Abstract
Genetic variants of the immunophilin FKBP5 have been implicated in susceptibility to post-traumatic stress disorder (PTSD) and other stress-related disorders. We examined the relationship between mushroom, stubby, thin and filopodial spine densities measured with Golgi staining and FKBP5 gene expression in the medial orbitofrontal cortex (BA11) in individuals diagnosed with PTSD and normal controls (n = 8/8). ANCOVA revealed PTSD cases had a significantly elevated density of stubby spines (29%, P < 0.037) and a trend for a reduction in mushroom spine density (25%, p < 0.082). Levels of FKBP5 mRNA were marginally elevated in the PTSD cases (z = 1.94, p = 0.053) and levels correlated inversely with mushroom (Spearman's rho = −0.83, p < 0.001) and overall spine density (rho = −0.75, p < 0.002) and directly with stubby spine density (rho = 0.55, p < 0.027). These data suggest that FKBP5 may participate in a cellular pathway modulating neuronal spine density changes in the brain, and that this pathway may be dysregulated in PTSD.
Keywords: Post-traumatic stress disorder, Post-mortem, Dendritic spine, FKBP5
Highlights
-
•
The present study is one of the first human post-mortem PTSD studies to date.
-
•
Extreme stress has robust repercussions on glucocorticoids and dendritic spine morphology in animal models.
-
•
FKBP5, involved in glucocorticoid signaling, was inversely associated with mushroom spine density in frontal cortex.
-
•
These findings are consistent with alterations in glucocorticoid signaling in PTSD affecting synaptic plasticity.
1. Introduction
The medial orbitofrontal cortex (mOFC, Brodmann Area 11, BA11) is an integral hub of the limbic system which has been hypothesized to play an important role in depression and PTSD. The mOFC, together with the amygdala and mediodorsal thalamus, are critical for the management of emotional learning and for linking this learning with visceromotor responses. As outlined by Ongur and Price (2000), the mOFC, driven by inputs from the subgenual anterior cingulate cortex, integrates information from the limbic cortex, amygdala and mediodorsal thalamus and provides output to the striatum and brainstem/hypothalamus that can affect physiological systems such as heart rate and cortisol release (Angrilli et al., 2007, Eippert et al., 2007). Loss of plasticity in the mOFC could “lock” this pathway in the “on” position, preventing the brain from disengaging visceromotor responses to emotional stimuli, thus contributing to the maintenance of excessive physiological responding in posttraumatic stress disorder (PTSD).
Study of the relationship between brain cellular architecture and acquired response to stress has been investigated primarily in animal models. Severe stress is associated with both short-term and long-term changes in the central nervous system (CNS) and hypothalamic-pituitary-adrenal (HPA) axis, including alterations in dendritic spine architecture. For instance, after chronic restraint stress in mice, up to a 60% reduction in spine density in the anterior cingulate cortex can occur (Kassem et al., 2013). Acute predator stress in rodents, thought to have relevance to PTSD because it generates long-lasting hyperarousal and anxiety, has also been associated with decreased spine density in the hippocampus 16 days after a single traumatic exposure (Adamec et al., 2012). Cellular pathways that are involved in synaptic plasticity and remodeling may thus contribute to long-term changes in dendritic morphology that are likely to represent a final common pathway in PTSD for the behavioral persistence of PTSD symptoms.
FK506-Binding Protein 5 (FKBP5) is a gene that has been implicated in enhanced susceptibility to PTSD and depression (Szczepankiewicz et al., 2014, Boscarino et al., 2011). One possible mechanism contributing to this susceptibility is FKBP5's role in mediating glucocorticoid receptor turnover and sensitivity in the brain. High levels of FKBP5 in the brain can suppress glucocorticoid receptor-mediated enhancement of synaptic plasticity, and this action has been shown to have detrimental effects on dendritic architecture (Bennett and Lagopoulos, 2014). In addition to observing changes in dendritic spine density in PTSD, we observed a strong relationship between FKBP5 transcript levels and spine density, suggesting that this gene may play a role in mediating cell regulatory responses that are altered in PTSD.
2. Methods
2.1. Postmortem collection
Postmortem brain tissue from normal control (n = 8) and PTSD (n = 8) subjects was obtained from the Southwest Brain Bank (SWBB), Department of Psychiatry, University of Texas Health Science Center at San Antonio (UTHSCSA), with consent from the next-of-kin (NOK). The SWBB postmortem brain tissue collection operates under the jurisdiction of the State of Texas Anatomical Review Board and the UTHSCSA IRB oversees the interviews with the NOK. Trained clinicians interview the NOK about the donor and conduct a proxy DSM-IV based Mini-International Neuropsychiatric Interview (MINI). Diagnoses were determined by expert diagnostician group consensus (MINI inter-rater reliability = 0.8) after review of the interview materials and available medical records (Table 1). Both gross and microscopic neuropathology exams were conducted by a neuropathologist and all specimens were found to be free of confounding neuropathology. All analyses were performed in JMP 10.0.0 (SAS Software, Cary NC). Work was performed under the supervision of the Yale, UTHSCSA and Texas A&M University IRBs.
Table 1.
Demographics.
| PTSD (n = 8) |
Control (n = 8) |
p for 2-sided t-testˆ or |
|
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Fisher's Exact Testˆˆ | |
| Age (years) | 53.0 ± 4.7 | 60.0 ± 3.2 | 0.2380ˆ |
| Gender (M/F) | 7/1 | 7/1 | – |
| Race (W,H,AA) | (4,3,1) | (4,4,0) | – |
| Hemisphere (R/L) | 7/1 | 7/1 | – |
| PMI (hours) ˆ | 24.1 ± 2.0 | 24.4 ± 2.8 | 0.9338 |
| Tissue pH ˆ | 6.43 ± 0.10 | 6.22 ± 0.70 | 0.4102 |
| Smoking History ˆˆ | 8 | 4 | 0.0769 |
| Alcohol Use (heavy/light) ˆˆa | 8 (6,2) | 2 (0,2) | 0.0070 |
| Antidepressant Historyˆˆ | 7 | 0 | 0.00007 |
M = Male, F = Female, W = White, H = Hispanic, AA = African American.
Comparing cases with any alcohol history: PTSD = 8/8, controls = 2/8, DF = 14 for all comparisons.
2.2. Golgi analysis
Blocks of fresh frozen tissue from area BA11 were coded and all analyses were performed blind to diagnosis. Blocks were thawed overnight in 4% paraformaldehyde fixative and then processed with the FD Rapid GolgiStain™ kit as follows. Blocks were immersion fixed for approximately 2 weeks in a combination of solutions A (Potassium dichromate and mercuric chloride) and B (Potassium chromate) followed by one week of cryoprotection in solution “C” (proprietary recipe). Thick sections (200 μm) were cut on a Vibratome and mounted on gelatin-coated glass slides. The sections were treated with solutions D and E (proprietary recipe) for signal development before being dehydrated through graded alcohols and cover-slipped with Permount.
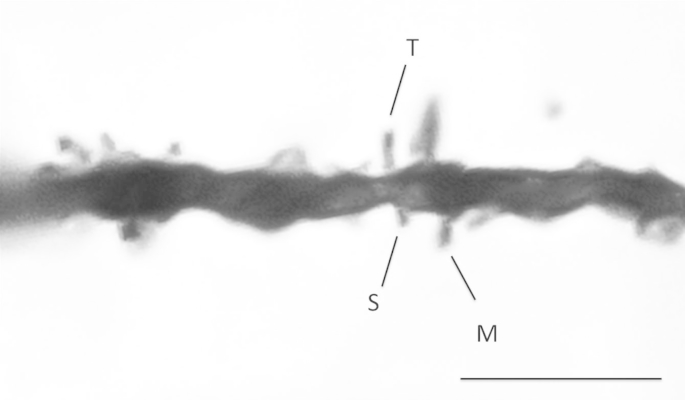
A sampling strategy based on the fractionator method developed for stereologic analysis (Gundersen et al., 1988) was employed; note however that the entire expanse of BA11 was not available for analysis as is required for truly unbiased sampling. Contours were drawn around the cortex in BA11 from the pial surface to the subjacent white matter and therefore including all six cortical layers in 3–5 sections of each brain. With the aid of a MicroBrightField Neurolucida system (Williston, VT), the fractionator sampling method was applied by placing a grid over the contour with counting frames randomly placed in such a manner that 20 sampling sites were generated for each brain area. All visible spine heads protruding from the shaft were counted along 20 μm-long dendritic segments in the counting frames (Fig. 1). Spines were classified into the following categories as described by Bourne and Harris (2007): thin, mushroom, stubby, filopodial and unclassified. Spine density was calculated as number of spines per micron.
Fig. 1.
Representative dendrites from a control brain are shown at 63× magnification. Stubby (S), Thin (T) and Mushroom (M) spines are labeled in this example. Scale bar = 5 μm.
2.3. FKBP5 analysis
FKBP5 mRNA was extracted from adjacent blocks of frozen BA11 using Qiagen RNeasy Lipid Tissue Mini Kit and stored at −80°. Whole genome expression analysis using Illumina Human WG-6 v3.0 Expression BeadChips (Illumina Inc, San Diego CA) “direct hybridization” assay was performed on blinded specimens. Arrays were scanned on an Illumina iScan System™ and processed on the Illumina GenomeStudio software. mRNA levels of FKBP5 in all cases were detectable with p < 0.0001.
3. Results
3.1. Spine density changes in PTSD
Approximately 500 spines were counted per brain (range 382–654). Only 1.39% of all observed spines could not be classified. In control brains, mushroom shaped spines accounted for 57% of the spines in BA11, followed by stubby (18%), thin (18%) and filopodial spines (7%). Shapiro–Wilk W test indicated that all sets of spine density data were normally distributed. ANCOVA's (two-tailed, DF = 1,15) with diagnosis, age, gender, PMI and pH as covariates revealed that PTSD cases had significantly elevated density of stubby spines (29%, PTSD LSMean [SE] = 0.212 [0.031], control = 0.299 [0.033], p < 0.037), and there was a trend for a decreased density of mushroom spines in these cases (25%, PTSD = 0.813 [0.090], control = 0.612 [0.010], p = 0.082). The stubby spine density finding did not survive Bonferroni correction.
3.2. FKBP5 levels and spine density
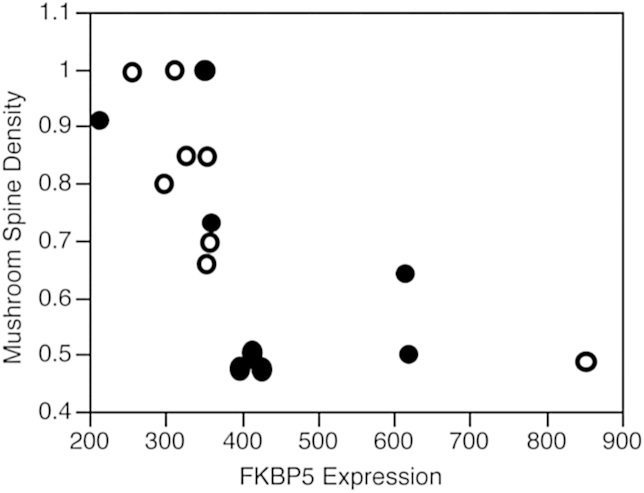
Shapiro–Wilk W test indicated that the FKBP5 data was not normally distributed. A Median Test found that levels of FKBP5 mRNA were borderline elevated in the PTSD cases (DF = 14, z = 1.94, p = 0.053). FKBP5 levels were inversely correlated to total spine density (Spearman's rho = −0.75, p < 0.002) and mushroom spine density (Spearman's rho = −0.83, p < 0.001 (Fig. 2)) and directly correlated with stubby spine density (Spearman's rho = 0.55, p < 0.027). FKBP5 correlations with mushroom and total spine densities survived Bonferroni correction.
Fig. 2.
FKBP5 gene expression levels in BA11 were inversely correlated to mushroom spine density (Spearman's rho = −0.83, p < 0.001) in PTSD (filled) and control cases (unfilled).
3.3. Clinical confounds
Smoking is a common confound in many post-mortem studies because of its high prevalence in psychiatric conditions. All PTSD cases had a history of smoking, while only 50% of the controls were smokers. Comparing smoking vs non-smoking controls, we did not observe any effect of smoking on spine density or FKBP5 levels (DF = 7, all t-test p's > 0.34). None of the controls had a history of heavy drinking, while a history of heavy alcohol use was common in the PTSD group (6/8), and it was not not possible to examine effects of this potential confound in the present data set.
4. Discussion
In the present study, we tentatively identified differences in dendritic spine architecture in samples of BA11 obtained from the frontal cortex of people who were affected by PTSD. An increase in density of stubby spines in BA11 of PTSD subjects was accompanied by trend reduction in the density of mushroom spines. These findings did not survive Bonferroni correction, and they will need to be validated in a larger sample. Levels of FKBP5 were significantly correlated with these changes, with the results being consistent with animal model data indicating that elevated levels of FKBP5 are associated with impaired synaptic function and increases in stress behaviors.
The observed alterations in spine density in PTSD are consistent with a regression of cortical synaptic architecture in BA11 to a less mature state (Harris et al., 1992). Some mature mushroom spines appear to have been replaced by simpler, stubby spines. Mushroom spines have constricted spine necks that act to contain the rise in calcium levels generated by synaptic activity locally within the spine head (Yuste et al., 2000). In contrast, stubby spines provide a relatively unrestricted path for calcium diffusion from the spine to the dendritic shaft due to the absence of the spine neck. Local concentration of calcium in the spine head creates an environment that is conducive to LTP-induced plasticity (Malenka et al., 1988, Holmes, 1990). Thin spines are thought to take part in plasticity-mediated learning and, under conditions that generate LTP, can enlarge into more stable mushroom spines believed to be important for long-term storage of memory (Kasai et al., 2003, Bourne and Harris, 2007). The enlarged head of a mushroom spine contains a large post-synaptic density, a spine apparatus and associated spine organelles, all important components of calcium regulation that are not found in other spine types including stubby spines (Sorra and Harris, 2000, Bourne and Harris, 2007). Thus, although the details of stubby spine physiology have yet to be fully established, most of the available data suggests that stubby spines in the mature brain would have a reduced capacity to make contributions to synaptic adaptability (i.e., LTD and LTP). Our finding provides evidence of a structural variation that could contribute to reduced plasticity in the mOFC in PTSD, making this area less capable of synaptic learning. New learning via LTP is thought to underlie conditioned fear extinction, the ability to quench adverse reactions to previously experienced dangerous situations that are no longer a threat, and failure to extinguish conditioned fear is the cardinal symptom of PTSD (Quirk et al., 2006).
One important observation is that the shift from mushroom to stubby spine morphology means that there may be reduced numbers of spine necks in PTSD. The constricted neck of a mushroom spine has multiple functional roles. In addition to affecting calcium signaling, spine necks may prevent the exodus of AMPA receptors from the spine head, thereby maintaining the strength of the synapse (Kusters et al., 2013). The spine neck also is an important site for shaping incoming excitatory signals passing from the PSD into the dendrite. For instance, dopamine and noradrenergic receptors have been localized to spine necks (Smiley et al., 1994, Bergson et al., 1995, Wang et al., 2007). The latter have also been shown to colocalize with hyperpolarization-activated cyclin nucleotide-gated (HCN) channels in the spine neck of long-thin spines (Wang et al., 2007, Paspalas et al., 2013), suggesting that spine necks are important for momentary changes in activation of prefrontal network activity associated with arousal, motivation and stress, as well as potentially for longer term effects mediated by LTP induced plasticity (Arnsten et al., 2010).
Alterations in dendritic spines have been found in association with neuropsychiatric illness. For example, using a normal, non-psychiatric human cohort and non-Golgi molecular techniques, Soetanto et al., 2010 found that psychological stress was associated with spine changes in the hippocampal CA3 region. Individuals reporting high levels of anxiety and depression had reduced density of both dendrites and spines in this area. In schizophrenia, robust reductions in dendritic spine density have been reported on pyramidal neurons in layer V and III of the frontal cortex (Broadbelt et al., 2002, Glantz and Lewis, 2000, Garey, 2010), subiculum (Rosoklija et al., 2000) and temporal cortex (Garey et al., 1998). In MDD, spine density was found to be reduced in the prefrontal cortex (Kang et al., 2012). These studies suggest that spine density changes may be a common final pathway of neuronal pathophysiology in several psychiatric conditions.
Dendritic spine alterations have been observed in a variety of animal models of potential relevance to PTSD. For instance, repeated restraint stress in rats alters spine morphology in the medial prefrontal cortex, resulting in fewer large spines (Radley et al., 2008). A chronic variable stress regimen in rats decreases spine density on pyramidal neurons in the prelimbic cortex and selectively reduces mushroom spines on neurons in the anterior bed nucleus of the stria terminalis (Radley et al., 2013). In this later model, animals displayed attenuated functional activation of prelimbic cortex neurons projecting to the anterior bed nucleus of the stria terminalis, which is an important center providing inhibitory control over the HPA axis. After chronic variable stress, these animals responded to an additional stressor by hyperactivation of HPA axis-related products such as plasma cortisol. One interesting observation is that animals susceptible to chronic defeat stress, in addition to having more stubby spines in the nucleus accumbens, display reduced post-synaptic density area and altered electrophysiology (Christoffel et al., 2011). Although miniature excitatory post-synaptic (mEPSC) amplitude was not changed, the frequency of mEPSCs was more than 50% higher in neurons with elevated numbers of stubby spines (Christoffel et al., 2011). These stress-related spine morphology changes were correlated with behavioral changes such as loss of social interactions and anhedonia-like behavior. Additional work is needed to determine whether changes in dendritic spine density in BA11, such as observed in our study, may contribute to long-term changes in HPA axis function in PTSD.
The pattern of reduced density of mushroom spines coupled with elevations in stubby spines has been reported for various stressors. For instance, in hippocampal culture, knockdown of SNX26, a synapse-related guanosine triphosphatase, significantly increased stubby spines at the expense of mushroom spines (Kim et al., 2013). In the hippocampus of adolescent rats, MK-801 reduced the density of mushroom spines and increased the density of stubby spines (Han et al., 2013). Finally, in the motor and somatosensory cortex of a model of Downs syndrome, mice had significantly fewer mushroom spines and significant increases in the number of stubby spines (Haas et al., 2013). Several animal models have thus found increases in stubby spines accompanied by reduced density of mushroom spines.
The mechanisms influencing the apparently enduring changes in OFC spine morphology are not well understood. One possibility is that the observed changes in spine architecture are driven by excessive glutamatergic drive through the OFC during the development of PTSD, and that the system has responded by reducing spine density, which would limit synaptic area available for excitatory signaling. The ability of ketamine (glutamatergic NMDA antagonist) to reverse spine loss in the prefrontal cortex of animal models of depression support this possibility (Nanxin et al., 2010). Furthermore, a recent MRI study provides support for loss of neuropil in the OFC in PTSD. Using pre and post-trauma MRI's, Sekiguchi et al. (2013) found that the OFC was the only region in the brain where PTSD symptoms were significantly correlated with grey matter volume reductions. In mice, stress has been observed to reduce grey matter volumes assessed with MRI, and anatomical studies have confirmed that this reduction is correlated with attenuation of alterations in dendritic spines (Kassem et al., 2013).
In addition to the association of FKBP5 polymorphisms with susceptibility to PTSD and depression (Szczepankiewicz et al., 2014, Boscarino et al., 2011), FKBP5 expression has been reported to be elevated in the cortex in individuals with depression (Sinclair et al., 2013). FKBP5 has been shown to be associated with impairments in stress resiliency and altered glucocorticoid signaling in rodent models (Scharf et al., 2011, Touma et al., 2011). Given that glucocorticoid receptor signaling is known to play an important role in mediating synaptic plasticity (Sarabdjitsingh et al., 2014), the present data supports the possibility that FKBP5 impacts synaptic processes through its relatively well-characterized interactions with glucocorticoid receptor signaling in neurons. However, research on FKBP5 is in its infancy, and this immunophilin has been linked to other cellular pathways and it is active in several cell types including glia. In astrocyte cell cultures, for instance, glucocorticoids induce a 2-fold elevation in FKBP5 levels (Carter et al., 2012). In its role as a molecular chaperone, FKBP5 participates in the construction and maintenance of the RISC complex that mediates microRNA processing (Martinez et al., 2013), and it is also involved in folding the microtubule associated protein Tau in Alzheimer's disease (Koren et al., 2011). Finally, it is worth noting that while FKBP5 may be present in elevated levels in brain in PTSD, several studies have indicated that reduced levels are present in blood, and that this may be associated with a long-term up-regulation of glucocorticoid sensitivity in blood cells (Yehuda et al., 2009, Sarapas et al., 2011). It is likely that many gene transcripts other than FKBP5 will be found to be correlated with spine density, however, the interesting biological phenotypes of FKBP5 knock out models (stress resilience and learning potentiation) argue for the possibility that this gene is a critical mediator of synaptic changes in the brain in stress-related conditions. If this is the case, then agents that can normalize FKBP5 levels in the brain may prove to be effective treatments for PTSD and other stress-related disorders.
The present study illustrates some of the strengths of human post-mortem studies in identifying initial pathophysiological pathways that deserve additional investigation in understanding the brain's response to stress. However, it should be considered that human post-mortem studies are commonly affected by multiple confounds, as was the case in the present study. This includes medication (7/8 antidepressant usage in PTSD, 0/8 in controls) and elevated rates of substance abuse and smoking in PTSD. It should also be remembered that there is abundant evidence for a substantial biological overlap between MDD and PTSD, and therefore, the present findings need to be further studied to determine whether they represent a shared pathophysiology, or to what extent changes may be limited to PTSD. Some of the variation observed in this study could be due to natural causes related to psychiatric condition at the time of death. If spine density changes are trait markers, it would be expected that theses changes might be exacerbated in individuals who were experiencing an episode of PTSD at the time of death. In the future, it will be interesting to study other brain regions to determine whether the observed findings are localized to frontal cortex or whether they represent a widespread phenomenon in PTSD. In one of the only other studies of post-mortem tissue in PTSD (Su et al., 2008), researchers focused on mitochondrial gene expression and did not measure spine density, so it will also be interesting to ascertain whether the mitochondrial pathways identified as abnormal in that study might be related to the changes in FKBP5 levels observed in this study.
In this first study of dendritic spine architecture in PTSD, the small number of specimens limits available power to withstand multiple comparison statistics with the PTSD phenotype, and replication will be important. However, the strong correlation of dendritic spine density to FKBP5 suggests that study of individual cellular pathways may provide a powerful tool for understanding the pathophysiology of stress in future studies of PTSD and other stress-related conditions.
Authors contributions
The authors equally shared in the design, implementation and analysis of the work. KAY wrote the initial draft of the manuscript and all authors shared in editing the manuscript.
Acknowledgments
This work was supported by grant W81XWH-11-2-0166 from the U.S. Army Materiel and Medical Command to Dr. Young and the Dielmann Family Genetic and Environmental Risk Endowment to Dr. Williamson. The open access publishing fees for this article have been covered by the Texas A&M University Online Access to Knowledge (OAK) Fund, supported by the University Libraries and the Office of the Vice President for Research. This manuscript is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care Systems, Temple, and the South Texas Veterans Health Care System, San Antonio, Texas. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the VA or the United States government.
References
- Adamec R., Hebert M., Blundell J., Mervis R.F. Dendritic morphology of amygdala and hippocampal neurons in more and less predator stress responsive rats and more and less spontaneously anxious handled controls. Behav. Brain Res. 2012;226:133–146. doi: 10.1016/j.bbr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrilli A., Bianchin M., Radaelli S., Bertagnoni G., Pertile M. Reduced startle reflex and aversive noise perception in patients with orbitofrontal cortex lesions. Neuropsychologia. 2007;46:1179–1184. doi: 10.1016/j.neuropsychologia.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F., Paspalas C.D., Gamo N.J., Yang Y., Wang M. Dynamic network connectivity: a new form of neuroplasticity. Trends Cogn. Sci. 2010;14:365–375. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M.R., Lagopoulos J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog. Neurobiol. 2014;112:80–99. doi: 10.1016/j.pneurobio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Bergson C., Mrzljak L., Smiley J.F., Pappy M., Levenson R., Goldman-Rakic P.S. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J. Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino J.A., Erlich P.M., Hoffman S.N., Rukstalis M., Stewart W.F. Association of FKBP5, COMT and CHRNA5 polymorphisms with PTSD among outpatients at risk for PTSD. Psychiatry Res. 2011;188:173–174. doi: 10.1016/j.psychres.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne J., Harris K.M. Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Broadbelt K., Byne W., Jones L.B. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr. Res. 2002;58:75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Carter B.S., Meng F., Thompson R.C. Glucocorticoid treatment of astrocytes results in temporally dynamic transcriptome regulation and astrocyte-enriched mRNA changes in vitro. Physiol. Genomics. 2012;44:1188–1200. doi: 10.1152/physiolgenomics.00097.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel D.J., Golden S.A., Dumitriu D., Robison A.J., Janssen W.G., Ahn H.F. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J. Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F., Veit R., Weiskopf N., Erb M., Birbaumer N., Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum. Brain Mapp. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey L. When cortical development goes wrong: schizophrenia as a neurodevelopmental disease of microcircuits. J. Anat. 2010;217:324–333. doi: 10.1111/j.1469-7580.2010.01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey L.J., Ong W.Y., Patel T.S., Kanani M., Davis A., Mortimer A.M. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J. Neurol. Neurosurg. Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz L.A., Lewis D.A. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Gundersen H.J., Bagger P., Bendtsen T.F., Evans S.M., Korbo L., Marcussen N. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Haas M.A., Bell D., Slender A., Lana-Elola E., Watson-Scales S., Fisher E.M. Alterations to dendritic spine morphology, but not dendrite patterning, of cortical projection neurons in Tc1 and Ts1Rhr mouse models of down syndrome. PLoS One. 2013;8:e78561. doi: 10.1371/journal.pone.0078561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D., Xu L., Xiao H., Prado Schmidt G.C., Shi S. Dizocilpine reduces head diameter of dendritic spines in the hippocampus of adolescent rats. Psychiatry Res. 2013;210:351–356. doi: 10.1016/j.psychres.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Harris K.M., Jensen F.E., Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J. Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W.R. Is the function of dendritic spines to concentrate calcium? Brain Res. 1990;519:338–342. doi: 10.1016/0006-8993(90)90098-v. [DOI] [PubMed] [Google Scholar]
- Kang H.J., Voleti B., Hajszan T., Rajkowska G., Stockmeier C.A., Licznerski P. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 2012;18:1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Matsuzaki M., Noguchi J., Yasumatsu N., Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kassem M.S., Lagopoulos J., Stait-Gardner T., Price W.S., Chohan T.W., Arnold J.C. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol. Neurobiol. 2013;47:645–661. doi: 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- Kim Y., Ha C.M., Chang S. SNX26, a GTPase-activating protein for Cdc42, interacts with PSD-95 protein and is involved in activity-dependent dendritic spine formation in mature neurons. J. Biol. Chem. 2013;288:29453–29466. doi: 10.1074/jbc.M113.468801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren J., Jinwal U.K., Davey Z., Kiray J., Arulselvam K., Dickey C.A. Bending tau into shape: the emerging role of peptidyl prolyl-isomerases in tauopathies. Mol. Neurobiol. 2011;44:65–70. doi: 10.1007/s12035-011-8182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusters R., Kapitein L.C., Hoogenraad C.C., Storm C. Shape-induced asymmetric diffusion in dendritic spines allows efficient synaptic AMPA receptor trapping. Biophys. J. 2013;105:2743–2750. doi: 10.1016/j.bpj.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R.C., Kauer J.A., Zucker R.S., Nicoll R.A. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988;242:81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Martinez N.J., Chang H.M., Borrajo Jde R., Gregory R.I. The co-chaperones Fkbp4/5 control Argonaute2 expression and facilitate RISC assembly. RNA. 2013;19:1583–1593. doi: 10.1261/rna.040790.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanxin L., Liu R., Dwyer J.M., Banasr M., Lee B., Son H. Glutamate NMDA receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry. 2010;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongür D., Price J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paspalas C.D., Wang M., Arnsten A.F. Constellation of HCN channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex: potential substrate for working memory deficits in schizophrenia. Cereb. Cortex. 2013;23:1643–1654. doi: 10.1093/cercor/bhs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G.J., Garcia R., Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol. Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Radley J.J., Anderson R.M., Hamilton B.A., Alcock J.A., Romig-Martin S.A. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J. Neurosci. 2013;33:14379–14391. doi: 10.1523/JNEUROSCI.0287-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Rocher A.B., Rodriguez A., Ehlenberger D.B., Dammann M., McEwen B.S. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J. Comp. Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoklija G., Toomayan G., Ellis S.P., Keilp J., Mann J.J., Latov N. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch. Gen. Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- Sarabdjitsingh R.A., Jezequel J., Pasricha N., Mikasova L., Kerkhofs A., Karst H. Ultradian corticosterone pulses balance glutamatergic transmission and synaptic plasticity. Proc. Natl. Acad. Sci. U. S. A. 2014;111:14265–14270. doi: 10.1073/pnas.1411216111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarapas C., Cai G., Bierer L.M., Golier J.A., Galea S., Ising M. Genetic markers for PTSD risk and resilience among survivors of the World Trade Center attacks. Dis. Markers. 2011;30:101–1010. doi: 10.3233/DMA-2011-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf S.H., Liebl C., Binder E.B., Schmidt M.V., Müller M.B. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS One. 2011;6:e16883. doi: 10.1371/journal.pone.0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi A., Sugiura M., Taki Y., Kotozaki Y., Nouchi R., Takeuchi H. Brain structural changes as vulnerability factors and acquired signs of post-earthquake stress. Mol. Psychiatry. 2013;18:618–623. doi: 10.1038/mp.2012.51. [DOI] [PubMed] [Google Scholar]
- Sinclair D., Stu G.F., Webster M.J., Weickert C.S. Dysregulation of glucocorticoid receptor co-factors FKBP5, BAG1 and PTGES3 in prefrontal cortex in psychotic illness. Sci. Rep. 2013;3:3539. doi: 10.1038/srep03539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J.F., Levey A.I., Ciliax B.J., Goldman-Rakic P.S. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetanto A., Wilson R.S., Talbot K., Un A., Schneider J.A., Sobiesk M. Association of anxiety and depression with microtubule-associated protein 2– and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch. Gen. Psychiatry. 2010;67:448–457. doi: 10.1001/archgenpsychiatry.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorra K.E., Harris K.M. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10:501–511. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Su Y.A., Wu J., Zhang L., Zhang Q., Su D.M., He P. Dysregulated mitochondrial genes and networks with drug targets in postmortem brain of patients with Posttraumatic Stress Disorder (PTSD) revealed by human mitochondria-focused cDNA microarrays. Int. J. Biol. Sci. 2008;4:223–235. doi: 10.7150/ijbs.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepankiewicz A., Leszczyńska-Rodziewicz A., Pawlak J., Narozna B., Rajewska-Rager A., Wilkosc M. FKBP5 polymorphism is associated with major depression but not with bipolar disorder. J. Affect Disord. 2014;164:33–37. doi: 10.1016/j.jad.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Touma C., Gassen N.C., Herrmann L., Cheung-Flynn J., Bull D.R., Ionescu I.A. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol. Psychiatry. 2011;70:928–936. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Wang M., Ramos B.P., Paspalas C.D., Shu Y., Simen A., Duque A. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Cai G., Golier J.A., Sarapas C., Galea S., Ising M. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol. Psychiatry. 2009;66:708–1110. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Yuste R., Majewska A., Holthoff K. From form to function: calcium compartmentalization in dendritic spines. Nat. Neurosci. 2000;3:653–659. doi: 10.1038/76609. [DOI] [PubMed] [Google Scholar]