Abstract
Objective
Low physical activity and high sedentary behavior levels are major concerns in persons with multiple sclerosis (MS) and these differ depending on the level of mobility disability. However, the manner in which daily activity is accumulated is currently unknown in this population.
Methods
A secondary analysis was performed on a combined data set of persons with MS from two previous investigations of physical activity and symptomatic or quality of life outcomes in the United States over a two year period (2007–2009). Mobility disability status was determined using the Patient Determined Disease Steps (PDDS) while activity behavior was objectively monitored using an ActiGraph accelerometer for 7 days.
Results
Persons with MS who have mobility disability were involved in sedentary behavior, light and moderate intensity activity for 65%, 34% and 1% of the day, respectively compared to 60%, 37%, and 3%, respectively in those without mobility disability (p < 0.05). Breaks in sedentary time did not differ by mobility disability status. Compared to those without mobility disability, the average number of sedentary bouts longer than 30 min was greater in those with mobility disability (p = 0.016).
Conclusion
Persons with MS with mobility disability are less active, engage in more sedentary behavior and accumulate prolonged sedentary bouts.
Keywords: Multiple sclerosis, Accelerometer, Sedentary behavior, Physical activity, Mobility disability
Highlights
-
•
MS patients with mobility disability had more daily sedentary time (65% vs. 60%).
-
•
MS patients with mobility disability had less time in light activity (34% vs. 37%).
-
•
MS patients with mobility disability had less time in MVPA (1% vs. 3%).
-
•
MS patients with mobility disability engage more in prolonged sedentary bouts (5.1 vs. 4.3).
Introduction
To date, there are a multitude of studies examining the total or overall amount of physical activity participation among those with multiple sclerosis (MS) (Cavanaugh et al., 2011, Motl et al., 2005, Ng and Kent-Braun, 1997). Very little is known about the way in which physical activity or sedentary behavior is accumulated (i.e. pattern of activity behavior) in MS. One recent study involving 24-hour monitoring of activity behavior reported differences in daily periods of activity between those with MS and healthy controls (Rietberg et al., 2014). That study did not include lengths of active or sedentary bouts or the influence of mobility disability on activity behavior.
Mobility disability (i.e., difficulty with walking) is one of the most frequent complications of MS, and is considered by a large majority of those affected as the “most challenging aspect of the disease” (LaRocca, 2011). The disease process results in progressive mobility disability that might be made worse by physiological deconditioning associated with being less physically active and more sedentary (LaRocca, 2011, Motl and Goldman, 2011). Activity levels differ depending on the level of mobility disability (Cavanaugh et al., 2011, Rietberg et al., 2010). The current approach to activity promotion is to encourage 30 min of moderate intensity aerobic or resistance training 2 or more times per week (Canadian Society for Exercise Physiology, 2013). This approach demands moving enough to ‘break a sweat’ and is challenging for people who may have difficulty with mobility.
One strategy that may be more feasible is to target sedentary behavior in people with MS through the whole-of-the-day approach for activity promotion (Owen et al., 2010). This whole-of-the-day approach includes recommendations to reduce sedentary time, increase non-exercise physical activity (i.e., light intensity activity), and, where possible, increase moderate-to-vigorous physical activity (MVPA) (Manns et al., 2012, Owen et al., 2010). For example, Klaren et al. (2014) reported a 99 minute reduction in sitting time with people with MS following social cognitive theory-based Internet interventions. The reduction of sedentary behavior in particular is important because it makes up a major part of the day in the general population (e.g., 50–60%) (Dunstan and Owen, 2012). Sedentary behavior is defined as “any waking behavior characterized by energy expenditure ≤ 1.5 metabolic equivalents (METs) while in a sitting or reclining posture” (Sedentary Behaviour Research Network, 2012). Available evidence in people without mobility disability indicates that individuals who sit for prolonged periods have a greater risk of diabetes, high blood pressure, increased blood lipids and poorer long-term mortality outcomes, independent of physical activity status (Katzmarzyk et al., 2009, Swartz et al., 2011).
The manner in which daily activity is accumulated (i.e., pattern of activity) is important. Healy et al. (2008) reported beneficial associations between regular interruptions in sedentary time (i.e., with light intensity activity) and cardiometabolic markers (e.g., adiposity levels, triglycerides and 2-hour plasma glucose) (Healy et al., 2008). Targeting the pattern of sedentary behavior in persons with MS and increasing light intensity physical activity (i.e., standing up and taking steps frequently) throughout the day is a novel strategy that holds promise for improving cardiometabolic outcomes and health in persons with MS.
The objective of this study was to extend previous work on physical activity and sedentary behaviors in persons with MS by exploring the volume (sedentary, light intensity, MVPA and mean activity intensity) and pattern (breaks in sedentary time, sedentary and activity bout lengths) of accelerometer-derived activity behavior over 1 week of monitoring in a large sample of persons with MS (n = 439). We classified persons with MS by mobility disability status to enhance our understanding of the volume and pattern of activity behavior and how it might differ depending on disability status.
Methods
Participants
The study involved a secondary analysis performed on a combined data set of persons with MS from two previous investigations of physical activity and symptomatic or quality of life outcomes from 2007 to 2009 (Motl et al., 2009, Motl et al., 2010). The data from each investigation have been previously de-identified before being considered for amalgamation and combined analysis. Participants with MS were recruited through a research advertisement that was posted on the website of the National Multiple Sclerosis Society (NMSS) and through research advertisements distributed to 12 chapters of the NMSS, including the Greater Illinois, Gateway, and Indiana chapters. The common inclusion criteria for both investigations were (1) diagnosis of MS that was confirmed in writing by the patient's neurologist; (2) relapse free in the last 30 days; and (3) ambulatory with minimal assistance (i.e., walk independently or walk with a cane). The final sample included 439 persons with MS, and all persons satisfied inclusion criteria and provided usable data for the analyses (i.e., ≥ 3 days of valid accelerometer data).
Measures
Activity behavior
Activity behavior was objectively measured with ActiGraph model 7164 accelerometers. All accelerometers were calibrated for accurate measurement prior to use by having laboratory staff members walk on a treadmill (4.8 km/h, 0% grade for 15 min) while wearing four to eight accelerometers on a belt around the waist. Accuracy was confirmed by < 10% difference in average counts per minute across the 15-minute period of walking among the batch of accelerometers worn simultaneously. This calibration was undertaken to minimize variation among devices and inaccuracy as sources of error in the study outcomes. The ActiGraph model 7164 is a device that measures activity behavior by using a piezoelectric bender element that produces an electric signal proportionate to the force acting on it during movement. The electrical signal is digitally converted into activity counts, and then activity counts are amalgamated over one minute sampling intervals (i.e., 60 s epochs) with a standard drop time of 2 min. The drop time represents the tolerance or allowable amount of time that can break a sedentary or activity bout (Table 1).
Table 1.
Description of sedentary behavior and physical activity terms with cut points.
| Term | Definition | Accelerometer cut-point |
|---|---|---|
| Volume | ||
| Sedentary time | Time in activities (sitting or lying) that do not increase energy expenditure substantially above the resting level (1.0–1.5 METs) (Sedentary Behaviour Research Network, 2012). | Total minutes < 100 counts/min (Healy et al., 2008, Matthews et al., 2008, Pate et al., 2008). |
| Light intensity activity | Activities that involve energy expenditure at the level of 1.6–2.9 METs. | 100 to 1721 counts/min (Sandroff et al., 2012). |
| Moderate-to-vigorous intensity activity (MVPA) | Activities that involve energy expenditure above 3.0 METs. | ≥ 1722 counts/min (Sandroff et al., 2012) |
| Mean activity intensity | Sum of daily activity counts/number of wear time minutes (Tudor-Locke et al., 2012). | N/A |
| Pattern | ||
| Break in sedentary time | A defined point in time where there is a change from sedentary to a non-sedentary activity or an interruption in sedentary time (Healy et al., 2008). A break signifies the start of a bout. | Activity count change from < 100 to ≥ 100 counts. A break only occurs if the change in counts is sustained for more than 2 min. |
| Sedentary bout length | Consecutive minutes when count values fall into the sedentary range (< 100 counts/min). In this study drop time was 2 min (i.e. sedentary bout ends if there is a transition from sedentary to non-sedentary activity that is sustained for greater than 2 min). | Consecutive minutes with recorded counts of < 100/min (if sustained for more than 2 min). |
| Activity bout length | Consecutive minutes when count values are above the sedentary range (≥ 100). Drop time was again 2 min (i.e. activity bout begins if there is a transition from sedentary to non-sedentary activity that is sustained for greater than 2 min). | Consecutive minutes with recorded counts of ≥ 100/min if sustained more than 2 min. |
MET, metabolic equivalent.
Volume of activity behavior
The data from each participant's accelerometer were processed using the ActiLife software and exported into Microsoft Excel files representing wear time, mean activity intensity, time spent in sedentary, light intensity activity, and MVPA (Table 1) based on activity count cut-points for people with MS (i.e., ≥ 1722 counts/min) (Sandroff et al., 2012).
Pattern of activity behavior
The number of daily breaks, lengths of sedentary and activity bouts (Fig. 1) as well as number of long sedentary bouts (> 30 min) were also determined from the Excel files. Definitions and cut points are provided in Table 1. Accelerometer wear time data were checked against participant recorded wear times from the log sheet, and only valid days (≥ 10 h of wear time without periods of continuous zeros exceeding 60 min indicative of compliance) were included in the analysis. Our analysis using ≥ 3 valid days of data is consistent with previous research by experts in the field that has yielded a median reliability estimate of 0.80 in persons with MS (Motl et al., 2007). This is considered acceptable for providing a reliable estimate of usual activity behavior (Masse et al., 2005).
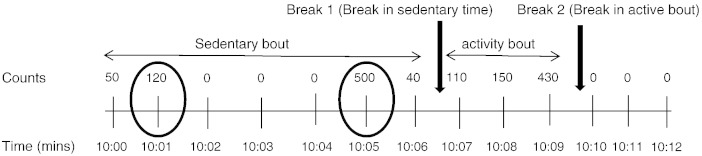
Fig. 1.
Illustration of the determination of breaks and bouts by ActiLife software.
This figure illustrates breaks (interruptions) in sedentary and active bouts. Counts above 100 sustained for a minimum of 2 min represent a break in a sedentary bout. Break 1 represents the end of the sedentary bout and the beginning of the active bout. Break 2 represents where the activity bout ends (because two or more minutes have counts that fall into the sedentary category), and the sedentary bout starts. The circled counts represent spikes of activity (minute with counts > 100) lasting 1 min but were not considered a break because of drop time of 2 min.
Mobility disability status
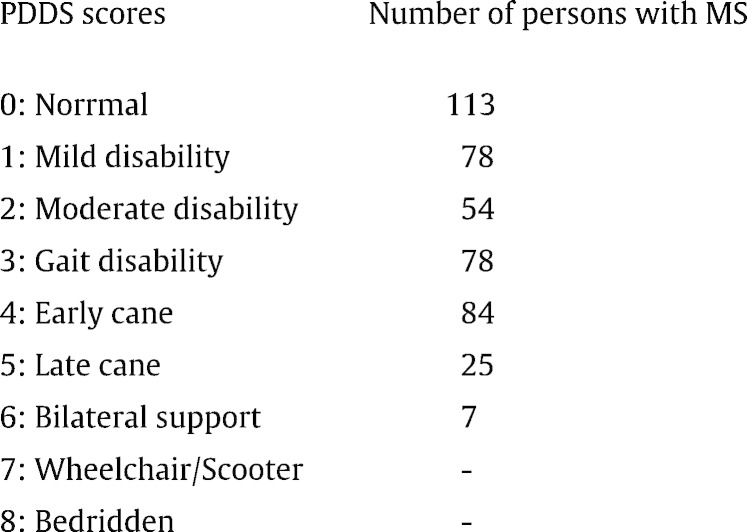
Mobility disability status was determined using the Patient Determined Disease Steps (PDDS) which has strong correlation (0.78) with the Expanded Disability Status Scale (EDSS) (Learmonth et al., 2013). The PDDS is a self-report questionnaire for measuring the degree of mobility impairment from neurological disease using an ordinal scale of 0 (Normal) through 8 (Bedridden) (Motl et al., 2014). Individuals with a PDDS score of ≥ 3 were classified as having mobility disability as this is consistent with an EDSS score of 4.0 (Marrie et al., 2010).
Procedure
The same university institutional review board approved both studies and associated procedures. After the initial telephone contact, screening for inclusion, and return of informed consent and diagnosis of MS verification documentation, participants were sent an accelerometer, log sheet, instructions for wearing the device, and demographic and clinical disability questionnaires. Participants were given written and graphic instructions to wear the accelerometer on the provided belt around the waist over the non-dominant hip during all waking hours of a seven-day period, except when swimming, bathing, or showering. The seven-day period of monitoring has been standard in studies of physical activity based on accelerometry (Masse et al., 2005). Waking hours were defined as the moment of getting out of bed in the morning until the moment of getting into bed in the evening. Participants were asked to maintain usual levels of activity during this one-week period. A member of the research staff called to make sure the participants received the materials and understood the instructions. We further provided pre-stamped and pre-addressed envelopes for return postal service. After completing the measures and wearing the accelerometer for the one-week period, participants returned the study materials through the U.S. postal service. We contacted participants by telephone and e-mail as a reminder to return the study materials up to three times. We further collected any missing questionnaire data based on follow-up telephone calls. All participants received $20 remuneration upon returning the study materials.
Data analysis
Participant characteristics (age, gender, ethnicity, marital status, occupation, MS type and duration) were reported by disability status. Chi square analysis was used to examine for differences between those with and without mobility disability. Further, multivariate regression analyses, adjusted for covariates (age, wear time and duration of MS) were used to determine if there were differences in accelerometer-derived variables by disability status. We used multiple regression to allow us to control for covariates that influence activity behavior (age, wear time and duration of MS). Significant covariates on univariate tests with activity behavior variables were included in the adjusted analyses. The variables sedentary time, light-intensity activity time and average sedentary bout length were additionally adjusted for MVPA whereas the average activity bout length and MVPA were adjusted for sedentary time. Further adjustments for MVPA and sedentary time were necessary as both could be potential confounders. All analyses were done using STATA version 13 (Stata Corporation, College Station, TX) at a significance level of p < 0.05.
Results
In the total sample of 439 participants, 44% (194) had mobility disability (Table 2). Most participants (68%) were aged between 40 and 59 years with an average age of 47.3 ± 10.0 years. The sample was largely Caucasian (92%). Of those with mobility disability, 73% were within the age range of 40–59 years. Persons with MS aged 40 years and above had higher levels of mobility disability than individuals younger than 40 years (p < 0.01). A greater percentage of those with mobility disability had secondary progressive MS. Individuals with shorter time since diagnosis (less than 10 years) had lower rates of mobility disability (72%) compared with those who had been diagnosed with MS for more than 10 years.
Table 2.
Participant characteristics.
| Full sample |
Mobility disability |
p-Value | ||
|---|---|---|---|---|
| Absent |
Present |
|||
| n = 439 |
n = 245 |
n = 194 |
||
| % (n) | % (n) | % (n) | ||
| Age (years) | ||||
| < 20 | 0.2 (1) | 0.4 (1.0) | 0 (0) | |
| 20–39 | 21.5 (94) | 29.1 (71) | 11.9 (23) | |
| 40–59 | 67.8 (297) | 63.9 (156) | 72.7 (141) | < 0.001 |
| 60–79 | 10.3 (45) | 6.6 (16) | 15.0 (29) | |
| > 80 | 0.2 (1) | 0 (0) | 0.5 (1) | |
| Gender | ||||
| Female | 84.7 (372) | 85.3 (209) | 84.0 (163) | 0.710 |
| Male | 15.3 (67) | 14.7 (36) | 16.0 (31) | |
| Ethnicity | ||||
| Caucasian | 92.5 (404) | 94.2 (229) | 90.2 (175) | 0.113 |
| Non-Caucasian | 7.6 (33) | 5.8 (14) | 9.8 (19) | |
| Marital status | ||||
| Married | 68.0 (298) | 64.3 (157) | 72.7 (141) | 0.063 |
| Non-married | 32.0 (140) | 35.7 (87) | 27.3 (53) | |
| Occupation | ||||
| Unemployed | 43.2 (188) | 29.2 (71) | 60.9 (117) | |
| Employed | 56.8 (247) | 70.8 (172) | 39.1 (75) | < 0.001 |
| Type of MS | ||||
| Relapsing–remitting | 90.4 (395) | 95.5 (232) | 84.0 (163) | |
| Secondary progressive | 6.2 (27) | 0.8 (2) | 12.9 (25) | < 0.001 |
| Primary progressive | 2.1 (9) | 1.2 (3) | 3.1 (6) | |
| Benign | 1.4 (6) | 2.5 (6) | 0 (0) | |
| Duration of MS | ||||
| 10 years or less | 63.7 (279) | 72.1 (176) | 53.1 (103) | < 0.001 |
| Greater than 10 years | 36.3 (159) | 27.9 (68) | 46.9 (91) | |
Mobility disability absent, Patient Determined Disease Steps ≤ 2; mobility disability present, Patient Determined Disease Steps ≥ 3.
p-Value represents difference between those with and without disability.
Volume of activity behavior
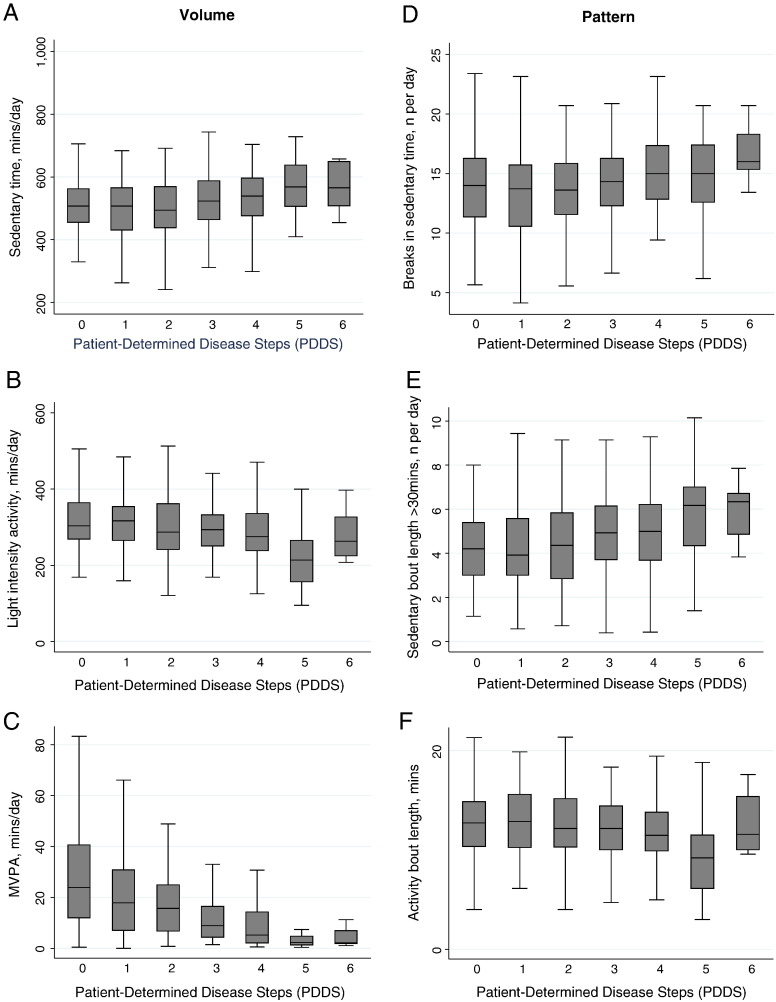
Activity variables for the full sample, stratified by mobility disability, are displayed in Table 3. Those with mobility disability were sedentary for 65% of the day (8.9 h) compared with 60% of the day (8.4 h) in those without mobility disability (p = 0.033). Participants with mobility disability were engaged in light intensity activity for an average of 34% of the day (4.7 h) as compared to 37% of the day (5.2 h) among those without mobility disability. MVPA made up only 1% (10 min) of the day among those with mobility disability as compared to 3% (25 min) in those without disability. The differences in light intensity activity and MVPA were significant. Mean activity intensity (average counts per minute over the monitored period) was significantly different between those with and without mobility disability (201.6 vs. 293.7 counts/min, respectively; p < 0.01). The influence of mobility disability (i.e. represented by individual PDDS scores) on activity behavior is illustrated in Fig. 2 (panels A, B, C). Time spent in sedentary behavior was longer while physical activity was shorter with higher levels of mobility disability except for PDDS of 6 (use of bilateral support or walker).
Table 3.
Accelerometry results by disability status (adjusted for age, wear time, duration of MS and MVPA or sedentary time).
| Mobility disability |
p-Value | ||
|---|---|---|---|
| Absent |
Present |
||
| n = 245 |
n = 194 |
||
| Mean (SD) | Mean (SD) | ||
| Accelerometer variables | |||
| Valid days | 6.2 (0.1) | 6.0 (0.1) | 0.047 |
| Wear time, min/day | 840.7 (6.7) | 827.5 (8.5) | 0.026 |
| Volume | |||
| Sedentary time, min/daya | 504.5 (4.8) | 533.3 (5.4) | 0.033 |
| Light intensity activity, min/daya | 311.4 (4.7) | 283.7 (5.3) | 0.035 |
| Moderate-to-vigorous intensity activity (MVPA), min/dayb | 24.8 (1.0) | 10.5 (1.2) | < 0.001 |
| Mean activity intensity, counts/min | 293.7 (7.5) | 201.6 (8.4) | < 0.001 |
| Pattern | |||
| Breaks in sedentary time, n per daya | 13.7 (0.2) | 14.7 (0.2) | 0.069 |
| Average sedentary bout length, mina | 23.8 (1.1) | 24.2 (1.3) | 0.752 |
| Sedentary bout length > 30 min, n per daya | 4.3 (0.1) | 5.1 (0.1) | 0.016 |
| Average activity bout length, minb | 12.8 (0.1) | 11.6 (0.1) | 0.187 |
Mobility disability absent, Patient Determined Disease Steps ≤ 2; mobility disability present, Patient Determined Disease Steps ≥ 3.
Sedentary time, light intensity, breaks in sedentary time, and sedentary bouts (adjusted for age, wear time, duration of MS and MVPA).
MVPA and activity bout (adjusted for age, wear time, duration of MS and sedentary time).
Fig. 2.
The influence of mobility disability, as characterized by individual PDDS scores, on activity behavior.
Notes: MVPA, moderate-to-vigorous physical activity; PDDS (Patient Determined Disease Steps).
Pattern of activity behavior
The number of daily breaks in sedentary time and average length of sedentary and activity bouts did not differ by mobility disability status. The average number of sedentary bouts longer than 30 min was greater in those with mobility disability as compared to those without disability (5.1 vs. 4.3, respectively; p = 0.016). The influence of mobility disability on the pattern of activity behavior is illustrated in Fig. 2 (panels D, E, F). Breaks in sedentary time and the number of sedentary bouts greater than 30 min were larger while activity bouts were smaller with higher levels of mobility disability except for PDDS of 6.
Discussion
The current analysis was conducted to examine the volume and pattern of sedentary and physical activity behavior in persons with MS, classified by mobility disability status. The main findings are that persons with MS with mobility disability: 1) spend a significantly greater percentage of their day in sedentary behavior; 2) are involved in significantly less physical activity (light intensity activity and MVPA); and 3) accumulate significantly more sedentary bouts greater than 30 min compared to persons with MS without mobility disability.
The percentage of sedentary behavior in persons with MS with mobility disability reported in this study is less than a previous study of persons with MS such that those with ambulatory limitation (Expanded Disability Status Scale (EDSS) scores > 4.5) were inactive for 85.3% of the day (Cavanaugh et al., 2011). However, different measurement devices were used and inactivity was defined as a stride count of zero, which includes standing (a non-sedentary behavior). Compared to persons with MS without mobility disability, data from a population representative sample shows that adults aged 46 years spend about 58% (8.4 h) of their day in sedentary behavior (Healy et al., 2011). This figure is similar to our findings in those without mobility disability.
Our results extend previous studies (Kos et al., 2007, Motl et al., 2005, Ng and Kent-Braun, 1997, Rietberg et al., 2014) demonstrating that physical activity levels are lower in persons with MS compared to the general population. In this study, we did not have a healthy control group, but reported the volume of physical activity (i.e. light intensity activity and MVPA) to be significantly less in those with mobility disability (PDDS ≥ 3) than those without disability. The mean activity intensity over the monitored period was particularly different between those with and without mobility disability (201.6 vs. 293.7 counts/min, respectively). This average intensity is also different from 62.8 counts/min reported in a study with stroke survivors (Rand et al., 2009), and 376 counts/min in healthy adults (Hagstromer et al., 2007). These findings suggest that persons with MS are generally more active than stroke survivors, but less active than the general population. An interesting finding in this study is that provision of gait aids that provide bilateral support (i.e. PDDS score of 6, use of walker) increases physical activity behavior in the MS population.
This study is the first to address the pattern of activity in people with MS. Although the number of daily breaks in sedentary time did not differ by mobility disability status, the number of breaks in both those with (14.7) and without (13.7) mobility disability was lower compared to healthy individuals. Healy et al. (2011) reported 92.5 daily breaks in sedentary time in healthy adults. The average lengths of sedentary and activity bouts did not differ by mobility disability status; however, the number of long sedentary bouts of length greater than 30 min was significantly higher in participants with mobility disability. Prolonged bouts of sedentary behavior have been described to be particularly harmful (Healy et al., 2008). There is evidence that prolonged sedentary time is negatively associated with several cardiometabolic markers (e.g., waist circumference, HDL-cholesterol, C-reactive protein, triglycerides and insulin sensitivity) (Healy et al., 2011). However, transitions from sedentary to non-sedentary activities have been beneficially associated with these cardiometabolic markers (Healy et al., 2011). With regard to energy expenditure, previous studies indicate that standing up and walking for 1-min within a sedentary bout of 30 min will result in a net energy expenditure of 3.0 kcal and if sustained for 1 week will accumulate to 120 kcal (Swartz et al., 2011). Frequently interrupting sedentary time with light intensity activity with particular emphasis on long sedentary bouts deserves special attention.
The method for defining breaks and bouts during the accelerometer data management and analysis requires discussion. A break bounds the beginning and end of a bout (sedentary or active). The ActiLife software uses the measurement of drop time (i.e. the allowable amount of time that can break a sedentary bout). Drop time was set to 2 min; therefore a sustained bout (either as sedentary or active) of more than 2 min (Fig. 1) was required for a break to be recorded. This is the default setting on the ActiLife software and was recommended in validation studies (Masse et al., 2005). For example, if counts were above 100 for more than 2 min during a 5 minute period, this was considered a break in sedentary time in our analysis. By contrast, Healy and colleagues defined a break as “interruption in sedentary time, or a transition from a sedentary (< 100 counts/min) to an active state (≥ 100 counts/min) for a minimum of 1 min” (Healy et al., 2008, Healy et al., 2011). The benefits of 2 minute interruption in sedentary time on postprandial glucose and insulin have been reported previously (Dunstan et al., 2012). Future studies will be required to examine differences in the pattern of activity (bouts and breaks) between drop times set at zero and at 2 min to see how this will improve our understanding of what truly constitutes a break.
The strengths of this study lie with the objective measurement of activity behavior and the large sample of persons with MS. Objective measurement reduces the bias associated with self-report, whereas the large sample allowed us to control for covariates in the analysis and provide more trustworthy estimates. Some limitations of ActiGraph accelerometers include misclassification based on cut-points. The commonly accepted cut-point of < 100 counts/min for sedentary behavior may actually represent light intensity activity for people with mobility disability. In a bid to avoid misclassification for physical activity behaviors, we used cut-points of ≥ 1722 counts/min for MVPA specific to the MS population (Sandroff et al., 2012), but researchers have not tested cut-points for sedentary behavior in MS. One limitation of this study is that the large sample allowed for the identification of statistically significant, yet small differences in sedentary and physical activity behaviors between disability categories, but such differences might not be biologically or behaviorally meaningful.
Conclusion
Persons with MS with mobility disability are less active, engage in more sedentary behavior and accumulate prolonged sedentary bouts compared to those without mobility disability. These findings underscore the need for interventions to reduce sedentary time and increase non-exercise physical activity in people with MS with mobility disability.
Funding
This research was funded, in part, by grants from the National Institute of Neurological Disorders and Stroke (NS054050) and National Multiple Sclerosis Society (RG 3926A2/1).
Conflict of interest statement
The authors declare that there are no conflicts of interests.
References
- Canadian Society for Exercise Physiology Canadian Physical Activity Guidelines for Adults with Multiple Sclerosis. 2013. http://www.csep.ca/english/view.asp?x=943
- Cavanaugh J.T., Gappmaier V.O., Dibble L.E., Gappmaier E. Ambulatory activity in individuals with multiple sclerosis. J. Neurol. Phys. Ther. 2011;35(1):26–33. doi: 10.1097/NPT.0b013e3182097190. [DOI] [PubMed] [Google Scholar]
- Dunstan D., Owen N. New exercise prescription: don't just sit there: stand up and move more, more often. Arch. Intern. Med. 2012;6:500. doi: 10.1001/archinternmed.2012.209. [DOI] [PubMed] [Google Scholar]
- Dunstan D.W., Kingwell B.A., Larsen R. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;(5):976. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstromer M., Oja P., Sjostrom M. Physical activity and inactivity in an adult population assessed by accelerometry. Med. Sci. Sports Exerc. 2007;39(9):1502–1508. doi: 10.1249/mss.0b013e3180a76de5. [DOI] [PubMed] [Google Scholar]
- Healy G.N., Dunstan D.W., Salmon J. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–666. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- Healy G.N., Matthews C.E., Dunstan D.W., Winkler E., Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur. Heart J. 2011;32(5):590–597. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmarzyk P.T., Church T.S., Craig C.L., Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med. Sci. Sports Exerc. 2009;41(5):998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- Klaren R., Hubbard E., Motl R. Efficacy of a behavioral intervention for reducing sedentary behavior in persons with multiple sclerosis: a pilot examination. Am. J. Prev. Med. 2014;47(5):613–616. doi: 10.1016/j.amepre.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Kos D., Nagels G., D'Hooghe M.B. Measuring activity patterns using actigraphy in multiple sclerosis. Chronobiol. Int. J. Biol. Med. Rhythm Res. 2007;24(2):345–356. doi: 10.1080/07420520701282364. [DOI] [PubMed] [Google Scholar]
- LaRocca N.G. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient Patient Centered Outcomes Res. 2011;3:189. doi: 10.2165/11591150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Learmonth Y.C., Motl R.W., Sandroff B.M., Pula J.H., Cadavid D. Validation of Patient Determined Disease Steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol. 2013;13:37. doi: 10.1186/1471-2377-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns P.J., Dunstan D.W., Owen N., Healy G.N. Addressing the nonexercise part of the activity continuum: a more realistic and achievable approach to activity programming for adults with mobility disability? Phys. Ther. 2012;92(4):614–625. doi: 10.2522/ptj.20110284. [DOI] [PubMed] [Google Scholar]
- Marrie R.A., Rudick R., Horwitz R. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74(13):1041–1047. doi: 10.1212/WNL.0b013e3181d6b125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse L.C., Fuemmeler B.F., Anderson C.B. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med. Sci. Sports Exerc. 2005;37(11):S544–s554. doi: 10.1249/01.mss.0000185674.09066.8a. [DOI] [PubMed] [Google Scholar]
- Matthews C.E., Chen K.Y., Freedson P.S. Amount of time spent in sedentary behaviours in the United States, 2003–2004. Am. J. Epidemiol. 2008;167(7):875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motl R.W., Goldman M. Physical inactivity, neurological disability, and cardiorespiratory fitness in multiple sclerosis. Acta Neurol. Scand. 2011;123(2):98–104. doi: 10.1111/j.1600-0404.2010.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motl R.W., McAuley E., Snook E.M. Physical activity and multiple sclerosis: a meta-analysis. Mult. Scler. (13524585) 2005;11(4):459–463. doi: 10.1191/1352458505ms1188oa. [DOI] [PubMed] [Google Scholar]
- Motl R.W., Zhu W., Park Y., McAuley E., Scott J.A., Snook E.M. Reliability of scores from physical activity monitors in adults with multiple sclerosis. Adapt. Phys. Act. Q. 2007;24(3):245–253. doi: 10.1123/apaq.24.3.245. [DOI] [PubMed] [Google Scholar]
- Motl R.W., McAuley E., Snook E.M., Gliottoni R.C. Physical activity and quality of life in multiple sclerosis: intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol. Health Med. 2009;14(1):111–124. doi: 10.1080/13548500802241902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motl R., McAuley E., Wynn D., Suh Y., Weikert M., Dlugonski D. Symptoms and physical activity among adults with relapsing–remitting multiple sclerosis. J. Nerv. Ment. Dis. 2010;198(3):213–219. doi: 10.1097/NMD.0b013e3181d14131. [DOI] [PubMed] [Google Scholar]
- Motl R.W., Mullen S., Suh Y., McAuley E. Does physical activity change over 24 months in persons with relapsing–remitting multiple sclerosis? Health Psychol. 2014;33(4):326–333. doi: 10.1037/a0032700. [DOI] [PubMed] [Google Scholar]
- Ng A.V., Kent-Braun J. Quantitation of lower physical activity in persons with multiple sclerosis. Med. Sci. Sports Exerc. 1997;29(4):517–523. doi: 10.1097/00005768-199704000-00014. [DOI] [PubMed] [Google Scholar]
- Owen N., Sparling P.B., Healy G.N., Dunstan D.W., Matthews C.E. Sedentary behaviour: emerging evidence for a new health risk. Mayo Clin. Proc. 2010;85(12):1138–1141. doi: 10.4065/mcp.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate R.R., O'Neill J., Lobelo F. The evolving definition of “sedentary”. Exerc. Sport Sci. Rev. 2008;36(4):173–178. doi: 10.1097/JES.0b013e3181877d1a. [DOI] [PubMed] [Google Scholar]
- Rand D., Eng J.J., Tang P.F., Jeng J.S., Hung C. How active are people with stroke?: use of accelerometers to assess physical activity. Stroke. 2009;40(1):163–168. doi: 10.1161/STROKEAHA.108.523621. [DOI] [PubMed] [Google Scholar]
- Rietberg M.B., Van Wegen E.E., Uitdehaag B.M., De Vet H.C., Kwakkel G. How reproducible is home-based 24-hour ambulatory monitoring of motor activity in patients with multiple sclerosis? Arch. Phys. Med. Rehabil. 2010;10:1537–1541. doi: 10.1016/j.apmr.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Rietberg M.B., van Wegen, Erwin E.H., Kollen B.J., Kwakkel G. Do patients with multiple sclerosis show different daily physical activity patterns from healthy individuals? Neurorehabil. Neural Repair. 2014;6:516–523. doi: 10.1177/1545968313520412. [DOI] [PubMed] [Google Scholar]
- Sandroff B.M., Motl R.W., Suh Y. Accelerometer output and its association with energy expenditure in persons with multiple sclerosis. J. Rehabil. Res. Dev. 2012;49(3):467–475. doi: 10.1682/jrrd.2011.03.0063. [DOI] [PubMed] [Google Scholar]
- Sedentary Behaviour Research Network Letter to the editor: standardized use of the terms ‘sedentary’ and ‘sedentary behaviours’. Appl. Physiol. Nutr. Metab. 2012;37(3):540–542. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- Swartz A.M., Squires L., Strath S.J. Energy expenditure of interruptions to sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 2011;8(1):69–75. doi: 10.1186/1479-5868-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor-Locke C., Camhi S.M., Troiano R.P. A catalog of rules, variables, and definitions applied to accelerometer data in the national health and nutrition examination survey, 2003–2006. Prev. Chronic Dis. 2012;9:1–16. doi: 10.5888/pcd9.110332. [DOI] [PMC free article] [PubMed] [Google Scholar]