Abstract
Silicone polymers are used for a wide array of applications from passive samplers in environmental studies, to implants used in human augmentation and reconstruction. If silicone sequesters toxicants throughout implantation, it may represent a history of exposure and potentially reduce the body burden of toxicants influencing the risk of adverse health outcomes such as breast cancer. Objectives of this research included identifying a wide variety of toxicants in human silicone implants, and measuring the in vivo absorption of contaminants into silicone and surrounding tissue in an animal model. In the first study, eight human breast implants were analyzed for over 1,400 organic contaminants including consumer products, chemicals in commerce, and pesticides. A total of 14 compounds including pesticides such as trans-nonachlor (1.2–5.9 ng/g) and p,p′-DDE (1.2–34 ng/g) were identified in human implants, 13 of which have not been previously reported in silicone prostheses. In the second project, female ICR mice were implanted with silicone and dosed with p,p′-DDE and PCB118 by intraperitoneal injection. After nine days, silicone and adipose samples were collected, and all implants in dosed mice had p,p′-DDE and PCB118 present. Distribution ratios from silicone and surrounding tissue in mice compare well with similar studies, and were used to predict adipose concentrations in human tissue. Similarities between predicted and measured chemical concentrations in mice and humans suggest that silicone may be a reliable surrogate measure of persistent toxicants. More research is needed to identify the potential of silicone implants to refine the predictive quality of chemicals found in silicone implants.
Keywords: silicone, implants, in vivo, biomonitoring, adipose, pesticides
Graphical abstract
1. Introduction
Due to initial health concerns regarding silicone implants used for breast reconstruction and augmentation, there have been numerous epidemiological studies conducted to evaluate adverse outcomes. Several studies have reported a protective effect for breast cancer in women with silicone implants (Brinton et al. 2006; Brisson et al. 2006; Deapen et al. 2007; Friis et al. 2006; Lipworth et al. 2009; McLaughlin et al. 2006; Villeneuve et al. 2006). Two studies found a 30–50 % reduction of risk in breast cancer with silicone augmentation (Brisson et al. 2006; Lipworth et al. 2009). If accumulation of contaminants in breast tissue is a risk factor for breast cancer (Brody et al. 2007), then silicone implants may function as a sink for organic contamination, resulting in unanticipated health benefits and warrants further investigation.
In the last decade, silicone polymers have been increasingly used as passive samplers to absorb contaminants in aqueous and atmospheric field deployments (Allan et al. 2009; Allan et al. 2013c; O’Connell et al. 2014a; Rusina et al. 2007; Seethapathy and Gorecki 2012). Organic compounds in air or water are sequestered into silicone media through passive diffusion, and can then be extracted from these samplers for chemical and biological assays (Allan et al. 2012; Seethapathy et al. 2008; Vrana et al. 2005; Zabiegala et al. 2010). Because passive samplers take up compounds in the dissolved phase (Anderson and Hillwalker 2008), much of the organic analytical interferences are excluded, simplifying subsequent extractions for chemical analysis (Namieśnik et al. 2005). Implant shells used in augmentation or reconstructive surgeries are constructed from similar silicone rubbers to those used in environmental passive sampling devices. We hypothesize that human implants will accumulate a wide range of organic compounds similar to those absorbed in environmental applications, and that in vivo partitioning in an animal model with and without silicone implants will test the significance of silicone influencing organic compound body burden.
We conducted two studies to evaluate the potential for silicone implants to sequester environmental chemicals. In the first study, we identified contaminants sequestered in silicone breast implant shells which had been removed from human tissue. Sample extracts from the implants were screened for over 1,400 compounds including consumer products, chemicals in commerce, and pesticides. Extracts were analyzed further in a quantitative pesticide method to compare levels of compounds between implants. In the second study, we implanted silicone into mice to evaluate in vivo silicone and tissue absorption for two model compounds, p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE) and 2,3′,4,4′,5-pentachlorobiphenyl (PCB118). Concentrations from mouse tissues and silicone allowed for comparisons between treatment groups, and distribution ratios between silicone and adipose tissue were used to predict mouse or human adipose tissue concentrations. If the absorption of contaminants in silicone and human tissue can be elucidated, then implants typically treated as waste might be a useful source of long-term human biomonitoring.
2. Methods
2.1 Breast implant collection and extraction
Implants were obtained in 2010 from Oregon Health and Science University (OSU IRB# 5851). All materials were numerically coded, and no personal demographic, occupational, or medical information was obtained or recorded. A total of 8 saline-filled implants were collected and stored at −20 °C prior to analyses (Figure 1A). In addition to implants, silicone-filled implant “sizers” were used as negative controls. Both saline and gel-filled implant shells are made with the same type of silicones (i.e. polydimethylsiloxane (Daniels 2012)), and sizers are used for demonstration or temporary intraoperative procedures to facilitate final size considerations (Figure 1B).
Figure 1.
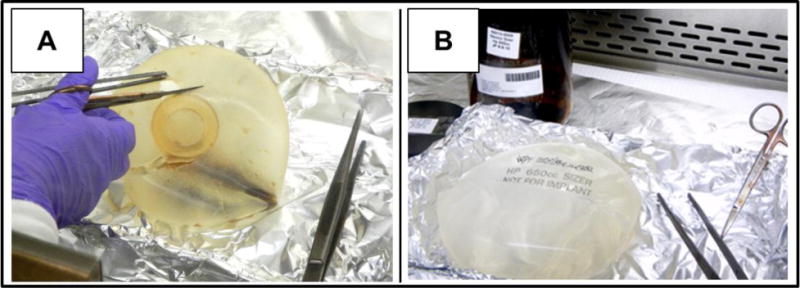
Silicone implant (A) and sizer (B). Sizers served as negative controls.
Small pieces of the silicone shell from both implants and sizers were excised for chemical analyses (1.8 – 4.4 g per piece). Pieces were selected from each side of the implant and sizer. Each piece was rinsed twice in purified water, and then with isopropyl alcohol, following methods for cleaning silicone used previously (O’Connell 2014a,b). Extraction consisted of placing each piece of rinsed silicone in 50 mL of ethyl acetate for at least two hours on an orbital shaker at 60 rotations per minute (rpm). The soaking process was repeated once more with additional solvent. Liquids from each soaking process were combined and reduced to 5 mL using closed-cell evaporators (TurboVaps®, Biotage, Charlotte, NC). For both laboratory extraction surrogates, tetrachloro-m-xylene (TCMX) and decachlorobiphenyl, 500 ng of each compound were added to the first round of extraction to assess loss due to evaporation or transfers between glassware. Concentrated samples were transferred to centrifuge tubes and stored at −20 °C. To increase the likelihood of identifying compounds in the analytical screening method, compounds in the extracts were further separated using gel permeation chromatography (GPC) to remove as much interference as possible while retaining compounds of interest. Details of the GPC method can be found in the Supporting Information.
2.2 Mouse implant study: silicone and cocktail preparation
Small discs (~0.5 cm2) of silicone were made from silicone sheeting (Stockwell Elastomerics Inc., Philadelphia, PA). The average weight of silicone discs was 0.02 +/− 0.001 g (n=25). Silicone discs were cleaned sequentially with water and mixes of ethyl acetate with hexane and methanol as described previously (O’Connell et al. 2014b). Discs were dried in a stainless steel keg (AEB Kegs, Delebio SO, Italy) under an air-filtered vacuum and stored in polytetrafluoroethylene (PTFE) air-tight bags until surgery.
Mice were dosed by intraperitoneal injection with p,p′-DDE and PCB118. These compounds were chosen because they are well-characterized lipophilic compounds with known resistance to metabolic processes (Berg et al. 2010; Falck et al. 1992). Both p,p′-DDE and PCB118 were dissolved in ethyl acetate and diluted with filtered (0.4 μm) peanut oil to 0.21 mg/mL and 0.16 mg/mL, respectively. Prior to injection, the mixture was further diluted by 10-fold with peanut oil in order to reduce ethyl acetate to less than 1% (v:v). Mice received 0.13 ± 0.002 and 0.10 ± 0.001 mg/Kg for p,p′-DDE and PCB118, respectively.
2.3 Animal care and surgery
Female ICR mice (Jackson Laboratory, Bar Harbor, ME) were maintained as a breeding colony and were held in the pathogen-free Laboratory Animals Resource Center (LARC) at Oregon State University. All experimental procedures and treatments were approved by the Institutional Animal Care and Use Committee. A total of 18 mice were used across 3 treatment groups, with 6 mice in each group. One treatment group received p,p′-DDE and PCB118 as well as subcutaneous silicone discs (hereafter referred to as “SIL”). As a vehicle control, a second group was dosed with peanut oil along with silicone discs (VEH). The third group was also dosed with p,p′-DDE and PCB118, but received sham surgeries with no silicone (SHAM) to determine if levels of contaminants differed due to the presence or absence of the implants.
On the day of surgery, animals were anesthetized with a mixture of isoflurane and oxygen, and implant areas were shaved and treated with betadine and alcohol. Two incisions were made on each mouse: a dorsal midline incision between shoulder blades, and a second ventral midline incision in the abdominal area. Two pieces of silicone were placed subcutaneously to the left and right of the shoulder incision and subsequently closed with sutures. Four additional pieces of silicone were placed to the left and right of the inguinal incision. In sum, six pieces of silicone were inserted subcutaneously per mouse in order to increase the likelihood of detecting chemicals in silicone (Figure 2). The total ratio of silicone to mouse body mass ranged from 1:330 to 1:500, and is within potential ratios of silicone implants to human body masses of 1:50 to 1:1500 (assuming an average body mass of 75.4 Kg (Centers for Disease Control and Prevention 2012), and implant combined weight ranging from 0.05 – 1.5 Kg (Mentor Worldwide 2013)).
Figure 2.
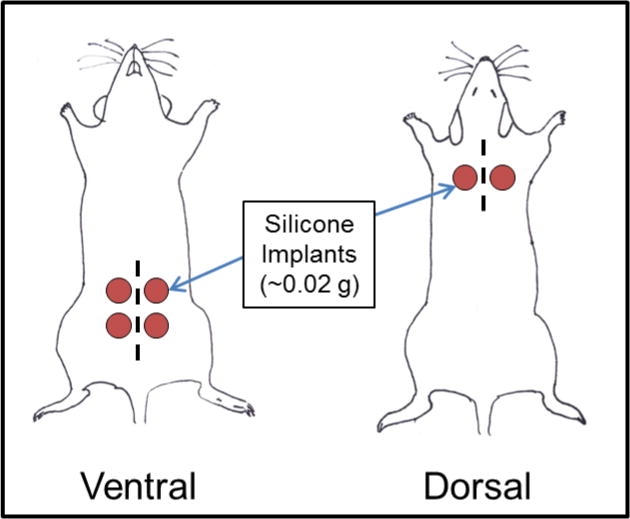
Silicone inserts in dorsal and ventral locations. All graphic representations are approximate and not necessarily to scale. Dashed lines represent approximate locations of incisions.
Following surgery, mice received 1 ml/10 g body weight of subcutaneous fluids before receiving the contaminant cocktail. Intraperitoneal injection was chosen as the route of exposure to ensure each mouse received a similar dose. Mice were monitored during recovery and for 24 hours post-surgery by a veterinary technician. After nine days, mice were euthanized via CO2 overdose and cervical dislocation. Previous research has suggested that 7-day exposures could be adequate to establish equilibrium between lipids and silicone (Jahnke and Mayer 2010; Jahnke et al. 2008). No gross organ malformations or changes in body weight were observed in any treatment group. Silicone pieces were composited into a single sample from each animal to ensure adequate analytical sensitivity. Adipose tissue samples were taken from the dorsal and abdominal region and stored separately. Mouse implants and tissues were stored in amber glass vials at −20 °C until laboratory processing.
2.4 Silicone and adipose extraction
Silicone pieces (n = 6) from each mouse were rinsed with filtered water and isopropyl alcohol and then combined into one extract (~0.12 g of total silicone). Extractions of silicone pieces were similar to that of the human implants pieces described in section 2.1, but scaled down to account for the smaller amount of total silicone. In total, three ethyl acetate extractions of 2 mL were combined and subsequently reduced to 0.5 mL. PCB180 and PCB100, each added at 500 ng, were used as laboratory surrogates for p,p′-DDE and PCB118, respectively. Sample extracts were stored in amber chromatography vials at 4 °C until analysis.
Adipose tissue samples from dorsal or ventral locations were extracted using a modified QuEChERS method (Forsberg et al. 2011), followed by solid-phase extraction (SPE) and solvent exchange. Details of the homogenization of the tissue, the QuEChERS method, SPE cleanup, and final solvent reduction can be found in the Supporting Information. Sample extracts were stored at 4 °C in chromatography vials until analysis.
2.5 Chemical analyses
Human study samples were qualitatively screened for 1,418 compounds using GC-MS with automated mass spectrum deconvolution identification software (AMDIS). An Agilent DB-5 (30m, 0.25mm, 0.25 μm) column was used on the GC-MS. Before and after target samples were screened on the GC-MS, a standard solution containing 24 compounds at 500 ng/mL was analyzed to provide an indication of instrument and software performance of the compound screen. No substantial changes in instrument or software performance were identified, and over 70% of the compounds were found in the standard solution before and after implant samples. Compounds in human implants were first identified by having at least a 60% spectral match, before additional confirmation by a trained analytical chemist. Additional criteria such as retention time and ion ratios were used for each compound presence/absence determination with more weight given to compounds that had matching spectra and ion ratios near parent and fragment ions with higher abundance. Any compounds identified in the sizers were considered background contaminants, and are not included in the human implant results (see SI-Table 1 for a full list of compounds identified in samples, sizer, and standards).
All samples from both studies were analyzed using a quantitative pesticide method for 43 compounds described elsewhere (Anderson et al. 2014). Before each injection, 4,4′-dibromooctafluorobiphenyl was added as an internal standard at 100 ng/mL. An Agilent DB-XLB (30m, 0.25mm, 0.25 μm) and a DB-17MS (30m, 0.25mm, 0.25 μm) were used for dual column confirmation coupled with dual micro-electron capture detection (GC-ECD, model 6890N, Agilent). All compounds were quantified using calibration curves of five concentrations or more, and all calibration curves had correlation coefficients of 0.99 or better. Contaminants were not reported if the sample was severely affected during laboratory processing (i.e., surrogate compounds were seen below 15% of starting amount), or were below signal to noise ratios of 3:1. Further details on laboratory equipment or chemicals can be found in the Supporting Information.
2.6 Quality control
Quality control samples represented over 39% of those analyzed. In the mouse implant study, pieces of non-deployed silicone were examined prior to surgery for any analytical background interferences. Silicone cleaning was considered successful if the highest peak on a full scan GC-MS analysis (range: 50–500 m/z) had an area less than 15-fold of a 500 ng standard. Other quality control samples included: non-deployed silicone, laboratory extraction blanks, and reagent blanks. Prior to quantitative analyses, all compounds were verified to be within +/− 20 % of the true value using certified standards. Certified standards were also run nominally every 10 samples as well as at the end of each analytical sample set. No detectable concentrations from the quantitative method were seen in any non-deployed silicone, sizers, laboratory extraction blanks, or reagent blanks.
3. Results
3.1 Organic contaminants in human implants
A total of 14 compounds were identified in human silicone implants including 5 consumer products, 3 chemicals used in commerce, 3 pesticides, 2 phthalates and 1 aromatic hydrocarbon (Table 1). Consumer products included several musk fragrances used in soaps, perfumes and detergents, as well as chemicals associated with food stuffs like caffeine and carvone (NLM 1993). Among chemicals in commerce, there were two compounds used as flame retardants: tris(2-chloroethyl) phosphate and tris(2-butoxyethyl) phosphate. Among all groups, several compounds were seen in more than one sample. For example, caffeine was seen in all 8 implants, and p,p′-DDE was the second-most identified chemical, detected in 5 implants (Table 1). Both galaxolide (a musk compound) and diisobutyl phthalate (a common commercial additive) were seen in 3 implant samples (Table 1). Alternatively, several compounds were seen in only one sample, including an oxygenated polycyclic aromatic hydrocarbon (OPAH), 9,10-anthraquinone.
Table 1.
Compounds identified in human implants from chemical screen of over 1,400 analytes.
| Categories | Compounds | CAS | Occurrence in implants (out of 8) | Possible source or use* |
|---|---|---|---|---|
| OPAHs | 9,10-anthraquinone | 84-65-1 | 1 | Breakdown product of polycyclic aromatic hydrocarbons (PAHs), from petrogenic and pyrogenic sources, also used in dye manufacturing |
| Consumer products | caffeine | 58-08-2 | 8 | Component of coffee, tea, and cocoa, used widely in food and pharmacological industries |
| galaxolide | 1222-05-5 | 3 | Artificial musk fragrance used in detergents, perfumes, soaps, and cosmetics | |
| phthalimide | 85-41-6 | 1 | Used as an intermediate for primary amines, dyes and a fungicide | |
| exaltolide | 106-02-5 | 1 | Artificial musk fragrance used in perfumes | |
| carvone | 99-49-0 | 1 | Found naturally in caraway and dill, used in confections, pharmaceuticals, perfumes, or soaps | |
| Pesticides | p,p′-DDE | 72-55-9 | 5 | An impurity in DDT production and degradation product of DDT |
| methoxychlor | 72-43-5 | 1 | Insecticide used to control mosquito larvae, house flies and other insect applications in agriculture | |
| trans-nonachlor | 39765-80-5 | 1 | Constituent of technical chlordane, an insecticide used for termite control and wood treatment | |
| Phthalates | diisobutyl phthalate | 84-69-5 | 3 | Used in manufacturing in polypropylene, fiberglass, polyvinyl chloride, nitrocellulose, and others |
| dicyclohexyl phthalate | 84-61-7 | 1 | Plasticizer for cellulose, chlorinated rubber, polyvinyl acetate or chloride, and other polymers | |
| Chemicals in Commerce | tris(2-chloroethyl) phosphate | 115-96-8 | 2 | Flame-retardant plasticizer found in vinyl resins, carpet backing, upholstery, thermosets, particle board |
| tris(2-butoxyethyl) phosphate | 78-51-3 | 1 | Flame-retardant plasticizer in synthetic rubber intended for food or drink consumption, among others | |
| 2,4-dimethylaniline | 95-68-1 | 1 | Intermediate in photographic chemicals, pesticides, dyes, and pharmaceutical products |
Source and use information was obtained from the National Library of Medicine, Hazardous Substances Data Bank (HSDB) – http://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm, accessed 1/7/15. Compounds in bold italics were also detected in the same extracts using a quantitative pesticide method described in the text and Anderson et al., 2014.
Implants containing p,p′-DDE and trans-nonachlor from the screening data were also found to contain these compounds in the quantitative pesticide analysis, providing confirmation from two independent analytical methods. Compounds p,p′-DDE and trans-nonachlor were quantified at or above reporting limits in at least one of the replicates from 7 and 4 implants, respectively (SI-Table 2). Implants had higher concentrations of p,p′-DDE than trans-nonachlor in most samples, ranging from 1.2–34 ng/g, and 1.2–5.9 ng/g, respectively (SI-Table 2). In two of the implants p,p′-DDE was found in only one of the replicates; however, in both cases the p,p′-DDE concentrations were near the detection limit (equivalent to ~0.7 ng/g). Similarly, trans-nonachlor was not consistently seen in implant replicates when close to the limit of detection (also equivalent to ~0.7 ng/g). Recovery of surrogates for silicone implants averaged 62%, indicating adequate extraction of the silicone.
3.2 Mouse study: silicone concentrations and percent uptake
All silicone samples from the SIL treatment group contained detectable levels of both p,p′-DDE and PCB118. Concentrations of p,p′-DDE in silicone ranged from 31–70 ng/g, averaging 49 ± 14 ng/g, while PCB118 ranged from 20–108 ng/g, averaging 57 ± 30 ng/g (Figure 3). The relative standard deviation (RSD) between silicone concentrations among mice were 34 % for p,p′-DDE and 54 % for PCB118. Nine days after the initial IP injection, percent uptake from the mouse into the silicone ranged from 0.05 % to 0.33 %, averaging 0.12 % for p,p′-DDE, and 0.18% for PCB118 (SI-Table 3). Excellent surrogate recoveries (all above 65 %) were seen in silicone from the mouse study.
Figure 3.
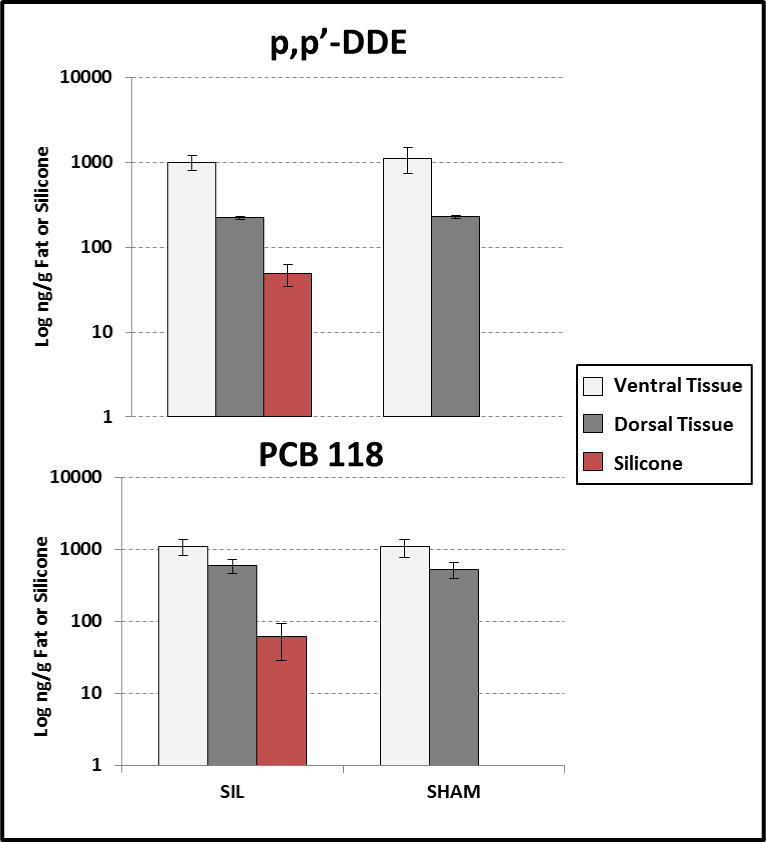
Concentrations in log scale of p,p′-DDE and PCB118 in silicone and surrounding tissues after nine days from an IP injection. Replication for each sample type ranged from 3-6. SIL groups received silicone and compounds, while SHAM received compounds and mock surgery. No compounds were detected in mice not given the compound injection, so VEH mice are not shown.
3.3 Mouse study: adipose tissue concentrations
Although the amount of tissue for analysis was small, p,p′-DDE and PCB118 were above detection limits in most adipose samples from mice that received the cocktail, and at much higher amounts than found in silicone. Tissue p,p′-DDE and PCB118 concentrations ranged from 210 to 1,700 ng/g, and 410 to 1,500 ng/g respectively. Dorsal tissue in SIL mice had an average p,p′-DDE concentration of 220 ± 11 ng/g, which was only slightly lower than the SHAM group (230 ± 11 ng/g), and not statistically different (p = 0.37, Figure 3). Ventral p,p′-DDE concentrations were also not significantly different between SIL and SHAM treatment groups (1,000 ± 200 ng/g compared to 1,100 ± 380 ng/g, respectively; p = 0.59) indicating that the silicone in the mice did not alter detectable p,p′-DDE residues in adipose tissue. Similarly, no difference in SIL over SHAM tissues were seen for PCB118 concentrations for either dorsal (600 ± 130 ng/g compared to 530 ± 140 ng/g) and ventral tissues (1,100 ± 280 ng/g versus 1,100 ± 300 ng/g), respectively (Figure 3). Slightly higher surrogate recovery was seen in dorsal tissues versus ventral tissues for PCB180 (averages: 52 % versus 31 %). For PCB100, surrogate recoveries were lower than PCB180, but similar from both dorsal and ventral tissues (averages: 29 % versus 24 %). When comparing mouse study samples collectively, surrogate recovery was lower for adipose tissue (34 % total average) when compared with silicone (80 % overall average).
4. Discussion
4.1 Human implants
Out of the 14 compounds reported in Table 1, thirteen are unique to this study compared with the only other report of environmental contaminants in silicone implants (Allan et al. 2013b). Caffeine was the only compound to be detected in all 8 implants, but is not surprising considering that caffeine is present in many widely consumed products (Somogyi 2010). Other compounds of interest include phosphate flame retardants, musk compounds from personal care products, and phthalates, which have all been previously detected in personal (external) silicone passive samplers (O’Connell et al. 2014a). Interestingly, 9,10-anthraquinone was seen in one explant and is present in petrogenic, pyrogenic, and dye manufacturing sources (NLM 1993), and has even been shown to migrate from pizza boxes into the food item (IARC 2012; NLM 1993)). Anthraquinone is one of the more commonly detected OPAHs in environmental samples (IARC 2012; O’Connell et al. 2013), but has not been previously measured in human samples to our knowledge. Another interesting observation was that many of the compounds detected (ex: caffeine, and some phosphate compounds) are known to be metabolized and excreted more rapidly than others. Sequestration of highly metabolized compounds in silicone may represent repeated exposures and/or elevated exposure levels. Trans-nonachlor, which has been identified in human adipose tissue along with p,p′-DDE, is a constituent of the insecticide chlordane (Brauner et al. 2012). Implants had roughly 3 to 6-fold less trans-nonachlor than p,p′-DDE (SI-Table 2), which is very similar to tissue data reported by Brauner et al (2012). Compared with the previous human implant study, p,p′-DDE is also the compound with the highest concentrations (Allan et al. 2013b). Furthermore, the magnitude and range of p,p′-DDE concentrations measured in this study (1.2–34 ng/g silicone) are very similar to Allan et al. (~0.2–37 ng/g silicone), despite potential differences in methodology and demographics of the population (2013b).
4.2 Mouse tissue and implant exposure
Significant differences were observed between ventral and dorsal tissues, with higher amounts in ventral adipose tissue (p,p′-DDE – p < 0.01; PCB118 – p < 0.01). This is expected because dorsal adipose tissue is composed of highly vascularized brown fat, and has lower lipid content (~55%) than abdominal adipose tissue (~90%) (Johansson 1959; Spencer and Dempster 1962). Brown fat also has a higher protein content (Johansson 1959), and may have a higher affinity for increasingly poly-chlorinated compounds (Patterson et al. 1989). This may explain why dorsal tissues had higher PCB118 concentrations (410–720 ng/g) than p,p′-DDE concentrations (210–240 ng/g) (Figure 3). Careful selection of adipose sampling locations is therefore critical for measuring compounds with more complex distribution and affinity than just lipid content would predict alone.
Even during a short exposure time of 9 days, percent uptake (0.05 to 0.33%) was adequate for analytical sensitivity (SI-Table 3). Direct comparisons of silicone percent uptake using our data with other studies is limited as the starting dose is unknown or difficult to determine (Allan et al. 2013a; Jahnke et al. 2009). While these studies indicate equilibrium between silicone and environmental contaminants may occur within lipid-rich tissue (living or deceased) from hours to days (Allan et al. 2013a; Jahnke et al. 2009), uptake rates of compounds will likely differ due to differences in dose mechanisms, lipid content of the tissue, mass of silicone implants, or other factors. Measuring discrete, differing locations in the body with silicone may help explain compound to compound differences in absorption, as well as reduce variability compared to pooled measurements.
4.3 Mouse adipose tissue limitations
Alternative methodology is often sought to measure organic contaminants in tissues due to low recoveries of surrogates, high variability, and labor intensive extractions (Jahnke et al. 2009; Jahnke et al. 2008; Musteata and Pawliszyn 2007). Recovering contaminants from fatty-tissues is challenging, with surrogate recoveries from a recent lipid extraction method ranging from 49–106% (Forsberg et al. 2011). However, better sensitivity and overall lower variability was observed using silicone implants as compared with some tissue samples that could not be reported due to low recovery of surrogates (<15%). This suggests a potential improved sampling alternative for contaminant measurements where implants might be relevant.
4.4 Comparing measured and predicted silicone distribution ratios in mice
From the mouse data presented in section 4.2, ratios between tissue and silicone can be calculated and used to predict human adipose concentrations. Ratios between silicone and adipose tissue can be useful when it is difficult or too invasive to collect tissue, but silicone implants are available or reasonable to use. If the system is known to be at equilibrium, partition ratios can be determined from the lipid phase and the silicone phase. If lipids may be present in the silicone and/or equilibrium is not necessarily achieved, distribution ratios are more appropriate ((IUPAC 1997; Jahnke et al. 2008)). The distribution ratio of these concentrations (Dtissue-silicone) can be used to predict contaminants in the tissue of other organisms. For example, estimates of ventral mouse tissue can be made using silicone and mammalian seal oil distribution ratios (Dlipid-silicone ≈ 21.2 for p,p-DDE, and ≈ 27.7 for PCB118, (Jahnke et al. 2008); Table 2). The predicted mouse tissue concentrations from both the published ratios and the measurements from the silicone implants in this study can be compared to the actual values measured in the ventral adipose tissue. When compared, there is considerable agreement (within 53%) between predicted values using ratios from Jahnke et al. (2008), to the actual mouse tissue measured in this study for both compounds (Table 2). Distribution ratios can also be calculated based on the measured silicone and the measured ventral tissue concentrations. The p,p′-DDE Dtissue-silicone value (22 ± 3.1) calculated from our data compare well to the Dlipid-silicone value (21 ± 1.3) from Jahnke et al., (2008) differing by only 5%. Since distribution ratios match closely with those from the other work which were determined to be at equilibrium, this provides some evidence that equilibrium in our mouse study might have taken place. Additionally, if assuming that uptake into the silicone is membrane controlled, the time to equilibrium between the tissue and silicone may be estimated to be less than 5 hours for either compound since the silicone pieces were so small (see Supporting Information for calculations). Together, it seems likely that equilibrium was established between the silicone pieces and the surrounding tissue, but empirically determining equilibrium should be a priority in future in vivo work. Other caveats are that silicone was aggregated from both locations, and adipose tissue was not lipid normalized since the purpose of this study was to directly compare contaminant concentrations between adipose tissues and silicone implants. However, even if tissues were lipid normalized, ventral values would likely be similar to current estimates based on the % lipid content (Spencer and Dempster 1962), while normalized dorsal values would likely more closely match concentrations from ventral data. Acknowledging these limitations, as well as differences in lipid type, capacity, and composition that may differ between tissues (van der Heijden and Jonker 2011), the observation that our results are similar to other studies encourages future work in this area that could potentially increase the accuracy in which lipid concentrations can be predicted from silicone. Future animal studies could use multiple time points of silicone implantation, use performance reference compounds (Huckins et al. 2002), or use implants with differing surface area to volume ratios to better characterize equilibrium. Alternatively, characterizing in vivo silicone uptake with activity measurements could also benefit future predictions and uses of silicone in relation to body burden.
Table 2.
Comparisons of measured and predicted adipose concentrations from in vivo silicone implants, and from mouse and human data
| p,p-DDE | PCB118 | |||||
|---|---|---|---|---|---|---|
|
|
||||||
|
Mousea ng/g Silicone or Tissue |
Silicone Implants |
Predicted Adiposec |
Measured Ventral |
Silicone Implants |
Predicted Adiposec |
Measured Ventral |
|
| ||||||
| 31–70 | 660–1500 | 730–1200 | 20–110 | 550–3000 | 710–1300 | |
|
| ||||||
| p,p-DDE | ||||||
|
|
||||||
|
Humanb ng/g Silicone or Tissue |
Silicone Implants |
Predicted Adiposec |
Predicted Adipose (using mouse data) |
Median (range) measured adipose tissue in literature
|
||
| Tabasco, Mexicod (n=150) |
Granada Province, Spaine (n=387) |
Antwerp, Belgiumf (n=52) |
||||
|
| ||||||
| 1.2–34 | 25–680 | 26–740 | 877 (50–5000) | 93 (2.0–2300) | 141 (15–8399) | |
Mouse implants included all those in the SIL treatment group
Human implants included all those containing p,p′-DDE
Based on Dlipid-silicone ≈ 21.2 for p,p-DDE, and ≈ 27.7 for PCB118 from (Jahnke et al. 2008)
4.5 Human Tissue Predictions
To examine how accurate predictions might be in human samples, adipose p,p′-DDE tissue concentrations were estimated from human silicone implant data using either seal oil distribution ratios (Jahnke et al. 2008), or mice tissue distribution ratios from this study (Table 2). After calculating predicted adipose tissue concentrations for each silicone implant that had detectable levels of p,p′-DDE, values were consistent with tissue concentrations reported in multiple studies around the world (Arrebola et al. 2013; Malarvannan et al. 2013; Waliszewski et al. 2012). Estimates using ventral mouse data and those using seal oil are near median levels of human cohorts, and well within the ranges of concentrations seen in these human populations (Table 2). More work would be necessary to reliably predict tissue concentrations with a high degree of accuracy, but these observations are well within real-world data. Future physiologically based pharmacokinetic modelling with silicone as an additional compartment may be able to link silicone concentrations to exposure if implant duration and other information are known.
5. Conclusions
Breast implants may represent long-term estimates of organic contaminant exposure. Over 23,000 implants were removed or replaced in 2013 within the United States alone (American Society of Plastic Surgeons 2014). Discarded implants are typically incinerated as waste, but these implants may actually be an important resource for exposure assessment and quantifying human body burden of organic pollutants. Our preliminary data suggests that in vivo silicone may be a reliable surrogate measure of persistent toxicants in humans. If a monitoring bank were to be established to archive routinely extracted breast implants, these specimens may be useful in characterizing silicone absorption of pollutants in vivo. In addition to bio-banking, implants may be used to further investigate whether there are potential health impacts of in vivo organic contaminant absorption to silicone. The reported protective effect for breast cancer in women with silicone implants is yet to be explained.
Supplementary Material
Highlights.
Human implants screened for over 1,400 chemicals
Silicone and tissue chemical concentrations compared in vivo using ICR mice
14 chemicals identified in human implants representing several chemical classes
All implants in dosed mice had p,p-DDE and PCB118 present above detection limits
Predicted adipose values using implant data within range of measured concentrations
Acknowledgments
This project was supported in part by award number P42 ES016465 and the associated Analytical Chemistry Facility Core, P30 ES000210 and R21 ES020120 from the National Institute of Environmental Health Sciences and the OSU Food Safety and Environmental Stewardship Program. Steven O’Connell was supported in part by NIEHS Training Grant Fellowship T32ES007060-32 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIEHS or the National Institutes of Health. The authors would also like to thank Kaiser Permanente and Mary Garrard for their help collecting breast implants, and Dr. Helen Diggs for silicone implantation in mice. In addition, the authors would like to thank Jennifer Przybyla, Melissa McCartney, Richard Scott, Jorge Padilla and Alan Bergmann for their help with laboratory methodology, and Dr. Gregory Sower for insightful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no competing financial interests.
References
- Allan IJ, Bæk K, Haugen TO, Hawley KL, Høgfeldt AS, Lillicrap AD. In Vivo Passive Sampling of Nonpolar Contaminants in Brown Trout (Salmo trutta) Environ Sci Technol. 2013a;47(20):11660–11667. doi: 10.1021/es401810r. [DOI] [PubMed] [Google Scholar]
- Allan IJ, Baek K, Kringstad A, Roald HE, Thomas KV. Should silicone prostheses be considered for specimen banking? A pilot study into their use for human biomonitoring. Environ Int. 2013b;59:462–468. doi: 10.1016/j.envint.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Allan IJ, Booij K, Paschke A, Vrana B, Mills G, Greenwood R. Field performance of seven passive sampling devices for monitoring of hydrophobic substances. Environ Sci Technol. 2009;43(14):5383–5390. doi: 10.1021/es900608w. [DOI] [PubMed] [Google Scholar]
- Allan IJ, Harman C, Ranneklev SB, Thomas KV, Grung M. Passive sampling for target and non-target analyses of moderately polar and nonpolar substances in water. Environ Toxicol Chem. 2013c;32(8):1718–1726. doi: 10.1002/etc.2260. [DOI] [PubMed] [Google Scholar]
- Allan SE, Smith BW, Tanguay RL, Anderson KA. Bridging environmental mixtures and toxic effects. Environ Toxicol Chem. 2012;31(12):2877–2887. doi: 10.1002/etc.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society of Plastic Surgeons. 2013 Cosmetic Plastic Surgery Statistics – Cosmetic Procedure Trends. 2014 Available: www.plasticsurgery.org [accessed 4/3/2014.
- Anderson KA, Hillwalker WE. Bioavailability. In: Jorgensen SE, Fath BD, editors. Encyclopedia of Ecology. Vol. 1. Oxford: Elsevier; 2008. pp. 348–357. [Google Scholar]
- Anderson KA, Seck D, Hobbie KA, Traore AN, McCartney MA, Ndaye A, et al. Passive sampling devices enable capacity building and characterization of bioavailable pesticide along the Niger, Senegal and Bani Rivers of Africa. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1639) doi: 10.1098/rstb.2013.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrebola JP, Fernandez MF, Olea N, Ramos R, Martin-Olmedo P. Human exposure to p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE) in urban and semi-rural areas in southeast Spain: A gender perspective. Sci Total Environ. 2013;458:209–216. doi: 10.1016/j.scitotenv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Berg V, Lyche JL, Gutleb AC, Lie E, Skaare JU, Aleksandersen M, et al. Distribution of PCB 118 and PCB 153 and hydroxylated PCB metabolites (OH-CBs) in maternal, fetal and lamb tissues of sheep exposed during gestation and lactation. Chemosphere. 2010;80(10):1144–1150. doi: 10.1016/j.chemosphere.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Brauner EV, Raaschou-Nielsen O, Gaudreau E, Leblanc A, Tjonneland A, Overvad K, et al. Predictors of adipose tissue concentrations of organochlorine pesticides in a general Danish population. J Expo Sci Environ Epidemiol. 2012;22(1):52–59. doi: 10.1038/jes.2011.39. [DOI] [PubMed] [Google Scholar]
- Brinton LA, Lubin JH, Murray MC, Colton T, Hoover RN. Mortality rates among augmentation mammoplasty patients – An update. Epidemiology. 2006;17(2):162–169. doi: 10.1097/01.ede.0000197056.84629.19. [DOI] [PubMed] [Google Scholar]
- Brisson J, Holowaty EJ, Villeneuve PJ, Xie L, Ugnat AM, Latulippe L, et al. Cancer incidence in a cohort of Ontario and Quebec women having bilateral breast augmentation. Int J Cancer. 2006;118(11):2854–2862. doi: 10.1002/ijc.21711. [DOI] [PubMed] [Google Scholar]
- Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer – Epidemiologic studies. Cancer. 2007;109(12):2667–2711. doi: 10.1002/cncr.22655. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. FastStats – Body Measurments (U.S.) 2012 Available: http://www.cdc.gov/nchs/fastats/bodymeas.htm [accessed 4/4/14 2014]
- Daniels AU. Silicone breast implant materials. Swiss Med Wkly. 2012;142:w13614. doi: 10.4414/smw.2012.13614. [DOI] [PubMed] [Google Scholar]
- Deapen DM, Hirsch EM, Brody GS. Cancer risk among Los Angeles women with cosmetic breast implants. Plast Reconstr Surg. 2007;119(7):1987–1992. doi: 10.1097/01.prs.0000260582.23971.02. [DOI] [PubMed] [Google Scholar]
- Falck F, Ricci A, Wolff MS, Godbold J, Deckers P. Pesticides and polychlorinated biphenyl residues in human breast lipids and their relation to breast-cancer. Arch Environ Health. 1992;47(2):143–146. [PubMed] [Google Scholar]
- Forsberg ND, Wilson GR, Anderson KA. Determination of Parent and Substituted Polycyclic Aromatic Hydrocarbons in High-Fat Salmon Using a Modified QuEChERS Extraction, Dispersive SPE and GC-MS. J Agric Food Chem. 2011;59(15):8108–8116. doi: 10.1021/jf201745a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis S, Holmich LR, McLaughlin JK, Kjoller K, Fryzek JP, Henriksen TF, et al. Cancer risk among Danish women with cosmetic breast implants. Int J Cancer. 2006;118(4):998–1003. doi: 10.1002/ijc.21433. [DOI] [PubMed] [Google Scholar]
- Huckins JN, Petty JD, Lebo JA, Almeida FV, Booij K, Alvarez DA, et al. Development of the permeability/performance reference compound approach for in situ calibration of semipermeable membrane devices. Environ Sci Technol. 2002;36(1):85–91. doi: 10.1021/es010991w. [DOI] [PubMed] [Google Scholar]
- Hunnego JN, Harrison DL. Metabolism of DDE, DDD, and DDT in sheep. New Zealand Journal of Agricultural Research. 1971;14(2):406–416. [Google Scholar]
- IARC. Anthraquinone. Monographs on the Evaluation of Cacinogenic Risks to Humans. 2012;101:41–70. [Google Scholar]
- IUPAC. Compendium of Chemical Terminology. 2nd. the “Gold Book”; 1997. Available: http://goldbook.iupac.org/D01817.html [accessed August 28th 2015] [Google Scholar]
- Jahnke A, Mayer P. Do complex matrices modify the sorptive properties of polydimethylsiloxane (PDMS) for non-polar organic chemicals? J Chromatogr A. 2010;1217(29):4765–4770. doi: 10.1016/j.chroma.2010.05.046. [DOI] [PubMed] [Google Scholar]
- Jahnke A, Mayer P, Broman D, McLachlan MS. Possibilities and limitations of equilibrium sampling using polydimethylsiloxane in fish tissue. Chemosphere. 2009;77(6):764–770. doi: 10.1016/j.chemosphere.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Jahnke A, McLachlan MS, Mayer P. Equilibrium sampling: Partitioning of organochlorine compounds from lipids into polydimethylsiloxane. Chemosphere. 2008;73(10):1575–1581. doi: 10.1016/j.chemosphere.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Johansson B. Brown Fat: A Review. Metabolism. 1959;8(3):221–240. [PubMed] [Google Scholar]
- Lipworth L, Tarone RE, Friis S, Ye WM, Olsen JH, Nyren O, et al. Cancer among Scandinavian women with cosmetic breast implants: A pooled long-term follow-up study. Int J Cancer. 2009;124(2):490–493. doi: 10.1002/ijc.23932. [DOI] [PubMed] [Google Scholar]
- Malarvannan G, Dirinck E, Dirtu AC, Pereira-Fernandes A, Neels H, Jorens PG, et al. Distribution of persistent organic pollutants in two different fat compartments from obese individuals. Environ Int. 2013;55(0):33–42. doi: 10.1016/j.envint.2013.02.012. [DOI] [PubMed] [Google Scholar]
- McLaughlin JK, Fryzek JP, Ye WM, Tarone RE, Nyren O. Long-term cancer risk among Swedish women with cosmetic breast implants: An update of a nationwide study. J Natl Cancer Inst. 2006;98(8):557–560. doi: 10.1093/jnci/djj134. [DOI] [PubMed] [Google Scholar]
- Mentor Worldwide. MENTOR® Volume Sizing System. 2013 Available: http://www.mentorwwllc.com/global-us/Breast.aspx [accessed April 4th 2014]
- Musteata FM, Pawliszyn J. In vivo sampling with solid phase microextraction. J Biochem Biophys Methods. 2007;70(2):181–193. doi: 10.1016/j.jbbm.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Namieśnik J, Zabiegała B, Kot-Wasik A, Partyka M, Wasik A. Passive sampling and/or extraction techniques in environmental analysis: a review. Anal Bioanal Chem. 2005;381(2):279–301. doi: 10.1007/s00216-004-2830-8. [DOI] [PubMed] [Google Scholar]
- NLM. TOXNET – Databases on toxicology, hazardous chemicals, environmental health, and toxic releases. 1993 Available: http://toxnet.nlm.nih.gov/ [accessed January 7 2015]
- O’Connell SG, Haigh T, Wilson G, Anderson KA. An Analytical Investigation of 24 Oxygenated-PAHs (OPAHs) using Liquid and Gas Chromatography-Mass Spectroscopy. Anal Bioanal Chem. 2013;405(27):8885–8896. doi: 10.1007/s00216-013-7319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell SG, Kincl LD, Anderson KA. Silicone Wristbands as Personal Passive Samplers. Environ Sci Technol. 2014a;48(6):3327–3335. doi: 10.1021/es405022f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell SG, McCartney M, Allan SE, Paulik LB, Tidwell LG, Wilson G, et al. Improvements in Pollutant Monitoring: Optimizing Silicone for Co-deployment with Polyethylene Passive Sampling Devices. Environ Pollut. 2014b;193:71–78. doi: 10.1016/j.envpol.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada A, Niwa Y, Katase T, Kai O. Controlled release of estradiol-17β and bisphenol A from a silicone tube for long-term administration in mice. Animal Science Journal. 2005;76(6):535–539. [Google Scholar]
- Okada A, Niwa Y, Katase T, Kai O. Release of 4-Nonylphenol from a Silicone Tube Implanted in Mice. Exp Anim. 2009;58(5):533–536. doi: 10.1538/expanim.58.533. [DOI] [PubMed] [Google Scholar]
- Patterson DG, Furst P, Henderson LO, Isaacs SG, Alexander LR, Turner WE, et al. Partitioning of in vivo bound PCDDs/PCDFs amoung various compartments in whole-blood. Chemosphere. 1989;19(1–6):135–142. [Google Scholar]
- Rusina TP, Smedes F, Klanova J, Booij K, Holoubek I. Polymer selection for passive sampling: A comparison of critical properties. Chemosphere. 2007;68(7):1344–1351. doi: 10.1016/j.chemosphere.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Seethapathy S, Gorecki T. Applications of polydimethylsiloxane in analytical chemistry: A review. Anal Chim Acta. 2012;750:48–62. doi: 10.1016/j.aca.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Seethapathy S, Górecki T, Li X. Passive sampling in environmental analysis. J Chromatogr A. 2008;1184(1–2):234–253. doi: 10.1016/j.chroma.2007.07.070. [DOI] [PubMed] [Google Scholar]
- Somogyi LP. Caffeine intake by the U.S. Population. The Food and Drug Administration; 2010. pp. 1–85. [Google Scholar]
- Spencer WA, Dempster G. The lipids of mouse brown fat. Canadian Journal of Biochemistry and Physiology. 1962;40(12):1705–1715. [PubMed] [Google Scholar]
- van der Heijden SA, Jonker MTO. Intra- and Interspecies Variation in Bioconcentration Potential of Polychlorinated Biphenyls: Are All Lipids Equal? Environ Sci Technol. 2011;45(24):10408–10414. doi: 10.1021/es2022158. [DOI] [PubMed] [Google Scholar]
- Villeneuve PJ, Holowaty EJ, Brisson J, Xie L, Ugnat AM, Latulippe L, et al. Mortality among Canadian women with cosmetic breast implants. Am J Epidemiol. 2006;164(4):334–341. doi: 10.1093/aje/kwj214. [DOI] [PubMed] [Google Scholar]
- Vrana B, Allan IJ, Greenwood R, Mills GA, Dominiak E, Svensson K, et al. Passive sampling techniques for monitoring pollutants in water. TrAC Trends in Analytical Chemistry. 2005;24(10):845–868. [Google Scholar]
- Waliszewski SM, Caba M, Diaz SSR, Saldarriaga-Norena H, Meza E, Zepeda R, et al. Levels of Organochlorine Pesticides Residues in Human Adipose Tissue, Data from Tabasco, Mexico. Bull Environ Contam Toxicol. 2012;89(5):1062–1067. doi: 10.1007/s00128-012-0803-8. [DOI] [PubMed] [Google Scholar]
- Zabiegala B, Kot-Wasik A, Urbanowicz M, Namiesnik J. Passive sampling as a tool for obtaining reliable analytical information in environmental quality monitoring. Anal Bioanal Chem. 2010;396(1):273–296. doi: 10.1007/s00216-009-3244-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


