Abstract
Metals are a common pollutant in the aquatic ecosystem. With global climate change, these levels are anticipated to rise as lower pH levels allow sediment bound metals to be released. The American alligator (Alligator mississippiensis) is an apex predator in the aquatic ecosystem and is considered a keystone species; as such it serves as a suitable monitor for localized pollution. One metal of increasing concern is hexavalent chromium (Cr(VI)). It is present in the aquatic environment and is a known human carcinogen and reproductive toxicant. We measured the cytotoxicity and genotoxicity of Cr(VI) in American alligator cells derived from scute tissue. We found that particulate and soluble Cr(VI) are both cytotoxic and genotoxic to alligator cells in a concentration-dependent manner. These data suggest that alligators may be used as a model for assessing the effects of environmental Cr(VI) contamination as well as for other metals of concern.
Keywords: American alligator, chromium, genotoxicity, cytotoxicity, aquatic model, hexavalent chromium
1. Introduction
The American alligator (Alligator mississippiensis) is a long lived, apex predator inhabiting primarily coastal areas of the southeastern US. Once listed as endangered due to overhunting, careful conservation efforts led to delisting in 1987. Localized populations of alligators are exposed to environmental contaminants through a variety of sources. Development in and near their ecosystem can lead to chemical and agricultural runoff. Since the alligator populations have successfully recovered after being listed as endangered in the 1970s, they can be used as a suitable monitor of environmental pollution (Delany et al., 1988).
Chromium (Cr) has recently been shown to be a metal of global concern (Wise, J. et al., 2009). Hexavalent chromium (Cr(VI)) is a known human carcinogen and can damage DNA and impair reproduction and development (Al-Hamood, et al., 1998, Bataineh et al., 1997, Chowdhury and Mitra, 1995, Holmes et al., 2008, IARC 1990, Mancuso 1997, Wise, S. et al., 2008, Witmer et al., 1989). A few studies have measured Cr in alligators and reveal a concern. A study of alligators in South Carolina found a cluster of alligators had relatively high concentrations of Cr in liver tissue with several animals having levels over 30 ug/g (Campbell et al., 2010). Horai et al (2014) showed that Cr accumulates in adult alligators based on comparisons between juvenile and adult alligator livers at 3 different sites in Florida. Interestingly of the three sites tested the Cr levels in adult alligators from Merritt Island National Wildlife Refuge (MINWR) were the highest and were 3 times higher than the other two sites suggesting localized pollution (Horai et al., 2014).
However, while these studies show alligators may be exposed to significant levels of Cr, no studies have considered potential adverse effects as a result of Cr exposure in alligators. In fact, consideration of the available literature shows Cr is poorly studied in aquatic reptiles and appears to be limited to two reports. One study, in green sea turtle cells, found Cr(VI) to be one of the most cytotoxic of four metals tested (Tan et al., 2010). The other considered hawksbill sea turtle cells and found both particulate and soluble Cr(VI) were cytotoxic and clastogenic (Wise, S. et al, 2014).
The explanation for the lack of data is presumably due to the lack of access to experimental models of aquatic reptiles. Many species are endangered and protected. However, it is possible to gain important species-specific insights into potential toxicological impacts through aquatic reptile cell cultures. Accordingly, to begin developing a better understanding of pollution impacts on alligators and crocodilians in general, we investigated the cytotoxicity and genotoxicity of chromium in fibroblasts developed from American alligator scute tissue. Because the major health concern in the environment is the hexavalent form of chromium, and since particulate Cr(VI) is considered to be a more potent carcinogen than soluble Cr(VI) (IARC, 1990; Holmes et al., 2008; Wise, S. et al., 2008), we focused our study on particulate and soluble Cr(VI) compounds.
2. Materials and Methods
2.1. Materials
All plasticware was purchased from BD Falcon. Dulbecco’s Phosphate-Buffered Saline (DPBS), RPMI media with Glutagro was purchased from Corning. Potassium chloride, demecolcine, lead chromate, and sodium chromate were purchased from Sigma/Aldrich. Crystal violet, methanol and acetic acid were purchased from JT Baker. Microscope slides were purchased from Thermo Scientific. Giemsa stain was manufactured by Rica Chemical Co. Fetal Bovine Serum (FBS), Gurr’s buffer, trypsin, penicillin-streptomycin and sodium pyruvate was purchased from GIBCO Invitrogen Corporation.
2.2. Cell Culture
Alligator fibroblasts were isolated from a scute sample obtained from a free-ranging alligator from the Yawkey Wildlife Preserve in South Carolina. The scute sample was placed in L-15 medium supplemented with 100 g/ml streptomycin, 100 U/ml penicillin, and 10 mg/ml gentamicin and then transported on cold packs to the laboratory. Upon receipt, tissue explants were rinsed several times in PBS with penicillin-streptomycin and gentamicin. Tissue samples were then sliced with a scalpel into small pieces (~1 mm), rinsed repeatedly and placed into T-25 flasks containing RMPI with Glutagro and supplemented with 10% FBS, 100 U/ml penicillin, 100 g/ml streptomycin. Flasks with tissues were placed in a 33°C (determined to be optimal growth temperature) humidified incubator with 5% CO2 and fibroblast cells were observed growing out of the explants. Cells were maintained as adherent subconfluent monolayers. They were fed at least twice a week and subcultured at least once a week. Cells were tested routinely for Mycoplasma contamination.
2.3. Chemical preparation
Lead chromate (CAS# 7758-97-6, ACS reagent minimum 98% purity), was used as a representative particulate Cr(VI) compound and administered as a suspension in water as previously described (Wise et al., 2002). Sodium chromate (CAS #7775-11-3, ACS reagent minimum 98% purity), was used as a soluble hexavalent chromium compound and was administered as a solution in water as previously described (Wise et al., 2002). Lead chromate is an insoluble compound and therefore treatment concentrations are expressed as weight per surface area (ug/cm2). Sodium chromate is fully soluble and therefore treatment concentrations are expressed as uM. Water was used as the vehicle control. Final concentrations ranged from 0–5 ug/cm2 for lead chromate, and 0–5 uM for sodium chromate. Based on comparisons with a potential sea turtle and whale exposures, we believe these concentrations to be environmentally relevant (Wise S et al., 2014; Wise J et al., 2008).
2.4. Cytotoxicity
Cytotoxicity was determined using a clonogenic assay based on our published methods (Wise, J. et al., 2002). Briefly, log phase cells were seeded into a 6-well culture plate and allowed to resume normal growth for 48 h. Cells were then treated with lead chromate and sodium chromate for 24 h. After treatment, cells were resuspended in fresh medium and reseeded at a colony forming density of 1,000 cells per 100 mm dish with four dishes per treatment group. When colony formation was sufficient (approximately 14 days) dishes were fixed and stained with crystal violet. Dishes were counted and averaged together to get a mean value for each dose in each experiment. Treatment dishes were compared to the control. Each experiment was repeated at least three times.
2.5. Clastogenicity
We used a chromosomal aberration assay to determine the clastogenicity of each chemical, based on our published methods (Wise, J. et al., 2002). Briefly, cells were seeded into 100 mm tissue culture dishes for 48 h. Dishes were treated with either lead chromate or sodium chromate for 24 h. One hour prior to the end of the treatment period cells were arrested in metaphase using 0.1 g/ml demecolcine. After the full 24 h treatment period, cells were harvested and resuspended in a potassium chloride hypotonic solution (KCl) for 17 m then fixed with 3:1 methanol:acetic acid. After two additional fixative changes, cells were dropped onto clean, wet microscope slides and stained with 5% Giemsa stain in Gurr’s Buffer. Slides were analyzed for chromosome aberrations in 100 metaphases per treatment concentration and reported as both percent of metaphases with damage where the metaphases is the unit of measure and as the total amount of damage seen in 100 metaphases where the chromosome is the unit of measure according to our published methods (Wise, J. et al., 2002).
2.6. Determination of intracellular chromium levels
Intracellular ion levels were measured using the ion uptake assay, as described previously (Holmes et al., 2005), with minor changes. Briefly, logarithmically growing cells were seeded into 100 mm tissue culture dishes, allowed to rest for 48 h and then treated with lead chromate and sodium chromate for 24 h. At the end of the treatment, 3 ml of treated culture media was saved for extracellular chromium analysis; cells were collected and the number and volume of cells were determined. Cells were washed twice with PBS, resuspended in 1 ml hypotonic solution followed by 1 ml 2% SDS. Finally, the solution was sheered through and 18 g needle and filtered to remove cellular debris. Samples were stored at −20 °C until analysis.
Intracellular Cr ion levels were determined using an Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES), equipped with a gem cone low flow nebulizer, according to previously published methods (Holmes et al., 2005). Solutions were introduced to the nebulizer using a peristaltic pump operating at 2 ml/min. Samples of intracellular fluids were diluted 5× in 0.16 M aqueous HNO3 prior to analysis. Chromium was determined using emission wavelength at 267.716 with a minimum detection limit of 2 ppb. Yttrium(Y) was used as an internal standard for chromium determinations. The intracellular concentrations were converted from ug/l to uM by dividing by the volume of the sample, the atomic weight of the chemical, the number of cells in the sample and the average cell volume. To account for the possibility of undissolved particulate Cr(VI) passing through the filter, 0 h treatments were performed for particulate lead chromate and these values were subtracted from the measurements obtained for the 24 h treatments.
2.7. Statistics
Analysis of variance was used to test the difference between different dose groups and the SCHEFFE test was used to make multiple comparisons. LC50's and EC20's were estimated using inverse prediction/estimation of linear regression analysis. Statistical analyses were performed using SAS version 9.3 and the minimum level of significance was 0.05 for each test.
3. Results
3.1. Cytotoxicity
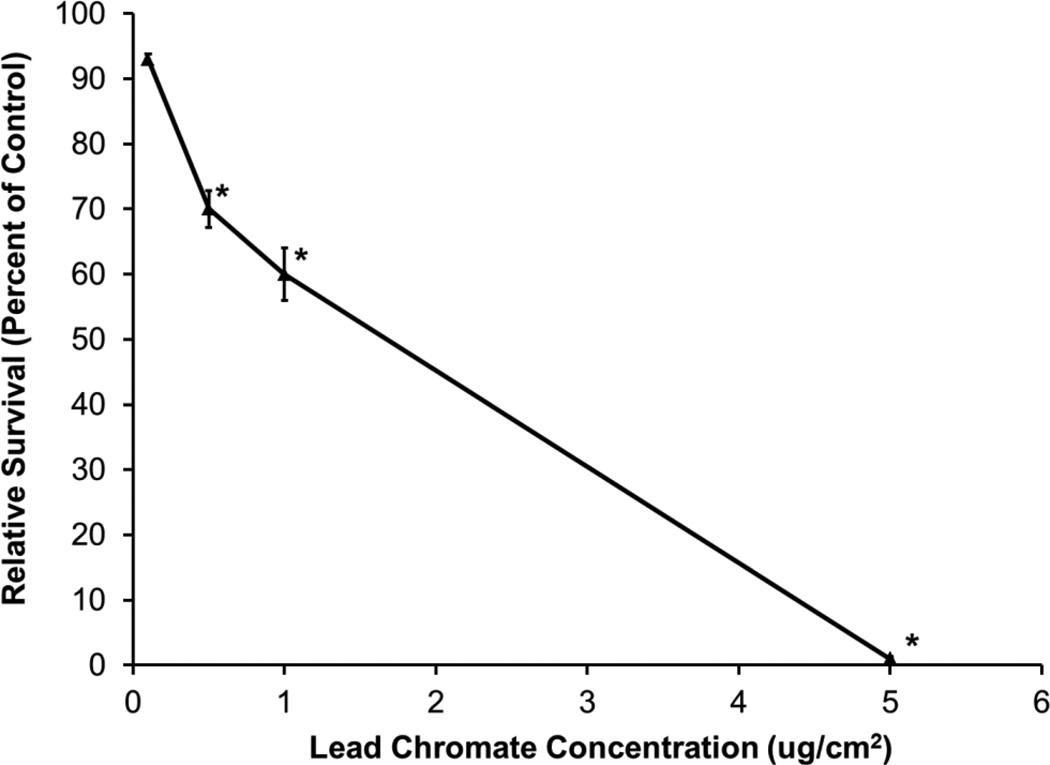
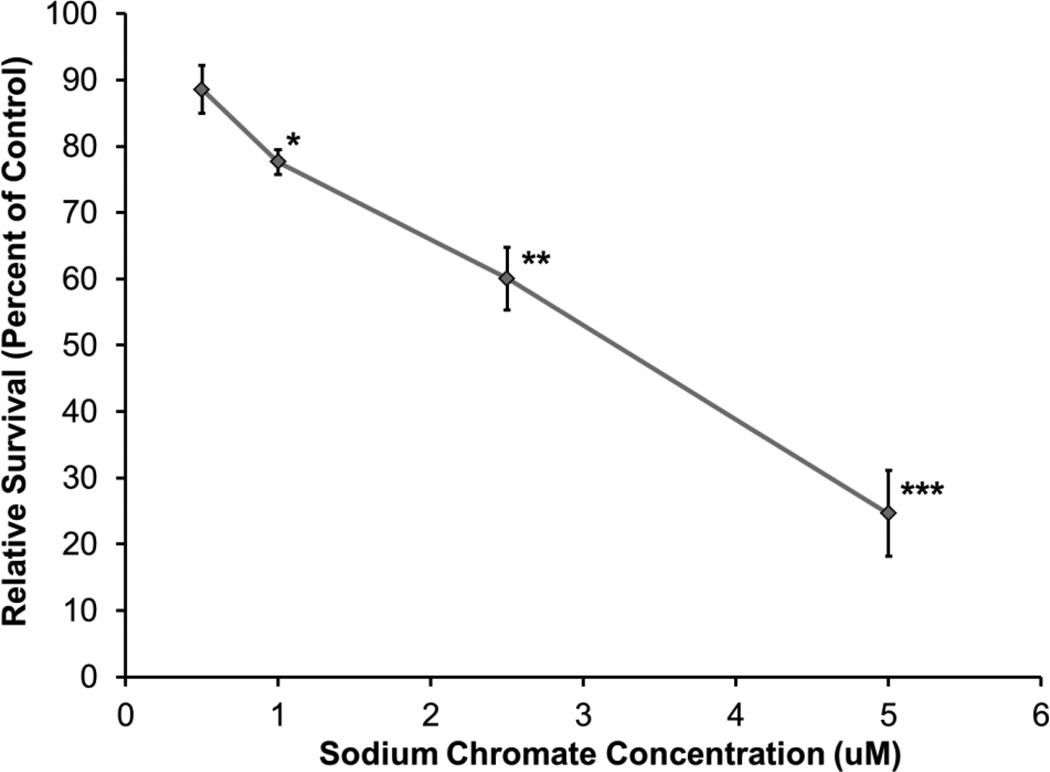
There was a concentration-dependent decrease in alligator cell survival after both lead chromate and sodium chromate treatment compared to the untreated controls. Treatments of 0.1, 0.5, 1, and 5 ug/cm2 lead chromate for 24 h induced 93, 70, 60, and 1 percent relative survival, respectively (Figure 1). Treatments of 0.5, 1, 2.5, and 5 uM sodium chromate induced 87, 78, 60, and 25 percent relative survival, respectively (Figure 2). The estimated LC50s for lead chromate and sodium chromate were 2.1 ug/cm2 (95% confidence interval: 0.9 to 3.4) and 3.2 uM (95% confidence interval: 2.1 to 4.2), respectively.
Figure 1.
The Cytotoxicity of Lead Chromate in Alligator Cells
Figure 2.
The Cytotoxicity of Sodium Chromate in Alligator Cells
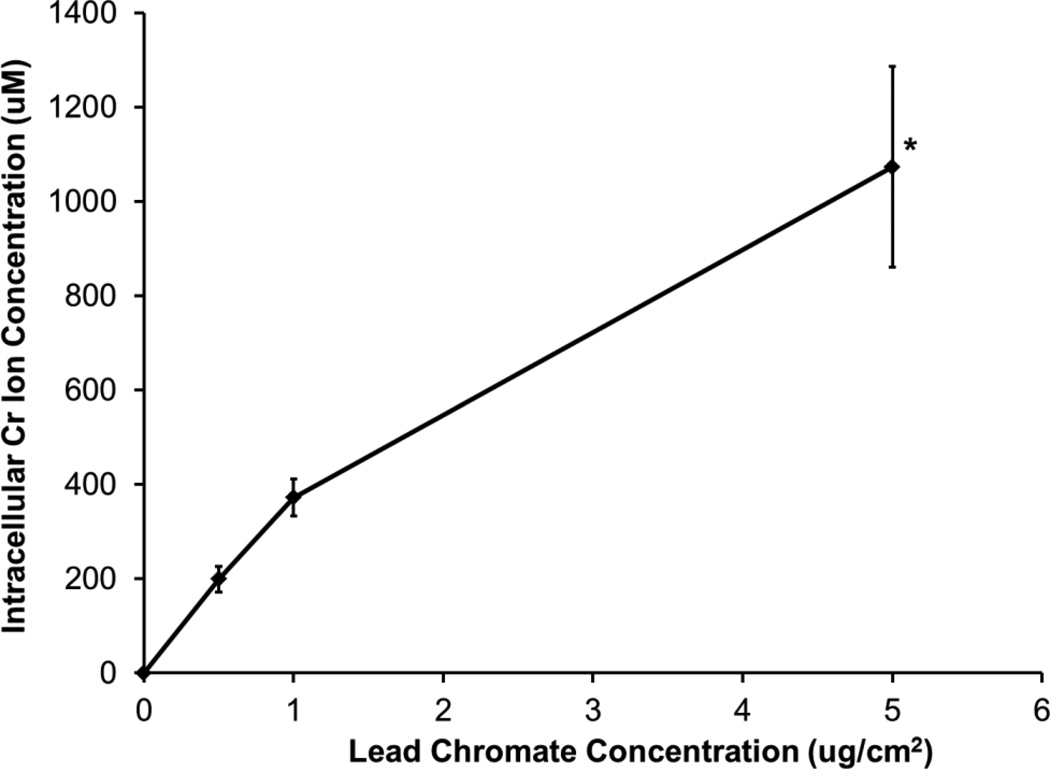
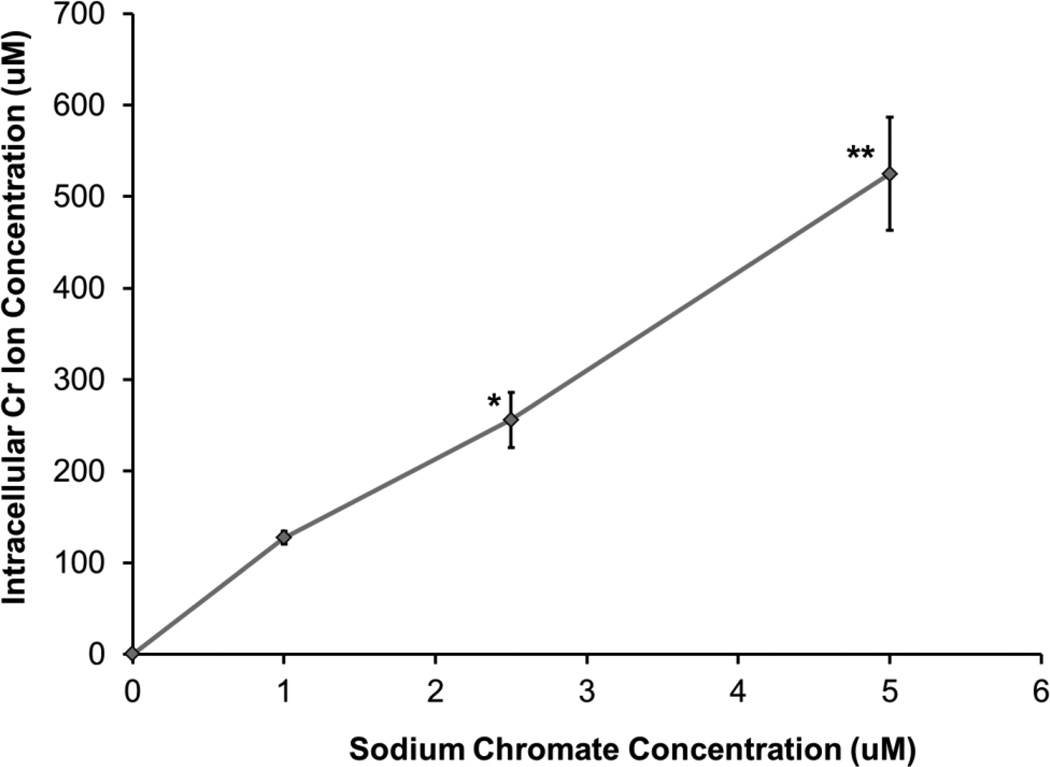
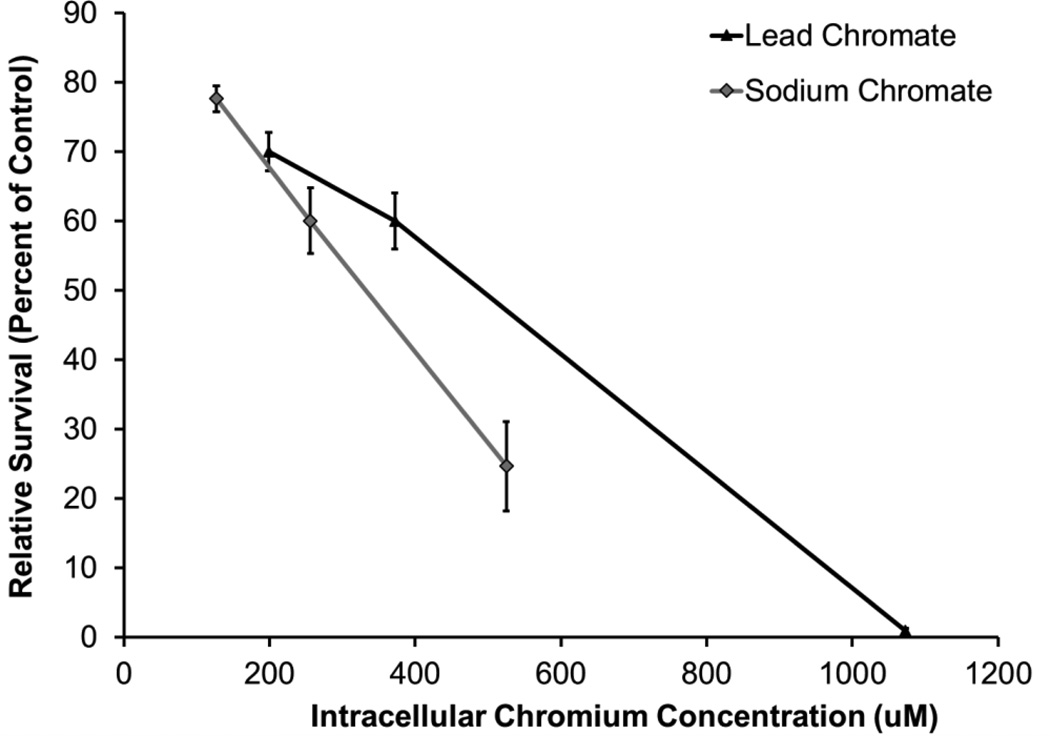
By measuring the intracellular Cr ion levels, these compounds can be compared more directly. Intracellular chromium concentrations increased with increasing concentration for both compounds compared to the untreated controls. Treatments of 0.5, 1, and 5 ug/cm2 lead chromate for 24 h resulted in intracellular Cr concentrations of 199, 372, and 1073 uM Cr, respectively (Figure 3). Treatments of 1, 2.5, and 5 uM sodium chromate resulted in intracellular Cr concentrations of 127, 256, and 525 uM Cr, respectively (Figure 4). When the cytotoxicity data is considered based on intracellular Cr level, soluble chromate is more cytotoxic to alligator cells than particulate chromate (Figure 5). For example, the LC50 for sodium chromate, based on intracellular concentration was 348.4 (120.9, 575.9) uM, while the LC50 for lead chromate, based on intracellular concentration is 513.1 (64.2, 961.9) uM.
Figure 3.
Intracellular Cr Ion Concentrations after Lead Chromate Treatment
Figure 4.
Intracellular Cr Ion Concentrations after Sodium Chromate Treatment
Figure 5.
Cytotoxicity Based on Intracellular Cr Concentrations
This figure shows that lead chromate is cytotoxic to alligator cells in a concentration-dependent manner after 24 h exposure. Data represent the average of 3 experiments ± the standard error of the mean. * = statistically different from control (p < 0.0001).
This figure shows that sodium chromate is cytotoxic to alligator cells in a concentration-dependent manner. Data represent the average of 3 experiments ± the standard error of the mean. * = statistically different from control (p < 0.05); ** = statistically different from control (p < 0.001); *** = statistically different from control (p < 0.0001)
This figure shows that lead chromate induces a concentration-dependent increase in the intracellular Cr ion concentration in alligator cells. Data represent the average of 3 experiments ± the standard error of the mean. * = statistically different from control (p < 0.001).
This figure shows that sodium chromate induces a concentration-dependent increase in the intracellular Cr ion concentration in alligator cells. Data represent the average of 3 experiments ± the standard error of the mean. * = statistically different from control (p < 0.01); ** = statistically different from control (p < 0.0001).
This figure shows that when adjusted for the amount of intracellular chromium, sodium chromate is more cytotoxic to alligator cells than lead chromate. The data represent an average of 3 experiments ± standard error of mean.
3.2. Clastogenicity
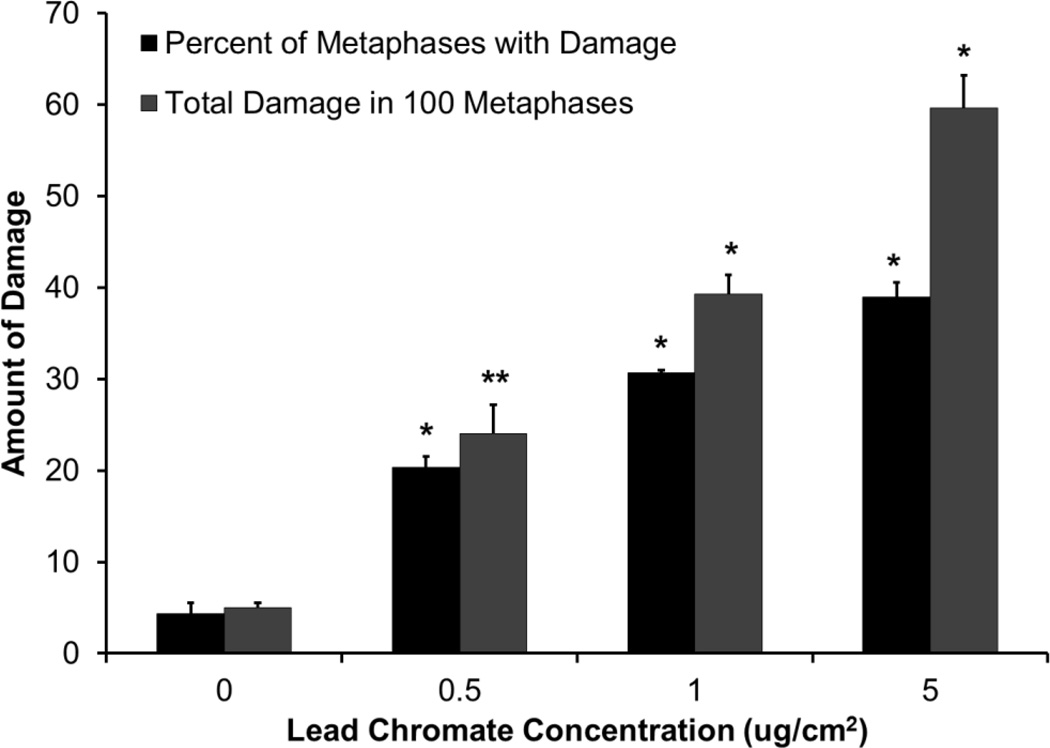
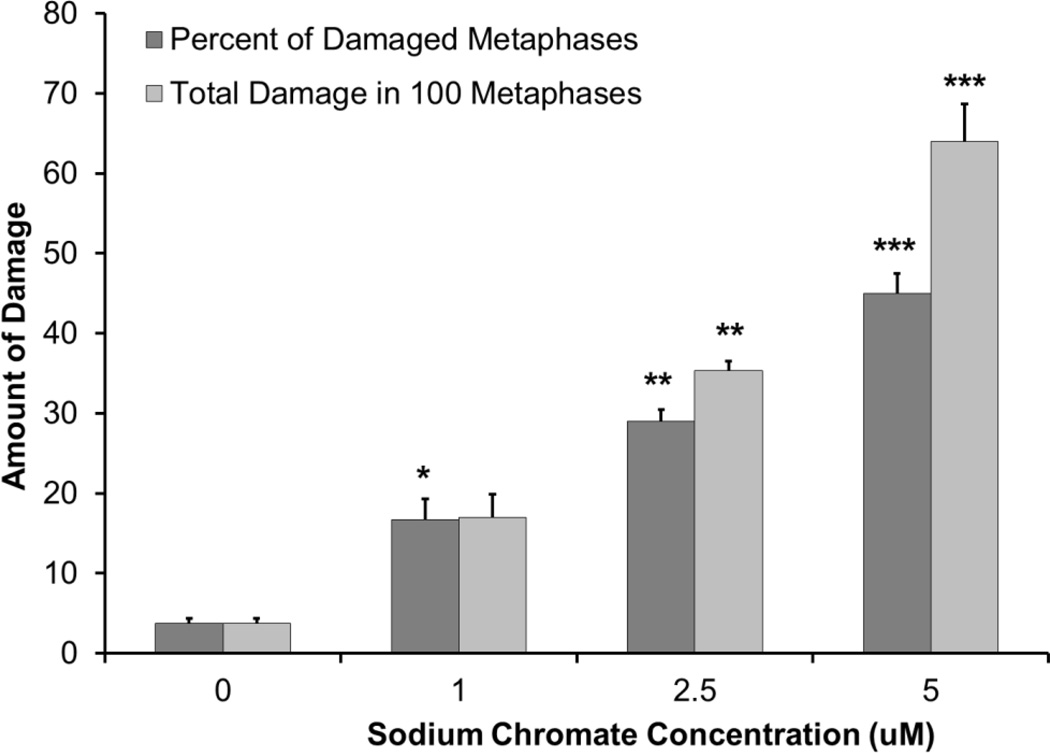
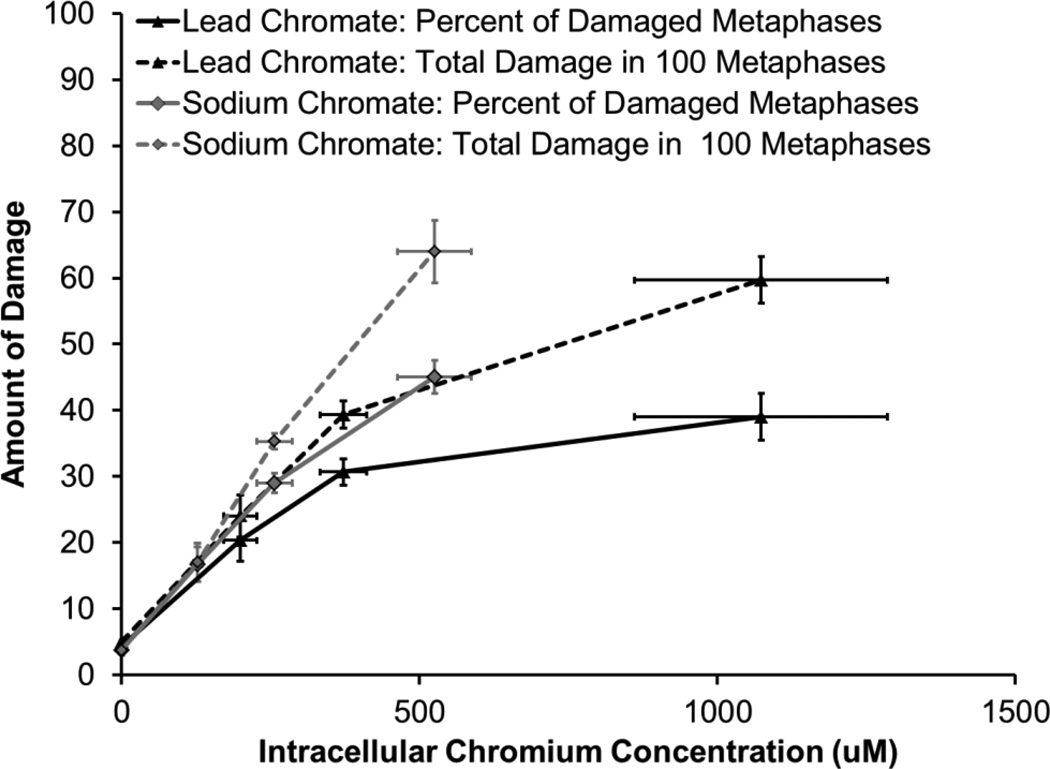
Clastogenicity was used as a measure of genotoxicity. Results are expressed as both the percent of damaged metaphases and as the total damage observed in 100 metaphases. Particulate and soluble Cr(VI) induced a concentration-dependent increase in genotoxicity compared to the untreated controls. Lead chromate concentrations of 0, 0.5, 1 and 5 ug/cm2 induced aberrations in 4, 20, 31 and 39 percent of metaphases and a total of 5, 24, 39 and 60 aberrations in 100 metaphases (Figure 6). Sodium chromate concentrations of 0, 1, 2.5, and 5 uM induced aberrations in 4, 17, 29 and 45 percent of metaphases and a total of 4, 17, 35 and 64 aberrations in 100 metaphases (Figure 7). When the clastogenicity data is considered based on intracellular Cr levels (Figure 8), soluble chromate is more potent than particulate chromate in alligator cells. For example, the EC20 for lead chromate and sodium chromate, based on intracellular concentration was 262.2 uM (−556.506, 1080.87) and 178.6 (29.2, 327.4)) uM, respectively.
Figure 6.
Lead Chromate Is Genotoxic to Alligator Cells
Figure 7.
Sodium Chromate Is Genotoxic to Alligator Cells
Figure 8.
Genotoxicity Based on Intracellular Cr Concentrations
This figure shows the lead chromate is genotoxic to alligator cells in a concentration-dependent manner. The data represent an average of 3 experiments ± standard error of mean. * = statistically different from control (p < 0.0001); ** statistically different from control (p < 0.01).
This figure shows the sodium chromate is genotoxic to alligator cells in a concentration-dependent manner. The data represent an average of 3 experiments ± standard error of mean. * = statistically different from control (p < 0.05); ** = statistically different from control (p < 0.001); *** = statistically different from control (p < 0.0001).
This figure shows that when adjusted for the amount of intracellular chromium, sodium chromate is more genotoxic to alligator cells than lead chromate. The data represent an average of 3 experiments ± standard error of mean.
4. Discussion
Our data show particulate and soluble Cr(VI) are cytotoxic to alligator cells. Our study is the first to report cytotoxicity in alligator cells and one of only a few to report cytotoxicity in any reptilian species. Our data are consistent with previous cytotoxicity studies in hawksbill sea turtle, loggerhead sea turtle, and green sea turtle cells (Tan et al., 2010, Wang et al., 2013, Webb et al., 2014, Wise S. et al., 2014). Three of these studies considered metal cytotoxicity (Tan et al., 2010, Wang et al., 2013, Wise S. et al., 2014) and two included cytotoxicity of Cr(VI) (Tan et al., 2010, Wise S. et al., 2014).
While our data are consistent with the general outcome of Cr(VI)-induced cytotoxicity, the potency differed with respect to the results in green sea turtle cells. Specifically, the most sensitive tissue tested in green sea turtles was brain cells with a reported LC50 of 22 uM of soluble Cr(VI); the least sensitive tissues was liver cells with a reported LC50 of over 100 uM. In alligator cells, we observed an LC50 of 3.2 uM for soluble Cr(VI), our results are consistent with those observed in hawksbill sea turtle cells, which reported and LC50 of 1.2 uM for soluble Cr(VI).
The explanation for the differences in cytotoxic response between alligator and green sea turtle brain are uncertain. The most likely explanation is that the differences are due to the type of assay used to detect cytotoxicity. The alligator and hawksbill cells were both tested using a rigorous clonogenic assay, whereas the green sea turtle study used the MTT and Coomassie blue assays, which are known to be less sensitive assays. It is possible the differences may reflect a species effect, however, given that our results are consistent with those observed in the hawksbill cells this explanation is unlikely. The differences may reflect differences in cell type, although the alligator cell results are consistent with the hawksbill cell results which were derived from scute and skin tissues, respectively. However, these two tissues are similar in nature and may be more sensitive than those tested in the green sea turtle which included brain, embryo, eye, heart, lung, liver, spleen, testes, bladder, and tumor cells. Finally, the observed differences may be due to the health of the animal from which the cells were derived. The alligator and hawksbill sea turtle cells were derived from healthy animals and both cell lines exhibited normal and stable karyotypes whereas the cells used in the green sea turtle study were obtained from an animal with severe fibropapilloma with abnormal chromosomes which may affect their sensitivity to Cr (Lu et al, 1999).
Our study is also the first to report genotoxicity in alligator cells. Genotoxicity for any chemical has only been considered in one other reptilian cell line, our results are consistent with those reported in the hawksbill sea turtle cells (Wise S et al., 2014). For example, in hawksbill cells, 0.5, 1, and 5 ug/cm2 particulate Cr(VI) induced a total of 17, 30, and 56 aberrations in 100 metaphases, respectively and in alligator cells we observed a total of 24, 39, and 60 aberrations in 100 metaphases at the same respective concentrations. Results were somewhat different with soluble chromium particularly at the higher concentrations; concentrations of 1, 2.5 and 5 uM sodium chromate induced a total of 17, 35 and 64 aberrations in 100 metaphases, respectively, in alligator cells and a total of 16, 26 and 39 aberrations in 100 metaphases, respectively, in hawksbill sea turtle skin cells. The explanation for this difference is uncertain particularly because alligator cells have a lower intracellular Cr ion concentration after soluble chromium treatment compared to hawksbill sea turtle cells (unpublished data). Hawksbill cells may have a more efficient way of sequestering intracellular Cr ions or they may have better DNA repair mechanisms compared to alligator cells. Further investigation into the differences in cellular uptake of Cr and the resulting DNA damage are necessary to fully elucidate these effects. It would be prudent to determine if they are Cr(VI)-, metal-, or contaminant-specific effects or if they are due to differences in DNA repair or other molecular mechanisms.
In sum, the current data show that both particulate and soluble Cr(VI) are cytotoxic and genotoxic to American alligator scute cells. Cr(VI) is emerging as a global health concern for the aquatic and marine environments and may be a more localized issue for particular regions (Wise, C. et al., in press, Wise, J. et al., 2009,). Cr(VI) is known to induce genotoxicity in humans and other terrestrial mammals and is known to target the respiratory and reproductive tissues. These data indicate that Cr has the potential for cytotoxic and genotoxic outcomes in the American alligator which could have important implications for the health of alligators and alligator populations in contaminated environments. The sensitivity of alligator cells to Cr(VI) suggest that alligators can serve as a good monitor for Cr contaminated ecosystems. Future work will address differences in DNA repair in alligator compared to other reptile and mammalian species and to determine if there are direct effects of Cr on disease or reproduction in alligators.
Highlights.
Particulate Cr(VI) is cytotoxic and clastogenic to American alligator cells.
Soluble Cr(VI) is cytotoxic and clastogenic to American alligator cells.
Cr(VI) may be a risk factor for American alligator health.
Acknowledgments
The authors would like to thank Matt Guillette for assistance in collecting alligator samples; Therry The and Christy Gianios, Jr. for technical support. Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number R01ES016893 (JPW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional funding was provided by the Maine Space Grant Consortium (JPW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hamood MH, Elbetieha A, Bataineh H. Sexual maturation and fertility of male and female mice exposed prenatally and postnatally to trivalent and hexavalent chromium compounds. Reprod. Fertil. Dev. 1998;10:179–183. doi: 10.1071/r97001. [DOI] [PubMed] [Google Scholar]
- Bataineh H, Al-Hamood MH, Elbetieha A, Bani Hani I. Effect of long-term ingestion of chromium compounds on aggression, sex behavior and fertility in adult male rat. Drug Chem. Toxicol. 1997;20:133–149. doi: 10.3109/01480549709003875. [DOI] [PubMed] [Google Scholar]
- Campbell JW, Waters MN, Tarter A, Jackson J. Heavy metal and selenium concentrations in liver tissue from wild American alligator (Alligator mississippiensis) livers near Charleston, South Carolina. J. Wildl. Dis. 2010;46(4):1234–1241. doi: 10.7589/0090-3558-46.4.1234. [DOI] [PubMed] [Google Scholar]
- Chowdhury AR, Mitram C. Spermatogenic and steroidogenic impairment after chromium treatment in rats. Indian J. Exp. Biol. 1995;33:480–484. [PubMed] [Google Scholar]
- Delany MF, Bell JU, Sundlof SF. Concentrations of contaminants in muscle of the American alligator in Florida. J. Wildl. Dis. 1988;24:62–66. doi: 10.7589/0090-3558-24.1.62. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Wise SS, Xie H, Gordon N, Thompson WD, Wise JP. Lead ions do not cause human lung cells to escape chromate-induced cytotoxicity. Toxicol. Appl. Pharmacol. 2005;203(2):167–176. doi: 10.1016/j.taap.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Wise SS, Wise JP., Sr Carcinogenicity of hexavalent chromium. Ind. J. Med. Res. 2008;128:353–372. [PubMed] [Google Scholar]
- Horai S, Itai T, Noguchi T, Yasuda Y, Adachi H, Hyobu Y, Riyadi AS, Boggs ASP, Lowers R, Guillette LJ, Tanabe S. Concentrations of trace elements in American alligators (Alligator mississippiensis) from Florida, USA. Chemosphere. 2014;108:159–167. doi: 10.1016/j.chemosphere.2014.01.031. [DOI] [PubMed] [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans: Chromium, Nickel and Welding. Vol. 49. Lyons, France: International Agency for Research on Cancer; 1990. [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Nerurkar VR, Aguirre AA, Work TM, Balazs GH, Yanagihara R. Establishment and characterization of 13 cell lines from a green turtle (Chelonia mydas) with fibropapillomas. In Vitro Cell. Dev. Biol. Anim. 1999;35(7):389–393. doi: 10.1007/s11626-999-0113-6. [DOI] [PubMed] [Google Scholar]
- Mancuso TF. Chromium as an industrial carcinogen. II. Chromium in human tissues. Am. J. Ind. Med. 1997;2:140–147. doi: 10.1002/(sici)1097-0274(19970204)31:2<140::aid-ajim2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Tan F, Wang M, Wang W, Alonso Aguirre A, Lu Y. Validation of an in vitro cytotoxicity test for four heavy metals using cell lines derived from a green sea turtle (Chelonia mydas) Cell Bio. Toxicol. 2010;26:255–263. doi: 10.1007/s10565-009-9130-1. [DOI] [PubMed] [Google Scholar]
- Wang H, Tong J, Bi Y, Wang C, Guo L, Lu Y. Evaluation of mercury mediated in vitro cytotoxicity among cell lines established from green sea turtles. Toxicol. in Vitro. 2013;27(3):1025–1030. doi: 10.1016/j.tiv.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Zychowski GV, Bauman SW, Higgins BM, Raudsepp T, Gollahon LS, Wooten KJ, Cole JM, Godard-Codding C. Establishment, Characterization, and Toxicological Application of Loggerhead Sea Turtle (Caretta caretta) Primary Skin Fibroblast Cell Cultures. Environ. Sci. Technol. 2014;48(24):14728–14737. doi: 10.1021/es504182e. [DOI] [PubMed] [Google Scholar]
- Wise CF, Wise SS, Thompson WD, Perkins C, Wise JP., Sr Chromium Is Elevated in Fin Whale (Balaenoptera physalus) Skin Tissue and Is Genotoxic to Fin Whale Skin Cells. Biol. Trace. Elem. Res. 2015 doi: 10.1007/s12011-015-0311-x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise JP, Sr, Wise SS, Little JE. The cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human lung cells. Mutat. Res. 2002;517:221–229. doi: 10.1016/s1383-5718(02)00071-2. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr, Wise SS, Kraus S, Fariba Shaffiey F, Grau M, Li Chen T, Perkins C, Thompson WD, Zheng T, Zhang Y, Romano T, O’Hara T. Hexavalent chromium is cytotoxic and genotoxic to the North Atlantic right whale (Eubalaena glacialis) lung and testes fibroblasts. Mutation Research. 2008;650:30–38. doi: 10.1016/j.mrgentox.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr, Payne R, Wise SS, LaCerte C, Wise J, Gianios C, Jr, Thompson WD, Perkins C, Zheng T, Zhu C, Benedict L, Kerr I. A global assessment of chromium pollution using sperm whales (Physeter macrocephalus) as an indicator species. Chemosphere. 2009;75(11):1461–1467. doi: 10.1016/j.chemosphere.2009.02.044. [DOI] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Wise JP., Sr Hexavalent chromium-induced DNA damage and repair mechanisms. Rev. Environ. Health. 2008;23:39–57. doi: 10.1515/reveh.2008.23.1.39. [DOI] [PubMed] [Google Scholar]
- Wise SS, Xie H, Fukuda T, Thompson WD, Wise JP. Hexavalent chromium is cytotoxic and genotoxic to Hawksbill Sea turtle cells. Toxicol. Appl. Pharmacol. 2014;279(2):113–118. doi: 10.1016/j.taap.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer CM, Park H-S, Shupack SI. Mutagenicity and disposition of chromium. Sci. Total Environ. 1989;86:131–148. doi: 10.1016/0048-9697(89)90200-3. [DOI] [PubMed] [Google Scholar]