Abstract
Interest in adolescence as a crucial stage of neurobehavioral maturation is growing, as is the concern of how stress may perturb this critical period of development. Though it is well recognized that stress-related vulnerabilities increase during adolescence, not all adolescent individuals are uniformly affected by stress nor do stressful experiences inevitability lead to negative outcomes. Indeed, many adolescents show resilience to stress-induced dysfunctions. However, relatively little is known regarding the mechanisms that may mediate resilience to stress in adolescence. The goal of this brief review is to bring together a few separate, yet related lines of research that highlight specific variables that may influence stress resilience during adolescence, including early life programming of the hypothalamic-pituitary-adrenal (HPA) axis, stress inoculation, and genetic predisposition. Though we are far from a clear understanding of the factors that mediate resistance to stress-induced dysfunctions, it is imperative that we identify and delineate these aspects of resilience to help adolescents reach their full potential, even in the face of adversity.
Keywords: Adolescence, HPA axis, Puberty, Resilience, Stress
1. Introduction
It is well established that prolonged or chronic exposure to stress can lead to a variety of adverse physiological and psychological consequences, including obesity, drug abuse, and mood disorders (McEwen, 2005, McEwen, 2007, de Kloet et al., 1998). Furthermore, a growing body of evidence indicates that periods marked by significant brain maturation and plasticity, such as perinatal and adolescent development, may be especially vulnerable to these disruptive effects of stress (Romeo et al., 2009, Eiland and Romeo, 2013). Less appreciated, however, is the fact that not all individuals exposed to extended or repeated stressors necessarily go on to develop neurobehavioral dysfunctions. The factors that mediate this resilience to stress-induced vulnerabilities are unclear, but likely involve an interaction between genetic and environmental variables (Rutter, 2013, Southwick and Charney, 2012). The purpose of this review is to discuss possible mechanisms that may contribute to stress resilience, particularly during the adolescent stage of development. Given the scarcity of data that directly addresses stress resilience during adolescence, this review will also suggest potential future lines of research to help fill this gap in our understanding.
2. Adolescence and stress-related vulnerabilities
An emergent body of research has begun to show the short- and long-term effects of exposure to stress during adolescence on a diverse set of negative physiological and neurobehavioral outcomes (Eiland and Romeo, 2013, McCormick and Green, 2013, McCormick, 2010, Hollis et al., 2013, McCormick and Mathews, 2010, McCormick et al., 2010). It has been proposed that adolescents may show a heightened sensitivity to stressors based on at least three converging factors (Romeo, 2013). First, animal studies have indicated that peripubertal individuals display greater hormonal stress responses compared to adults following a variety of physical and psychological stressors (Romeo, 2010a, Romeo, 2010b, McCormick and Mathews, 2007). Second, neuroanatomical studies have reported that the brain areas known to be highly sensitive to stressors in adulthood, namely the amygdala, hippocampus, and prefrontal cortex, all continue to mature during adolescence (Giedd and Rapoport, 2010). Third, the adolescent brain may be more responsive to the stress-related hormones than the more mature brain, as a previous study in rats showed that exposure to similar levels of corticosterone increased gene expression for glutamate receptor subunits to a greater degree in the adolescent compared to adult hippocampus (Lee et al., 2003). Taken together, these data argue that changes in hormonal stress reactivity coupled with the continued maturation of stress-sensitive neural circuits contribute to the marked increase in stress-related vulnerabilities often observed during adolescence (Fig. 1).
Fig. 1.
A schematic representation of factors that may contribute to the increase in stress-related vulnerabilities observed during adolescence. Specifically, adolescent changes in hormonal stress reactivity, brain maturation, and sensitivity to stress-related hormones may lead to the marked increase in stress-related dysfunctions often associated with adolescence, such as obesity, psychological disorders, and drug abuse.
3. Adolescence and stress resilience
Despite the convergence and interaction of these hormonal and neurobiological variables that may render the adolescent particularly vulnerable to stressors, not all adolescents are adversely affected by stress and experiencing stressors during adolescence does not inevitability result in negative outcomes. However, it is unclear what may account for the different reactions that adolescents show in response to stress exposure.
Some differences in the neurobehavioral responses to adolescent stress across studies are undoubtedly mediated by subtle or significant differences in the specific experimental paradigms and/or assays used. For instance, studies that exposed adolescent rats to social defeat stress found either increased or decreased anxiety-like behaviors in adulthood (Watt et al., 2009, Weathington et al., 2012), but these diametrically opposed results can likely be explained by experimental differences, such as the length and frequency of the social defeat and the animal housing conditions (i.e., single vs. group) used in these two studies. More intriguing, however, is the difference in how individual animals respond to a stressor within an experiment. A greater understanding and appreciation of this variation may potentially shed light on what makes some animals more or less resistant to stressful experiences.
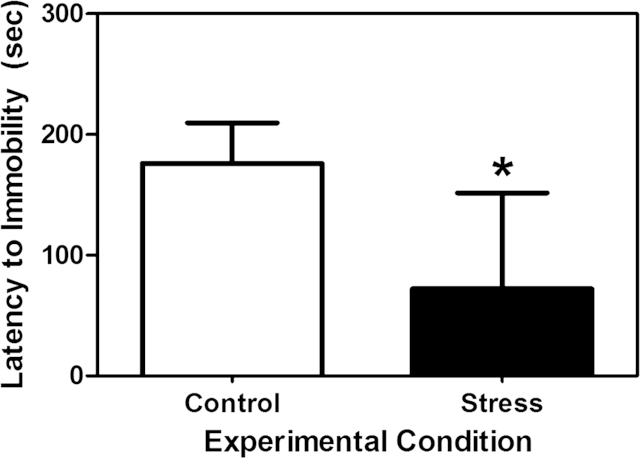
To illustrate this stress-induced variability, I present a specific example from a pilot study we recently conducted. Briefly, in this study we exposed adolescent male rats to 1 h of restraint stress every other day from postnatal day (PND) 28–49. This age span was used as this 3 week period in rodents is associated with the most significant changes in physiological, neurobiological, and behavioral parameters as animals transition into adulthood (Spear, 2000). We then tested these animals in the forced swim test in young adulthood to measure depressive-like behaviors (Porsolt et al., 1977). We found that the rats exposed to restraint stress during adolescence showed a shorter latency to immobility than age-matched non-stressed controls (Fig. 2; unpublished observation). Though these results suggest that adolescent stress exposure leads to depressive-like behaviors in adulthood, these data are presented here to provide an example of the relatively high degree of variability in the experimental group. Specifically, the mean and standard deviation of the control group are 176.0 and 33.6, respectively, while the stress group is 72.2 and 79.3, respectively. This high standard deviation in the experimental group indicates a rather large spread around the mean. Though not surprising that individual differences exist in responsiveness to a relatively complex set of stimuli experienced throughout adolescence, data such as these do lead to the simple question: why do some animals appear to be more affected than others? Here, we will highlight three interrelated factors that may impart greater resilience to an adolescent facing a stressful environment: early life programming of the hypothalamic-pituitary-adrenal (HPA) axis, stress inoculation, and genetic predisposition. It is important to note that these factors are neither unique to stress resilience during adolescence, nor the only elements likely at work modulating an individual's resilience to stress. Instead, these factors are discussed to illustrate potential mechanisms through which resilience to adolescent stress may be realized and provide examples of future lines of research that could be investigated.
Fig. 2.
Mean (±SEM) latency to immobility in the forced swim test exhibited by male rats in young adulthood that had previously been exposed to either 1 h of restraint stress every other day throughout adolescence (postnatal days 28–49; stress) or no restraint stress (control). Asterisk indicates a significant difference between the groups (t (15) = 3.81, p = 0.03). Note the relative large variance around the mean in the stress group (unpublished observation).
4. Early life programming of the HPA axis
The HPA axis is the primary neuroendocrine axis that mediates stress-induced hormonal responses. This response is driven by a cascade of signals beginning with the release of corticotropin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus. CRH is released into the hypophyseal portal system, which in turn leads to the release of adrenocorticotropin hormone (ACTH) from the anterior pituitary. ACTH then stimulates the secretion of the glucocorticoids (i.e., cortisol in primates and corticosterone in many rodent species) from the adrenal cortex (Herman and Cullinan, 1997, Herman et al., 2003, Ulrich-Lai and Herman, 2009).
In the short-term, release of these hormones mediate many beneficial effects, such as mobilization of energy stores, reduced inflammation, and enhanced immune activity and memory formation (McEwen, 2007, Roozendaal, 2000, Sapolsky et al., 2000, Dhabhar, 2009). However, if individuals experience prolonged or repeated exposure to these stress-related hormones, then negative effects may emerge, including altered metabolism and cognitive deficits (McEwen, 2005, McEwen and Stellar, 1993, McEwen, 2003, Sapolsky, 1999, Herbert et al., 2006, McEwen, 2004, van Praag, 2004). Therefore, factors that modulate the responsiveness of the HPA axis may have significant and widespread consequences for the individual.
Many experiments have addressed how experiences early in life shape HPA axis function and the implications these changes may have on an individual's later physiology and behavior (Korosi and Baram, 2010). One salient influence on early life programming of the HPA axis is the relative presence or absence of a caregiver, usually the mother in rodent studies, and the quantity and quality of parental care. Data derived from the “handling” paradigm (Levine, 1957), in which brief periods of maternal separation lead to enhanced maternal behavior, have led to numerous discoveries about the role of maternal care on the offspring's HPA function (Caldji et al., 2000, Tang et al., 2014). It has been shown that increased quantity of arch backed nursing and licking and grooming (Liu et al., 1997), as well as the consistency of these maternal behaviors (Akers et al., 2008), are important variables in reducing stress reactivity in adulthood.
Neonatal handling has also been shown to modify HPA function in adolescent animals. Specifically, daily separating the dams and pups for 15 min throughout the entire pre-weaning period (PND 1-22) resulted in decreased basal corticosterone levels in handled compared to non-handled prepubertal male and female rats (Papaioannou et al., 2002). In response to an acute restraint stress, however, neonatal handling was shown to result in sex-specific effects, such that restraint-induced corticosterone responses are lower in handled males, but higher in handled females, compared to controls (Park et al., 2003). Thus, it appears that neonatal handling, and presumably subsequent changes in maternal care, lead to changes in adolescent HPA stress reactivity in a sex-dependent manner.
It is unclear if this early life handling manipulation would protect males specifically from adolescent stress-induced changes in neurobehavioral function, but such manipulations have been shown to reduce anxiety-like behaviors, while increasing active coping behaviors, in adult males later exposed to stress (Papaioannou et al., 2002, Meerlo et al., 1999). Moreover, whether neonatally handled females would show greater vulnerability to adolescent stress exposure is also unknown. Future studies will need to parse out these effects of early life experiences and sex, and whether they contribute to resilience (or vulnerability) to subsequent stress exposure during adolescence. For instance, do male or female offspring receiving greater levels of maternal licking and grooming, due to either natural variations in care or experimental manipulation, show greater resilience to stress-induce perturbations during adolescence? If so, would these effects of maternal care be mediated by reduced HPA reactivity in the adolescent offspring?
Though not studying early life experiences on later stress reactivity per se, a recent experiment in male mice did show an association between reduced HPA function following adolescent stress and changes in adult emotionality. Schmidt and colleagues found that adult male mice that were able to maintain lower basal corticosterone levels following chronic adolescent social stress (cage mates changed twice a week) showed less anxiety- and depressive-like behaviors in adulthood than mice that responded to the adolescent stress with elevated basal levels of corticosterone (Schmidt et al., 2010). Therefore, it appears that animals with lower HPA reactivity to adolescent stress exposure experience fewer negative outcomes in adulthood, at least in the context of these emotional behaviors. Though not reported in the study (Schmidt et al., 2010), it would be interesting to know whether differential levels of the quantity or consistency of maternal care predicted which mice showed less reactivity to chronic stress during adolescence.
5. Stress inoculation
Another factor that may impart resilience to stress during adolescence may be previously experiencing stress itself. The notion that stressful experiences early in life can inoculate the individual against later stressors comes in part from the “hygiene hypothesis” borrowed from immunology (von Mutius, 2007). This hypothesis, which was based on the observed negative correlation between the likelihood of developing hay fever allergies and the number of siblings one had (Strachan, 1989), suggests that previous infections or exposure to pathogens may inoculate one against future immunological insults. In the context of stress research, the “inoculation model” suggests that levels of early life stress can be represented by an inverted-U function, such that too little or too much early life stress can lead to later stress-induced dysfunction, while intermediate, moderate levels of stress may immunize one against later adversity (Lyons et al., 2010, Bock et al., 2014) (Fig. 3).
Fig. 3.

A schematic representation of a “stress inoculation” model as an inverted-U function such that moderate levels of early life stress lead to better future health outcomes compared very low or high levels of early life stress.
There is experimental support for this idea that mild to moderate levels of stress early in life can alter HPA function later in adulthood. For instance, in both rodent and primate studies, neonatal exposure to reoccurring bouts of novelty (Tang et al., 2006) or brief intermittent maternal separations (Parker et al., 2006) result in a more tightly regulated HPA axis in adolescence or adulthood. In adult rats, this is manifest by lower basal corticosterone levels and a faster stress-induced rise in corticosterone (Akers et al., 2008, Tang et al., 2006), while juvenile squirrel monkeys show lower basal cortisol and reduced cortisol responses to social stress tests (Parker et al., 2006). It is important to note that these effects of neonatal stimulation on later HPA function occur in the absence of changes in maternal care (Tang et al., 2006, Parker et al., 2006). Instead, the effect on the young appears to be mediated by a rise in their own stress-related hormones caused by the neonatal experience, as well as the increase in stress-related hormones transmitted to the young through their mother's milk (Tang et al., 2014, Macri et al., 2011, Catalani et al., 2011).
Similar to the lack of studies directly investigating how changes in maternal care may affect stress responsiveness and resilience to adversity during adolescence, it is currently unknown if neonatal challenges would modify adolescent HPA function and later adult physiological and neurobehavioral dysfunctions. Thus, whether early life stress inoculates adolescent animals against later stressors remains unclear. However, there are a number of provocative studies in rats that suggest intermittent and predictable exposure to stressors during adolescence may insulate and protect the animals from stress-related vulnerabilities in adulthood. For instance, male rats exposed to predictable chronic mild stress (PCMS; 5 min of restraint every day) from PND 28-55 showed less anxiety- and depressive-like behaviors in young adulthood, such that compared to controls, adolescent rats exposed to PCMS showed more open arm entries in the elevated plus maze and less immobility in the forced swim test (Suo et al., 2013). Similarly, male rats exposed to predator odor (i.e., cat fur) every other day from PND 33-57 show less depressive-like behavior in adulthood than controls (Kendig et al., 2011). The study employing PCMS during adolescence also examined whether this experience protected against further stress exposures in adulthood. Interestingly, they found rats given PCMS during adolescence were resistant to anxiety- and depressive-like behaviors induced by chronic unpredictable stress (CUS) later in adulthood (Suo et al., 2013). These data suggest that repeated exposure to mild, predictable stressors during adolescence could immunize the animals against the negative behavioral effects often observed in adult animals induced by CUS (Willner, 1997).
Along these lines, Buwalda and colleagues have investigated the short- and long-term effects of adolescent social stress on adult behaviors by exposing Wistar rats to older, more aggressive wild type Groningen (WTG) rats in either social defeat (Buwalda et al., 2013) or visible burrow system (VSB) paradigms (Buwalda et al., 2011). They find that when these Wistar rats are again exposed to social defeat by WTG rats in adulthood, the Wistar rats that had experienced adolescent stress are attacked less and show greater resistance to anhedonia compared to Wistar rats that did not receive the aggressive, stressful interactions during adolescence (Buwalda et al., 2013, Buwalda et al., 2011). These data add to the adolescent stress inoculation idea and broaden it to include aspects of the “match-mismatch hypothesis”, which basically states that the long-term costs of early life adversity are dependent on how well early life and later life environments match (less cost) or mismatch (greater cost) (Schmidt, 2011, Nederhof and Schmidt, 2012, Daskalakis et al., 2013). Thus, adolescent stress exposure may instill greater resilience in an individual that will also have to experience similar stressors later in their adult environment.
6. Gene–Environment interactions
Gene and environment (G × E) interactions are another set of variables that need to be taken into consideration when discussing resilience and vulnerability to stressors (Nugent et al., 2011, Caspi and Moffitt, 2006). That is, genetic differences can significantly influence the likelihood of developing a physiological or neurobehavioral dysfunction following exposure to stress. For instance, a notable G × E interaction study showed that the effect of early life stress on development of depression in adulthood was moderated in part by a polymorphism in the promoter region of the serotonin transporter gene (5-HTT). In this study it was found that individuals with one or two copies of the short allele of 5-HTT had greater levels of depression and suicidal ideation following early life stress than individuals homozygous for the long allele of 5-HTT (Caspi et al., 2003).
In the context of adolescent stress, a series of studies have examined the interaction of single nucleotide polymorphisms (SNPs) within the gene encoding the CRH receptor (CRHR1) and stressful life events during early and late adolescence on alcohol initiation and use. Previous studies had shown two particular SNPs of the CRHR1 gene, namely rs1876831 and rs242938, were associated with binge drinking specifically, and amount of alcohol intake in general, in both adolescent and adult populations ((Treutlein et al., 2006), except see (Dahl et al., 2005)). This group more recently reported that stressful life events occurring between either 12–15 years of age (Blomeyer et al., 2008) or between 15-19 years of age (Schmid et al., 2010) resulted in heavier and earlier initiation of alcohol use in subjects that had either the rs1876831 or rs242938 SNP in the CRHR1 gene. Though it is currently unknown what functional implications the rs242938 SNP has on CRHR1, the rs1876831 SNP has been implicated in elevated transcriptional activation of CRHR1 (Treutlein et al., 2006). It is important to note that experiments using genetically selected rats with a high alcohol preference show increased Crhr1 expression levels in the brain compared to unselected rats with little alcohol preference (Hansson et al., 2006). These human and non-human animal data suggest that adolescent stress and variations in CRH receptor activity can lead to alcohol abuse vulnerability.
From a resilience perspective, unfortunately not much is known regarding G × E interactions on adolescent alcohol use patterns. However, there has been recent research conducted on the H2 haplotype at chromosome 17q21.31 and protection against stress-induced alcohol dependence (Nelson et al., 2010). The CRHR1 gene is located in this chromosomal region (Koolen et al., 2008) and the H2 haplotype has been noted to influence recombination at this site, modifying the risk of various neurological disorders such as mental retardation and progressive supranuclear palsy (Stefansson et al., 2005, Pastor et al., 2004). It was found that carriers of the H2 haplotype appeared to be protected from alcohol dependence in adulthood when exposed to early life adversity in the form childhood sexual abuse. Whether this H2 haplotype would be protective against significant life stressors experienced during adolescence is currently unknown. Given the involvement of CRHR1 genetic alterations in stress-related vulnerabilities to alcohol use and abuse during adolescence, this would be an interesting association for future experiments to explore. Regardless, these G × E interaction studies are making it increasingly clear that it will be informative to take genetic background into consideration when addressing why some adolescents are more resistant they others to stressful life events.
7. Conclusions and future directions
As research moves forward and we continue to elucidate the mechanisms through which adolescents show heightened susceptibility to stress-induced dysfunctions, it will be equally important to appreciate the mechanisms that confer resilience to these stress-induced vulnerabilities. Understanding these mechanisms will undoubtedly benefit both the basic science and translational potential of this multifaceted line of research. It is will also be important to continue to explore the variance often observed in experimental data. In particular, assuming proper experimental designs are employed and precise execution of experiments are achieved, the variance within experimental groups could prove to be informative and engender valuable insights into individual differences in vulnerability and resistance to stress.
Though the current review highlighted early life programing of the HPA axis, stress inoculation, and G × E interactions in modulating resilience to stress in adolescence, this is certainly not an exhaustive list of meditators (Fig. 4). For instance, stress-induced epigenetic changes, either during perinatal and/or adolescent stages of development (McGowan and Szyf, 2010, Chakraverty et al., 2014, Lo and Zhou, 2014, Diwadkar et al., 2014), will need to be examined and whether these alterations in the individual's epigenetic landscape are context- or germline-dependent (Crews, 2008). Furthermore, future experiments will need to investigate sex differences in these potential mechanisms mediating stress resilience, given the significant role of sex in modulating responsiveness to stressors (Becker et al., 2007, Bangasser and Valentino, 2014). Taking factors such as these into account will certainly enrich our understanding of stress in general, and resilience to stress during adolescence specifically.
Fig. 4.
A schematic representation of the many potential environmental, genetic, and epigenetic factors that converge during adolescence to mediate resilience to stressors experienced in adolescence. Question marks represent those currently unknown factors that influence resilience and the vast potential for future research.
Acknowledgments
R.D.R. was supported in part by a grant from the National Science Foundation (IOS-1022148).
References
- Akers K.G., Yang Z., DelVecchio D.P., Reeb B.C., Romeo R.D., McEwen B.S. Social competitiveness and plasticity of neuroendocrine function in old age: influence of neonatal novelty exposure and maternal care reliability. PLoS ONE. 2008;3:e2840. doi: 10.1371/journal.pone.0002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Monteggia L.M., Perrot-Sinal T.S., Romeo R.D., Taylor J.R., Yehuda R. Stress and disease: is being female a predisposing factor? J. Neurosci. 2007;27:11851–11855. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeyer D., Treutlein J., Esser G., Schmidt M.H., Schumann G., Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol. Psychiatry. 2008;63:146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Bock J., Rether K., Groger N., Xie L., Braun K. Perinatal programming of emotional brain circuits: an intergrative view from systems to molecules. Front. Neurosci. 2014;8:11. doi: 10.3389/fnins.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B., Geerdink M., Vidal J., Koolhaas J.M. Social behavior and social stress in adolescence: a focus on animal models. Neurosci. Biobehav Rev. 2011;35:1713–1721. doi: 10.1016/j.neubiorev.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Buwalda B., Stubbendorff C., Zickert N., Koolhaas J.M. Adolescent social stress does not necessarily lead to a compromised adaptive capacity during adulthood: a study on the consequences of social stress in rats. Neuroscience. 2013;249:258–270. doi: 10.1016/j.neuroscience.2012.12.050. [DOI] [PubMed] [Google Scholar]
- Caldji C., Diorio J., Meaney M.J. Variations in maternal care in infancy regulate the development of stress reactivity. Biol. Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Caspi A., Moffitt T.E. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat. Rev. Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Caspi A., Sugden K., Moffitt T.E., Taylor A.R., Craig I.W., Harrington H. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Catalani A., Alema G.S., Cinque C., Zuena A.R., Casolini P. Maternal corticosterone effects on hypothalamus-pituitary-adrenal axis regulation and behavior of the offspring in rodents. Neurosci. Biobehav Rev. 2011;35:1502–1517. doi: 10.1016/j.neubiorev.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Chakraverty S., Pathak S.S., Maitra S., Khandelwal N., Chandra K.B., Kumar A. Epigenetic regulatory mechanisms in stress-induced behavior. Int. Rev. Neurobiol. 2014;115:117–154. doi: 10.1016/B978-0-12-801311-3.00004-4. [DOI] [PubMed] [Google Scholar]
- Crews D. Epigenetics and its implications for behavioral neuroendocrinology. Front. Neuroendocrinol. 2008;29:344–357. doi: 10.1016/j.yfrne.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J.P., Doyle G.A., Oslin D.W., Buono R.J., Ferraro T.N., Lohoff F.W. Lack of association between single nucleotide polymorphisms in the corticotropin releasing hormone receptor 1 (CRHR1) gene and alcohol dependence. J. Psychiatr. Res. 2005;39:475–479. doi: 10.1016/j.jpsychires.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Daskalakis N.P., Bagot R.C., Parker K.J., Vinkers C.H., de Kloet E.R. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38:1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E.R., Vreugdenhil E., Oitzl M.S., Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Dhabhar F.S. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar V.A., Bustamante A., Rai H., Uddin M. Epigenetics, stress, and their potential impact on brain network function: a focus on the schizophrenia diatheses. Front. Psychiatry. 2014;5:71. doi: 10.3389/fpsyt.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L., Romeo R.D. Stress and the developing adolscent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson A.C., Cippitelli A., Sommer W.H., Fedeli A., Bjork K., Soverchia L. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by enrivonmental stress. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert J., Goodyer I., Grossman A.B., Hastings M.H., de Kloet E.R., Lightman S.L. Do corticosteroids damage the brain? J. Neuroendocrinol. 2006;18:393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Cullinan W.E. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trend Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Figueiredo H., Mueller N.K., Ulrich-Lai Y., Ostander M.M., Choi D.C. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamic-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hollis F., Isgor C., Kabbaj M. The consequences of adolescent chronic unpredictable stress exposure on brain and behavior. Neuroscience. 2013;249:232–241. doi: 10.1016/j.neuroscience.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Kendig M.D., Bowen M.T., Kemp A.H., McGregor I.S. Predatory threat induces huddling in adolsecnt rats and residual changes in early adulthood suggestive of increased resilience. Behav. Brain Res. 2011;225:406–414. doi: 10.1016/j.bbr.2011.07.058. [DOI] [PubMed] [Google Scholar]
- Koolen D.A., Sharp A.J., Hurst J.A., Firth H.V., Knight S.J.L., Goldenberg A. Clinical and molecular delineation of the 17q21.31 microdeletion syndrome. J. Med. Genet. 2008;45:710–720. doi: 10.1136/jmg.2008.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A., Baram T.Z. Plasticity of the stress response early in life: mechanisms and significance. Dev. Psychobiol. 2010;52:661–670. doi: 10.1002/dev.20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.R., Brandy D., Koenig J.I. Corticosterone alters N-methyl-D-aspartate receptor subunit mRNA expression before puberty. Mol. Brain Res. 2003;115:55–62. doi: 10.1016/s0169-328x(03)00180-3. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405–406. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lo C.-L., Zhou F.C. Environmental alterations of epigenetics prior to the birth. Int. Rev. Neurobiol. 2014;115:1–49. doi: 10.1016/B978-0-12-801311-3.00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons D.M., Parker K.J., Schatzberg A.F. Animal models of early life stress: implications for understanding resilience. Dev. Psychobiol. 2010;52:616–624. doi: 10.1002/dev.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S., Zoratto F., Laviola G. Early-stress regulates resilience, vulnerability and experimental validity in laboratory rodents through mother-offspring hormonal transfer. Neurosci. Biobehav Rev. 2011;35:1534–1543. doi: 10.1016/j.neubiorev.2010.12.014. [DOI] [PubMed] [Google Scholar]
- McCormick C.M. An animal model of social instability stress in adolescence and risk for drugs of abuse. Physiol. Behav. 2010;99:194–203. doi: 10.1016/j.physbeh.2009.01.014. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Green M.R. From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience. 2013;249:242–257. doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Mathews I.Z. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol. Biochem. Behav. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Mathews I.Z. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog. Neurosychopharmacol Biol. Psychiatry. 2010;34:756–765. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Mathews I.Z., Thomas C., Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Mood disorders and allostatic load. Biol. Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Protection and damage from acute and chronic stress: allostasis and allostatic overland and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Stellar E. Stress and the individual: mechanisms leading to disease. Arch. Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McGowan P.O., Szyf M. The epigenetics of social adversity in early life: implications for mental health outcomes. Neurobiol. Dis. 2010;39:66–72. doi: 10.1016/j.nbd.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Meerlo P., Horvath K.M., Nagy G.M., Koolhaas J.M. The influence of postnatal handling no adult neuroendocrine and behavioral stress reactivity. J. Neuroendocrinol. 1999;11:925–933. doi: 10.1046/j.1365-2826.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- Nederhof E., Schmidt M.V. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol. Behav. 2012;106:691–700. doi: 10.1016/j.physbeh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Nelson E.C., Agrawal A., Pergadia M.L., Wang J.C., Whitfield J.B., Sassone F.S. H2 haplotype at chromosome 17q21.31 protects against childhood sexual abuse-associated risk for alcohol consumption and dependence. Addict. Biol. 2010;15:1–11. doi: 10.1111/j.1369-1600.2009.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent N.R., Tyrka A.R., Capenter L.L., Price L.H. Gene–environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacology. 2011;214:175–196. doi: 10.1007/s00213-010-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou A., Gerozissis K., Prokopiou A., Bolaris S., Stylianopoulou F. Sex differences in the effects of neonatal handling on the animal's response to stress and the vulnerability for depressive behaviour. Behav. Brain Res. 2002;129:131–139. doi: 10.1016/s0166-4328(01)00334-5. [DOI] [PubMed] [Google Scholar]
- Park M.K., Hoang T.A., Belluzzi J.D., Leslie F.M. Gender specific effect of neonatal handling on stress reactivity in adolescent rats. J. Neuroendocrinol. 2003;15:289–295. doi: 10.1046/j.1365-2826.2003.01010.x. [DOI] [PubMed] [Google Scholar]
- Parker K.J., Buckmaster C.L., Sundlass K., Schatzberg A.F., Lyons D.M. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor P., Ezquerra M., Perez J.C., Chakraverty S., Norton J., Racette B.A. Novel haplotypes in 17q21 are associated with progressive supranuclear palsy. Ann. Neurol. 2004;56:249–258. doi: 10.1002/ana.20178. [DOI] [PubMed] [Google Scholar]
- Porsolt R.D., Le Pichon M., Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Romeo R.D. Adolescence: a central event in shaping stress reactivity. Dev. Psychobiol. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Romeo R.D. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front. Neuroendocrinol. 2010;31:232–240. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Romeo R.D. The teenage brain: the stress response and the adolescent brain. Curr. Dir. Psychol. 2013;22:140–145. doi: 10.1177/0963721413475445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo R.D., Tang A.C., Sullivan R.M. Early-life experiences: enduring behavioral, neurological, and endocrinological consequences. In: Pfaff D.W., Etgen A.M., Fahrbach S.E., Rubin R.T., editors. Hormones, Brain and Behavior. second ed. Elsevier; New York: 2009. pp. 1975–2004. [Google Scholar]
- Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Rutter M. Annual research review: resilience-clinical implications. J. Child. Psychol. Psychiatry. 2013;54:474–487. doi: 10.1111/j.1469-7610.2012.02615.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp. Gerontol. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M., Romero L.M., Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schmid B., Blomeyer D., Treutlein J., Zimmermann U.S., Buchmann A.F., Schmidt M.H. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int. J. Neuropsychopharmacol. 2010;13:703–714. doi: 10.1017/S1461145709990290. [DOI] [PubMed] [Google Scholar]
- Schmidt M.V. Animal models of depression and the mismatch hypothesis of disease. Psychoneuroendocrinology. 2011;36:330–338. doi: 10.1016/j.psyneuen.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Schmidt M.V., Scharf S.H., Sterlemann V., Ganea K., Liebl C., Holsboer F. High susceptibility to chronic social stress is associated with a depression-like phenotype. Psychoneuroendocrinology. 2010;35:635–643. doi: 10.1016/j.psyneuen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Southwick S.M., Charney D.S. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338:79–82. doi: 10.1126/science.1222942. [DOI] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stefansson H., Helgason A., Thorleifsson G., Steinthorsdottir V., Masson G., Barnard J. A common inversion under selection in Europeans. Nat. Genet. 2005;37:129–137. doi: 10.1038/ng1508. [DOI] [PubMed] [Google Scholar]
- Strachan D.P. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo L., Zhao L., Si J., Liu J., Zhu W., Chai B. Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology. 2013;38:1387–1400. doi: 10.1038/npp.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A.C., Akers K.G., Reeb B.C., Romeo R.D., McEwen B.S. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A.C., Reeb-Sutherland B.C., Romeo R.D., McEwen B.S. On the causes of early life experience effects: evaluating the role of mom. Front. Neuroendocrinol. 2014;35:245–251. doi: 10.1016/j.yfrne.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Treutlein J., Kissling C., Frank J., Wiemann S., Dong L., Depner M. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol. Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H.M. Can stress cause depression? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:891–907. doi: 10.1016/j.pnpbp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- von Mutius E. Allergies, infections and the hygiene hypothesis–the epidemiological evidence. Immunobiology. 2007;212:433–439. doi: 10.1016/j.imbio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Watt M.J., Burke A.R., Renner K.J., Forster G.L. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav. Neurosci. 2009;123:564–576. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington J.M., Arnold A.R., Cooke B.M. Juvenile social subjugation induces a sex-specific pattern of anxiety and depression-like behaviors in adult rats. Horm. Behav. 2012;61:91–99. doi: 10.1016/j.yhbeh.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Willner P. The chronic mild stress procedure as an animal model of depression: valid, reasonably reliable, and useful. Psychopharmacology. 1997;134:371–377. [Google Scholar]