Abstract
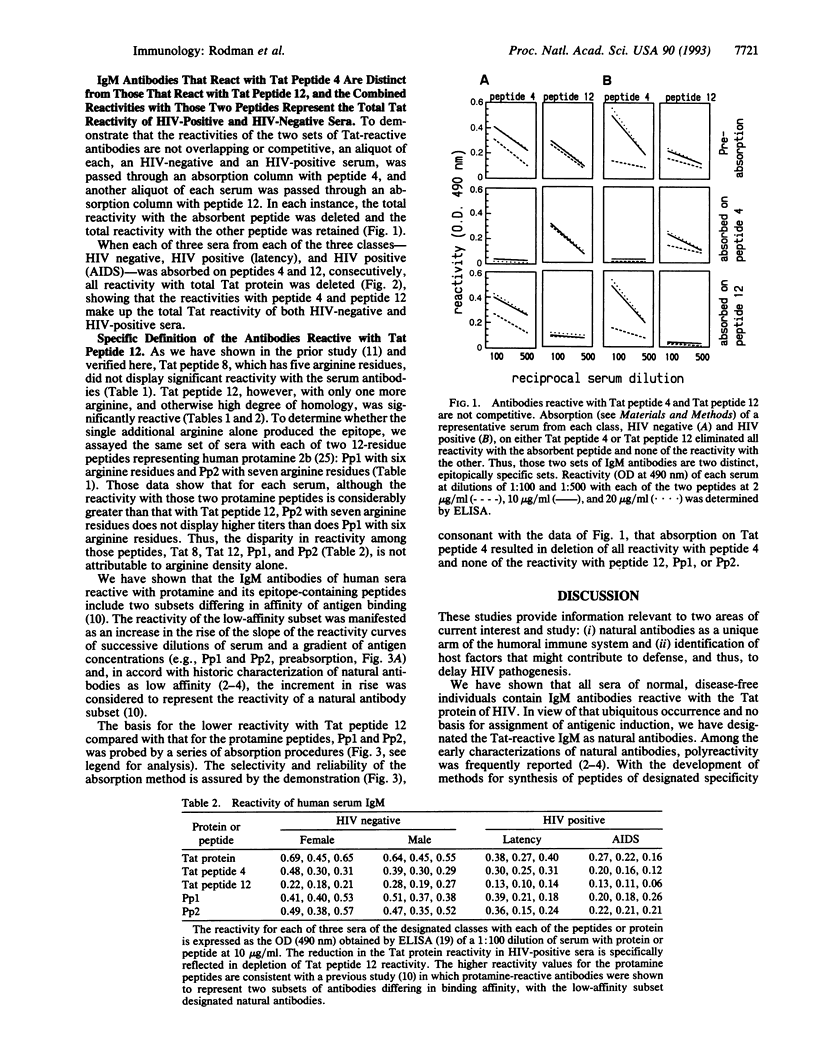
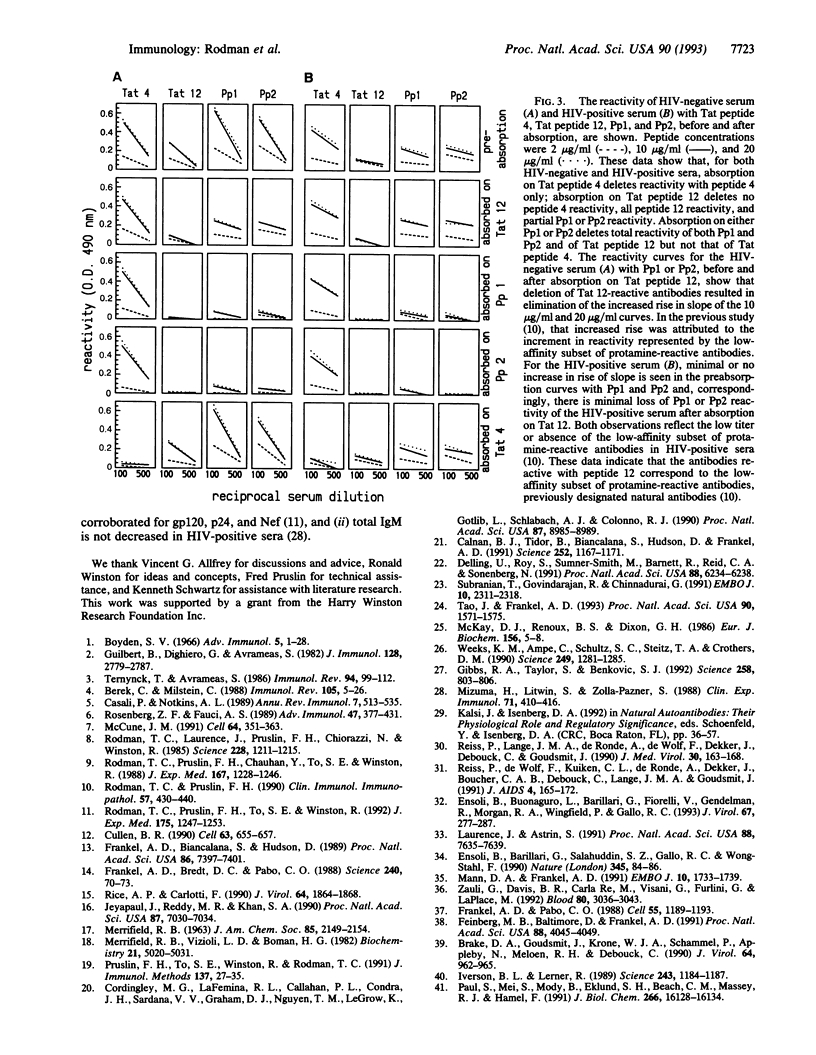
We have previously shown that IgM antibodies that react with human immunodeficiency virus (HIV) Tat, a regulatory protein essential for viral replication, are present in sera of all normal, HIV-negative individuals and deficient in sera of HIV-positive individuals at progressively greater frequency as diagnosis of AIDS nears. That IgM was designated as a set of natural antibodies, a repertoire of the normal humoral immune system believed to provide early defense against infectious invaders. In the prior study, by means of a series of synthetic peptides representing the amino acid sequence of HIV-1 Tat, one epitope for the IgM natural antibodies was defined within the cysteine-rich domain, shown in cell transfection studies to participate in Tat function. In this study we have defined another epitope, within the basic domain, with which the natural antibodies react. The specific sequence and amino acid residues required for that epitope are coincident with those required for the role of Tat in viral replication. The IgM antibodies reactive with the two epitopes of Tat make up two distinct sets, which, together, account for the total Tat reactivity of both HIV-negative and HIV-positive sera. The striking coincidence of the two epitopes with the two functional sequences of Tat suggests a potential role of those natural antibodies in control of HIV pathogenesis. By inference from the extensive evidence for the presence of extracellular Tat in cultures of HIV-infected cells, Tat may be expected to be present in the circulating plasma of infected people. We propose, therefore, that the Tat-reactive natural antibodies, documented in these studies to be present in the circulating plasma in the pre-AIDS stages of HIV infection, may inhibit cell entry of plasma-borne Tat and thereby curtail HIV propagation. Thus, those natural antibodies may be a host factor for delay in HIV pathogenetic progression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berek C., Milstein C. The dynamic nature of the antibody repertoire. Immunol Rev. 1988 Oct;105:5–26. doi: 10.1111/j.1600-065x.1988.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Boyden S. V. Natural antibodies and the immune response. Adv Immunol. 1966;5:1–28. doi: 10.1016/s0065-2776(08)60271-0. [DOI] [PubMed] [Google Scholar]
- Brake D. A., Goudsmit J., Krone W. J., Schammel P., Appleby N., Meloen R. H., Debouck C. Characterization of murine monoclonal antibodies to the tat protein from human immunodeficiency virus type 1. J Virol. 1990 Feb;64(2):962–965. doi: 10.1128/jvi.64.2.962-965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali P., Notkins A. L. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol. 1989;7:513–535. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- Cordingley M. G., LaFemina R. L., Callahan P. L., Condra J. H., Sardana V. V., Graham D. J., Nguyen T. M., LeGrow K., Gotlib L., Schlabach A. J. Sequence-specific interaction of Tat protein and Tat peptides with the transactivation-responsive sequence element of human immunodeficiency virus type 1 in vitro. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8985–8989. doi: 10.1073/pnas.87.22.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. The HIV-1 Tat protein: an RNA sequence-specific processivity factor? Cell. 1990 Nov 16;63(4):655–657. doi: 10.1016/0092-8674(90)90129-3. [DOI] [PubMed] [Google Scholar]
- Delling U., Roy S., Sumner-Smith M., Barnett R., Reid L., Rosen C. A., Sonenberg N. The number of positively charged amino acids in the basic domain of Tat is critical for trans-activation and complex formation with TAR RNA. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6234–6238. doi: 10.1073/pnas.88.14.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Buonaguro L., Barillari G., Fiorelli V., Gendelman R., Morgan R. A., Wingfield P., Gallo R. C. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993 Jan;67(1):277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg M. B., Baltimore D., Frankel A. D. The role of Tat in the human immunodeficiency virus life cycle indicates a primary effect on transcriptional elongation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4045–4049. doi: 10.1073/pnas.88.9.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. D., Biancalana S., Hudson D. Activity of synthetic peptides from the Tat protein of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7397–7401. doi: 10.1073/pnas.86.19.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. D., Bredt D. S., Pabo C. O. Tat protein from human immunodeficiency virus forms a metal-linked dimer. Science. 1988 Apr 1;240(4848):70–73. doi: 10.1126/science.2832944. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Pabo C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988 Dec 23;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Taylor S., Benkovic S. J. Antibody-catalyzed rearrangement of the peptide bond. Science. 1992 Oct 30;258(5083):803–805. doi: 10.1126/science.1439788. [DOI] [PubMed] [Google Scholar]
- Guilbert B., Dighiero G., Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982 Jun;128(6):2779–2787. [PubMed] [Google Scholar]
- Iverson B. L., Lerner R. A. Sequence-specific peptide cleavage catalyzed by an antibody. Science. 1989 Mar 3;243(4895):1184–1188. doi: 10.1126/science.2922606. [DOI] [PubMed] [Google Scholar]
- Jeyapaul J., Reddy M. R., Khan S. A. Activity of synthetic tat peptides in human immunodeficiency virus type 1 long terminal repeat-promoted transcription in a cell-free system. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7030–7034. doi: 10.1073/pnas.87.18.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence J., Astrin S. M. Human immunodeficiency virus induction of malignant transformation in human B lymphocytes. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7635–7639. doi: 10.1073/pnas.88.17.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D. A., Frankel A. D. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991 Jul;10(7):1733–1739. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M. HIV-1: the infective process in vivo. Cell. 1991 Jan 25;64(2):351–363. doi: 10.1016/0092-8674(91)90644-e. [DOI] [PubMed] [Google Scholar]
- McKay D. J., Renaux B. S., Dixon G. H. Human sperm protamines. Amino-acid sequences of two forms of protamine P2. Eur J Biochem. 1986 Apr 1;156(1):5–8. doi: 10.1111/j.1432-1033.1986.tb09540.x. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B., Vizioli L. D., Boman H. G. Synthesis of the antibacterial peptide cecropin A (1-33). Biochemistry. 1982 Sep 28;21(20):5020–5031. doi: 10.1021/bi00263a028. [DOI] [PubMed] [Google Scholar]
- Mizuma H., Litwin S., Zolla-Pazner S. B-cell activation in HIV infection: relationship of spontaneous immunoglobulin secretion to various immunological parameters. Clin Exp Immunol. 1988 Mar;71(3):410–416. [PMC free article] [PubMed] [Google Scholar]
- Paul S., Mei S., Mody B., Eklund S. H., Beach C. M., Massey R. J., Hamel F. Cleavage of vasoactive intestinal peptide at multiple sites by autoantibodies. J Biol Chem. 1991 Aug 25;266(24):16128–16134. [PubMed] [Google Scholar]
- Pruslin F. H., To S. E., Winston R., Rodman T. C. Caveats and suggestions for the ELISA. J Immunol Methods. 1991 Mar 1;137(1):27–35. doi: 10.1016/0022-1759(91)90390-2. [DOI] [PubMed] [Google Scholar]
- Reiss P., Lange J. M., de Ronde A., de Wolf F., Dekker J., Debouck C., Goudsmit J. Speed of progression to AIDS and degree of antibody response to accessory gene products of HIV-1. J Med Virol. 1990 Mar;30(3):163–168. doi: 10.1002/jmv.1890300303. [DOI] [PubMed] [Google Scholar]
- Reiss P., de Wolf F., Kuiken C. L., de Ronde A., Dekker J., Boucher C. A., Debouck C., Lange J. M., Goudsmit J. Contribution of antibody response to recombinant HIV-1 gene-encoded products nef, rev, tat, and protease in predicting development of AIDS in HIV-1-infected individuals. J Acquir Immune Defic Syndr. 1991;4(2):165–172. [PubMed] [Google Scholar]
- Rice A. P., Carlotti F. Mutational analysis of the conserved cysteine-rich region of the human immunodeficiency virus type 1 Tat protein. J Virol. 1990 Apr;64(4):1864–1868. doi: 10.1128/jvi.64.4.1864-1868.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman T. C., Laurence J., Pruslin F. H., Chiorazzi N., Winston R. Naturally occurring antibodies reactive with sperm proteins: apparent deficiency in AIDS sera. Science. 1985 Jun 7;228(4704):1211–1215. doi: 10.1126/science.3890184. [DOI] [PubMed] [Google Scholar]
- Rodman T. C., Pruslin F. H., Chauhan Y., To S. E., Winston R. Protamine-reactive natural IgM antibodies in human sera. Characterization of the epitope demonstrates specificity of antigenic recognition; occurrence indicates obscurity of origin and function. J Exp Med. 1988 Mar 1;167(3):1228–1246. doi: 10.1084/jem.167.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman T. C., Pruslin F. H. Identification of a low-affinity subset of protamine-reactive IgM antibodies present in normal, deficient in AIDS, sera: implications for HIV latency. Clin Immunol Immunopathol. 1990 Dec;57(3):430–440. doi: 10.1016/0090-1229(90)90117-9. [DOI] [PubMed] [Google Scholar]
- Rodman T. C., Pruslin F. H., To S. E., Winston R. Human immunodeficiency virus (HIV) Tat-reactive antibodies present in normal HIV-negative sera and depleted in HIV-positive sera. Identification of the epitope. J Exp Med. 1992 May 1;175(5):1247–1253. doi: 10.1084/jem.175.5.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. The immunopathogenesis of HIV infection. Adv Immunol. 1989;47:377–431. doi: 10.1016/s0065-2776(08)60665-3. [DOI] [PubMed] [Google Scholar]
- Subramanian T., Govindarajan R., Chinnadurai G. Heterologous basic domain substitutions in the HIV-1 Tat protein reveal an arginine-rich motif required for transactivation. EMBO J. 1991 Aug;10(8):2311–2318. doi: 10.1002/j.1460-2075.1991.tb07768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Frankel A. D. Electrostatic interactions modulate the RNA-binding and transactivation specificities of the human immunodeficiency virus and simian immunodeficiency virus Tat proteins. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1571–1575. doi: 10.1073/pnas.90.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternynck T., Avrameas S. Murine natural monoclonal autoantibodies: a study of their polyspecificities and their affinities. Immunol Rev. 1986 Dec;94:99–112. doi: 10.1111/j.1600-065x.1986.tb01166.x. [DOI] [PubMed] [Google Scholar]
- Weeks K. M., Ampe C., Schultz S. C., Steitz T. A., Crothers D. M. Fragments of the HIV-1 Tat protein specifically bind TAR RNA. Science. 1990 Sep 14;249(4974):1281–1285. doi: 10.1126/science.2205002. [DOI] [PubMed] [Google Scholar]
- Zauli G., Davis B. R., Re M. C., Visani G., Furlini G., La Placa M. tat protein stimulates production of transforming growth factor-beta 1 by marrow macrophages: a potential mechanism for human immunodeficiency virus-1-induced hematopoietic suppression. Blood. 1992 Dec 15;80(12):3036–3043. [PubMed] [Google Scholar]



