Abstract
Overall health has been linked to socioeconomic status, with the gap between social strata increasing each year. Studying the impact of social position on health and biological functioning in nonhuman primates has allowed researchers to model the human condition while avoiding ethical complexities or other difficulties characteristic of human studies. Using female cynomolgus macaques (Macaca fascicularis), our lab has examined the link between social status and stress for 30 years. Female nonhuman primates are especially sensitive to social stressors which can deleteriously affect reproductive health, leading to harmful consequences to their overall health. Subordinates have lower progesterone concentrations during the luteal phase of menstrual cycle, which is indicative of absence or impairment of ovulation. Subordinate animals receive more aggression, less affiliative attention, and are more likely to exhibit depressive behaviors. They also express higher stress-related biomarkers such as increased heart rates and lower mean cortisol. While no differences in body weight between dominant and subordinate animals are observed, subordinates have lower bone density and more visceral fat than their dominant counterparts. The latter increases risk for developing inflammatory diseases. Differences are also observed in neurological and autonomic function. A growing body of data suggests that diet composition may amplify or diminish physiological stress responses which have deleterious effects on health. More experimental investigation of the health effects of diet pattern is needed to further elucidate these differences in an ongoing search to find realistic and long-term solutions to the declining health of individuals living across the ever widening socioeconomic spectrum.
Keywords: Social stress, Social status, Nonhuman primates, Western diet, Mediterranean diet
Abbreviations: HR, Heart rate; HPA, Hypothalamic-pituitary-adrenal; ACTH, Adrenocorticotropic hormone; TPH, Tryptophan hydroxylase; 5-HT, Serotonin; HVA, Homovanillic acid; CSF, Cerebrospinal fluid; PET, Positron emission tomography; CAA, Coronary artery atherosclerosis; CRH, Corticotropin-releasing hormone; IGF-1, Insulin-like growth factor-1; TPC, Total plasma cholesterol; HDL-C, High-density lipoprotein cholesterol; ANS, Autonomic nervous system
1. Introduction
1.1. Socioeconomic status and health
A strong gradient in health parallels the socioeconomic gradient in human society. Health disparities across social strata grow larger each year, and there have been a great deal of clinical and epidemiological research directed toward understanding the causes of this growing inequality. Important contributors that have been identified include social determinants such as health-related features of neighborhoods (e.g. walkability, recreational areas, accessibility to healthy food), socioeconomic factors (e.g. income, wealth, and education) (Braveman and Gottlieb, Jan–Feb 2014), discrimination, and social capital (Braveman et al., 2011) (Uphoff et al., 2013). The proximal effect these factors have in common is that when experienced chronically they may promote or buffer physiological responses which damage health (Braveman et al., 2011) (Chen and Miller, 2013). Socioeconomic status is inversely associated with level of chronic social stress (AdlerRehkoph, 2008). Several decades of research, spanning basic science to epidemiological levels of analysis, have repeatedly identified a sense of control over the environment and social supports as important moderators of the physiological impact of stressful life events (Matthews and Gallo, 2011).
1.2. Characteristics of primate social status hierarchies
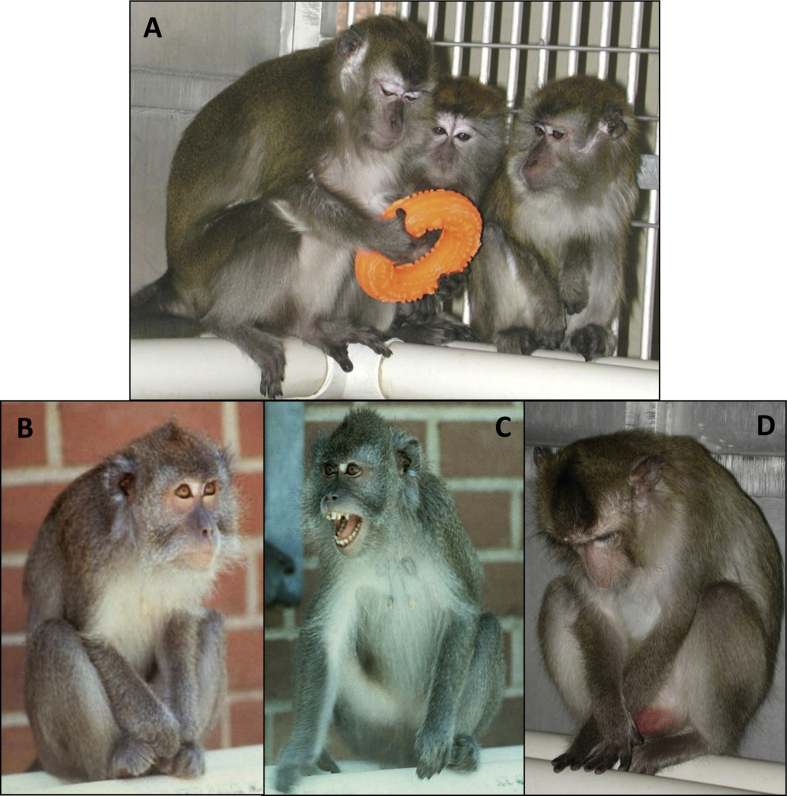
The social status hierarchy is a central organizing feature in the societies of most species living in groups larger than the nuclear family. Some characteristics of social status are shared across species. For example, high social status confers priority of access to resources such as food, water, safe resting sites, and mates (Fig. 1A). When resources are abundant there is little difference between high and low status individuals in access to resources. However, when resources become scarce, such as during drought or famine, social status may determine whether an individual can obtain enough food or water to maintain the degree of good health necessary to reproduce, or survive (Sapolsky, Apr 29 2005). High social status also confers a relatively more predictable social environment – dominants can have what they want, when they want it. Subordinates depend upon the largess of dominant animals for access to necessary resources which may be withdrawn at any time. Subordinates also may be subject to aggression at any given moment (Fig. 1B, C). In general the offspring of subordinates are also subordinate, at least while dependent on their parent(s), and share low priority of access to resources and a relatively unpredictable social environment (Shively, 1985). This situation creates the opportunity for both genetic and nongenetic transmission of traits along social status lines. These basic characteristics of social status set the stage for social inequalities in health.
Fig. 1.
Behaviors Associated with Social Status. A: High social status confers priority of access to resources; B: Vigilant scanning of the social environment is characteristic of subordinates; C: Subordinates receive more aggression from more dominant monkeys; D: Subordinates are more likely than dominants to exhibit depressive behavior which includes a slumped or collapsed body posture (head below shoulders), with open eyes, accompanied by a lack of responsivity to environmental events (Shively et al., Apr 15 1997).
1.3. Social suppression of reproductive function
It is imperative for female mammals to be sensitive to the current physical and social environment because of the enormous investment they make in each offspring. When resources are scarce it is a better strategy to divert energy from reproduction to physiologic processes designed to keep the individual alive; when resources are plentiful reproduction is favored. Compared to dominants, subordinate female mammals may experience more reproductive system dysfunction, which in turn may impact other aspects of health. Thus, females appear to be sensitive to environmental characteristics which may influence reproductive outcomes (Beehner and Lu, Sep–Oct 2013).
1.4. Translational studies of social status effects on health
Social status hierarchies in human societies share most of these basic characteristics. Studying the impact of social position on health and biological functioning in other gregarious species may give us insight on the human condition without the ethical complexities of studying human behavior in situ. Studying the impact of social status on health in a laboratory environment affords tighter controls over confounding factors such as status differences in physical environments, food quality and accessibility, ethnicity, and health care allowing for a focused evaluation of the biological impact of social status differentials.
2. Social status and stress in cynomolgus macaque social groups
2.1. Social status in cynomolgus macaque society
In the wild, cynomolgus monkeys (Macaca fascicularis) live in groups comprised of one or more adult males, multiple adult females, and their dependent offspring. Males are usually not related and emigrate between groups one to several times during their lifetime. Adult females are related through one or more matrilines and typically remain in their natal group for life. Female offspring have the same social status as their mother; maternal social status determines number of pregnancies, infant survival, and lifetime reproductive success (v. S. and van Noordwijk, 1999). Thus, this species experiences suppression of reproductive function by social status relationships.
We have studied the effects of social status on the health of adult female cynomolgus monkeys (M. fascicularis) in the laboratory for nearly 30 years. These monkeys were wild-caught as adults, and in recent years came from a purpose-bred free-ranging colony in Indonesia. The monkeys were housed in small social groups of 3–5 females in rooms approximately 8–10 m3 and enriched with perches, barrels, and manipulanda such as mirrors and toys. The monkeys were fed a diet containing moderate amounts of fat and cholesterol to mimic key dietary constituents consumed in Western societies. When placed in these groups, the monkeys quickly organize themselves into linear social status hierarchies which are usually stable over time (Shively and Kaplan, 1991). Social status is evaluated by recording the outcomes of agonistic interactions. The animal to which all others in the group direct submissive behaviors is considered dominant. The monkey that all but the most dominant submits to is considered second-ranking, and so on.
2.2. Behavioral indicators of stress in subordinates
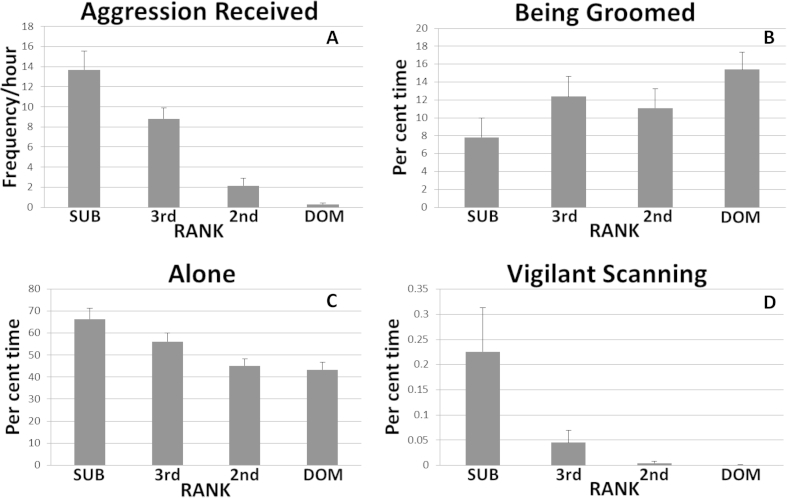
Compared to dominant females subordinates receive more aggression (Fig. 2A), are groomed less (Fig. 2B), and spend more time alone out of arm's reach of another monkey (Fig. 2C). Thus, subordinates appear to be subject to more hostility and have less social support than their dominant counterparts. Vigilant scanning (Fig. 2D) of the social environment, a behavior which consists of head swiveling to visually scan the home pen while in a crouched posture, is also a characteristic of subordinate female cynomolgus monkeys in these small groups. These monkeys appear fearful and anxious when engaged in vigilant scanning, as it is often accompanied by lip smacking and grimacing (fear and appeasement behaviors in macaques) (Shively et al., Apr 15 1997) (Shively, Nov 1 1998).
Fig. 2.
Differences in Behavior by Quartiles of Social Status. These behaviors were recorded from 42 adult female cynomolgus monkeys housed in small social groups (3-5/group) during 10 min focal animal observations made 6 times/month for 18 months. A: Rate (frequency/hour) of aggression received (main effect of social status: p < 0.0001); B: Per cent time being groomed (most subordinate versus most dominant quartile p < 0.02); C: Per cent time spent alone out of arm's reach of another monkey (main effect of social status: p < 0.001); D: Vigilant scanning accompanied by fear/appeasement behaviors (lipsmack, grimace) (main effect of social status: p = 0.007). Sub = Subordinate; Dom = Dominant.
2.3. Physiological indicators of stress in subordinates
We have used telemetered heart rate as an indicator of autonomic function. In an open field test, subordinates respond to a standardized stressor with a higher initial heart rate (HR) and recover more slowly than dominants (Shively, Nov 1 1998). Hypothalamic-pituitary-adrenal (HPA) function also differs by social status. Subordinates have higher morning cortisol concentrations than dominants (Shively et al., Apr 15 1997), are hypercortisolemic in adrenocorticotropic hormone (ACTH) challenge tests (Shively, Nov 1 1998) (Kaplan et al., 1986), and are insensitive to glucocorticoid-negative feedback in dexamethasone suppression tests (Kaplan et al., Dec 2010) (Shively et al., Apr 15 1997). Hypercortisolemia has been reported in association with social subordination in a number of primate species (Abbott et al., Jan 2003).
2.4. Social status differences in ovarian function
Cynomolgus monkeys have menstrual cycles similar to those of women in length, sex steroid and gonadotropin variations. The peak progesterone concentration in the luteal phase is used as an index of the quality of ovarian function. High values indicate that ovulation occurred, whereas low values indicate impaired ovulation or an anovulatory cycle. We have characterized luteal phase progesterone concentrations in multiple experiments and found that subordinates have lower mean peak levels than their dominant counterparts (Kaplan et al., Dec 2010, 1985; Adams et al., Dec 1985, Shively and Clarkson, May 1994). Cycles in which luteal phase progesterone concentrations are low are also characterized by lower follicular phase estradiol concentrations (Adams et al., Dec 1985). Thus, subordinate females are estrogen deficient relative to their dominant counterparts. These observations are consistent with those of Cameron and Bethea in stress sensitive cynomolgus macaques (Bethea et al., Dec 2008).
2.5. The stress of social subordination
This behavioral and physiological profile indicates that socially subordinate female cynomolgus monkeys in these small laboratory social groups are stressed relative to their dominant counterparts. Acute social defeat is a social stressor used in some rodent and tree shrew stress models of depression. While social subordination includes instances of social defeat, it also includes four other features that are likely important to the nature of the stressor: 1) cynomolgus monkeys normally live in social groups which are characterized by stable linear social status hierarchies throughout their lives; 2) these hierarchies are usually established in a matter of hours or days and do not generally involve much overt aggression; 3) while subordinates appear stressed relative to dominants, it is a level of physiological stress to which they can accommodate throughout their lifetime; and 4) time spent being groomed is positively correlated with social status while time spent fearfully scanning is negatively correlated with social status, suggesting that fear and a lack of positive social interaction are as important as hostility received in the experience of social subordination stress. These characteristics suggest that social subordination in laboratory groups is a chronic mild social stressor with social validity in respect to the natural history of the species.
3. Other characteristics of dominants and subordinates
3.1. Body composition
We have not observed differences in body weight between dominant and subordinate female cynomolgus macaques. Higher body weights have been observed in dominant male and female rhesus monkeys (Macaca mulatta), and male baboons (Papio anubis) (Michopoulos et al., Dec 2009) (Sapolsky and Mott, Nov 1987) (Zehr et al., May 2005). The social status differences in body weight of captive monkeys may depend on laboratory feeding practices. To reduce food competition we feed 10% in excess of consumption which helps to attenuate status differences in body weight. Bone mineral density is lower in subordinate monkeys, which may be due to reduced estradiol exposure from suppressed ovarian function (Kaplan et al., Dec 2010). There are also social status differences in fat deposition patterns. Dominants are more likely to deposit fat in the subcutaneous abdominal depot, while subordinates deposit fat in the visceral depot (Wallace et al., May 1999) (Shively et al., Sep 2009). Visceral fat produces a relative abundance of cytokines and inflammatory adipokines, which may be one mechanistic pathway through which social subordination increases risk of inflammatory diseases.
3.2. Neurobiological characteristics
Social status differences are apparent in central monoaminergic function. Tryptophan hydroxylase (TPH) activity is the rate limiting factor for serotonin (5-HT) production which mostly occurs in the raphe nucleus. The raphe nucleus of ovariectomized subordinate cynomolgus monkeys contains lower TPH concentrations than the same region of dominant conspecifics, supporting differences in central serotonergic function (Shively et al., 2003). The prolactin response to fenfluramine is an indicator of central serotonergic function. Ovariectomized subordinate cynomolgus monkeys have a lower prolactin response to fenfluramine then their dominant counterparts (Shively, Oct 1998). Likewise, in a community study low socioeconomic status was associated with a blunted prolactin response to fenfluramine, indicating diminished serotonergic responsivity in men and women (Manuck et al., Apr 2005). Social status differences are also apparent in central dopaminergic function. The prolactin response to haloperidol is an indicator of central dopaminergic function; subordinate female cynomolgus monkeys have lower prolactin responses to haloperidol than dominants (Shively, Nov 1 1998). Subordinate male and female macaques also have lower cerebrospinal fluid (CSF) concentrations of the dopamine metabolite homovanillic acid (HVA) (Kaplan et al., 2002), another indication of differences in dopaminergic tone. These observations were followed by multiple observations of lower striatal dopamine D2 receptor binding availability, as measured by positron emission tomography (PET), in subordinate male and female cynomolgus monkeys relative to their dominant counterparts (GrantShively et al., 1998) (Morgan et al., Feb 2002). Subsequently, socioeconomic status was also observed to be positively associated with striatal D2 receptor binding availability in men and women (Martinez et al., Feb 1 2010). Striatal D2 receptor binding availability was also positively associated with perceived social support in this study, emphasizing the importance of positive social relationships (Martinez et al., Feb 1 2010).
4. Social status, disease risk and mortality
4.1. Cardiovascular disease
Coronary heart disease is caused by coronary artery atherosclerosis (CAA) and its sequelae. Cynomolgus monkeys have been useful models to study factors that affect the development of CAA. Among female cynomolgus macaques, subordinates have about twice as extensive CAA as dominants, a difference which has been observed in multiple studies (Kaplan et al., Sep 2009). Both poor ovarian function and exaggerated heart rate responses to acute stress are associated with increased CAA extent. These characteristics of subordinates may provide mechanistic paths to increased atherogenesis.
4.1.1. Depressive behavior
About 25 years ago, we began observing and recording the frequency and percent time spent in a behavior termed “depressive”, in which the monkeys sat in a slumped or collapsed body posture with open eyes, accompanied by a lack of responsivity to environmental events (Fig. 1D). This behavior was reminiscent of that described in infant macaques removed from their mothers and adults following separation from their family environment (Suomi et al., 1975). We have observed this depressive behavior in three separate groups of female monkeys (a total of 120 animals). Interobserver agreement in the identification of depressive behavior was greater than 92% in all experiments. Rates of depression were similar in the three experiments (38–45%) (Shively et al., Apr 15 1997, Shively et al., Apr 2005, Shively et al., 2014). Depressive behavior was more common in subordinate females; 61% of subordinates displayed depressive behavior while only 10% of dominants exhibited this behavior (Shively et al., Apr 15 1997). Social subordination and depression are not homologous; subordinate and depressed monkeys differ in neurobiological and behavioral characteristics (Shively and Willard, Jan 2012) and 39% of subordinates did not display depressive behavior and a few dominants did, suggesting individual differences in stress sensitivity and resilience (Shively et al., Apr 15 1997). We concluded that the stress associated with low social status may increase the likelihood of depressive behavior. Rates of depression in the human population are also inversely related to socioeconomic status (AdlerRehkoph, 2008, Lorant et al., Jan 15 2003).
4.1.2. Variability in sensitivity and responses to stress
The fact that many, but not all, socially subordinate females and only a few dominant females exhibit depressive behavior indicates unexplained variability that may be due to variation in the social environment, or to individual differences in sensitivity or resilience to social stress (Bethea et al., Dec 2008). At the social group level it is reasonable to posit that some groups may be more stressful to live in than others. Unfortunately, the available data that address this hypothesis are sparse due to the challenge of studying an adequate number of social groups. Depressive behavior may be only one in a range of potential responses to social subordination stress. Studying the attributes of subordinates that do not become depressed may provide valuable insights about alternative stress responses.
4.1.3. Predictors of depression
Single caging may be considered a stressor as it increases heart rate in adult female cynomolgus monkeys (Watson et al., Apr 1998). We measured circulating biomarkers and heart rate (HR) in single caged monkeys immediately prior to social housing. Females that had higher overnight HRs in single cages were later more likely to exhibit behavioral depression in social groups, suggesting that stress sensitivity may increase the likelihood of a depressive response to social stress (Shively et al., Sep–Oct 2002). Likewise the monkeys that later developed behavioral depression in social groups had decreased cortisol secretion in a corticotropin-releasing hormone (CRH) challenge test, decreased circulating insulin-like growth factor-1 (IGF-1) concentrations, lower activity levels, and higher total plasma cholesterol (TPC) concentrations and ratios of TPC:high-density lipoprotein cholesterol (HDL-C) concentrations while singly caged. These data suggest that individuals at increased risk for a depressive response to social stress also differ in a number of physiological systems associated with increased disease risk (Shively et al., Apr 2005).
4.2. Cancer risk
In a study of 46 ovariectomized cynomolgus monkeys, socially subordinate females had increased cell proliferation and proportions of glandular and epithelial tissue, and less stroma in endometrium, and increased breast tissue thickness than their dominant counterparts (Shively et al., Jul–Aug 2004).
These tissue characteristics are associated with increased risk of endometrial and breast cancer in women (Nucci et al., Mar 2003, Ursin et al., Apr 2003).
4.3. Mortality
Socially dominant rhesus macaques live longer than their subordinate counterparts (Blomquist et al., 2011). Likewise, low social status is associated with increased mortality in the human population (Adler, Nov 2009, Adler et al., Jan 1994).
5. Dietary mitigation of psychosocial stress effects on health in female primates
5.1. Stress and diet
There is reason to believe that diet composition may modulate stress responses. For example, rats consuming a high fat diet have a higher cortisol response to stress compared to rats consuming a low fat diet (Legendre and Harris, Nov 2006). Likewise, chronic variable stress exaggerates the lipid response to a high fat diet (Manting et al., 2011). In clinical studies, consuming a high fat meal (mostly saturated animal fat) acutely exaggerates cardiovascular responses to stress (Jakulj et al., Apr 2007). Such responses have been shown to be attenuated in short term studies by consuming diets rich in polyunsaturated fats derived from plant sources (e.g. walnuts and flaxseed oil) which increase the ratio of omega-3: omega-6 fatty acids (West et al., Dec 2010). While these observations are intriguing, they derive from small and short-term studies and evaluate dietary manipulations that do not recapitulate diets that human beings generally eat.
5.2. Western versus Mediterranean diet patterns and stress
There is growing recognition that studying dietary patterns rather than single nutrients may result in a better understanding of the relationship between diet and health (Hu, Feb 2002). Recently, there has been much interest in differential health effects associated with Mediterranean versus Western diet patterns. The proportion of calories that come from protein, carbohydrates, and fats in Western and Mediterranean diets are similar. However, Western diets contain protein and fat derived mainly from animal sources, thus the diet is high in saturated fats and low in monounsaturated and omega-3 fatty acids. The Mediterranean diet pattern contains protein and fats derived mainly from plant sources. Compared to the Western diet pattern, the Mediterranean diet is high in monounsaturated fatty acids, omega-3 fatty acids, complex carbohydrates, and fiber, and low in refined sugars (A. R. S. U.S Department of Agriculture, 2007-2008, Bedard et al., Oct 28 2012). In population studies, the Western diet pattern is associated with greater perceived stress and higher urinary cortisol levels (Laugero et al., Feb 2011), whereas the Mediterranean diet pattern is associated with lower perceived stress (Hodge et al., Mar 2013).
5.3. 24 hour heart rates in female cynomolgus macaques consuming prudent or Western diets
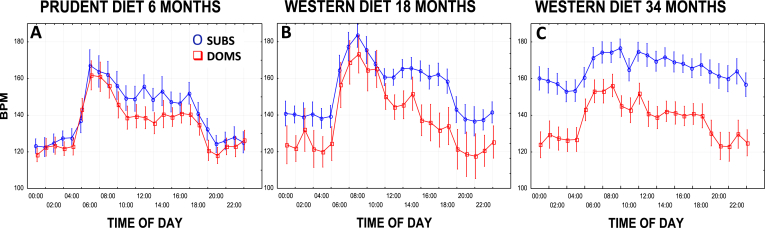
Recently we gathered 24 h HR data via telemetry from 42 socially housed monkeys at 3 time points: six months after consuming a low-fat plant-based prudent diet (monkey chow), and 18 and 34 months after consuming a Western diet. Subordinate HRs were higher on average, but not statistically different while consuming the prudent diet (Fig. 3A: p = 0.34). Social status differences emerged over time while consuming the Western diet (Fig. 3B, C: 18 months p = 0.13, 34 months p = 0.002). Subordinates also lost much of their HR circadian rhythm by 34 months (Fig. 3C: time × status interaction p = 0.005). In contrast, dominant HRs changed little with changes in diet. These data suggest that the Western diet may deleteriously affect the autonomic nervous system (ANS) of subordinates but not dominants (Shively, unpublished data). However, confirmation of these diet-by-social status interactions requires a parallel arm study in which a prudent diet is compared to a Western diet.
Fig. 3.
Diet, Social Status, and Autonomic Function. 24 h heart rates (HRs) were collected via telemetry from 42 socially housed monkeys at 3 time points: after consuming a low-fat plant-based prudent diet (monkey chow) for 6 months, and after consuming a Western diet for 18 and 34 months. A: Subordinate HRs were higher on average while consuming the prudent diet but not statistically different (p = 0.34); B, C: Social status differences emerged with time consuming the Western diet (main effect of social status B: 18 months p = 0.13, C: 34 months p = 0.002). C. Subordinates also lost much of their HR circadian rhythm by 34 months (C: time × status interaction p = 0.005). Sub = Subordinate; Dom = Dominant.
5.4. Cortisol responses to ACTH in female macaques consuming prudent or Western diet effects
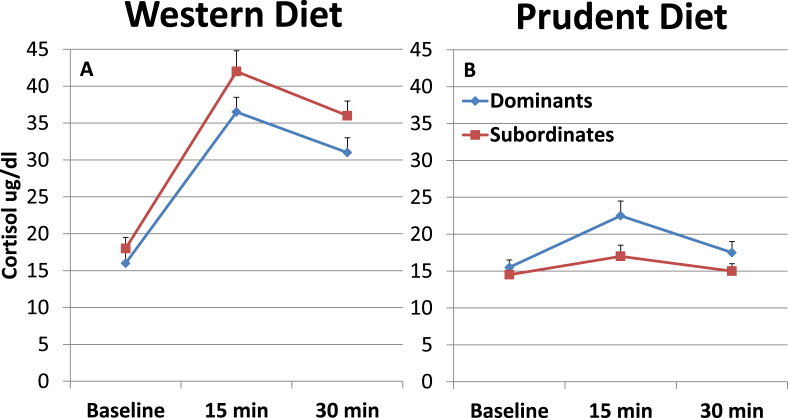
The cortisol response to ACTH challenge indicates adrenal responsivity to hypothalamic-pituitary activation. In intact and ovariectomized cynomolgus macaques consuming a Western diet, we have observed that dominants have lower cortisol responses to ACTH than subordinates (Shively, Nov 1 1998, Kaplan et al., 1986) (Fig. 4A). These observations were interpreted as indicating that the adrenal glands of subordinates are hyperresponsive and contribute to hypercortisolemia. However in a recent report, dominant female rhesus macaques consuming a prudent diet had higher cortisol responses to ACTH than subordinates (Michopoulos et al., Sep 2012a) (Fig. 4B). These results were interpreted as indicating that subordinates were unable to mount an appropriate glucocorticoid response. Furthermore, cortisol responses overall appeared higher in monkeys consuming a Western versus those consuming a Prudent diet. While these studies utilized different species (M. fascicularis vs. M. mulatta), the species are genetically similar as evidenced by more than one million years of interbreeding (Osada et al., 2010).
Fig. 4.
Diet and Cortisol Response to Adrenocorticotropin (ACTH) Challenge in Socially Housed Adult Female Macaques. Cortisol responses to ACTH in adult female macaques: A. Cynomolgus macaques consuming a Western diet (Shively, Nov 1 1998); B. Rhesus macaques consuming a low-fat, plant –based Prudent diet (monkey chow) (Michopoulos et al., Sep 2012a). Both studies used the same ACTH challenge test protocol (Shively, Nov 1 1998) and cortisol from both experiments was assayed in the Yerkes National Primate Research Center Biomarkers Core Lab. Monkeys consuming the Western diet appear to have higher cortisol responses to ACTH than those consuming a Prudent diet. Among those consuming a Western diet, the cortisol response was highest in subordinates, whereas among those consuming a Prudent diet, the cortisol response was highest in dominants. Figure adapted from (Shively, Nov 1 1998, Michopoulos et al., Sep 2012a).
Given the previous observations of diet effects on stress physiology, these seemingly opposite findings could be the result of the major differences between the diets. The Western-like diet consumed by monkeys in the aforementioned HR and HPA studies contained 40% of calories from fat (mostly saturated), and 0.25–0.40 mg cholesterol per kcal (350–500 mg cholesterol/day human equivalent), with protein and fat mostly from animal sources. The Prudent diet in all studies was standard monkey chow: low in fat (12% of calories) and cholesterol (trace amounts), with protein and fat from vegetable sources. These data suggest that long term consumption of a Western versus a Prudent diet may alter HPA stress responses in female primates. Supporting this interpretation, Michopoulos et al. (Sep 2012a) also observed in female macaques that cortisol responses to an acute stressor are higher in those consuming a high fat and sugar diet than those consuming a low fat and sugar diet (standard monkey chow) (Michopoulos et al., Sep 2012b).
6. Conclusions
Social status hierarchies are a central organizing feature of the societies of most gregarious mammals. Group-living macaques have been valuable in understanding the impact of social status on health. Social status differences are found in most physiologic systems examined, and social inequalities in health are characteristic of group-living macaques. These differences appear to be due to the physiological impact of the stress of low social status. In human studies, women consistently report more stress than men, and stress deleteriously impacts reproductive function in females which in turn has detrimental effects on other aspects of health. Thus, it is important to understand sex-specific social status-health relationships. It also appears that diet may contribute to stress vulnerability/resistance. A growing library of research suggests that our Western diet is exacerbating physiological stress responses, particularly among those who experience the most psychosocial stress. Thus healthier diets may contribute to stress resistance whereas Western-like diets may contribute to stress vulnerability. In human beings, the socioeconomic gradient in health continues to grow. Lower social position is associated with higher stress levels, less healthy diets, and higher body weight, and these relationships are more pronounced in women than in men (Moore and Cunningham, Apr 2012). Thus, changing our overall diet pattern might be most beneficial to those with the greatest psychosocial stress, who have the least healthful diet, and are least able to afford dietary supplements.
Acknowledgments
This research was supported by the National Institutes of Health, RO1HL087103 [to CAS]. We would like to thank Vasiliki Michopoulos and Mark Wilson for sharing their cortisol data.
References
- A. R. S. U.S Department of Agriculture . NHANES; 2007-2008. Energy Intakes: Percentages of Energy from Protein, Carbohydrate, Fat and Alcohol, by Gender and Age, What We Eat in America.http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0708/Table_5_EIN_GEN_07.pdf [Online]. Available: [Google Scholar]
- Abbott D.H., Keverne E.B., Bercovitch F.B., Shively C.A., Mendoza S.P., Saltzman W. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm. Behav. Jan 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Adams M.R., Kaplan J.R., Koritnik D.R. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis. Physiol. Behav. Dec 1985;35:935–940. doi: 10.1016/0031-9384(85)90262-8. [DOI] [PubMed] [Google Scholar]
- Adler N.E., Boyce T., Chesney M.A., Cohen S., Folkman S., Kahn R.L. Socioeconomic status and health. The challenge of the gradient. Am. Psychol. Jan 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Adler N.E. Health disparities through a psychological lens. Am. Psychol. Nov 2009;64:663–673. doi: 10.1037/0003-066X.64.8.663. [DOI] [PubMed] [Google Scholar]
- Adler N.E., Rehkoph D.H. U.S. disparities in health: descriptions, causes and mechanisms. Annu. Rev. Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- Bedard A., Riverin M., Dodin S., Corneau L., Lemieux S. Sex differences in the impact of the Mediterranean diet on cardiovascular risk profile. Br. J. Nutr. Oct 28 2012;108:1428–1434. doi: 10.1017/S0007114511006969. [DOI] [PubMed] [Google Scholar]
- Beehner J.C., Lu A. Reproductive suppression in female primates: a review. Evol. Anthropol. Sep-Oct 2013;22:226–238. doi: 10.1002/evan.21369. [DOI] [PubMed] [Google Scholar]
- Bethea C.L., Centeno M.L., Cameron J.L. Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol. Neurobiol. Dec 2008;38:199–230. doi: 10.1007/s12035-008-8042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomquist G.E., Sade D.S., Berard J.D. Rank-related fitness differences and their demographic pathways in semi-free-ranging rhesus macaques (Macaca mulatta) Int. J. Primatol. 2011;32:193–208. [Google Scholar]
- Braveman P., Gottlieb L. The social determinants of health: it's time to consider the causes of the causes. Public Health Rep. Jan-Feb 2014;129(Suppl. 2):19–31. doi: 10.1177/00333549141291S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P., Egerter S., Williams D.R. The social determinants of health: coming of age. Annu Rev. Public Health. 2011;32:381–398. doi: 10.1146/annurev-publhealth-031210-101218. [DOI] [PubMed] [Google Scholar]
- Chen E., Miller G.E. Socioeconomic status and health: mediating and moderating factors. Annu Rev. Clin. Psychol. 2013;9:723–749. doi: 10.1146/annurev-clinpsy-050212-185634. [DOI] [PubMed] [Google Scholar]
- Grant K.A., Shively C.A., Nader M.A., Ehrenkaufer R.L., ine S.W., Morton T.E., Gage H.D., Mach R.H. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29:80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Hodge A., Almeida O.P., English D.R., Giles G.G., Flicker L. Patterns of dietary intake and psychological distress in older Australians: benefits not just from a Mediterranean diet. Int. Psychogeriatr. Mar 2013;25:456–466. doi: 10.1017/S1041610212001986. [DOI] [PubMed] [Google Scholar]
- Hu F.B. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr. Opin. Lipidol. Feb 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Jakulj F., Zernicke K., Bacon S.L., van Wielingen L.E., Key B.L., West S.G. A high-fat meal increases cardiovascular reactivity to psychological stress in healthy young adults. J. Nutr. Apr 2007;137:935–939. doi: 10.1093/jn/137.4.935. [DOI] [PubMed] [Google Scholar]
- Kaplan J.R., Adams M.R., Koritnik D.R., Rose J.C., Manuck S.B. Adrenal responsiveness and social status in intact and ovariectomized Macaca fascicularis. Am. J. Primatol. 1986;11:181–193. doi: 10.1002/ajp.1350110209. [DOI] [PubMed] [Google Scholar]
- Kaplan J.R., Manuck S.B., Fontenot M.B., Mann J.J. Central nervous system Monoamine correlates of social dominance in cynomolgus monkeys (Macaca fascicularis) Neuropsychopharmacology. 2002;26:431–443. doi: 10.1016/S0893-133X(01)00344-X. [DOI] [PubMed] [Google Scholar]
- Kaplan J.R., Chen H., Appt S.E., Lees C.J., Franke A.A., Berga S.L. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Hum. Reprod. Dec 2010;25:3083–3094. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J.R., Chen H., Manuck S.B. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. Am. J. Primatol. Sep 2009;71:732–741. doi: 10.1002/ajp.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugero K.D., Falcon L.M., Tucker K.L. Relationship between perceived stress and dietary and activity patterns in older adults participating in the Boston Puerto Rican Health Study. Appetite. Feb 2011;56:194–204. doi: 10.1016/j.appet.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre A., Harris R.B. Exaggerated response to mild stress in rats fed high-fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. Nov 2006;291:R1288–R1294. doi: 10.1152/ajpregu.00234.2006. [DOI] [PubMed] [Google Scholar]
- Lorant V., Deliege D., Eaton W., Robert A., Philippot P., Ansseau M. Socioeconomic inequalities in depression: a meta-analysis. Am. J. Epidemiol. Jan 15 2003;157:98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- Manting L., Haihong Z., Jing L., Shaodong C., Yihua L. The model of rat lipid metabolism disorder induced by chronic stress accompanying high-fat-diet. Lipids Health Dis. 2011;10:153. doi: 10.1186/1476-511X-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck S.B., Bleil M.E., Petersen K.L., Flory J.D., Mann J.J., Ferrell R.E. The socio-economic status of communities predicts variation in brain serotonergic responsivity. Psychol. Med. Apr 2005;35:519–528. doi: 10.1017/s0033291704003757. [DOI] [PubMed] [Google Scholar]
- Martinez D., Orlowska D., Narendran R., Slifstein M., Liu F., Kumar D. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol. Psychiatry. Feb 1 2010;67:275–278. doi: 10.1016/j.biopsych.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K.A., Gallo L.C. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu Rev. Psychol. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V., Berga S.L., Kaplan J.R., Wilson M.E. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biol. Reprod. Dec 2009;81:1154–1163. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V., Reding K.M., Wilson M.E., Toufexis D. Social subordination impairs hypothalamic-pituitary-adrenal function in female rhesus monkeys. Horm. Behav. Sep 2012;62:389–399. doi: 10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V., Toufexis D., Wilson M.E. Social stress interacts with diet history to promote emotional feeding in females. Psychoneuroendocrinology. Sep 2012;37:1479–1490. doi: 10.1016/j.psyneuen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C.J., Cunningham S.A. Social position, psychological stress, and obesity: a systematic review. J. Acad. Nutr. Diet. Apr 2012;112:518–526. doi: 10.1016/j.jand.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Morgan D., Grant K.A., Gage H.D., Mach R.H., Kaplan J.R., Prioleau O. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat. Neurosci. Feb 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Nucci M.R., Castrillon D.H., Bai H., Quade B.J., Ince T.A., Genest D.R. Biomarkers in diagnostic obstetric and gynecologic pathology: a review. Adv. Anat. Pathol. Mar 2003;10:55–68. doi: 10.1097/00125480-200303000-00001. [DOI] [PubMed] [Google Scholar]
- Osada U.Y.N., Mineta K., Kameoka Y., Takahashi I., Terao K. Ancient genome-wide admixture extends beyond the current hybrid zone between Macaca fascicularis and M. mulatta. Mol. Ecol. 2010;19:2884–2895. doi: 10.1111/j.1365-294X.2010.04687.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M., Mott G.E. Social subordinance in wild baboons is associated with suppressed high density lipoprotein-cholesterol concentrations: the possible role of chronic social stress. Endocrinology. Nov 1987;121:1605–1610. doi: 10.1210/endo-121-5-1605. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. The influence of social hierarchy on primate health. Science. Apr 29 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Shively C.A. Springer-Verlag; New York: 1985. The Evolution of Dominance Hierarchies in Nonhuman Primate Society. [Google Scholar]
- Shively C.A., Clarkson T.B. Social status and coronary artery atherosclerosis in female monkeys. Arterioscler. Thromb. May 1994;14:721–726. doi: 10.1161/01.atv.14.5.721. [DOI] [PubMed] [Google Scholar]
- Shively C.A., Kaplan J.R. Stability of social status rankings of female cynomolgus monkeys, of varying reproductive condition, in different social groups. Am. J. Primatol. 1991;23:239–245. doi: 10.1002/ajp.1350230404. [DOI] [PubMed] [Google Scholar]
- Shively C.A., Willard S.L. Behavioral and neurobiological characteristics of social stress versus depression in nonhuman primates. Exp. Neurol. Jan 2012;233:87–94. doi: 10.1016/j.expneurol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively M.S.C.A., Lu N.Z., Henderson J.A., Bethea C.L. Soy and social stress affect serotonin neurotransmission in primates. Pharm. J. 2003;3:114–121. doi: 10.1038/sj.tpj.6500166. [DOI] [PubMed] [Google Scholar]
- Shively R.T.C.A., Appt S.E., Clarkson T.B. 2014. Long Term Sertraline Treatment Exacerbates Coronary Artery Atherosclerosis in Depressed Premenopausal Female Primates. submitted. [Google Scholar]
- Shively C.A., Laber-Laird K., Anton R.F. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol. Psychiatry. Apr 15 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Shively C.A., Register T.C., Friedman D.P., Morgan T.M., Thompson J., Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol. Psychol. Apr 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Shively C.A., Register T.C., Grant K.A., Johnson J.L., Cline J.M. Effects of social status and moderate alcohol consumption on mammary gland and endometrium of surgically postmenopausal monkeys. Menopause. Jul-Aug 2004;11:389–399. doi: 10.1097/01.gme.0000109312.11228.62. [DOI] [PubMed] [Google Scholar]
- Shively C.A., Register T.C., Clarkson T.B. Social stress, visceral obesity, and coronary artery atherosclerosis: product of a primate adaptation. Am. J. Primatol. Sep 2009;71:742–751. doi: 10.1002/ajp.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively C.A., Williams J.K., Laber-Laird K., Anton R.F. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosom. Med. Sep-Oct 2002;64:699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- Shively C.A. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biol. Psychiatry. Nov 1 1998;44:882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Shively C.A. Behavioral and neurobiological effects of estrogen replacement therapy and a history of triphasic oral contraceptive exposure. Psychoneuroendocrinology. Oct 1998;23:713–732. doi: 10.1016/s0306-4530(98)00039-0. [DOI] [PubMed] [Google Scholar]
- Suomi S.J., Eisele C.D., Grady S.A., Harlow H.F. Depressive behavior in adult monkeys following separation from family environment. J. Abnorm. Psychol. 1975;84:576–578. doi: 10.1037/h0077066. [DOI] [PubMed] [Google Scholar]
- Uphoff E.P., Pickett K.E., Cabieses B., Small N., Wright J. A systematic review of the relationships between social capital and socioeconomic inequalities in health: a contribution to understanding the psychosocial pathway of health inequalities. Int. J. Equity Health. 2013;12:54. doi: 10.1186/1475-9276-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin G., Ma H., Wu A.H., Bernstein L., Salane M., Parisky Y.R. Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol. Biomarkers Prev. Apr 2003;12:332–338. [PubMed] [Google Scholar]
- v. S C., van Noordwijk M.A. The effects of dominance rank and group size on female lifetime reproductive success in wild long-tailed macaques, Macaca fascicularis. Primates. 1999;40:105–130. doi: 10.1007/BF02557705. [DOI] [PubMed] [Google Scholar]
- Wallace J.M., Shively C.A., Clarkson T.B. Effects of hormone replacement therapy and social stress on body fat distribution in surgically postmenopausal monkeys. Int. J. Obes. Relat. Metab. Disord. May 1999;23:518–527. doi: 10.1038/sj.ijo.0800865. [DOI] [PubMed] [Google Scholar]
- Watson S.L., Shively C.A., Kaplan J.R., Line S.W. Effects of chronic social separation on cardiovascular disease risk factors in female cynomolgus monkeys. Atherosclerosis. Apr 1998;137:259–266. doi: 10.1016/s0021-9150(97)00277-3. [DOI] [PubMed] [Google Scholar]
- West S.G., Krick A.L., Klein L.C., Zhao G., Wojtowicz T.F., McGuiness M. Effects of diets high in walnuts and flax oil on hemodynamic responses to stress and vascular endothelial function. J. Am. Coll. Nutr. Dec 2010;29:595–603. doi: 10.1080/07315724.2010.10719898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr J.L., Van Meter P.E., Wallen K. Factors regulating the timing of puberty onset in female rhesus monkeys (Macaca mulatta): role of prenatal androgens, social rank, and adolescent body weight. Biol. Reprod. May 2005;72:1087–1094. doi: 10.1095/biolreprod.104.027755. [DOI] [PubMed] [Google Scholar]