deliberately focusing on a sample with subthreshold symptoms, we may be elucidating patterns predictive of recovery more so than PTSD.
Risk stratification is the first step toward reducing PTSD symptoms in SMs who have been deployed. Recent evidence suggests early evidence-based intervention after a trauma may reduce the adverse effect of biological risk factors (Rothbaum et al., 2014). Within a clinical context, biological markers help elucidate a more detailed profile of functioning and help fill in gaps where self-report accuracy declines in the face of recall bias, symptom minimization, and alexithymia. As such, these markers could be used to identify those in need of more intense treatment or resiliency training prior to meeting clinical criteria for PTSD, thereby preventing progression to a PTSD diagnosis.
Conflicts of interest
The authors report no conflicts of interest.
Acknowledgments
This research was supported by a grant from the Center for Neuroscience and Regenerative Medicine (CNRM) (CNRM-83-2265), Uniformed Services University of the Health Sciences, Bethesda, MD, USA. Any opinions, views, or assertions expressed are solely those of the authors and do not necessarily represent those of CNRM, the Uniformed Services University, the Department of Defense, Department of Army/Navy/Air Force, or the U.S. Government.
References
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a Clinician-Administered PTSD Scale. J. Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Cahill L., Alkire M.T. Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol. Learn. Mem. 2003;79(2):194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cukor J., Wyka K., Jayasinghe N., Difede J. The nature and course of subthreshold PTSD. J. Anxiety Disord. 2010;24(8):918–923. doi: 10.1016/j.janxdis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Delahanty D.L., Raimonde A.J., Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol. Psychiatr. 2000;48(9):940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- Frank M.J., Hutchison K. Genetic contributions to avoidance-based decisions: striatal D2 receptor polymorphisms. Neuroscience. 2009;164(1):131–140. doi: 10.1016/j.neuroscience.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geracioti T.D., Jr., Baker D.G., Ekhator N.N., West S.A., Hill K.K., Bruce A.B. CSF norepinephrine concentrations in posttraumatic stress disorder. Am. J. Psychiatr. 2001;158(8):1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- Gold P.E., van Buskirk R. Posttraining brain norepinephrine concentrations: correlation with retention performance of avoidance training and with peripheral epinephrine modulation of memory processing. Behav. Biol. 1978;23(4):509–520. doi: 10.1016/s0091-6773(78)91614-0. [DOI] [PubMed] [Google Scholar]
- Grubaugh A.L., Magruder K.M., Waldrop A.E., Elhai J.D., Knapp R.G., Frueh B.C. Subthreshold PTSD in primary care: prevalence, psychiatric disorders, healthcare use, and functional status. J. Nerv. Ment. Dis. 2005;193(10):658–664. doi: 10.1097/01.nmd.0000180740.02644.ab. [DOI] [PubMed] [Google Scholar]
- Heinsbroek R.P., van Haaren F., Feenstra M.G., van Galen H., Boer G., van de Poll N.E. Sex differences in the effects of inescapable footshock on central catecholaminergic and serotonergic activity. Pharmacol. Biochem. Behav. 1990;37(3):539–550. doi: 10.1016/0091-3057(90)90025-d. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., Castro C.A. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Hoge E.A., Worthington J.J., Nagurney J.T., Chang Y., Kay E.B., Feterowski C.M. Effect of acute posttrauma propranolol on PTSD outcome and physiological responses during script-driven imagery. CNS Neurosci. Ther. 2012;18(1):21–27. doi: 10.1111/j.1755-5949.2010.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatr. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kolassa I.T., Kolassa S., Ertl V., Papassotiropoulos A., De Quervain D.J. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biol. Psychiatr. 2010;67(4):304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Krystal J.H., Neumeister A. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res. 2009;1293:13–23. doi: 10.1016/j.brainres.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magruder K.M., Frueh B.C., Knapp R.G., Johnson M.R., Vaughan J.A., 3rd, Carson T.C. PTSD symptoms, demographic characteristics, and functional status among veterans treated in VA primary care clinics. J. Trauma. Stress. 2004;17(4):293–301. doi: 10.1023/B:JOTS.0000038477.47249.c8. [DOI] [PubMed] [Google Scholar]
- Neylan T.C., Lenoci M., Samuelson K.W., Metzler T.J., Henn-Haase C., Hierholzer R.W. No improvement of posttraumatic stress disorder symptoms with guanfacine treatment. Am. J. Psychiatr. 2006;163(12):2186–2188. doi: 10.1176/appi.ajp.163.12.2186. [DOI] [PubMed] [Google Scholar]
- Norrholm S.D., Jovanovic T. Tailoring therapeutic strategies for treating posttraumatic stress disorder symptom clusters. Neuropsychiatr. Dis. Treat. 2010;6:517–532. doi: 10.2147/NDT.S10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm S.D., Jovanovic T., Smith A.K., Binder E., Klengel T., Conneely K. Differential genetic and epigenetic regulation of catechol-O-methyltransferase is associated with impaired fear inhibition in posttraumatic stress disorder. Front. Behav. Neurosci. 2013;7:30. doi: 10.3389/fnbeh.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M.F., Shear M.K., Fox R., Skritskaya N., Campbell B., Ghesquiere A. Catecholamine predictors of complicated grief treatment outcomes. Int. J. Psychophysiol. 2013;88(3):349–352. doi: 10.1016/j.ijpsycho.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak R.H., Gallezot J.D., Ding Y.S., Henry S., Potenza M.N., Southwick S.M. Association of posttraumatic stress disorder with reduced in vivo norepinephrine transporter availability in the locus coeruleus. JAMA Psychiatr. 2013;70(11):1199–1205. doi: 10.1001/jamapsychiatry.2013.399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pietrzak R.H., Goldstein M.B., Malley J.C., Rivers A.J., Johnson D.C., Southwick S.M. Risk and protective factors associated with suicidal ideation in veterans of operations enduring freedom and Iraqi freedom. J. Affect. Disord. 2010;123(1–3):102–107. doi: 10.1016/j.jad.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A., Evins A.E., Schetter E.C., Frank M.J., Pajtas P.E., Santesso D.L. Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl) 2008;196(2):221–232. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind M.A., Peterson K., Williams T., Hoff D.J., Hart K., Holmes H. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am. J. Psychiatr. 2013;170(9):1003–1010. doi: 10.1176/appi.ajp.2013.12081133. [DOI] [PubMed] [Google Scholar]
- Reger M.A., Gahm G.A., Swanson R.D., Duma S.J. Association between number of deployments to Iraq and mental health screening outcomes in US army soldiers. J. Clin. Psychiatr. 2009;70(9):1266–1272. doi: 10.4088/JCP.08m04361. [DOI] [PubMed] [Google Scholar]
- Rona R.J., Jones M., Iversen A., Hull L., Greenberg N., Fear N.T. The impact of posttraumatic stress disorder on impairment in the UK military at the time of the Iraq war. J. Psychiatr. Res. 2009;43(6):649–655. doi: 10.1016/j.jpsychires.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Rothbaum B.O., Kearns M.C., Reiser E., Davis J.S., Kerley K.A., Rothbaum A.O. Early intervention following trauma may mitigate genetic risk for PTSD in civilians: a pilot prospective emergency department study. J. Clin. Psychiatr. 2014;75(12):1380–1387. doi: 10.4088/JCP.13m08715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers S.L., Farrow V.A., Ross J., Oslin D.W. Family problems among recently returned military veterans referred for a mental health evaluation. J. Clin. Psychiatr. 2009;70(2):163–170. doi: 10.4088/jcp.07m03863. [DOI] [PubMed] [Google Scholar]
- Schnurr P.P., Lunney C.A., Bovin M.J., Marx B.P. Posttraumatic stress disorder and quality of life: extension of findings to veterans of the wars in Iraq and Afghanistan. Clin. Psychol. Rev. 2009;29(8):727–735. doi: 10.1016/j.cpr.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Seal K.H., Metzler T.J., Gima K.S., Bertenthal D., Maguen S., Marmar C.R. Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using department of veterans affairs health care, 2002–2008. Am. J. Public Health. 2009;99(9):1651–1658. doi: 10.2105/AJPH.2008.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton K., Ressler K.J., Norrholm S.D., Jovanovic T., Bradley-Davino B. PTSD and gene variants: new pathways and new thinking. Neuropharmacology. 2012;62(2):628–637. doi: 10.1016/j.neuropharm.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.C., Ryan M.A., Wingard D.L., Slymen D.J., Sallis J.F., Kritz-Silverstein D. New onset and persistent symptoms of post-traumatic stress disorder self reported after deployment and combat exposures: prospective population based US military cohort study. BMJ. 2008;336(7640):366–371. doi: 10.1136/bmj.39430.638241.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick S.M., Davis M., Horner B., Cahill L., Morgan C.A., 3rd, Gold P.E. Relationship of enhanced norepinephrine activity during memory consolidation to enhanced long-term memory in humans. Am. J. Psychiatr. 2002;159(8):1420–1422. doi: 10.1176/appi.ajp.159.8.1420. [DOI] [PubMed] [Google Scholar]
- Steckler T., Risbrough V. Pharmacological treatment of PTSD – established and new approaches. Neuropharmacology. 2012;62(2):617–627. doi: 10.1016/j.neuropharm.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M.B., Kerridge C., Dimsdale J.E., Hoyt D.B. Pharmacotherapy to prevent PTSD: results from a randomized controlled proof-of-concept trial in physically injured patients. J. Trauma. Stress. 2007;20(6):923–932. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Yoshida M., Emoto H., Ishii H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur. J. Pharmacol. 2000;405(1–3):397–406. doi: 10.1016/s0014-2999(00)00569-0. [DOI] [PubMed] [Google Scholar]
- Tsai J., Harpaz-Rotem I., Pietrzak R.H., Southwick S.M. The role of coping, resilience, and social support in mediating the relation between PTSD and social functioning in veterans returning from Iraq and Afghanistan. Psychiatry. 2012;75(2):135–149. doi: 10.1521/psyc.2012.75.2.135. [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Proctor S.P., Friedman M.J., Hoge C.W., Heeren T., King L.A. PTSD symptom increases in Iraq-deployed soldiers: comparison with nondeployed soldiers and associations with baseline symptoms, deployment experiences, and postdeployment stress. J. Trauma. Stress. 2010;23(1):41–51. doi: 10.1002/jts.20487. [DOI] [PubMed] [Google Scholar]
- Weathers F.W., Keane T.M., Davidson J.R. Clinician-administered PTSD scale: a review of the first ten years of research. Depress. Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Young E.A., Breslau N. Cortisol and catecholamines in posttraumatic stress disorder: an epidemiologic community study. Arch. Gen. Psychiatr. 2004;61(4):394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- Ziegenhorn A.A., Roepke S., Schommer N.C., Merkl A., Danker-Hopfe H., Perschel F.H. Clonidine improves hyperarousal in borderline personality disorder with or without comorbid posttraumatic stress disorder: a randomized, double-blind, placebo-controlled trial. J. Clin. Psychopharmacol. 2009;29(2):170–173. doi: 10.1097/JCP.0b013e31819a4bae. [DOI] [PubMed] [Google Scholar]