Abstract
Mice deficient in the Polycomb repressor Bmi1 develop numerous abnormalities including a severe defect in stem cell self-renewal, alterations in thymocyte maturation and a shortened lifespan. Previous work has implicated de-repression of the Ink4a/Arf (also known as Cdkn2a) locus as mediating many of the aspects of the Bmi1–/– phenotype. Here we demonstrate that cells derived from Bmi1–/– mice also have impaired mitochondrial function, a marked increase in the intracellular levels of reactive oxygen species and subsequent engagement of the DNA damage response pathway. Furthermore, many of the deficiencies normally observed in Bmi1–/– mice improve after either pharmacological treatment with the antioxidant N-acetylcysteine or genetic disruption of the DNA damage response pathway by Chk2 (also known as Chek2) deletion. These results demonstrate that Bmi1 has an unexpected role in maintaining mitochondrial function and redox homeostasis and indicate that the Polycomb family of proteins can coordinately regulate cellular metabolism with stem and progenitor cell function.
Bmi1 is a member of the Polycomb family of transcriptional repressors that mediate gene silencing by regulating chromatin structure and is essential for the maintenance and self-renewal of both haematopoietic and neural stem cells1,2. Mice deficient in Bmi1 have a number of defects including severe neurological abnormalities, alterations in various haematopoietic cell lineages, a generalized failure-to-thrive and a markedly shortened lifespan. Previous studies have documented that approximately 50% of the knockout mice die before the completion of weaning and the remaining 50% succumb anywhere from 3 to 20 weeks of age3. In an effort to understand how the effects of Bmi1 are mediated, significant attention has been placed on the repression of the Ink4a/Arf locus, which encodes two separate tumour suppressors and cell cycle regulators, p16Ink4a and p19Arf (refs 4–7). The importance of the transcriptional repression of the Ink4a/Arf locus is underscored by the observation that mice deficient in Bmi1 and also lacking p16Ink4a, p19Arf or both of these gene products develop less severe neurological and haematological abnormalities than Bmi1–/– animals4–6.
Although improved, mice lacking Bmi1 as well as Ink4a/Arf remain significantly smaller than wild-type littermates and their overall survival is similar to that of Bmi1–/– mice6. In addition, examination of the number of peripheral nucleated thymocytes or splenocytes, as well as the cellular composition of the bone marrow microenvironment itself, remains significantly altered in combined Bmi1/Ink4a/Arf-deficient mice8. These results therefore indicate that, besides repression of the Ink4a/Arf locus, additional Bmi1-regulated pathways undoubtedly exist. Here we demonstrate that Bmi1 can separately regulate mitochondrial function, reactive oxygen species (ROS) levels and the activation of the DNA damage response (DDR) pathway.
Bmi1 regulates ROS levels
Mice deficient in either the ataxia telangiectasia mutated (Atm) gene product or the FOXO family of transcription factors demonstrate a rapid postnatal decline in haematopoietic stem cell (HSC) number that in each case is associated with a rise in ROS levels within the c-KIT1Sca-11Lin– (LSK) bone marrow cell population that includes HSCs9,10. We therefore sought to test whether a similar rise in ROS levels was evident in Bmi1–/– cells. Assessment of unfractionated bone marrow, purified LSK cells and bone marrow cells expressing SLAM family receptors previously shown to allow for enrichment of long-term HSC cells (LT-HSCs)11 demonstrated that the absence of Bmi1–/– resulted in increased levels of ROS (Fig. 1a and Supplementary Fig. 1). A similar increase in ROS levels was also evident in various other cell types known to be impaired in Bmi1–/– mice, including freshly isolated thymocytes (Fig. 1b and Supplementary Fig. 1).
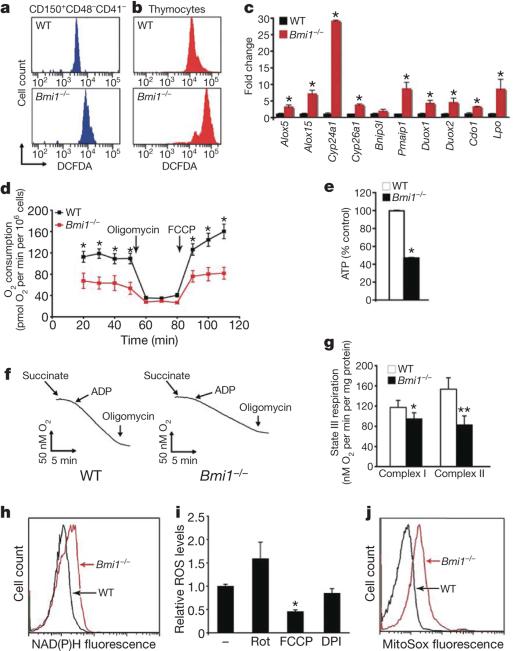
Figure 1. The absence of Bmi1 increases ROS levels and alters mitochondrial function.
a, Levels of ROS as assessed by DCFDA fluorescence in purified wild-type (WT) or Bmi1–/– bone marrow cells. b, ROS levels in freshly isolated thymocytes. c, Quantitative rtPCR expression analysis of gene products involved in redox homeostasis in either WT or Bmi1–/– thymocytes. Results are normalized to Gapdh expression (n = 3 animals per group, *P < 0.05). d, Oxygen consumption in intact thymocytes under basal conditions, following the addition of the mitochondrial inhibitor oligomycin (0.5 μg ml–1) or in the presence of the uncoupler FCCP (1 μM). Cells were obtained from n = 4 animals per genotype; *P < 0.02. e, Relative ATP levels in freshly isolated thymocytes. Measurements were made in triplicate (mean and s.d.) with 4 animals per group; *P < 0.01. f, Representative oxygen consumption from isolated heart mitochondria. g, Measured state III respiration for either complex-I- or complex-II-dependent respiration (mean and s.d.; n = 4 animals per group; *P < 0.05, **P < 0.01). h, NAD(P)H levels as assessed by endogenous fluorescence in WT and Bmi1–/– thymocytes. i, ROS levels in Bmi1–/– thymocytes in the presence or absence of the complex I inhibitor rotenone (Rot), the chemical uncoupler FCCP or the NADPH oxidase inhibitor DPI (mean and s.d.; n = 3; *P < 0.05). j, Analysis of thymocytes using the redox fluorophore MitoSox Red.
In an effort to explain this observed rise in ROS, we made use of several previous gene expression studies that have identified a multitude of Polycomb target genes1,12,13. Among these identified targets were numerous genes either involved directly in ROS generation or that localize to the mitochondria and are known to affect mitochondrial function. Given the scarcity of LT-HSCs in Bmi1–/– mice, we chose to analyse gene expression in Bmi1–/– thymocytes, because previous reports have demonstrated a marked perturbation of this cell population in Bmi1-deficient mice3,4 and our data indicated that these cells had marked differences in ROS levels. As noted in Fig. 1c, Bmi1–/– thymoctyes de-repressed a number of previously identified Polycomb-regulated gene products that can regulate intracellular redox homeostasis. Given that mitochondria can produce oxidants and are in turn quite sensitive to their damaging effects14, we sought to analyse the mitochondrial function of Bmi1–/– cells. Intact Bmi1–/– thymocytes had both reduced basal mitochondrial oxygen consumption and reduced mitochondrial oxidative capacity (Fig. 1d). Basal ATP levels were also significantly reduced in Bmi1–/– thymocytes (Fig. 1e) as well as in other tissues (Supplementary Fig. 2). The observed differences in mitochondrial respiration and intracellular energetics were not however accompanied by any obvious differences in mitochondrial number, structure, bio-genesis or rate of degradation (Supplementary Fig. 3).
Consistent with our intact cellular respiration measurements, we observed significant impairment in the function of purified Bmi1–/– mitochondria (Fig. 1f, g). The interruption of flow down the electron transport chain (ETC) evident in Bmi1–/– mitochondria is believed to be a major source for ROS generation. Impaired electron flow increases the likelihood of superoxide formation and is often accompanied by a build-up of mitochondrial reducing equivalents (for example, NADH). Consistent with such a mechanism, NAD(P)H levels were increased in Bmi1–/– thymocytes (Fig. 1h). To substantiate further the connection between Polycomb activity, mitochondrial function and oxidant stress, we next measured ROS levels in the setting of various inhibitors of mitochondrial function. As expected, using the complex I inhibitor rotenone to reduce electron flow increased ROS levels (Fig. 1i). In contrast, treatment with the pharmacological uncoupler FCCP, which specifically lowers mitochondrial membrane potential and hence augments ETC flux, markedly reduced ROS levels in Bmi1–/–cells. Consistent with the observed rise in Duox1 and Duox2 expression (Fig. 1c), we also observed a small but non-significant decrease in ROS levels after treatment with the NADPH oxidase inhibitor DPI (Fig. 1i). Finally, the use of the mitochondrial redox fluorophore MitoSox Red provided additional support for the hypothesis that the mitochondria are the major source of increased ROS levels observed in Bmi1–/– cells (Fig. 1j and Supplementary Fig. 3).
Antioxidants can rescue Bmi1–/– mice
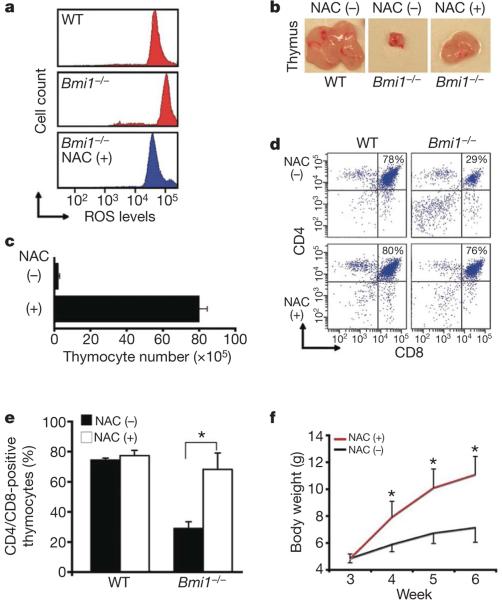
We next sought to assess whether the increase in ROS levels might contribute to the various in vivo phenotypic defects observed in Bmi1–/– mice. We randomizedfour-week-old Bmi1–/– mice to treatment with and without the antioxidant scavenger N-acetylcysteine (NAC). After one week of treatment, ROS levels in Bmi1–/– thymocytes had been reduced to near wild-type levels (Fig. 2a). Coincident with this reduction, we noted a marked increase in the overall size of the thymus in the antioxidant-treated Bmi1–/– mice (Fig. 2b). This increase in thymus size was also accompanied by a significant increase in the overall number of total thymocytes in the antioxidant-treated Bmi1–/– animals, although Bmi1–/– mice treated with NAC still had reduced number of thymocytes when compared to wild-type animals (Fig. 2c). Previous studies have also demonstrated that Bmi1–/– mice also have defects in thymocyte maturation3,4. As noted in Fig. 2d, approximately 80% of cells isolated from wild-type mice were CD4+CD8+ double-positive thymocytes. A one-week administration of NAC to wild-type mice did not appreciably alter this distribution. In contrast, the frequency of CD4+CD8+ double-positive thymocytes observed in untreated Bmi1–/– mice was markedly reduced, yet normalized after NAC administration (Fig. 2d, e). Finally, NAC administration had effects beyond altering thymocyte function, because this intervention also appeared to partially correct the failure-to-thrive phenotype seen in Bmi1–/– animals (Fig. 2f).
Figure 2. Antioxidant treatment rescues Bmi1–/– thymocytes.
a, ROS in thymocytes isolated from four-week-old wild-type (WT) mice or Bmi1–/– mice randomized to NAC treatment for one week before collection. b, Overall thymus size in 4-week-old mice either treated for one week with NAC (+) of left untreated (–). c, Total number of thymocytes recovered in 4-week-old Bmi1–/– mice either treated for one week with NAC or left untreated (mean and s.d.; n = 4 animals per group). d, Representative assessment of thymocyte maturation (CD4+CD8+ cells) in 4-week-old WT or Bmi1–/– mice that were treated with NAC for one week or left untreated before collection. e, Quantitative assessment of thymocyte maturation in 4-week-old mice randomized for one week of antioxidant treatment (mean and s.d.; n = 4 animals per group). f, Weight of Bmi1–/– mice with or without antioxidant treatment beginning after weaning (mean and s.d.; n = 5–7 animals per group). *P < 0.05.
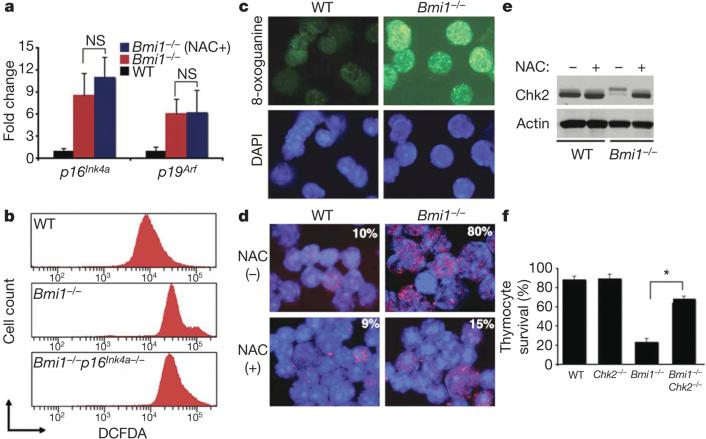
Quantitative real-time polymerase chain reaction (rtPCR) analysis demonstrated that the improvement seen in Bmi1–/– mice after antioxidant treatment was not accompanied by any apparent alteration in Ink4a/Arf transcription (Fig. 3a). Thus, the Bmi1-mediated rise in ROS does not seem to be required for the subsequent induction of Ink4a/Arf. There is evidence that, in certain situations, a rise in p16Ink4a expression can be associated with, and appears to be required for, a subsequent increase in oxidant levels15. Thus, it seemed possible that NAC was rescuing Bmi1–/– mice by scavenging oxidants that were produced as a result of p16Ink4a expression. To test this possibility formally, we analysed the levels of ROS in thymocytes obtained from Bmi1–/– mice or from mice doubly deficient in both Bmi1 and p16Ink4a (Fig. 3b and Supplementary Fig. 4). The induction of p16Ink4a does not seem to be required for the rise in ROS levels observed in Bmi1–/– cells.
Figure 3. Activation of the DDR pathway in Bmi1–/– thymocytes occurs through a redox-sensitive pathway.
a, Quantitative rtPCR analysis of Ink4a/Arf expression in thymocytes obtained from mice randomized to antioxidant therapy (mean and s.d.; n = 3 animals per group). NS, not significant. b, ROS levels in thymocytes obtained from WT, Bmi1–/– or combined Bmi1/p16Ink4a-deleted mice. c, Levels of the oxidatively modified nucleotide 8-oxoguanine in isolated thymocytes. DAPI (4,6-diamidino-2-phenylindole) staining (blue) was used to visualize nuclei. d, 53BP1 nuclear foci thymocytes obtained from mice treated for one week before collection with the antioxidant NAC or left untreated. Overall percentage of thymocytes demonstrating activation of the DDR in each condition is shown. e, Western blot analysis for Chk2 activation in thymocytes obtained from WT or Bmi1–/– mice. f, Assessment of primary thymocyte survival in culture 24 h after isolation.
Bmi1 regulates the DDR pathway
To understand which other pathways might be activated by the observed increase in ROS levels, we took advantage of previous observations demonstrating that oxidative stress can trigger activation of the DDR pathway16. As noted in Fig. 3c, Bmi1–/– thymocytes had increased levels of 8-oxoguanine, a stable marker of oxidatively damaged DNA. In addition, consistent with ongoing DNA damage, we observed increased nuclear foci of 53BP1 (also known as Trp53bp1), another known hallmark of DNA damage and DDR activation17. In Bmi1–/– mice treated with NAC for one week before collection, the number of 53BP1 nuclear foci was markedly reduced (Fig. 3d). Other DDR components including Chk2 were also activated in Bmi1–/– cells, and again this activation was reduced by antioxidant treatment (Fig. 3e). These results indicate that the sustained levels of ROS seen in Bmi1–/– mice are sufficient to damage DNA directly and to engage the DDR pathway. To underscore this point further, we observed that treatment of thymocytes with exogenous hydrogen peroxide could activate the DDR pathway and that cells obtained from Chk2–/– mice were largely protected from ROS-induced cell death (Supplementary Fig. 5). These results indicated that interruption of the DDR pathway by deletion of Chk2 may provide some benefit to the oxidatively stressed Bmi1–/– cells and tissues. To begin to address this hypothesis formally, we assessed the survival in culture of wild-type, Chk2–/–, Bmi1–/– or combined Bmi1–/–Chk2–/– thymocytes. Whereas Bmi1–/– thymocytes rapidly lost viability in culture, the survival of thymocytes lacking both Bmi1 and Chk2 was similar to that in wild-type cells (Fig. 3f). A similar rescue of Bmi1–/– thymocytes could also be obtained by supplementing the media with NAC (Supplementary Fig. 6).
Chk2 deletion rescues Bmi1–/– mice
To understand further how the activation of the DDR pathway might contribute to the overall Bmi1–/– phenotype, we asked whether mice lacking both Bmi1 and Chk2 demonstrated any phenotypic improvement compared to Bmi1–/– mice. Previous results have demonstrated that, whereas Chk2-deficient cells have an increase in radioresistance, Chk2–/– mice do not exhibit a high rate of spontaneous tumour formation and appear, for the most part, to be phenotypically identical to wild-type mice18,19. We first examined in vivo thymocyte maturation in either Bmi1–/– or combined Bmi1–/–Chk2–/– animals. As previously noted with antioxidant treatment, the overall size of the thymus, as well as the spleen, was significantly larger in the Bmi1–/–Chk2–/– mice than in Bmi1–/–-only mice (Supplementary Fig. 7). Similarly, the previously observed reduction in the number of CD4+CD8+ double-positive thymocytes in Bmi1–/– mice was significantly less marked in mice lacking both Bmi1 and Chk2 (Fig. 4a). We also noted significant alterations in the overall architecture of the thymus. In contrast to wild-type mice, Bmi1–/– mice lacked a distinct separation between thymic cortex and medulla (Fig. 4b). A similar change in overall thymus architecture has been noted in other animal models with impaired thymocyte maturation20,21. In contrast, a near normal compartmentalization of thymic cortex and medulla was observed in the Bmi1–/–Chk2–/– mice (Fig. 4b). Given that Chk2 deletion seems to protect thymocytes from ROS-mediated cell death (Fig. 3f and Supplementary Fig. 5), we postulated that the absence of Chk2 could be rescuing thymocyte number and maturation by inhibiting apoptosis. Consistent with this notion, levels of apoptosis were increased in the cortex of Bmi1–/– compared to wild-type animals, whereas this level of overall apoptosis was reduced in animals deficient in both Bmi1 and Chk2 (Fig. 4c). As previously observed with antioxidant treatment, the improvement observed in the Bmi1–/–Chk2–/– mice did not seem to be the result of alterations in the induction of the Ink4a/Arf locus (Fig. 4d and Supplementary Fig. 8). Similarly, deletion of Chk2 did not directly alter the level of ROS induced by the absence of Bmi1 expression (Supplementary Fig. 9). Interestingly, the combination of NAC treatment and Chk2 deletion was slightly more effective at rescuing Bmi1–/– thymocyte number and maturation than Chk2 deletion alone, indicating that some of the effects of ROS may be independent of the DDR response (Supplementary Fig. 10).
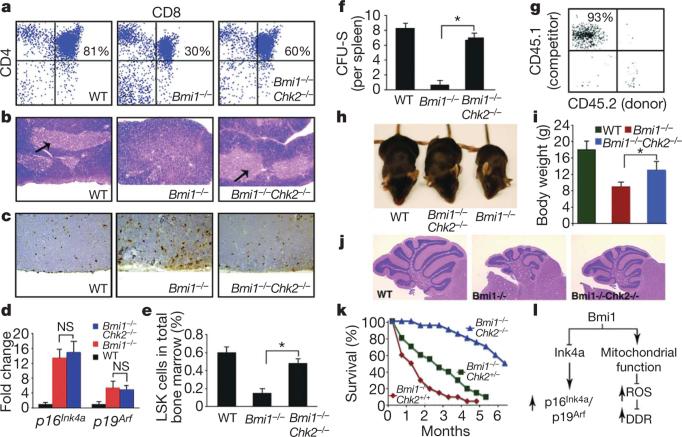
Figure 4. Inhibition of the DDR pathway by Chk2 deletion rescues multiple defects in Bmi1–/– mice.
a, Chk2 deletion restores in vivo thymocyte maturation of Bmi1–/– mice. WT, wild type. b, Architecture of the thymus in WT, Bmi1–/– and combined Bmi1–/–Chk2–/– mice. The arrows point to a normal medulla region. c, Representative TUNEL staining in the cortex region of the thymus. d, Quantitative rtPCR analysis of Ink4a/Arf induction in Bmi1–/– or combined Bmi1–/–Chk2–/– thymocytes (mean and s.d.; n = 3 animals per group). e, Frequency of LSK cells intotal bonemarrow (*P < 0.01; mean and s.d., n = 6 animals per group). f, In vivo CFU-S in lethally irradiated mice infused with equal numbers of bone marrow cells obtained from WT, Bmi1–/– or combined Bmi1–/–Chk2–/– mice (*P < 0.01; mean and s.d., n = 5 animals per group). g, Peripheral blood composition four months after competitive repopulation in which equal amounts of WT competitor bone marrow cells (CD45.1) and combined Bmi1–/–Chk2–/– donor bone marrow (CD45.2) were transplanted into an irradiated host (CD45.2). We observed little to no contribution of the donor-derived Bmi1–/–Chk2–/– cells after such competitive repopulation experiments. h, Representative appearance of WT, Bmi1–/– or combined Bmi1–/–Chk2–/– mice. i, Analysis of body weight at 6 weeks of age (*P < 0.01; mean and s.d.; n = 6 per group). j, Defects in cerebellar architecture observed in Bmi1–/– mice are rescued by Chk2 deletion. k, Lifespan of Bmi1–/– mice with varying Chk2 status. P < 0.05 Chk2+/– versus Chk2+/+ and P < 0.001 for Chk2–/– versus Chk2+/+; n ≥ 15 animals per genotype. l, The Polycomb protein Bmi1 normally simultaneously represses the Ink4a/Arf locus leading to reduced p16Ink4a and p19Arf expression as well as modulating mitochondrial function to lower ROS levels and suppress activation of the DDR pathway. Activation of both Ink4a/Arf and the DDR have been separately linked to tumour suppression and stem cell ageing.
We next assessed whether the absence of Chk2 could also improve the number or functional capacity of Bmi1–/– haematopoietic stem or progenitor cells. To begin to address this issue, we measured the number of LSK cells in either Bmi1–/– or combined Bmi1–/–Chk2–/– mice. This cell population is generally believed to contain abundant progenitor cells, as well as rarer, long-term repopulating cells. As noted in Fig. 4e, the number of LSK cells in mice lacking both Bmi1 and Chk2 was significantly higher than that seen in Bmi1–/– animals. We next sought to assess the functional capacity of the progenitor cells isolated from Bmi1–/–Chk2–/– mice. Bone marrow cells from Bmi1–/– mice were markedly impaired in their in vitro colony-forming ability whereas the colony-forming capacity of Bmi1–/–Chk2–/– bone marrow cells was significantly improved (Supplementary Fig. 11). In a further in vivo test of progenitor function, we measured spleen colony-forming units (CFU-S) that formed after injection of total bone marrow (1 × 105 cells) into irradiated hosts. Again, using this in vivo test of progenitor cell function, we noted a marked impairment of Bmi1–/– bone marrow that was significantly less pronounced in the Bmi1–/–Chk2–/– mice (Fig. 4f).
Although these results indicate that Chk2 deletion results in improvement in the number and function of Bmi1–/– progenitor cells, they do not address whetherthere is also a concomitant improvement in long-term repopulating ability. To test this, we performed competitive repopulation studies using wild-type, Bmi1–/–, Bmi1+/– or combined Bmi1–/–Chk2–/– bone marrow. As previously reported1,8, consistent with a defect in stem cell maintenance and self-renewal, we observed that Bmi1–/– bone marrow was unable to contribute in such long-term repopulation assays (Supplementary Fig. 12). We also observed essentially no contribution to long-term repopulation from the Bmi1–/–Chk2–/– bone marrow (Fig. 4g and Supplementary Fig. 12). We therefore conclude that, whereas Bmi1–/–Chk2–/– mice have an increased number of LSK cells and an apparent improvement in progenitor cell function, deletion of Chk2 does not rescue the self-renewal defect seen in Bmi1–/– mice.
The improvement in Bmi1–/– mice that was observed by concomitant deletion of Chk2 extended beyond thymocytes and haematopoietic progenitors. Indeed, the overall appearance of these doubly deficient mice was improved compared to the Bmi1–/– animals (Fig. 4h). This was also accompanied by a significant increase in overall body weight (Fig. 4i). In addition, whereas the Bmi1–/– mice had a severe ataxia, this phenotype also appeared to be mitigated in the Bmi1–/–Chk2–/– animals. Consistent with such improvement, the cerebellum of the double-deficient mice showed a marked increase in cellularity and was more developed than what was observed in Bmi1–/– mice (Fig. 4j). Similarly, whereas Bmi1–/– mice were infertile, both male and female combined Bmi1–/–Chk2–/– mice were capable of generating viable offspring. In contrast, the deletion of Chk2 did not rescue previously described changes in the axial skeleton, because we observed that approximately 80% of the Bmi1–/–Chk2–/– mice had 6 as opposed to the normal 7 vertebrosternal ribs3.
Finally, we noted a number of progeriod features in the Bmi1–/– mice that were largely absent in age-matched Bmi1–/–Chk2–/– mice (Supplementary Fig. 13). On the basis of these observations, we asked whether the absence of Chk2 could extend the median and maximum lifespan of animals lacking Bmi1. In our colony, the median survival of Bmi1–/– mice was roughly 1 month and essentially all mice succumbed by 3–4 months (Fig. 4k). Deletion of one copy of Chk2 provided a survival advantage, whereas deletion of both copies of Chk2 was even more effective and extended the median survival of the Bmi1–/–Chk2–/– mice to approximately 6 months.
Conclusions
We demonstrate that the absence of Bmi1 leads to increased expression of a collection of gene products involved in mitochondrial function and ROS homeostasis. Cells lacking Bmi1 have significant mitochondrial dysfunction accompanied by a sustained increase in ROS that is sufficient to engage the DDR pathway. Treatment with an antioxidant or interruption of the DDR by Chk2 deletion substantially improves some, but not all, aspects of the Bmi1–/– mice. Our observations further indicate that, in Bmi1–/– mice, ROS act independently of the Ink4a/Arf pathway. In addition, although in the context of the Bmi1–/– mouse deletion of the Ink4a/Arf locus results in substantial rescue of the LT-HSC defect, it is interesting to note that the beneficial effects of Chk2 deletion are exclusively confined to very early haematopoietic progenitor cells.
Activation of the DDR response has been invoked as a natural consequence of age-associated damage. For instance, when compared to younger HSCs, purified HSCs from old mice demonstrate increased levels of γ-H2AX nuclear foci—a marker of DNA damage22. Although such old HSCs do not decline in absolute number, the activation of the DDR does correlate with impairment of stem cell function. Similarly, in a number of mouse genetic models in which there are inherited defects in various components of the DNA repair apparatus, there is also evidence for a reduction in functional capacity of transplanted HSCs23,24. In addition, recent evidence indicates that the self-renewal of cancer stem cells can also be limited by oncogene-induced DNA damage25. In other animal models, the age-dependent increase in p16Ink4a expression has been linked to age-dependent decline in stem cell function26. Furthermore, in several independent models, deletion of p16Ink4a leads to improved functional capacity of neural, pancreatic and haematopoietic stem cells27–29. Thus, both the DDR pathway and a rise in p16Ink4a have been linked to stem and progenitor cell ageing and dysfunction. Outside of the stem and progenitor compartment, both activation of the Ink4a/Arf locus and engagement of the DDR pathway have also been separately implicated in mediating cellular senescence, growth arrest and/or cell death induced by various stresses30,31. Activation of these two pathways has been invoked as an important tumour suppressor barrier, because both pathways act as potent inhibitors of the proliferation or propagation of damaged cells. Interestingly, our data indicate that the absence of Bmi1 results in the simultaneous engagement of both of these stress-activated and tumour-suppressive pathways and that both of these pathways appear to separately contribute to the overall phenotype of the Bmi1–/– mice (Fig. 4l). Furthermore, the necessity of stem cells to persist throughout the entire lifespan of the organism indicates that these cells might have particularly stringent requirements to limit ROS production. Our results imply that the Polycomb family of proteins might be uniquely positioned to coordinately regulate mitochondrial function and redox homeostasis with overall stem cell biology.
METHODS
Cells and mice
Bmi1+/– and Chk2+/– mice have been described previously3,18. Bmi1+/– C57BL/6 mice and Chk2+/–mice on a mixed 129/Black Swiss genetic background were crossed to generate Bmi1+/+ and Bmi1–/– mice with various Chk2 status. Littermate controls were used for analysis and all mice were geno-typed routinely by PCR with mouse tail DNA. Except where indicated, histological and biological analysis was performed for both male and female mice at 1–3 months of age. Mice deleted specifically for p16Ink4a have been described35 and were obtained from the National Cancer Institute (MMHCC repository, Fredrick MD). For the in vivo administration of NAC, we randomized animals to normal drinking water, or water containing NAC at 1 mg ml–1 as previously described10. In addition, owing to the overall frailty of Bmi1–/– mice, the food of animals treated or untreated with antioxidant was soaked in NAC-treated or -untreated drinking water and placed inside the cage.
Intracellular ROS analysis
Total bone marrow cells, LSK cells and cells stained for SLAM family members (CD150 and CD48; BioLegend) as well as CD41 (BioLegend) were isolated as previously described11,36. In general, thymocytes and splenocytes were isolated from 6–8-week-old mice and were plated at an initial density of 1 × 106 cells per ml in Dulbecco Modified Essential Medium (DMEM) supplemented with 10% fetal bovine serum. Thymocyte populations were analysed where indicated by CD4 and CD8 staining (BD Biosciences). For analysis of intracellular ROS, bone marrow cells enriched for LT-HSCs, thymocytes and other cell types were incubated with 5 μM DCFDA (Invitrogen) and placed in a shaker at 37 °C for 30 min, followed immediately by flow cytometry analysis using an LSRII instrument (Becton-Dickinson). Viable cells (1 × 106) as determined by 7-AAD staining were used for the analysis of ROS levels. Where indicated, cells were treated with either rotenone, carbonyl cyanide p-trifluor-methoxyphenylhydrazone (FCCP) or diphenyleneiodonium chloride (DPI) for 10 min before assessment of ROS levels.
Mitochondrial isolation, functional studies and ATP determination
For isolation of functional mitochondria from mouse tissues, we rapidly collected, washed and minced mouse hearts in ice-cold Isolation Buffer I containing 210 mM mannitol, 70 mM sucrose, 5 mM HEPES (pH 7.4), 1 mM EGTA and 0.5 mg ml–1 BSA. The hearts were then homogenized in this buffer with a glass-Teflon motorized homogenizer. The mitochondrial fraction was isolated by differential centrifugation and subsequently washed twice before resuspension in Isolation Buffer I at a concentration of 0.5 mg ml–1 before functional assessment.
Mitochondrial respiration from intact mitochondria was measured by standard protocols using a respiration buffer containing 225 mM mannitol, 75 mM sucrose, 10 mM KCl, 10 mM Tris-HCl and 5 mM KH2PO4 at pH 7.2. Glutamate (5 mM), malate (5 mM) and ADP (1 mM) were used to assay respiration through complex I. Succinate (5 mM), rotenone (1 μM) and ADP (1 mM) were used to assay complex II respiration. Respiratory control ratios (RCRs; state 3/state 4) were determined after inhibition of mitochondrial ATPase with oligomycin. Measurement of intact cellular respiration was performed using the Seahorse XF24 analyser32. Respiration was measured under basal condition, in the presence of the mitochondrial inhibitor oligomycin (0.5 μM) and in the presence of the mitochondrial uncoupler FCCP (1 μM) to assess maximal oxidative capacity as we have described previously37.
ATP was measured using the ATP-determination kit (Molecular Probes). For in vivo measurements, mice tissues were rapidly collected and immediately placed in ice-cold ATP buffer (20 mM Tris, pH 7.5, 0.5% Nonidet P-40, 25 mM NaCl, 2.5 mM EDTA) for 5 min. Tissue samples then underwent one five-second round of sonication. Lysates were than centrifuged at 13,000g for 30 min and the supernatant measured for protein concentration. ATP concentration was measured from 0.2 μg of this protein lysate. We always measured ATP from freshly isolated (not frozen) tissues.
HSC cultures and colony-forming assays
CFU-GM in vitro assays were performed according to the manufacturer's instructions (StemCell Technologies). In brief, freshly isolated total bone marrow cells were cultured in semisolid MethoCult media supplemented with 10 ng ml–1 GM-CSF. Cells were plated in triplicate at an initial density of 2 × 104 cells per well of a 12-well plate. After 5–7 days of culture, CFU-GM colonies were assessed. The CFU-S assay was performed as previously described38. In brief, 1 × 105 bone marrow cells from five donors of each group (Bmi1–/–, combined Bmi1–/–Chk2–/– and wild-type controls) were transplanted into each of three 12-week-old normal B6 recipients pre-irradiated at 950 rad. Six B6 mice that received the same dose of irradiation and no subsequent bone marrow transplantation were used as negative controls. The spleens of all animals were fixed on day 12 after transplantation, and the total numbers of colonies per spleen were assessed.
For competitive repopulation experiments, lethally irradiated C57BJ/6 mice (B6-CD45.2) were competitively reconstituted with 1 × 106 total bone morrow cells from Bmi1+/+, Bmi1+/–, Bmi1–/– or combined Bmi1/Chk2-deficient mice. We used five recipients in each group and each recipient also received competitive repopulation with 1 × 106 bone marrow cells obtained from C57BJ/6 mice congenic for the CD45 locus (B6-CD45.1). Analysis was performed on a monthly basis after transplantation up to month four. Peripheral blood cells of the recipients were stained with antibodies against CD45.1, CD45.2, B220, CD3 and Mac-1 to monitor the reconstitution of both myeloid and lymphoid lineages.
Gene expression
Total RNA was isolated by using Trizol reagent and subsequently treated with DNase I (Invitrogen). Complementary DNA was prepared using Taqman reverse transcription reagents and oligo-dT primers. Quantitative rtPCR for Ink4a/Arf expression or for the expression of other Bmi1-regulated genes was performed with 50 ng cDNA on an MxP3005P real-time PCR system (Stratagene) using SYBR Green PCR Mastermix (Applied Biosystems) according to the manufacturer's instructions. For primer sequences, see Supplementary Information and Supplementary Methods.
Supplementary Material
Acknowledgements
We are grateful to M. Clarke for providing the initial supply of Bmi1–/– mice, to J. Moss for the gift of anti-PARP antibodies and to M. Daniels and the NHLBI electron microscope core for their assistance. This work was supported by funding from the NIH intramural program and the Ellison Medical Foundation.
Footnotes
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Park IK, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 2.Molofsky AV, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Lugt NM, et al. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 protooncogene. Genes Dev. 1994;8:757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 5.Bruggeman SW, et al. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev. 2005;19:1438–1443. doi: 10.1101/gad.1299305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bracken AP, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oguro H, et al. Differential impact of Ink4a and Arf on hematopoietic stem cells and their bone marrow microenvironment in Bmi1-deficient mice. J. Exp. Med. 2006;203:2247–2253. doi: 10.1084/jem.20052477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 11.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006;20:1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fasano CA, et al. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell. 2007;1:87–99. doi: 10.1016/j.stem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi A, et al. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nature Cell Biol. 2006;8:1291–1297. doi: 10.1038/ncb1491. [DOI] [PubMed] [Google Scholar]
- 16.Lombard DB, et al. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Adams MM, Carpenter PB. Tying the loose ends together in DNA double strand break repair with 53BP1. Cell Div. 2006;1:19. doi: 10.1186/1747-1028-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takai H, et al. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 2002;21:5195–5205. doi: 10.1093/emboj/cdf506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirao A, et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol. Cell. Biol. 2002;22:6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okada H, et al. Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J. Exp. Med. 2004;199:399–410. doi: 10.1084/jem.20032092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaugg K, et al. Cross-talk between Chk1 and Chk2 in double-mutant thymocytes. Proc. Natl Acad. Sci. USA. 2007;104:3805–3810. doi: 10.1073/pnas.0611584104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi DJ, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 23.Reese JS, Liu L, Gerson SL. Repopulating defect of mismatch repair-deficient hematopoietic stem cells. Blood. 2003;102:1626–1633. doi: 10.1182/blood-2002-10-3035. [DOI] [PubMed] [Google Scholar]
- 24.Nijnik A, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 25.Viale A, et al. Cell-cycle restriction limits DNA damage and maintains self-renewal of leukaemia stem cells. Nature. 2009;457:51–56. doi: 10.1038/nature07618. [DOI] [PubMed] [Google Scholar]
- 26.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 27.Janzen V, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 28.Molofsky AV, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnamurthy J, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 30.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 32.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov. Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Struthers L, Patel R, Clark J, Thomas S. Direct detection of 8-oxodeoxyguanosine and 8-oxoguanine by avidin and its analogues. Anal. Biochem. 1998;255:20–31. doi: 10.1006/abio.1997.2354. [DOI] [PubMed] [Google Scholar]
- 34.Neumann CA, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 35.Sharpless NE, et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 36.Smith AL, Ellison FM, McCoy JP, Jr, Chen J. c-Kit expression and stem cell factor-induced hematopoietic cell proliferation are up-regulated in aged B6D2F1 mice. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:448–456. doi: 10.1093/gerona/60.4.448. [DOI] [PubMed] [Google Scholar]
- 37.Schieke SM, et al. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J. Biol. Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- 38.TeKippe M, Harrison DE, Chen J. Expansion of hematopoietic stem cell phenotype and activity in Trp53-null mice. Exp. Hematol. 2003;31:521–527. doi: 10.1016/s0301-472x(03)00072-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.