Abstract
Context
The importance of physical activity to paediatric health warrants investigation into its determinants. Objective measurement allows a robust examination of genetic and environmental influences on physical activity.
Objective
To systematically review the evidence on the extent of genetic and environmental influence on children's objectively-measured activity levels from twin studies.
Data sources and search terms
Medline, EMBASE, PsycINFO, Health and Psychosocial Instruments and all Ovid Databases. Search terms: “accelerometer” OR “actometer” OR “motion sensor” OR “heart rate monitor” OR “physical activity energy expenditure” AND “twin”. Limited to Human, English language and children (0–18 years).
Results
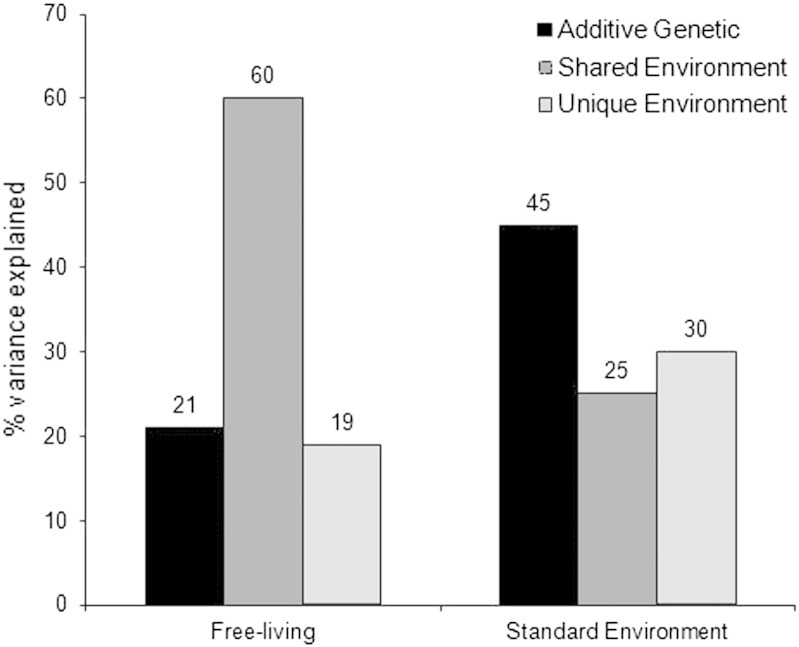
Seven sets of analyses were included in the review. Six analyses examined children's daily-life activity and found that the shared environment had a strong influence on activity levels (weighted mean 60%), with a smaller contribution from genetic factors (weighted mean 21%). Two analyses examined short-term, self-directed activity in a standard environment and found a smaller shared environment effect (weighted mean 25%) and a larger genetic estimate (weighted mean 45%).
Conclusions
Although genetic influences may be expressed when children have brief opportunities for autonomous activity, activity levels in daily-life are predominantly explained by environmental factors. Future research should aim to identify key environmental drivers of childhood activity.
Keywords: Twin, Child, Activity, Genetic, Environment
Highlights
-
•
Genetic influences may be expressed when children have brief opportunities for autonomous activity.
-
•
Activity levels in daily-life are predominantly explained by environmental factors.
-
•
Future research should aim to identify key environmental drivers of childhood activity.
Introduction
Regular participation in physical activity is protective against many chronic diseases including cardiovascular disease, some cancers, and diabetes (Booth et al., 2002, Cooper et al., 2005, Cooper et al., 2006). There is evidence that activity ‘tracks’ from childhood into adult life, with active children being more likely to become active adults (Craigie et al., 2011, Telama et al., 2005). Childhood physical activity is therefore important for health over the life-course. However, surprisingly little is known about the causes of variation between children's levels of physical activity.
Several studies have demonstrated that physical activity levels aggregate within families (Craig et al., 2013, Freedson and Evenson, 1991, Jago et al., 2010, Moore et al., 1991, Spinath et al., 2002). Most have depended on self-report or parent-report measures. The three studies that used objective measures of free-living activity found inconsistent effects. One study found that 4–7 year olds with two active parents were around six times more likely to be sufficiently active than those whose parents were sedentary (Moore et al., 1991). Similarly, a large study of 539 parent–child pairs found that children with more active mothers or fathers were more likely to be active themselves (Craig et al., 2013). However, another study measuring activity over 3 days found no parent–child correlations for activity levels (Jago et al., 2010); it is possible that a shorter measurement period (compared with 7 + days in the former studies) reduced the reliability of these results.
Studies of parent–child resemblance cannot distinguish between genetic interpretations (e.g. activity level is a heritable trait) and environmental interpretations (e.g. children's activity is influenced by parental modelling). However, twin studies make it possible to estimate the relative extent of genetic and environmental influence by comparing the similarity between genetically identical pairs (monozygotic; MZ) and fraternal pairs (dizygotic; DZ) who share on average, half of their segregating genes (Plomin et al., 2008). In addition, by assessing the extent to which twin similarity exceeds the correlation expected by the heritability of the trait, it is possible to divide the environmental component into the shared environment effect (which makes twins reared in the same home more similar) and the non-shared (or unique) environment effect (which makes the children different from one another).
Most twin studies on physical activity have used data from adult twin cohorts and have relied on self-reported measures of physical activity. This literature was reviewed by Beunen and Thomas (1999) who concluded that heritability of self-reported sports participation ranged from 35 to 85%, while the heritability of self-reported daily physical activity was slightly lower, at 29–62%. Two large twin studies published since this review have supported the conclusion that there is a moderate-to-strong genetic influence on adult physical activity (Stubbe et al., 2005, Stubbe et al., 2006), although one study that used the criterion of meeting adult guidelines for physical activity (≥ 150 min per week) found that the environmental effect predominated and the genetic effect was non-significant (Duncan et al., 2008).
Fewer adult studies have used objective measures of physical activity. A small exploratory study using accelerometers in 20 twin pairs suggested strong genetic influence of 78% (57–87%) on free-living daily physical activity (Joosen et al., 2005). A larger study involving 225 twin pairs, which measured activity over a 6 hour period in a controlled setting in which participants carried out a variety of tasks, such as psychological testing, role playing and giving presentations, found that the genetic effect explained almost half of the variation (Spinath et al., 2002).
In infants and young children there are a number of studies using parent-reported activity (often from activity subscales on temperament questionnaires), which generally suggest a slightly lower genetic influence (in the region of 19–40%, as reviewed in Hwang and Rothbart, 2003). However, parent-reported child activity can be unreliable (Corder et al., 2009), and a particular critique of its use in twin studies is the possibility of ‘contrast bias’ where parents over-report the differences between their DZ twins, or ‘assimilation bias’, whereby parents over-report the similarity of their MZ twins (Neale and Stevenson, 1989, Saudino, 2003, Saudino et al., 2000). These biases are indicated when DZ correlations are less than half of the MZ correlations and they generate inflated heritability estimates (Neale and Stevenson, 1989, Saudino, 2003, Saudino et al., 2000).
Objective measurements of physical activity avoid contrast or assimilation bias and provide more robust estimates of genetic and environmental effects. This paper systematically reviews paediatric twin studies that have used objective measures to quantify the extent of genetic and environmental influences on physical activity.
Materials and methods
Literature search
The following databases were searched simultaneously for peer-reviewed journals; Medline, PsycINFO, EMBASE, Health and Psychosocial Instruments and all OVID Databases in April 2015. Results were limited to human, English language and “all child” (0 to 18 years). Searched terms used were “twin” AND “accelerometer” OR “actometer” OR “heart rate monitor” OR “physical activity energy expenditure” OR “motion sensor.” A total of 3134 papers were identified. Titles and abstracts were scanned for relevance by three reviewers (AF, AS, LS), after removing papers based on title 74 were carried forward to abstract screening. Seven potentially eligible papers were identified. References lists of these papers were searched for relevant articles and reference lists of those papers and so on until no more articles could be identified. Four additional papers were included. Eleven papers were taken forward to full text review (Fig. 1).
Fig. 1.
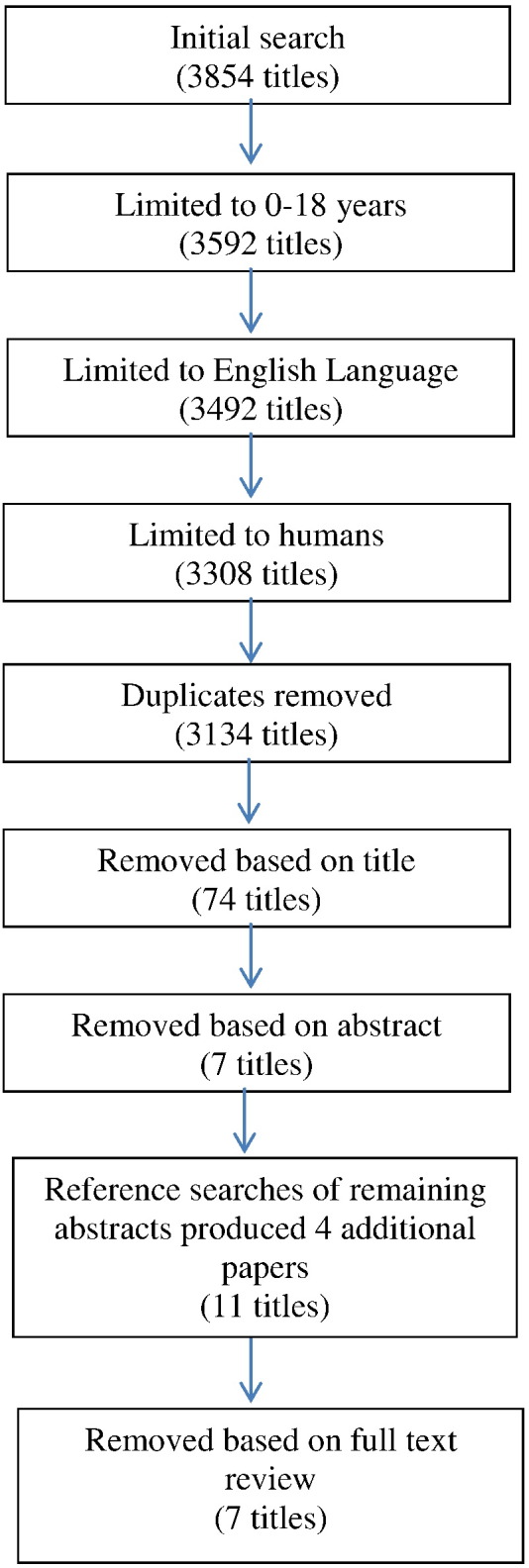
Flow of citations.
We identified eleven papers that provided heritability estimates of objectively-measured activity in children, but two sets of reports used the same twin sample with data examined in different ways. One set reported analyses of accelerometer data in one paper (Wood et al., 2007) and analyses of a composite index of accelerometer plus subjective measures in another (Wood et al., 2008). The other set reported analyses of activity level in different settings using a composite score for each setting (Saudino, 2009) or multiple individual scores (Saudino and Zapfe, 2008), and compared data with measures of attention deficit hyperactivity disorder (ADHD) (Ilott et al., 2010), and baseline data (Saudino, 2012). We selected one study from each set to include in this review, based on their relevance to our research question (Wood et al., 2007, Saudino, 2009). One of the selected studies had data both on free-living activity and activity in a standard environment; both sets of data were included because they contributed to separate analyses (Saudino, 2009). One small study assessed motor activity (waving of arms and legs) in infants who were not yet walking, but because it is unclear whether this behaviour is analogous to later childhood locomotor activity (Saudino and Eaton, 1991), this was not included. However, the findings were similar to those observed in the other studies included in the review.
Seven papers were therefore included in the final review (Fig. 1). The quality of all studies was then assessed using the Newcastle–Ottawa Scale adapted for cross-sectional designs (Hezrog et al., 2013). Each paper received a score out of 10 stars, with 10 being the highest possible score (Table 1).
Table 1.
Study quality assessment score.
| Study | Representativeness of the sample | Sample size | Non-respondents | Ascertainment of the exposure | The subjects in different outcome groups are comparable | Assessment of the outcome | Statistical test | Score (out of 10 with 10 being the best possible score) |
|---|---|---|---|---|---|---|---|---|
| Saudino (2009) | * | – | – | ** | * | ** | * | 7 |
| Saudino and Eaton (1995) | – | – | – | ** | * | ** | * | 6 |
| Franks et al. (2005) | * | – | – | ** | * | ** | * | 7 |
| Plomin and Foch (1980) | * | – | – | ** | Not enough information to score | Not enough information to score | _ | 3 |
| Fisher et al. (2010) | * | – | * | ** | * | ** | * | 8 |
| Hopkins et al. (2010) | – | – | – | ** | * | ** | * | 6 |
| Wood et al. (2007) | * | – | * | ** | * | ** | * | 8 |
Six analyses assessed free-living activity over several days (Fisher et al., 2010, Franks et al., 2005, Hopkins et al., 2010, Plomin and Foch, 1980, Saudino, 2009,Saudino and Eaton, 1995) and two assessed activity over a short period in a standard controlled environment (children were taking part in psychological testing in a research centre, then were allowed to play freely in breaks without parental direction) (Saudino, 2009, Wood et al., 2007). The different settings might expose real differences in the determinants; for example heritable genetic effects might be revealed more strongly when family influence is taken away. The methods were also very different, with a much shorter measurement period in the standard environment (hours opposed to days), which might be less reliable than the data collected over several days in the free-living setting (Penpraze et al., 2006). The two types of data were therefore included separately.
All studies measured physical activity objectively, using motion sensors such accelerometers, actometers or pedometers (Fisher et al., 2010, Franks et al., 2005, Hopkins et al., 2010, Plomin and Foch, 1980, Saudino, 2009,Saudino and Eaton, 1995, Wood et al., 2007) or by assessing physical activity energy expenditure (PAEE) with doubly-labelled water (DLW). PAEE was calculated as (Total Energy Expenditure − (Resting Metabolic Rate + 0.1 × Total Energy Expenditure)) (Franks et al., 2005).
A summary of the twin design
Central to understanding the genetic and environmental estimates provided in this paper is an appreciation of the twin design. Intraclass correlations give an estimate of effects: if a phenotype is entirely under genetic influence, the correlation between MZ pairs would be 1.0 and between DZ pairs would be 0.5. A correlation between DZ twins that is over half of the MZ correlation indicates shared environment influence (being reared in the same home inducing similarity). Difference between MZ twins denotes unique environment, but also includes measurement error. Structural equation modelling generates quantitative parameter estimates including confidence intervals (a = the genetic component, c = the shared environment component, e = the non-shared environment component). Measurement error is included in the e parameter.
In extracting results from the studies reviewed, if the paper only reported correlations, we have calculated genetic and environmental contributions using a simple formula by Falconer (11): a2 = 2(rMZ − rDZ); c2 = rMZ − a2; e2 = 1 − (a2 + c2) for ease of comparison. Because studies differed greatly in sample size, a weighted mean was calculated for summary purposes using the predefined weighted mean formula in Microsoft Excel (https://support.microsoft.com/en-us/kb/214049).
Results
Quality assessment scores were ranked out of 10 stars and ranged from 3 to 8 with a mean score of 6 (Table 1). A total of four papers scored well (7 to 8 stars) on quality assessment (Saudino, 2009, Franks et al., 2005, Fisher et al., 2010, Wood et al., 2007). Studies examining genetic and environmental influences on objectively-assessed activity level in children are summarised in Table 2, Table 3. Five of the six estimates for daily-life activity in children identified shared environmental effects as the dominant influence (Fisher et al., 2010, Franks et al., 2005, Hopkins et al., 2010, Plomin and Foch, 1980, Saudino, 2009). In these studies, the overall environmental (shared and non-shared) effect accounted for nearly 80% of the variation in children's activity levels. One smaller study involving 2-year old twin pairs (n = 53) produced a lower shared environmental estimate, but overall the environmental effect still accounted for half of the variance (Saudino and Eaton, 1995). The two adolescent studies both found that the shared environment explained most of the variance in total daily activity (Fisher et al., 2010, Hopkins et al., 2010). However, one examined different intensities of activity (moderate/vigorous physical activity and sedentary time) and showed that time spent in more intense activity had stronger genetic influence (Fisher et al., 2010).
Table 2.
Estimates of genetic and environmental effects of objectively-measured physical activity in children, ordered by age (in a free-living setting).
| Ref | n MZ pairs | n DZ pairs | Age (years) | Method | Duration | Activity behaviour | rMZ | rDZ | a2 | c2 | e2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Saudino (2009) | 144 | 168 | 2.07 | Accelerometer | 2 days | Free-living | 0.87 | 0.70 | 0.32 (0.18, 0.48) | 0.54 (0.39, 0.66) | 0.14 (0.11, 0.18) |
| Saudino and Eaton (1995) | 37 | 16 | 2.92 | Actometer | 2 days | Free-living | 0.76 | 0.51 | 0.50a | 0.26 | 0.24 |
| Franks et al. (2005) | 62 | 38 | 4–10 | PAEE by DLW | 14 days | Free-living | 0.87 | 0.76 | – | 0.69 (0.33,0.77) | 0.31 (0.23,0.29) |
| Plomin and Foch (1980) | 30 | 18 | 6–10 | Pedometer | 7 days | Free-living | 0.99 | 0.94 | 0.14a | 0.85 | 0.01 |
| Fisher et al. (2010) | 57 | 60 | 9–12 | Accelerometer | 6 days | Free-living | 0.76 | 0.71 | – | 0.73 (0.63, 0.81) | 0.27 (0.19, 0.37) |
| Hopkins et al. (2010) | 11 | 11 | 13 | Accelerometer | 7 days | Free-living | 0.68 | 0.60 | 0.16a | 0.52 | 0.32 |
MZ = monozygotic, DZ = dizygotic; rMZ = intra class correlation between MZ pairs, rDZ = intra class correlation between DZ pairs; a2 = genetic estimate, c2 = shared environment estimate, e2 = non-shared environment estimate; PAEE by DLW = physical activity energy expenditure by doubly labelled water. The best fitting model on which the authors based their conclusions is presented in each case.
Genetic and environmental influences were not provided so were calculated by the review authors using the equation by Falconer (11).
Table 3.
Estimates of genetic and environmental effects of objectively-measured physical activity in children, ordered by age (in a standard, laboratory setting without parental direction).
| Ref | n MZ pairs | n DZ pairs | Age (years) | Method | Duration | Activity behaviour | rMZ | rDZ | a2 | c2 | e2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Saudino (2009) | 144 | 168 | 2.07 | Accelerometer | 15 min + test administration | Standard environment | 0.63 | 0.36 | 0.59 (0.37, 0.70) | 0.03 (0.00, 0.21) | 0.38 (0.30, 0.48) |
| Wood et al. (2007) | 150 | 224a | 7–9 | Accelerometer | 2.5 h | Standard environment | 0.73 | 0.58 | 0.36 (0.17,0.56) | 0.39 (0.21,0. 55) | 0.25 (0.20, 0.32) |
MZ = monozygotic, DZ = dizygotic; rMZ = intra class correlation between MZ pairs, rDZ = intra class correlation between DZ pairs; a2 = genetic estimate, c2 = shared environment estimate, e2 = non-shared environment estimate. The best fitting model on which the authors based their conclusions is presented in each case.
Including 111 opposite sex pairs — 89 ‘singleton’ twins were also included in the twin modelling analysis in this study.
The weighted mean proportions of variance explained by genetic, shared and unique environmental factors for daily-life activity are shown in the first set of bars in Fig. 2. The shared environment was the main influence (weighted mean estimate 60%) while genetic effects were much smaller (weighted mean estimate 21%).
Fig. 2.
Genetic and environmental influences on objectively-measured physical activity in children. Data are weighted mean estimates of variance from six studies of objectively-measured physical activity in childhood in a free-living setting and two studies conducted in a standard, laboratory setting.
Two fairly large studies examined activity levels in a standard environment, with the children taking part in psychological tests as well as being allowed to play freely during break sessions without parental direction (Saudino, 2009, Wood et al., 2007). In this context, the genetic effects on activity level were higher, with a weighted mean heritability of 45% (Saudino, 2009, Wood et al., 2007). In one of these studies, data had also been collected under free-living conditions, allowing for direct comparison (Saudino, 2009) and showed that heritability was higher in the standard setting (59%) than the daily-life setting (32%), while the shared environment effect explained more variance in daily-life setting (54%) than in the standard setting (3%) (Saudino, 2009).
Genetic factors had the strongest influence on children's objectively-measured physical activity in a standard environment, explaining around half of the variance (see Fig. 2); but shared and unique environmental factors were also important.
Discussion
This review quantified genetic and environmental effects on children's physical activity using twin studies in which activity was measured objectively, thereby avoiding the biases that may influence parent-reported child activity. In the free-living setting, shared environmental influences predominated in all the studies, with no more than a small genetic effect. In contrast, the two studies which assessed activity over the short-term in a standard environment, without parental direction, found a substantially higher genetic influence (Saudino, 2009, Wood et al., 2007).
Heritability estimates based on short snapshots of children's activity in a standard environment were similar to estimates of genetic influence on self-reported leisure-time activity or sports participation in adults (reviewed in Beunen and Thomas, 1999). This could suggest that the key issue to allow expression of genetic tendencies is the opportunity to choose their level of activity freely. This would be more likely in adults, but also more likely when children are playing on their own outside the home, and therefore outside of parental and sibling influence. Although studies testing this in physical activity are required, there is a well-established significant increase in heritability of intelligence with age and a proposed mechanisms is a gene–environment correlation (whereby children select, modify and create environments associated with their genetic propensities) (Plomin and Deary, 2015, Bouchard, 2013).
Recent longitudinal research investigating the drivers of continuity and change in objectively measured activity level from 2 to 3 years in 304 same-sex twins reported a strong environmental underpinning to change in activity level when measured in the home environment, but a stronger genetic underpinning when measured in a standard, laboratory setting (Saudino, 2012). The authors posited that the predominant role of environment in activity level change within the home might relate to the introduction of age-dependent family activities.
One study also found that in the transition from adolescence to adulthood there was a shift from environmental to genetic determinants of leisure time physical activity (Stubbe et al., 2005). A recent study examining self-reported activity data from 8355 adolescent twins, which stratified the sample by age and sex, found a predominant genetic effect overall, but a stronger shared environment effect in the younger girls (van der Aa et al., 2010). Objective physical activity data in adolescents are lacking, and a valuable contribution to the literature would be to assess habitual activity during important transition periods (e.g. from childhood to adolescence, or adolescence to adulthood) in a large twin sample to determine whether genetic influences change over time. In the current review, the number of eligible studies was too small and age-range too varied to draw inferences about whether the importance of the environment versus genes changes with age (as is observed in other phenotypes such as intelligence (Plomin and Deary, 2015, Bouchard, 2013) and body mass index (Haworth et al., 2008)). However, a longitudinal twin study using objective measures of physical activity is warranted.
A large proportion of children's daily activities are directed by parents, teachers, or even co-twins. Indeed there is some evidence from singleton children that the modern environment suppresses activity (Esliger et al., 2010), and that parental factors, such as support and encouragement, are required to promote children's physical activity (Hinkley et al., 2008, Sallis et al., 2000). An exploratory study in which 24 children wore accelerometers compared a novel ‘active learning environment’ (where they were allowed to move about freely during lessons), a ‘standing classroom’ (where vertical work stations were used), and a standard sitting desk-based classroom. Average activity levels were substantially and significantly higher in the active learning environment, providing preliminary evidence that children move more in an activity-permissive environment, but it did not address individual differences (Lanningham-Foster et al., 2008). At the same time ‘activity-permissive’ environments might allow greater expression of subtle differences in preference for active vs. sedentary activities; a suggestion that is supported to some extent by evidence for significant genetic influence on children's self-reported activity preferences (Fisher et al., 2010).
The evidence for environmental effects is also consistent with findings that children who walk or cycle to school have higher overall daily activity levels and are fitter than those who travel by car, supporting an environmental influence (Cooper et al., 2005, Cooper et al., 2006). It is also likely that children who are genetically predisposed to being more physically active are more likely to choose more active transport options, and indeed choose other options that are conducive to physical activity, like spending more time outdoors, and this should be further explored. However, external factors that cannot be influenced by the individual child, such as season or school physical activity policy, have also been shown to have a significant impact on children's total daily activity, making a case for environmental effects (Ferreira et al., 2006, Fisher et al., 2005, Kolle et al., 2009, Sallis et al., 2000). A recent review identified attributes of the physical environment that were correlated with children's activity (such as availability and access to leisure facilities) (Krahnstoever Davidson and Lawson, 2009) but further research is required in this area.
This review is limited by a shortage of research. Further research measuring activity in both structured and unstructured environments could elucidate the comparative contribution of environment and genes to activity levels in children, investigating whether different settings might manipulate the effect genes have on activity. It cannot be discounted that differences in the findings reported in this review are in part due to increased error arising from the reduced period of measurement in laboratory-based studies which measure activity in a standard environment. However, overall a key finding is that the environment is an important influence on children's activity in any situation.
Although the twin design is very valuable in allowing quantification of genetic and environmental contributions to a phenotype, it is not without limitations. It is feasible that MZ twins share their environments to a greater extent than DZ twin, because they are treated more similarly. However, in the current context this would lead to a greater proportion of the variance in physical activity being explained by genes, rather than the shared environment. Additionally, there is empirical support for the equal environment assumption from twin studies of psychiatric illness (Kendler et al., 1993). Complimentary study designs that would further enhance the elucidation of the genetic and environmental influences on physical activity, and importantly, gene–environment interactions, include adoption studies, inclusion of siblings in a twin design, and genome-wide complex trait analyses (Boomsma et al., 2002). However, to the best of our knowledge these have not been carried out in objectively measured physical activity in childhood. This review highlights the need for further research in this important field. Findings from twin studies may not be fully generalisable to singleton populations. However, similar levels of objectively measured activity between young twin and singleton cohorts are encouraging (Fisher et al., 2010).
The Falconer formula was useful in giving an estimate of the variance explained by genes and the shared and unique environments where this had not been provided in the manuscript. However, a limitation of the equation is that it does not give model fit statistics or confidence intervals. An alternative approach would be to request all original data from authors and conduct formal quantitative genetic analyses. However this was not feasible for the current review, and mean estimates are generally very similar between those calculated using the Falconer equation and those acquired by more formal heritability analyses.
In comparing our findings to those from studies using parent-report in early childhood, there is a problem of matching measures. A review of twin studies using activity subscales on temperament questionnaires estimate significant heritability estimates of 19–40% (Hwang and Rothbart, 2003), which are similar to those found in the standard environment studies. However, these data were generally derived from infant studies using questions like ‘during the past week while dressing how often did your baby wave their arms and kick’ from the Infant Behaviour Questionnaire (Gartstein and Rothbard, 2003, Hwang and Rothbart, 2003, Rothbart, 1981). A small objective study of early infant motor activity level also provided heritability estimates of around 40%, suggesting the high genetic effects found in parent-report studies might be particular to infant activity (Saudino and Eaton, 1991). There are no twin studies assessing children's free-living habitual activity level using parent-report as this cannot be measured reliably using subjective measures (Corder et al., 2008, Reilly et al., 2008).
Conclusions
This review of paediatric twin studies using objective measures of activity, demonstrates that physical activity in children shows modest genetic influence when measured over a short time-period in a standard environment, but in everyday life is predominantly determined by the environment in which they live. These results highlight the need for research into the modifiable environmental drivers of physical activity in children's home and school environments.
Contributors statement
Conception and design of review: AF, CHMvJ, LS, AS and JW. Review of the literature: AF, CHMvJ, LS and AS. Analysis and interpretation of data: AF, CHMvJ, LS, AS and JW. Drafting of paper: AF and AS. Critical revision of paper for intellectual content: CHMvJ, LS, and JW. Final approval of the version to be published: AF, CHMvJ, LS, AS and JW.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
We would like to thank the Cancer Research UK (grant number C1418/A141) for funding this work.
Footnotes
Funding source: Cancer research UK.
Contributor Information
Abigail Fisher, Email: Abigail.fisher@ucl.ac.uk.
Lee Smith, Email: lee.smith@ucl.ac.uk.
Cornelia H.M. van Jaarsveld, Email: ellen.van.jaarsveld@kcl.ac.uk.
Alexia Sawyer, Email: alexia.sawyer@ucl.ac.uk.
Jane Wardle, Email: jane.wardle@ucl.ac.uk.
References
- Beunen G., Thomas M. Genetic determinants of sports participation and daily physical activity. Int. J. Obes. Relat. Metab. Disord. 1999;23:s55–s63. doi: 10.1038/sj.ijo.0800885. [DOI] [PubMed] [Google Scholar]
- Boomsma D., Bussahn A., Pletonen L. Classical twin studies and beyond. Nat. Rev. 2002;3:827–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Booth F.W., Chakravarthy M.V., Gordon S.E. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J. Appl. Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- Bouchard T. The Wilson effect: the increase in heritability of IQ with age. Twin Res. Hum. Genet. 2013;16:923–930. doi: 10.1017/thg.2013.54. [DOI] [PubMed] [Google Scholar]
- Cooper A.R., Andersen L.B., Wedderkopp N. Physical activity levels of children who walk, cycle, or are driven to school. Am. J. Prev. Med. 2005;29:179–184. doi: 10.1016/j.amepre.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Cooper A.R., Wedderkopp N., Wang H. Active travel to school and cardiovascular fitness in Danish children and adolescents. Med. Sci. Sports Exerc. 2006;38:1724–1731. doi: 10.1249/01.mss.0000229570.02037.1d. [DOI] [PubMed] [Google Scholar]
- Corder K., Ekelund U., Steele R.M. Assessment of physical activity in youth. J. Appl. Physiol. 2008;105:977–987. doi: 10.1152/japplphysiol.00094.2008. [DOI] [PubMed] [Google Scholar]
- Corder K., van Sluijs E.M.F., Wright A. Is it possible to assess free-living physical activity and energy expenditure in young people by self-report? Am. J. Clin. Nutr. 2009;89:862–870. doi: 10.3945/ajcn.2008.26739. [DOI] [PubMed] [Google Scholar]
- Craig C.L., Cameron C., Tudor-Locke C. Relationship between parent and child pedometer-determined physical activity: a sub-study of the CANPLAY surveillance study. Int. J. Behav. Nutr. Phys. Act. 2013;10:8. doi: 10.1186/1479-5868-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie A.M., Lake A.A., Kelly S.A. Tracking of obesity-related behaviours from childhood to adulthood: a systematic review. Maturitas. 2011;70:266–284. doi: 10.1016/j.maturitas.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Duncan G.E., Goldberg J., Noonan C. Unique environmental effects on physical activity participation: a twin study. PLoS ONE. 2008:e2019. doi: 10.1371/journal.pone.0002019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esliger D.W., Tremblay M.S., Copeland J.L. Physical activity profile of Old Order Amish, Mennonite, and contemporary children. Med. Sci. Sports Exerc. 2010;42:296–303. doi: 10.1249/MSS.0b013e3181b3afd2. [DOI] [PubMed] [Google Scholar]
- Ferreira I., van der Horst K., Wendel-Vos W. Environmental correlates of physical activity in youth — a review and updates. Obes. Rev. 2006:129–154. doi: 10.1111/j.1467-789X.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- Fisher A., Reilly J.J., Montgomery C. Seasonality in physical activity and sedentary behavior in young children. Pediatr. Exerc. Sci. 2005;17:31–40. doi: 10.1123/pes.19.1.51. [DOI] [PubMed] [Google Scholar]
- Fisher A., van Jaarsveld C.H.M., Llewellyn C.H. Environmental influences on children's physical activity: quantitative estimates using a twin design. PLoS ONE. 2010;5:e10110. doi: 10.1371/journal.pone.0010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks P.W., Ravussin E., Hanson R.L. Habitual physical activity in children: the role of genes and the environment. Am. J. Clin. Nutr. 2005;82:901–908. doi: 10.1093/ajcn/82.4.901. [DOI] [PubMed] [Google Scholar]
- Freedson P.S., Evenson S. Familial aggregation in physical activity. Res. Q. Exerc. Sport. 1991;62:384–389. doi: 10.1080/02701367.1991.10607538. [DOI] [PubMed] [Google Scholar]
- Gartstein M.A., Rothbard M.K. Studying infant temperament via the Revised Infant Behaviour Questionnaire. Infant Behav. Dev. 2003;26:64–86. [Google Scholar]
- Haworth C., Carnell S., Meaburn E. Increasing heritability of BMI and stronger associations with the FTO gene over childhood. Obesity. 2008;16:2663–2668. doi: 10.1038/oby.2008.434. [DOI] [PubMed] [Google Scholar]
- Hezrog R., Alvarez-Pasquin J., Diaz C. Are health care workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley T., Crawford D., Salmon J. Preschool children and physical activity: a review of correlates. Am. J. Prev. Med. 2008;34:435–441. doi: 10.1016/j.amepre.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Hopkins N., Stratton G., Maia J. Heritability of arterial function, fitness and physical activity in youth: a study of monozygotic and dizygotic twins. J. Paediatr. 2010;157:943–948. doi: 10.1016/j.jpeds.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Hwang J., Rothbart M.K. Behaviour genetics studies of infant temperament: findings vary across parent-report instruments. Infant Behav. Dev. 2003;26:113–114. [Google Scholar]
- Ilott N., Saudino K.J., Wood A. A genetic study of ADHD and activity level in infancy. Genes Brain Behav. 2010;9:296–304. doi: 10.1111/j.1601-183X.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jago R., Fox K.R., Page A.S. Parent and child physical activity and sedentary time: do active parents foster active children? BMC Public Health. 2010;10:194. doi: 10.1186/1471-2458-10-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosen A.M., Gielen M., Vlietinck R. Genetic analysis of physical activity in twins. Am. J. Prev. Med. 2005;82:1253–1259. doi: 10.1093/ajcn/82.6.1253. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Neale M.C., Kessler R.C. A test of equal-environment assumption in twin studies of psychiatric illness. Behav. Genet. 1993;23:21–27. doi: 10.1007/BF01067551. [DOI] [PubMed] [Google Scholar]
- Kolle E., Steene-Johannessen J., Andersen L.B. Seasonal variation in objectively assessed physical activity among children and adolescents in Norway: a cross-sectional study. Int. J. Behav. Nutr. Phys. Act. 2009;6:36. doi: 10.1186/1479-5868-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahnstoever Davidson K., Lawson C.T. Do attributes in the physical environment influence children's physical activity?; A review of the literature. Int. J. Behav. Nutr. Phys. Act. 2009;3:19. doi: 10.1186/1479-5868-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanningham-Foster L., Foster R.C., McCrady S.K. Changing the school environment to increase physical activity in children. Obesity. 2008;16:1849–1853. doi: 10.1038/oby.2008.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L.L., Lombardi D.A., White M.J. Influence of parents' physical activity levels on activity levels of young children. J. Paediatr. 1991;118:215–219. doi: 10.1016/s0022-3476(05)80485-8. [DOI] [PubMed] [Google Scholar]
- Neale M.C., Stevenson J. Rater bias in the EASI temperament scales: a twin study. J. Pers. Soc. Psychol. 1989;56:446–455. doi: 10.1037//0022-3514.56.3.446. [DOI] [PubMed] [Google Scholar]
- Penpraze V., Reilly J.J., MacLean C.M. Monitoring of physical activity in young children: how much is enough? Pediatr. Exerc. Sci. 2006;18:483–491. doi: 10.1123/pes.18.4.483. [DOI] [PubMed] [Google Scholar]
- Plomin R., Deary I.J. Genetics and intelligence differences: five special findings. Mol. Psychiatry. 2015;20:98–108. doi: 10.1038/mp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R., Foch T.T. A twin study of objectively assessed personality in childhood. J. Pers. Soc. Psychol. 1980;39:680–688. doi: 10.1037//0022-3514.45.3.633. [DOI] [PubMed] [Google Scholar]
- Plomin R., DeFries J.C., McLean G.E. 5th ed. Worth Publishers; New York: 2008. Behavioural Genetics. [Google Scholar]
- Reilly J.J., Penpraze V., Hislop J. Objective measurement of physical activity and sedentary behaviour: review with new data. Arch. Dis. Child. 2008;93:614–619. doi: 10.1136/adc.2007.133272. [DOI] [PubMed] [Google Scholar]
- Rothbart M.K. Measurement of temperament in infancy. Child Dev. 1981;52:569–578. [Google Scholar]
- Sallis J.F., Prochaska J.J., Taylor W.C. A review of correlates of physical activity of children and adolescents. Med. Sci. Sports Exerc. 2000;32:963–975. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Saudino K. Parent ratings of infant temperament: lessons from twin studies. Infant Behav. Dev. 2003;26:100–107. [Google Scholar]
- Saudino K.J. Do different measures tap the same genetic influences? A multi-method study of activity level in twins. Dev. Sci. 2009:626–633. doi: 10.1111/j.1467-7687.2008.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudino K.J. Sources of continuity and change in activity level in early childhood. Child Dev. 2012;83:266–281. doi: 10.1111/j.1467-8624.2011.01680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudino K.J., Eaton W.O. Infant temperament and genetics: an objective twin study of motor activity level. Child Dev. 1991;62:1167–1174. [PubMed] [Google Scholar]
- Saudino K.J., Eaton W.O. Continuity and change in objectively assessed temperament: a longitudinal twin study of activity level. Br. J. Soc. Psychol. 1995;13:81–95. [Google Scholar]
- Saudino K.J., Zapfe J.A. Genetic influences on activity level in early childhood: do situations matter? Child Dev. 2008;79:930–943. doi: 10.1111/j.1467-8624.2008.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudino K.J., Cherny S.S., Plomin R. Parent ratings of temperament in twins: explaining the ‘too low’ DZ correlations. Twin Res. 2000;3:224–233. doi: 10.1375/136905200320565193. [DOI] [PubMed] [Google Scholar]
- Spinath F.M., Wolf H., Angleitner A. Genetic and environmental influences on objectively assessed activity in adults. Personal. Individ. Differ. 2002;33:633–645. [Google Scholar]
- Stubbe J.H., Boomsma D.I., De Geus E.J. Sports participation during adolescence: a shift from environmental to genetic factors. Med. Sci. Sports Exerc. 2005;37:563–570. doi: 10.1249/01.mss.0000158181.75442.8b. [DOI] [PubMed] [Google Scholar]
- Stubbe J.H., Boomsma D.I., Vink J.M. Genetic influences on exercise participation in 37,051 twin pairs from seven countries. PLoS ONE. 2006;1:e22. doi: 10.1371/journal.pone.0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telama R., Yang X., Viikari J. Physical activity from childhood to adulthood: a 21-year tracking study. Am. J. Prev. Med. 2005;28:267–273. doi: 10.1016/j.amepre.2004.12.003. [DOI] [PubMed] [Google Scholar]
- van der Aa N., De Gues E.J., van Beijsterveldt T.C. Genetic influence on individual differences in exercise behaviour during adolescence. Int. J. Pediatr. 2010:138345. doi: 10.1155/2010/138345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A.C., Saudino H.R., Asherton P., Kuntsi J. Genetic influences on mechanically-assessed activity level in children. J. Child Psychol. Psychiatry. 2007;48:695–702. doi: 10.1111/j.1469-7610.2007.01739.x. [DOI] [PubMed] [Google Scholar]
- Wood A.C., Rijsdijk F., Saudino K.J. High heritability for a composite index of children's activity level measures. Behav. Genet. 2008;38:266–276. doi: 10.1007/s10519-008-9196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]