Abstract
The incidence of oropharyngeal carcinoma, involving palatine and lingual tonsils, is increasing globally. This significant rise is driven by human papillomavirus. Whether palatine tonsillectomy affects risk of diagnosis with oropharyngeal carcinoma is unknown. The association between tonsillectomy and incidence of oropharyngeal carcinoma was explored in the Danish Cancer Registry. The association between tonsillectomy and oropharyngeal carcinoma was analyzed by time since first registration of tonsillectomy. Tonsillectomy was a time-dependent variable. Individuals were censored for death, emigration, or tonsillectomy within incident year of diagnosis. Incidence rate ratios (RR) were estimated by Poisson regression models and adjusted for confounders. Kaplan-Meier survival analyses were compared by the log-rank test, and HRs were estimated by Cox proportional hazards models. From 1977 to 2012, the incidence of tonsillectomies significantly decreased, whereas the incidence of oropharyngeal carcinoma significantly increased. Tonsillectomy was not associated with risk of oropharyngeal carcinoma or malignancies of other anatomic sites, including base of tongue. However, tonsillectomy significantly reduced risk of diagnosis with tonsil carcinoma [RR, 0.40; 95% confidence interval (CI), 0.22–0.70]. The risk of diagnosis with tonsil carcinoma at age <60 years was significantly decreased (RRadj, 0.15; 95% CI, 0.06–0.41) after tonsillectomy. Tonsillectomy within 1 year of diagnosis with tonsil carcinoma was associated with significantly improved overall survival (HR, 0.53; 95% CI, 0.38–0.74). In conclusion, remote history of tonsillectomy reduces the risk of diagnosis with tonsil carcinoma. These data inform risk and benefit of tonsillectomy, a common procedure and design of secondary prevention trials.
Introduction
The incidence of oropharyngeal carcinoma is increasing significantly worldwide, predominantly in developed countries among younger age cohorts and men (1). These epidemiologic changes are driven by human papillomavirus (HPV), a sexually transmitted infection that is etiologically responsible for the majority of oropharyngeal carcinomas (2). Indeed, approximately 77% of tonsil carcinomas diagnosed in Denmark between 2000 and 2010 were HPV-related (3, 4). HPV-related oropharyngeal carcinomas characteristically arise from the palatine and lingual tonsils of Waldeyer’s ring (5).
The rapid increase in HPV-related oropharyngeal carcinomas in recent decades has been attributed to the sexual revolution (6). Early sexual debut and increased number of lifetime sexual partners, surrogates for exposure to sexually transmitted infection, are well-recognized risk factors for HPV-related oropharyngeal carcinoma (5). Concurrent with a change in sexual behavior, tonsillectomy for hypertrophic tonsils and recurrent tonsillitis have lost previously held general acceptance (7). In light of the risks of tonsillectomy, including potential for catastrophic hemorrhage, and potential loss of immune “organs,” more stringent criteria have been adopted in recent years (8–11). Whether changes in tonsillectomy practices have contributed to the incidence trends is unknown.
Therefore, this study was designed to describe the incidence of tonsillectomies and oropharyngeal carcinoma in Denmark and to determine whether tonsillectomy reduces the future risk of oropharyngeal carcinoma.
Materials and Methods
Danish residents born between 1920 and 1972 were eligible for analysis. The Danish Cancer Registry a is population-based prospective nationwide program. Vital status and dates of emigration or immigration are maintained separately by the Central Population Register, which is linked to the Danish Cancer Registry. The association between tonsillectomy and oropharyngeal carcinomas was analyzed by time since first registration of tonsillectomy. Tonsillectomy was considered as a time-dependent variable and categorized into three categories a priori: (i) no history of tonsillectomy, (ii) tonsillectomy within 1 year, and (iii) remote history of tonsillectomy, defined as more than 1 year before diagnosis of tonsil carcinoma. Tonsillitis without history of tonsillectomy was also modeled as a separate exposure group. This definition of time was previously used to evaluate risk of Hodgkin lymphoma after tonsillectomy in the Danish Cancer Registry (12). Follow-up began on January 1st, 1977, and ended on December 31st, 2012, date of emigration, death, or date of tonsil carcinoma, whichever came first. Incidence rate ratios (RR) were estimated by Poisson regression models and adjusted for available potential confounders (attained age, calendar period, education, gender). RRs were determined for oropharyngeal carcinoma at any age and before median age of diagnosis given the reported differences in HPV tumor status by age of diagnosis (3–5, 13).
Analyses evaluating any oropharyngeal carcinoma included malignancies arising from the base of tongue, tonsil, oropharynx, pharynx, Waldeyers’ ring, and branchial cleft. Prior population-based analyses in Denmark (4) and the United States have used this anatomic site classification for HPV-related oropharyngeal carcinomas (1, 2, 14), which has been validated using gold-standard HPV detection methods (2). ICD-0 codes included C01, C09, C10, C14, C02.4, C02.8, C9.0, C09.1, C09.8, C09.9, C10.2, C10.3, C10.4, C10.8, C10.9, C14.0, C14.2, and C14.8. Subset analyses were restricted to oropharynx subsites relevant to HPV-related tumors, namely base of tongue and tonsil carcinomas. ICD-O codes C01, C02.4, and C02.8 were considered base of tongue specifically. For evaluations of tonsil carcinoma specifically, analyses were restricted to codes C09.0, C09.1, C09.8, and C09.9.
Survival by tonsillectomy group was compared with the Kaplan-Meier method and log-rank tests. Inverse probability weighting by age was applied to adjust for potential differences in age of diagnosis. Cox proportional hazards models were used to obtain hazard ratios (HRs) with multivariate adjustment for available confounders. At risk time was from diagnosis until death, censoring for emigration or December 2013, whichever came first. All statistical tests were two-sided, and a significance level of 5% was applied. Analyses were conducted using R statistical software (15).
Results
Trends in incidence of tonsillectomies
Danish residents (3,859,867) were followed for 72,701,956 person-years. In this population, 90,755 individuals had a history of tonsillectomy (with a previous diagnosis of tonsillitis in 41% of these cases). Since 1977, the incidence of tonsillectomy in Denmark has significantly decreased (P < 0.001; Fig. 1), most markedly after 1995. The rate of tonsillectomies has declined by 1.1% [95% confidence interval (CI), 1.0–1.2] annually, on average. Over a 35-year period (1977 to 2012), this corresponds to a 33.8% (95% CI, 32.9–34.8) decrease in the incidence of tonsillectomies. Tonsillectomy was significantly less frequent in more recent than earlier age cohorts (P < 0.001). The majority of tonsillectomies were in persons under age of 20 with a rate of >200 per 100,000 person-years.
Figure 1.
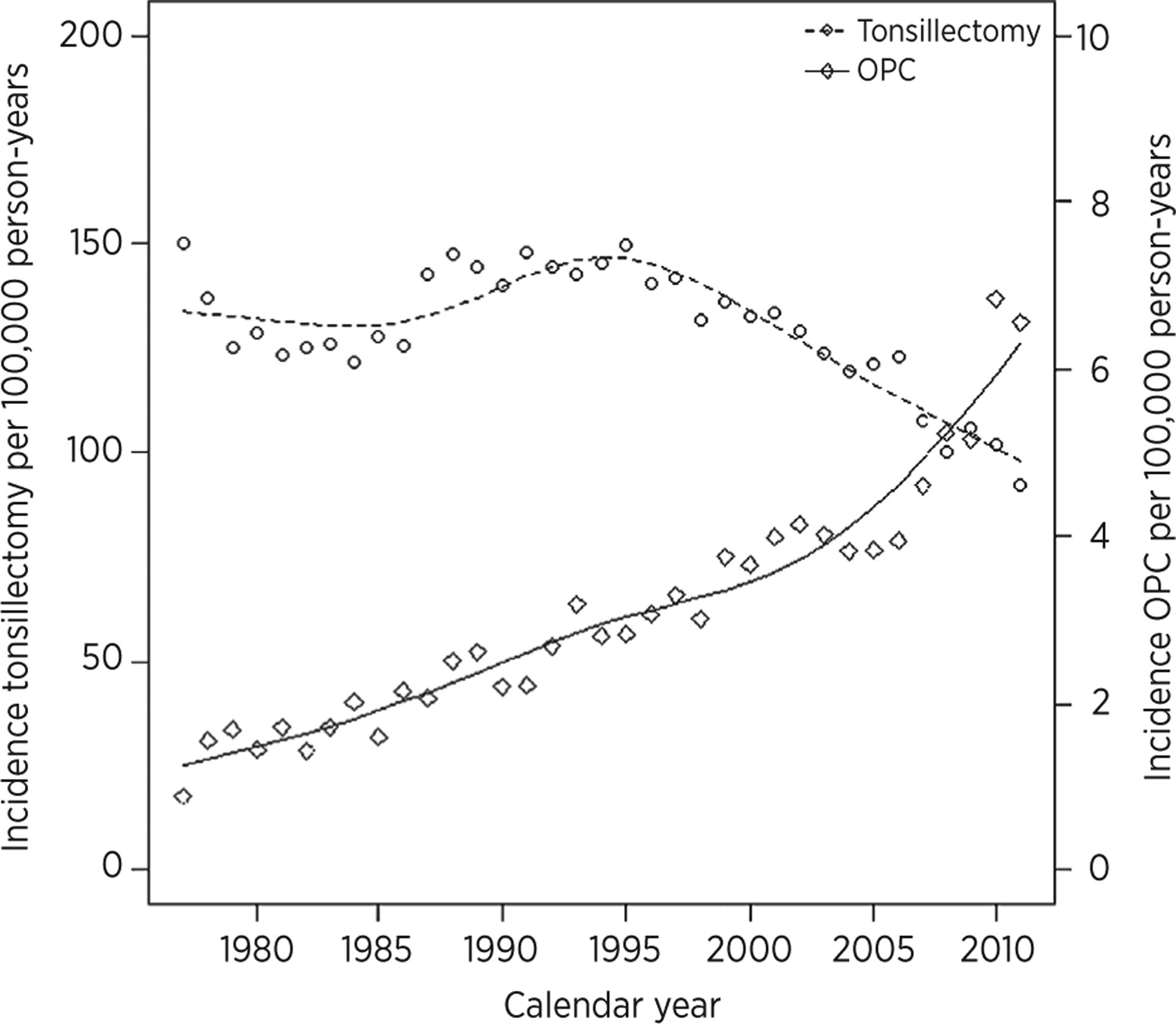
Incidence of tonsillectomy and oropharyngeal carcinoma (OPC) in Denmark 1977–2012. The x-axis represents the calendar year. The incidence of tonsillectomy and oropharyngeal carcinoma per 100,000 person-years is depicted on the y-axes.
Incidence of oropharyngeal carcinoma
During the same time period, the incidence of oropharyngeal carcinoma has dramatically increased (Fig. 1). The incidence of oropharyngeal carcinoma overall was 6.9 (95% CI, 6.8–7.1) per 100,000 person-years. Median age at the time of oropharyngeal carcinoma diagnosis was 59.0 years (95% CI, 45.1–75.9). Median age of individuals diagnosed with oropharyngeal carcinoma has decreased consistently from the 1970s to 2010s from 64 to 59 years. The incidence of oropharyngeal carcinoma was highly correlated with age, and only a few cases occurred below the age of 40. In the follow-up period, 5,111 individuals (with or without tonsillectomy) developed oropharyngeal carcinoma. An age-period-cohort analysis showed that oropharyngeal carcinomas increased by 6.0% (5.7–6.3) per year, most prominently in recent calendar periods. The majority of oropharyngeal carcinomas diagnosed were tonsillar (2,788 of 5,111, 54.5%).
In total, 52 (1.0%) of the individuals diagnosed with oropharyngeal carcinoma had a remote history of tonsillectomy (more than 1 year before oropharyngeal carcinoma diagnosis). A small subset of individuals (174, 3.4%) had a history of tonsillectomy within 1 year of oropharyngeal carcinoma diagnosis. The remaining 4,885 (95.6%) had no history of tonsillectomy.
Incidence of oropharyngeal carcinoma within 1 year of tonsillectomy
Tonsillectomies within 1 year of oropharyngeal carcinoma were considered separately given the increased potential for diagnostic or therapeutic procedure. In this subset, median time from tonsillectomy to diagnosis of oropharyngeal carcinoma was 7 days. Tonsillectomy was strongly associated with increased incidence of any oropharyngeal carcinoma within 1 year (RR, 200.09; 95% CI, 172.0–232.8). Similarly, the incidence of base of tongue (122.11; 95% CI, 74.41–200.4) and tonsil (RR, 261.68; 95% CI, 218.4–313.6) carcinomas was significantly increased within 1 year after tonsillectomy. The diagnosis of tonsillitis within 1 year of oropharyngeal carcinoma was significantly associated with increased incidence of oropharyngeal carcinoma. After adjustment for confounders, estimates remained robust (Table 1).
Table 1.
Risk of oropharyngeal, base of tongue, and tonsil carcinoma diagnosis after tonsillectomya
| Any age | <60 years | ≥60 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| History of tonsillectomy | Ncases | Pyrs (×103) | RRb (95% CI) | RRc (95% CI) | Ncases | Pyrs (×103) | RRb (95% CI) | RRc (95% CI) | Ncases | Pyrs (×103) | RRb (95% CI) | RRc (95% CI) |
| Oropharyngeal carcinoma overall | ||||||||||||
| No tonsillectomy or tonsillitis | 4,825 | 71,653 | 1.0 | 1.0 | 2,593 | 48,105 | 1.00 | 1.00 | 2,232 | 23,548 | 1.00 | 1.00 |
| Tonsillectomy <1 year before OPC | 174 | 13 | 200.1 (172.0–232.8) | 194.5 (167.1–226.3) | 106 | 10 | 191.6 (157.8–232.7) | 172.8 (142.3–209.9) | 68 | 3 | 270.8 (212.7–344.7) | 238.6 (187.4–303.8) |
| Tonsillitisd <1 year before OPC | 43 | 12 | 55.3 (41.0–74.7) | 49.8 (36.9–67.3) | 27 | 9 | 57.8 (39.6–84.5) | 47.5 (32.5–69.4) | 16 | 3 | 58.6 (35.8–95.8) | 53.8 (32.9–87.9) |
| Tonsillectomy ≥1 year before OPC | 52 | 791 | 1.0 (0.7–1.3) | 0.9 (0.7–1.2) | 29 | 709 | 0.8 (0.5–1.1) | 0.6 (0.4–0.9) | 23 | 82 | 3.0 (2.0–4.5) | 2.5 (1.7–3.8) |
| Tonsillitis ≥1 year before OPC | 17 | 234 | 1.1 (0.7–1.7) | 0.9 (0.5–1.4) | 8 | 183 | 0.8 (0.4–1.6) | 0.6 (0.3–1.2) | 9 | 51 | 1.9 (1.0–3.6) | 1.6 (0.8–3.0) |
| BOT carcinoma | ||||||||||||
| No tonsillectomy or tonsillitis | 727 | 71,653 | 1.00 | 1.00 | 386 | 48,105 | 1.00 | 1.00 | 341 | 23,548 | 1.00 | 1.00 |
| Tonsillectomy <1 year before BOT carcinoma | 16 | 13 | 122.1 (74.4–200.4) | 117.4 (71.5–192.8) | 10 | 10 | 121.4 (64.8–227.5) | 107.6 (57.4–201.6) | 6 | 3 | 156.4 (69.8–350.6) | 135.7 (60.5–304.2) |
| Tonsillitis <1 year before BOT carcinoma | 2 | 12 | 17.1 (4.3–68.4) | 15.1 (3.8–60.7) | 0 | 9 | - | - | 2 | 3 | 47.9 (11.9–192.4) | 43.3 (10.8–173.9) |
| Tonsillectomy ≥1 year before BOT carcinoma | 10 | 791 | 1.3 (0.7–2.3) | 1.1 (0.6–2.1) | 4 | 709 | 0.7 (0.3–1.9) | 0.5 (0.2–1.4) | 6 | 82 | 5.1 (2.3–11.3) | 4.2 (1.9–9.3) |
| Tonsillitis ≥1 year before BOT carcinoma | 2 | 234 | 0.8 (0.2–3.4) | 0.7 (0.2–2.7) | 0 | 183 | - | - | 2 | 51 | 2.7 (0.7–10.9) | 2.2 (0.5–9.0) |
| Tonsil carcinoma | ||||||||||||
| No tonsillectomy or tonsillitis | 2,608 | 71,653 | 1.00 | 1.00 | 1,462 | 48,105 | 1.00 | 1.00 | 1,146 | 23,548 | 1.00 | 1.00 |
| Tonsillectomy <1 year before tonsil carcinoma | 123 | 13 | 261.7 (218.4–313.6) | 252.2 (210.3–302.3) | 75 | 10 | 240.4 (190.6–303.2) | 216.9 (172.0–273.6) | 48 | 3 | 372.3 (278.9–496.9) | 332.5 (249.0–443.8) |
| Tonsillitis <1 year before tonsil carcinoma | 37 | 12 | 88.1 (63.7–121.8) | 79.3 (57.3–109.7) | 24 | 9 | 91.2 (60.9–136.5) | 75.8 (50.6–113.5) | 13 | 3 | 92.7 (53.7–160.1) | 85.6 (49.6–147.9) |
| Tonsillectomy ≥1 year before tonsil carcinoma | 12 | 791 | 0.4 (0.2–0.7) | 0.4 (0.2–0.7) | 4 | 709 | 0.2 (0.07–0.5) | 0.2 (0.06–0.4) | 8 | 82 | 2.0 (1.0–4.0) | 1.8 (0.9–3.6) |
| Tonsillitis ≥1 year before tonsil carcinoma | 8 | 234 | 0.9 (0.5–1.9) | 0.8 (0.4–1.6) | 4 | 183 | 0.7 (0.3–1.9) | 0.5 (0.2–1.5) | 4 | 51 | 1.6 (0.6–4.3) | 1.4 (0.5–3.8) |
NOTE: Bolded estimates are significant.
Abbreviations: BOT, Base of tongue; OPC, oropharyngeal carcinoma.
Estimates have been rounded to one significant digit for presentation in this table.
Crude.
Adjusted for attained age, calendar period, education, and gender.
Tonsillitis without history of tonsillectomy.
Incidence of oropharyngeal carcinoma after remote history of tonsillectomy
The incidence of diagnosis with any oropharyngeal carcinoma or base of tongue carcinoma was similar for individuals with a history of tonsillectomy compared with no tonsillectomy (Table 1). However, tonsillectomy significantly reduced the risk of tonsil carcinoma (RR, 0.42; 95% CI, 0.24–0.74). After adjustment for available demographic variables, the protective effect of tonsillectomy remained robust (RR, 0.40; 95% CI, 0.22–0.70).
Given the strong association between younger age and HPV-positive tumor status, analyses were stratified by age of diagnosis with oropharyngeal carcinoma. The risk of diagnosis with oropharyngeal carcinoma at age younger than 60 years was reduced (adjusted RR, RRadj, 0.60; 95% CI, 0.42–0.87) following tonsillectomy. Tonsillectomy was associated with a nonsignificant reduction of risk for diagnosis with base of tongue carcinoma at age younger than 60 years (RRadj, 0.53; 95% CI, 0.20–1.43). Notably, the risk of diagnosis of tonsil carcinoma at age less than 60 years (RRadj, 0.15; 95% CI, 0.06–0.41) was significantly reduced after tonsillectomy as compared with no tonsillectomy. Therefore, tonsillectomy was independently associated with a reduction in the risk diagnosis before age 60 of any oropharyngeal carcinoma, and specifically, tonsil carcinoma (Table 1).
Conversely, diagnosis with any oropharyngeal carcinoma at age greater than 60 years was increased after tonsillectomy (RRadj, 2.52; 95% CI, 1.67–3.80). Risk of base of tongue carcinoma after age of 60 was significantly increased after tonsillectomy (RRadj, 4.16; 95% CI, 1.85–9.33). Similarly, risk of tonsil carcinoma after age 60 was increased after tonsillectomy (RRadj, 1.78; 95% CI, 0.89–3.56), however nonsignificantly.
Whether the effect of tonsillectomy on risk of oropharyngeal carcinoma was modified by calendar time was explored. There was no evidence of interaction between tonsillectomy and calendar time (as a continuous variable; P = 0.64). Therefore, the effect of tonsillectomy was similar over the observation period from 1977 to 2012.
The effect of tonsillectomy on risk of nonoropharyngeal carcinomas was explored. The risk of larynx carcinoma diagnosis at any age (RR, 1.32; 95% CI, 0.96–1.81), before (1.09; 95% CI, 0.96–1.81), and after age 60 (RR, 1.46; 95% CI, 0.92–2.32) was statistically similar after tonsillectomy. Tonsillectomy was not associated with increased risk of lung carcinoma at any age (RR, 1.64; 95% CI, 0.92–2.91), before (RR, 1.84; 95% CI, 0.94–3.59), or after age 60 (RR, 0.98; 95% CI, 0.32–3.01). The incidence of malignancies of other major sites, including colon, breast, and prostate, was not associated with tonsillectomy.
Of note, the remote history of tonsillitis (without tonsillectomy) was not associated with risk of oropharyngeal carcinoma overall, base of tongue, or tonsil carcinomas (Table 1).
Survival analysis
In light of the interest in primary surgical therapy for oropharyngeal carcinoma, the survival of individuals who had tonsillectomy within 1 year of diagnosis with oropharyngeal carcinoma was compared with survival of individuals with either no tonsillectomy or remote history of tonsillectomy. Oropharyngeal carcinoma patients with a remote history of tonsillectomy and no history of tonsillectomy had similar survival (P > 0.05). Tonsillectomy within 1 year of diagnosis with oropharyngeal carcinoma, however, was independently associated with significantly improved overall survival (HRadj = 0.55; 95% CI, 0.42–0.71; Table 2) compared with no tonsillectomy. Median survival for oropharyngeal carcinoma with tonsillectomy within 1 year of diagnosis was greater than for oropharyngeal carcinoma with no tonsillectomy (11.2 vs. 2.8 years, P < 0.01). Other factors associated with improved overall survival included younger age at diagnosis (HR, 0.70; 95% CI, 0.67–0.72 per 10 years) and calendar period of diagnosis (HR, 0.86; 95% CI, 0.83–0.90 per 10 years). Tonsillectomy was not associated with overall survival for base of tongue carcinoma. For tonsil carcinoma specifically, tonsillectomy within 1 year of diagnosis was associated with significantly improved survival (HRadj, 0.53; 95% CI, 0.38–0.74; Fig. 2). Median survival for tonsil carcinoma patients was greater after tonsillectomy within 1 year of diagnosis than no tonsillectomy (14.3 vs. 3.8 years, P < 0.01).
Table 2.
Overall survival after oropharyngeal, base of tongue, and tonsil carcinoma by history of tonsillectomya
| Any age | <60 years | ≥60 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| History of tonsillectomy | Ncases | Dead | HRb (95% Cl) | HRc (95% Cl) | Ncases | Dead | HRb (95% Cl) | HRc (95% Cl) | Ncases | Dead | HRb (95% Cl) | HRc (95% Cl) |
| Oropharyngeal carcinoma overall | ||||||||||||
| No tonsillectomy or tonsillitis | 4,825 | 3,064 | 1.00 | 1.00 | 2,593 | 1,601 | 1.00 | 1.00 | 2,232 | 1,463 | 1.00 | 1.00 |
| Tonsillectomy <1 year before OPC | 174 | 45 | 0.5 (0.4–0.6) | 0.6 (0.4–0.7) | 106 | 24 | 0.4 (0.3–0.6) | 0.5 (0.3–0.8) | 68 | 21 | 0.5 (0.4–0.7) | 0.6 (0.4–0.8) |
| Tonsillectomy ≥1 year before OPC | 52 | 25 | 1.0 (0.7–1.5) | 1.3 (0.9–1.9) | 29 | 13 | 1.4 (0.8–2.4) | 1.9 (1.1–3.2) | 23 | 12 | 0.7 (0.4–1.3) | 1.0 (0.6–1.6) |
| Base of tongue carcinoma | ||||||||||||
| No tonsillectomy or tonsillitis | 727 | 413 | 1.00 | 1.00 | 386 | 213 | 1.00 | 1.00 | 341 | 200 | 1.00 | 1.00 |
| Tonsillectomy <1 year before base of tongue carcinoma | 16 | 7 | 0.8 (0.4–1.5) | 1.0 (0.5–2.1) | 10 | 4 | 0.8 (0.3–2.1) | 1.2 (0.4–3.3) | 6 | 3 | 0.9 (0.3–2.4) | 1.0 (0.4–2.8) |
| Tonsillectomy ≥1 year before base of tongue carcinoma | 10 | 5 | 0.7 (0.3–1.8) | 1.0 (0.4–2.3) | 4 | 1 | 0.5 (0.07–3.7) | 0.7 (0.09–5.2) | 6 | 4 | 0.7 (0.3–1.9) | 1.1 (0.4–2.9) |
| Tonsil carcinoma | ||||||||||||
| No tonsillectomy or tonsillitis | 2,608 | 1,623 | 1.00 | 1.00 | 1,462 | 868 | 1.00 | 1.00 | 1,146 | 755 | 1.00 | 1.00 |
| Tonsillectomy <1 year before tonsil carcinoma | 123 | 26 | 0.4 (0.3–0.6) | 0.5 (0.4–0.7) | 75 | 15 | 0.4 (0.2–0.7) | 0.5 (0.3–0.8) | 48 | 11 | 0.5 (0.3–0.7) | 0.5 (0.3–0.8) |
| Tonsillectomy ≥1 year before tonsil carcinoma | 12 | 6 | 1.1 (0.5–2.3) | 1.3 (0.6–2.6) | 4 | 3 | 2.4 (0.8–7.3) | 2.1 (0.7–6.5) | 8 | 3 | 0.7 (0.3–1.8) | 0.9 (0.3–2.4) |
NOTE: Bolded estimates are significant.
Abbreviation: OPC, oropharyngeal carcinoma.
Estimates have been rounded to one significant digit for presentation in this table.
Crude.
Adjusted for attained age, calendar period, education and gender.
Figure 2.
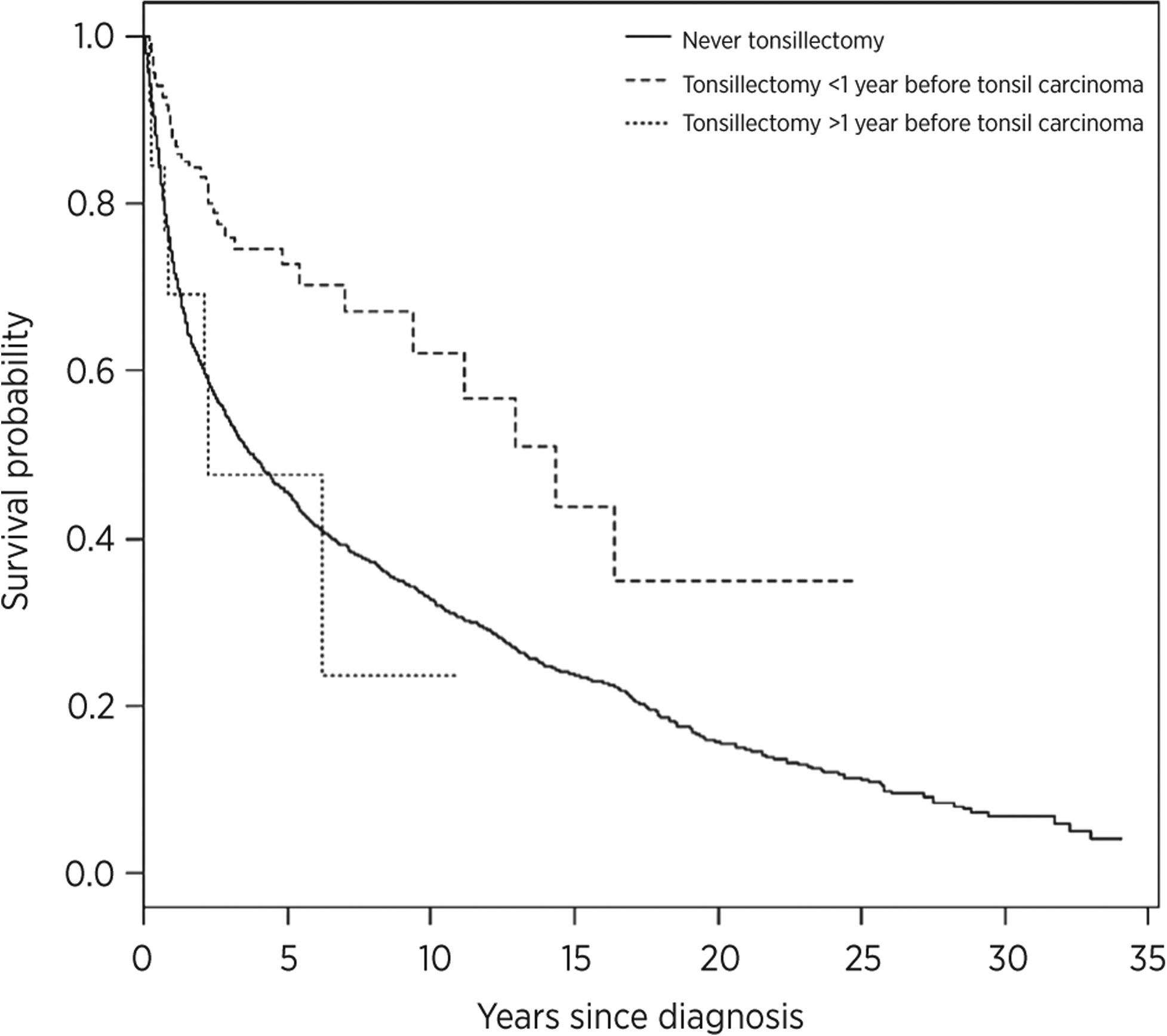
Survival analysis of tonsil carcinomas after tonsillectomy. The survival of tonsil carcinoma cases with history of no tonsillectomy or remote tonsillectomy was compared with tonsillectomy within 1 year of diagnosis. Tonsillectomy within 1 year of diagnosis was associated with significantly improved overall survival.
Discussion
In this population-based study, the incidence of tonsil carcinoma was significantly reduced after tonsillectomy. These findings have important implications for informed counseling of patients regarding risks and benefits of tonsillectomy, a common surgical procedure and design of future clinical trials evaluating secondary prevention of oropharyngeal carcinoma.
Despite the reduced risk of tonsil carcinoma observed herein, prophylactic tonsillectomies in the general population are not supported by these data. The risk of tonsil carcinoma remains rare irrespective of tonsillectomy; the incidence of tonsil carcinoma with versus without a history of tonsillectomy was 1.52 cases per 100,000 person-years versus 3.64 cases per 100,000 person-years, respectively. However, if biomarkers of high predictive value for development of oropharyngeal carcinoma were identified, tonsillectomy could be part of an intervention-based clinical trial to reduce incidence of malignancy. For example, in cervical cancer screening, removal of precursor lesions has reduced the burden of cervical cancer in the United States. If early tonsil lesions could be identified through a combination of biomarkers for risk stratification (16, 17), imaging (18, 19), and cytologic evaluation (20), then tonsillectomy in a select well-defined population may reduce the incidence of tonsil carcinoma.
Tonsillectomy likely reduces the palatine lymphoid tissue susceptible to carcinogenic factors, and subsequent potential for malignant transformation. Analogous precedents include prophylactic oophorectomy and mastectomy in high-risk populations. For tonsil carcinoma, the absence of palatine lymphoid tissue in the general population reduces the risk of tonsil carcinoma development by 60% (Table 1). As expected, this protective effect is specific to tonsil carcinoma and was not observed for malignancies of other anatomic sites or oropharyngeal subsites.
The global increase in oropharyngeal carcinoma incidence in recent age cohorts and calendar periods due to HPV has been attributed to the sexual revolution (1, 6). Other potential cofactors, however, have not been identified. In this study, a dramatic reduction in the incidence of tonsillectomies in recent cohorts and calendar periods accompanied a concurrent rise in the incidence of oropharyngeal carcinoma. It is important to note that the incidence of tonsillectomy remains 20-fold higher than the incidence of oropharyngeal carcinoma. Therefore, although the decreased performance of tonsillectomy, a common surgical procedure, may have contributed to the rise in incidence in recent calendar periods, it is merely another factor and not the primary driver of the increase in incidence. This analysis also provides population-based trends for rates of tonsillectomies which have not previously been available. In the United States, patterns in volume and rates of tonsillectomies have been estimated with inpatient registries, state-based revenue reports, or limited to cross-sectional data (21–23).
Of note, the risk for diagnosis of oropharyngeal carcinoma before age 60, a characteristic of HPV-related oropharyngeal carcinoma, was reduced after tonsillectomy, whereas the risk for diagnosis of oropharyngeal carcinoma after age 60 was significantly increased (Table 1). The inverse associations of tonsillectomy with oropharyngeal carcinoma diagnosed below and greater than median age may reflect distinct entities with different risk factors and biology (13, 24). HPV-related carcinomas more commonly arise in younger individuals with greater lifetime sexual exposure and decreased smoking history (13, 24, 25). In contrast, HPV-unrelated carcinomas more commonly arise in older individuals with an extensive tobacco and alcohol history (13, 25). A tonsillectomy in the context of HPV likely represents a reduction in the tissue susceptible to HPV infection upon exposure to the virus and, therefore, decreased potential for subsequent malignant transformation. This is consistent with recent natural history data that showed a significant reduction in the incidence of oral HPV infections among individuals with a history of a tonsillectomy (26). The absence of tonsil tissue in the context of smoking, however, may correspond to a loss of the local immune response and, in effect, may create a locally immunosuppressed environment. This may be very pronounced in the context of tobacco which is independently immunosuppressive (27). HPV tumor status is unavailable and therefore the interpretation of this observation is limited and speculative.
Tonsillectomy within 1 year of diagnosis of tonsil carcinoma (diagnostic or therapeutic) was associated with improved overall survival (Table 2, Fig. 2). With the increasing incidence of oropharyngeal carcinoma in younger age cohorts (1) and improved long-term survival (28, 29), there is interest in reducing the long-term sequelae of therapy (30). Therefore, primary surgical therapy (therapeutic palatine or lingual tonsillectomy) is being explored in clinical trials. In this study, we observed at a population level an improved overall survival among patients who had tonsillectomy within a year of diagnosis with tonsil carcinoma. Whether these tonsillectomies were performed as diagnostic or therapeutic procedures is unknown. These observations are consistent with U.S. data which found tonsillectomy before radiotherapy to be associated with improved survival (31, 32), and may be validated by ongoing and future well-designed prospective trials. However, it important to consider that smaller primary tumors are more amenable to tonsillectomy for either diagnostic or therapeutic purposes and associated with improved survival. Nevertheless, removal of a primary tumor before adjuvant therapy may have prognostic significance but cannot be extrapolated from the present data.
There are several limitations to this study. Clinically relevant information including HPV tumor status, stage, tobacco use, margin status, and treatment strategies (primary and adjuvant) was unavailable in this registry. The absence of these clinically significant covariates introduces the potential for residual confounding. However, the analysis for oropharyngeal carcinoma was limited to HPV-related sites consistent with site-classification approaches used by prior population-based studies of incidence trends for Denmark, United States, and Cancer Incidence in Five Continents databases. In addition, available variables associated with HPV-positive tumor status, including age of diagnosis, and calendar period were accounted for. Education level is associated with tobacco and alcohol consumption and was therefore used to adjust for unmeasured confounders. While the Danish Cancer Registry provides population-based data, it is limited to follow-up since 1977 and a relatively small population. Therefore, it is not surprising that there are few cases of oropharyngeal carcinoma, a rare malignancy, in this population-based cohort study. The limited number of oropharyngeal carcinoma cases overall (n = 52) and tonsil carcinomas (n = 12) following tonsillectomy could imply that the observed associations were by chance. Although the estimates of the effect of tonsillectomy may have low precision and relatively wide CIs, the significant point estimates are far from the null and highly significant, therefore supporting a true association. In order to evaluate whether the significant effect estimates could be chance findings, a sensitivity analysis regarding the estimate for the smallest group, i.e., the 12 tonsil cancer patients more than 1 year after tonsillectomy, was conducted. For the effect of tonsillectomy to vanish, 7 additional cases in the tonsillectomy group—i.e., a total of 19 cases—an increase of more than 50%—would need to have been observed. The validity of the Danish Cancer Registry is high and secured by the application of manual quality control routines, and an underreporting of 50% is unlikely. Therefore, this sensitivity analysis indicates that it is unlikely that the results are a reflection of chance alone.
In conclusion, tonsillectomy reduces tonsil carcinoma diagnosed before age 60 by 85% and any tonsil carcinoma by 60%. This observation is made with caution. Tonsillectomy is not a surgical procedure to be discounted and can lead to both minor and major complications, including death. Careful consideration of the relatively low incidence of oropharyngeal carcinoma, potential morbidity and mortality of tonsillectomy, and promising potential impact of the prophylactic HPV vaccine is warranted.
Grant Support
The study was supported by Oral Cancer Foundation.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 2013;31:4550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garnaes E, Kiss K, Andersen L, Therkildsen MH, Franzmann MB, Filtenborg-Barnkob B, et al. A high and increasing HPV prevalence in tonsillar cancers in Eastern Denmark, 2000–2010: the largest registry-based study to date. Int J Cancer 2015;136:2196–203. [DOI] [PubMed] [Google Scholar]
- 4.Blomberg M, Nielsen A, Munk C, Kjaer SK. Trends in head and neck cancer incidence in Denmark, 1978–2007: focus on human papillomavirus associated sites. Int J Cancer 2011;129:733–41. [DOI] [PubMed] [Google Scholar]
- 5.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007;356:1944–56. [DOI] [PubMed] [Google Scholar]
- 6.D’Souza G, Cullen K, Bowie J, Thorpe R, Fakhry C. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS One 2014;9:e86023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glover JA. The incidence of tonsillectomy in school children. 1938. Int J Epidemiol 2008;37:9–19. [DOI] [PubMed] [Google Scholar]
- 8.Lescanne E, Chiron B, Constant I, Couloigner V, Fauroux B, Hassani Y, et al. Pediatric tonsillectomy: clinical practice guidelines. Eur Ann Otorhinolaryngol Head Neck Dis 2012;129:264–71. [DOI] [PubMed] [Google Scholar]
- 9.Baugh RF, Archer SM, Mitchell RB, Rosenfeld RM, Amin R, Burns JJ, et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg 2011;144:S1–30. [DOI] [PubMed] [Google Scholar]
- 10.Ogra PL. Effect of tonsillectomy and adenoidectomy on nasopharyngeal antibody response to poliovirus. N Engl J Med 1971;284:59–64. [DOI] [PubMed] [Google Scholar]
- 11.Wood CB. Immunological factors and tonsillectomy. Proc R Soc Med 1973;66:411–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vestergaard H, Westergaard T, Wohlfahrt J, Hjalgrim H, Melbye M. Tonsillitis, tonsillectomy and Hodgkin’s lymphoma. Int J Cancer 2010;127: 633–7. [DOI] [PubMed] [Google Scholar]
- 13.Gillison M, D’souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomaviurs type 16-positive and human papillomavirus 16-negative head and neck cancers. J Natl Cancer Inst 2008;100:407–20. [DOI] [PubMed] [Google Scholar]
- 14.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 2008;26: 612–9. [DOI] [PubMed] [Google Scholar]
- 15.R Core Team R. A Language and environment for statistical computing. Available from: http://www.R-project.org/ 2013.
- 16.Kreimer AR, Johansson M, Waterboer T, Kaaks R, Chang-Claude J, Drogen D, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol 2013;31:2708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castle PE. Teaching moment: why promising biomarkers do not always translate into clinically useful tests. J Clin Oncol 2014;32:359–61. [DOI] [PubMed] [Google Scholar]
- 18.Fakhry C, Agrawal N, Califano J, Messing B, Liu J, Saunders J, et al. The use of ultrasound in the search for the primary site of unknown primary head and neck squamous cell cancers. Oral Oncol 2014;50:640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco RG, Califano J, Messing B, Richmon J, Liu J, Quon H, et al. Transcervical ultrasonography is feasible to visualize and evaluate base of tongue cancers. PLoS One 2014;9:e87565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fakhry C, Rosenthal BT, Clark DP, Gillison ML. Associations between oral HPV16 infection and cytopathology: evaluation of an oropharyngeal “pap-test equivalent” in high-risk populations. Cancer Prev Res (Phila) 2011; 4:1378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wennberg JE, Blowers L, Parker R, Gittelsohn AM. Changes in tonsillectomy rates associated with feedback and review. Pediatrics 1977;59: 821–6. [PubMed] [Google Scholar]
- 22.Derkay CS. Pediatric otolaryngology procedures in the United States: 1977–1987. Int J Pediatr Otorhinolaryngol 1993;25:1–12. [DOI] [PubMed] [Google Scholar]
- 23.Boss EF, Marsteller JA, Simon AE. Outpatient tonsillectomy in children: demographic and geographic variation in the United States, 2006. J Pediatr 2012;160:814–9. [DOI] [PubMed] [Google Scholar]
- 24.Benson E, Li R, Eisele D, Fakhry C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol 2014;50: 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rettig E, Kiess AP, Fakhry C. The role of sexual behavior in head and neck cancer: implications for prevention and therapy. Expert Rev Anticancer Ther 2015;15:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beachler DC, Sugar EA, Margolick JB, Weber KM, Strickler HD, Wiley DJ, et al. Risk factors for oral HPV infection acquisition and clearance among HIV-infected and HIV-uninfected adults. Am J Epidemiol 2015;181:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sopori M Effects of cigarette smoke on the immune system. Nat Rev Immunol 2002;2:372–7. [DOI] [PubMed] [Google Scholar]
- 28.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008;100:261–9. [DOI] [PubMed] [Google Scholar]
- 29.Ang K, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adelstein D, Ridge J, Gillison M, Chaturvedi AK, D’Souza G, Gravitt PE, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9–10, 2008. Head Neck 2009;31:1393–422. [DOI] [PubMed] [Google Scholar]
- 31.Holliday MA, Tavaluc R, Zhuang T, Wang H, Davidson B. Oncologic benefit of tonsillectomy in stage I and II tonsil cancer: a surveillance epidemiology and end results database review. JAMA Otolaryngol Head Neck Surg 2013;139:362–6. [DOI] [PubMed] [Google Scholar]
- 32.Yildirim G, Morrison WH, Rosenthal DI, Sturgis EM, Papadimitrakopoulou VA, Schwartz DL, et al. Outcomes of patients with tonsillar carcinoma treated with post-tonsillectomy radiation therapy. Head Neck 2010;32:473–80. [DOI] [PubMed] [Google Scholar]


