Abstract
Pituitary neoplasias can occur as part of a complex inherited disorder, or more commonly as sporadic (non-familial) disease. Studies of the molecular and genetic mechanisms causing such pituitary tumours have identified dysregulation of >35 genes, with many revealed by studies in mice, rats and zebrafish. Strategies used to generate these animal models have included gene knockout, gene knockin and transgenic over-expression, as well as chemical mutagenesis and drug induction. These animal models provide an important resource for investigation of tissue-specific tumourigenic mechanisms, and evaluations of novel therapies, illustrated by studies into multiple endocrine neoplasia type 1 (MEN1), a hereditary syndrome in which ∼30% of patients develop pituitary adenomas. This review describes animal models of pituitary neoplasia that have been generated, together with some recent advances in gene editing technologies, and an illustration of the use of the Men1 mouse as a pre clinical model for evaluating novel therapies.
Keywords: Pituitary, Adenoma, Carcinoma, Mouse, Rat, Multiple endocrine neoplasia type 1
Highlights
-
•
Over 40 animal models of pituitary neoplasia have been reported.
-
•
Animal models have confirmed roles of genes implicated in pituitary tumourigenesis.
-
•
Animal models have revealed novel genes involved in pituitary tumourigenesis.
-
•
Menin is a key protein in suppressing pituitary tumour development.
1. Introduction
Pituitary tumours represent 10–15% of all intracranial tumours. The standardised incidence rate of pituitary tumours is ∼4/1,00,000, with the higher incidence being in females (∼5/1,00,000) (Tjornstrand et al., 2014, Raappana et al., 2010, Gittleman et al., 2014). Pituitary tumours may occur as part of hereditary syndromes (e.g. Multiple Endocrine Neoplasia Type 1 (MEN1) or Type 4 (MEN4)), or as an isolated (non-syndromic) disorder which may be inherited (e.g. Familial Isolated Pituitary Adenomas (FIPA)), or more commonly (>95%) as non-familial (i.e. sporadic) neoplasms (Yates et al., 2014). Pituitary tumours are also classified according to their hormonal production as: lactotrophinomas (secreting prolactin), that comprise ∼50% of tumours; gonadotrophinomas (secreting follicle stimulating hormone (FSH) or lutenising hormone (LH), but predominantly non-functioning), comprising ∼30% of tumours; somatotrophinomas (secreting growth hormone (GH)), comprising 15–20% of tumours; corticotrophinomas (secreting adrenocorticotropic hormone (ACTH)), comprising 5–10% of tumours; and thyrotrophinomas (secreting thyroid stimulating hormone (TSH)), comprising <1% of tumours. However, it is important to note that pituitary adenomas may secrete more than one hormone (Levy, 2008). Over 99% of pituitary tumours are benign adenomas with <0.2% being carcinomas that may metastasise (Scheithauer et al., 2006).
To date, over 30 animal models of pituitary tumourigenesis, usually mouse models, have been generated, using gene knockout and over-expression approaches. Evaluation of these models has increased our understanding of pituitary tumour biology and of the roles of oncogenic and tumour suppressor genes. This review will discuss these animal models of pituitary neoplasia, focussing on rodent models, together with the methods used in their generation. In addition, use of a Men1 mouse model in evaluating approaches to targeted therapies will be reviewed.
2. Pituitary neoplasia models
Pituitary neoplasia may result from mutations involving either activation of a dominant gain-of-function oncogene, or inactivation of a recessive loss-of-function tumour suppressor gene. These mutations have been discovered by studies of pituitary tumours from patients, or from animal models generated for other disorders. To date, human studies of familial syndromes and sporadic disease have indicated the involvement of >35 genes in the development and progression of pituitary neoplasias (Table 1). Animal models harbouring mutations of ∼35% of these genes have been generated, and animal models of mutations in genes not previously implicated in pituitary neoplasia have also been generated, such that over 40 animal models of pituitary neoplasia have been generated, with the majority of these animal models being mutant mice (Table 2). Many of these models represent human syndromes e.g. MEN1 (Crabtree et al., 2001, Bertolino et al., 2003a, Biondi et al., 2002, Loffler et al., 2007a, Loffler et al., 2007b, Harding et al., 2009) and MEN4 (Kiyokawa et al., 1996, Nakayama et al., 1996, Fero et al., 1996), as well as representing a range of pituitary neoplasms that include hyperplasia, adenomas and carcinomas (Table 2). These pituitary tumours may secrete hormones such as prolactin, GH, ACTH, FSH, LH and TSH, or they may be non-secreting, which is also referred to as non-functioning adenomas (Table 2). These models have been generated using different methods, which will be briefly reviewed below.
Table 1.
Genetic abnormalities identified from human studies to be associated with pituitary neoplasias.
| Gene | Tumour type/Syndrome | Gene defect | Reference |
|---|---|---|---|
| AIP | FIPA | Germline inactivating mutation | (Beckers et al., 2013) |
| Young onset sporadic pituitary macroadenomas | |||
| BMP-4 | Corticotrophinomas | Gene down-regulation | (Giacomini et al., 2006) |
| Somatotrophinomas | Gene over-expression | ||
| Prolactinomas | Gene over-expression | ||
| CASP8 | Functioning and non-functioning adenomas | Methylation mediated gene silencing | (Bello et al., 2006) |
| CCNA2 | MEN1 patients without MEN1, CASR or HRPT2 mutations | Gene over-expression | (Agarwal et al., 2009) |
| CCNB1 | MEN1 patients without MEN1, CASR or HRPT2 mutations | Gene over-expression | (Agarwal et al., 2009) |
| CCNB2 | MEN1 patients without MEN1, CASR or HRPT2 mutations | Gene over-expression | (Agarwal et al., 2009) |
| CCND1 | Non-functioning adenomas | Gene over-expression | (Jordan et al., 2000) |
| CCNE1 | Cushing's syndrome | Gene over-expression | (Jordan et al., 2000, Jaffrain-Rea et al., 2013) |
| MEN1 patients without MEN1, CASR or HRPT2 mutations | |||
| CDH1 | Somatotrophinomas with prominent fibrous bodies | Methylation-mediated gene silencing | (Zhou et al., 2013) |
| CDH13 | Functioning and non-functioning adenomas | Methylation-mediated gene silencing, correlating with tumour aggressiveness | (Qian et al., 2007) |
| CDKN1A | Functioning and non-functioning adenomas | Gene down-regulation | (Hiyama et al., 2002) |
| CDKN1B | MEN4 patients | Germline inactivating mutation | (Pellegata et al., 2006) |
| CDKN2A | Functioning and non-functioning adenomas | Methylation-mediated gene silencing | (Zhou et al., 2013) |
| CDKN2B | Functioning and non-functioning adenomas | Methylation-mediated gene silencing | (Zhou et al., 2013) |
| CDKN2C | Functioning and non-functioning adenomas | Methylation-mediated gene silencing | (Zhou et al., 2013) |
| DAPK family | Functioning and non-functioning adenomas | Loss of expression | (Simpson et al., 2002) |
| FGFR2 | Functioning adenomas | Methylation-mediated gene silencing | (Zhu et al., 2007) |
| FGFR4 | Functioning adenomas | Constitutively phosphorylated | (Ezzat et al., 2002) |
| GADD45B | Gonadotrophinoma | Gene silencing | (Michaelis et al., 2011) |
| GADD45G | Functioning, but more commonly in non-functioning adenomas | Gene silencing | (Zhou et al., 2013) |
| GNAS | Somatotrophinomas | Mutations detected | (Mantovani et al., 2010) |
| HMGA-1 | Prolactinomas | Gene over-expression | (De Martino et al., 2009) |
| HMGA-2 | Prolactinomas | Gene over-expression | (Fedele et al., 2006) |
| LGALS3 | Lactotrophinomas | Gene over-expression | (Righi et al., 2010) |
| Corticotrophinomas | |||
| MEG3 | Non-functioning adenomas | Methylation-mediated gene silencing | (Zhang et al., 2010) |
| MEN1 | MEN1 | Inactivating mutations and gene deletions | (Thakker, 2010) |
| Young onset sporadic pituitary adenomas | |||
| MGMT | Carcinomas | Methylation-mediated gene silencing | (Zhou et al., 2013) |
| PLAGL1 | Non-functioning adenomas | Methylation-mediated gene silencing | (Pagotto et al., 2000) |
| PRKAR1A | Somatotrophinomas | Gene down-regulation | (Kirschner, 2010) |
| Non-functioning adenomas | |||
| PTAG | Adenomas (subtype not defined) | Methylation-mediated gene silencing | (Bahar et al., 2004) |
| PTTG1 | Functioning and non-functioning adenomas | Gene over-expression | (Salehi et al., 2008) |
| RAS family | Functioning and non-functioning adenomas | Activating mutations | (Karga et al., 1992) |
| RASSF1 | Functioning and non-functioning adenomas | Methylation-mediated gene silencing | (Qian et al., 2005) |
| RASSF3 | Somatotrophinomas | Methylation-mediated gene silencing | (Peng et al., 2013) |
| RB1 | Aggressive adenomas | Rare inactivating mutations, methylation-mediated gene silencing | (Pei et al., 1995) |
| Carcinoma | |||
| SOCS1 | Somatotrophinomas | Methylation-mediated gene silencing | (Buslei et al., 2006) |
| Corticotrophinomas | |||
| Non-functioning adenomas | |||
| SOX2 | Early onset pituitary adenomas | Rare gene deletion | (Alatzoglou et al., 2011) |
| TP53 | Carcinoma | Rare inactivating mutations | (Tanizaki et al., 2007) (Kawashima et al., 2009) |
| Atypical corticotrophinoma | Rare inactivating mutation in one patient | ||
| USP8 | Corticotroph adenomas | Dominant gain of function mutations | (Reincke et al., 2015, Jian et al., 2015) |
Table 2.
Mouse models of pituitary neoplasia.
| Tumour type | Tumour site | Syndrome/Disorder | Mouse model (gene – method) | Gender phenotype | Reference |
|---|---|---|---|---|---|
| Prolactinoma (secreting prolactin) | Isolated | Carney Complex |
Prkar1a – Tissue-specific homozygous knockout |
__ | (Yin et al., 2008) |
| Acromegaly/ Gigantism |
Ghrh – Transgenic over-expression |
__ | (Asa et al., 1992a, Asa et al., 1992b) | ||
| Non-syndromic |
Prl – Heterozygous and homozygous knockout |
Female only | (Cruz-Soto et al., 2002) | ||
|
Prlr – Homozygous knockout |
Male and female | (Schuff et al., 2002) | |||
|
TGFα - Tissue-specific transgenic Over-expression |
Females only | (McAndrew et al., 1995) | |||
|
ptd-FGFR4 – Tissue-specific transgenic Over-expression |
Male and female | (Ezzat et al., 2002) | |||
|
Prop1 – Tissue specific transgenic Over-expression |
Female only | (Egashira et al., 2008, Cushman et al., 2001) | |||
|
Drd2 – Homozygous knockout |
Females only | (Kelly et al., 1997, Asa et al., 1999) | |||
|
Cyclin E – Tissue-specific transgenic over-expression |
__ | (Roussel-Gervais et al., 2010) | |||
| Multiple | MEN1 |
Men1 – Heterozygous knockout |
Higher incidence in females | (Crabtree et al., 2001, Bertolino et al., 2003a, Bertolino et al., 2003b, Biondi et al., 2002, Loffler et al., 2007a, Loffler et al., 2007b, Harding et al., 2009, Lemos et al., 2009) | |
|
Men1 – Tissue-specific homozygous knockout |
Higher incidence in females | (Biondi et al., 2004, Crabtree et al., 2003) | |||
| Non-syndromic |
Pttg:Rb – Tissue-specific transgenic Pttg over-expression: Heterozygous Rb knockout |
Male and female NB Pttg knockout x Rb knockout is protective for pituitary adenomas |
(Donangelo et al., 2006, Chesnokova et al., 2005) | ||
|
p19 – Homozygous knockout |
Male and female | (Bai et al., 2014) | |||
|
Hmga1 – Transgenic over- expression |
Higher incidence in females | (Fedele et al., 2005) | |||
|
Hmga2 – Transgenic over- expression |
Higher incidence in females | (Fedele et al., 2002) | |||
|
AVP-SV40 large T antigen – Transgenic expression |
__ | (Stefaneanu et al., 1992) | |||
| Gonadotrophinoma (secreting follicle stimulating hormone, lutenising hormone, or non-functioning) | Isolated | Non-syndromic | FSHβ-SV40 temperature sensitive T antigen –Transgenic expression | Male only | (Kumar et al., 1998) |
|
Pttg – Tissue-specific transgenic over-expression |
Male and Female | (Donangelo et al., 2006, Abbud et al., 2005) | |||
|
Prop1 – Tissue-specific transgenic Over-expression |
Female only | (Egashira et al., 2008, Cushman et al., 2001) | |||
|
Cyclin E – Tissue-specific transgenic over-expression |
__ | (Roussel-Gervais et al., 2010) | |||
| Multiple | MEN1 |
Men1 – Heterozygous knockout |
Higher incidence in females | (Crabtree et al., 2001, Bertolino et al., 2003a, Bertolino et al., 2003b, Biondi et al., 2002, Loffler et al., 2007a, Loffler et al., 2007b, Harding et al., 2009, Lemos et al., 2009) | |
| Non-syndromic |
Pttg:Rb – Tissue-specific transgenic Pttg over-expression: Heterozygous Rb knockout |
Male and female NB Pttg knockout x Rb knockout is protective for pituitary adenomas |
(Donangelo et al., 2006, Chesnokova et al., 2005) | ||
|
p19 – Homozygous knockout |
Male and female | (Bai et al., 2014) | |||
| Somatotrophinoma (secreting growth hormone) | Isolated | FIPA |
Aip – Heterozygous knockout |
Male and female | (Raitila et al., 2010) |
| Carney Complex |
Prkar1a – Tissue-specific homozygous knockout |
__ | (Yin et al., 2008) | ||
| Acromegaly/ Gigantism |
Ghrh – Transgenic over- expression |
__ | (Asa et al., 1992a, Asa et al., 1992b) | ||
| Non-syndromic |
Prop1 – Tissue-specific transgenic over-expression |
Female only | (Egashira et al., 2008, Cushman et al., 2001) | ||
|
Cyclin E – Tissue-specific transgenic over-expression |
__ | (Roussel-Gervais et al., 2010) | |||
| Multiple | MEN1 |
Men1 – Heterozygous knockout |
Higher incidence in females | (Crabtree et al., 2001, Bertolino et al., 2003a, Bertolino et al., 2003b, Biondi et al., 2002, Loffler et al., 2007a, Loffler et al., 2007b, Harding et al., 2009, Lemos et al., 2009) | |
| Non-syndromic |
Pttg:Rb – Tissue-specific transgenic Pttg over-expression: Heterozygous Rb knockout |
Male and female NB Pttg knockout x Rb knockout is protective for pituitary adenomas |
(Donangelo et al., 2006, Chesnokova et al., 2005) | ||
|
p19 – Homozygous knockout |
Male and female | (Bai et al., 2014) | |||
|
Hmga1 – Transgenic over- expression |
Higher incidence in females | (Fedele et al., 2005) | |||
|
Hmga2 – Transgenic over- expression |
Higher incidence in females | (Fedele et al., 2002) | |||
|
AVP-SV40 large T antigen – Transgenic over-expression |
__ | (Stefaneanu et al., 1992) | |||
| Corticotrophinoma (secreting adrenocorticotropic hormone) | Isolated | Acromegaly/ Gigantism |
p18 – Homozygous knockout |
Male and female | (Franklin et al., 1998) |
|
p18:p27 – Homozygous p18 knockout: Homozygous p27 knockout |
Male and female Accelerated rate of adenomas |
(Franklin et al., 1998) | |||
| Cushing's |
PyLT – Transgenic over- expression |
Male and female | (Helseth et al., 1992) | ||
|
Crh – Transgenic over- expression |
Male and female | (Stenzel-Poore et al., 1992) | |||
|
Crh – Heterozygous gain-of-function mutation (ENU) |
Male and female | (Bentley et al., 2014) | |||
| Non-syndromic |
Pttg:Rb – Tissue-specific transgenic Pttg over-expression: Heterozygous Rb knockout |
Male and female NB Pttg knockout x Rb knockout is protective for pituitary adenomas |
(Donangelo et al., 2006, Chesnokova et al., 2005) | ||
| Multiple | Non-syndromic |
Rb:Ini1 – Heterozygous Rb knockout: Heterozygous Ini1 knockout |
__ Accelerated carcinoma development |
(Guidi et al., 2006) | |
| SV40 large T antigen –Transgenic expression under Pomc promoter | __ | (Low et al., 1993) | |||
|
AVP-SV40 large T antigen – Transgenic over- expression |
__ | (Stefaneanu et al., 1992) | |||
| Thyrotrophinoma (secreting thyroid stimulating hormone) | Isolated | Carney Complex |
Prkar1a – Tissue-specific homozygous knockout |
__ | (Yin et al., 2008) |
| Acromegaly/ Gigantism |
Ghrh – Transgenic over- expression |
__ | (Asa et al., 1992a, Asa et al., 1992b) | ||
| Non-syndromic |
p18:αSU – Homozygous p18 knockout: Homozygous αSU knockout |
__ | (Lloyd et al., 2002) | ||
|
Prop1 – Tissue-specific transgenic over-expression |
Female only | (Egashira et al., 2008, Cushman et al., 2001) | |||
| Multiple | Non-syndromic |
AVP-SV40 large T antigen – Transgenic over- expression |
__ | (Stefaneanu et al., 1992) | |
| Craniopharyngiomas | Isolated | Non-syndromic |
Ctnnb1 – Tissue-specific knockout of exon 3, rendering Ctnnb1 degradation resistant |
__ | (Gaston-Massuet et al., 2011) |
| Undefined adenoma subtype | Isolated | Non-syndromic |
Bmi1 – Tissue-specific transgenic over-expression |
__ | (Westerman et al., 2012) |
| Multiple | MEN4 |
Cdkn1b – Homozygous knockout |
Male and female - females infertile | (Kiyokawa et al., 1996, Nakayama et al., 1996, Fero et al., 1996) | |
|
Cdkn1b – Homozygous inactivating mutation knockin |
__ | (Besson et al., 2006) | |||
| Non-syndromic |
Cdk4 – mutation (rendering protein insensitive to INK4 inhibitors) knockin |
Male and female | (Sotillo et al., 2001) | ||
| Cdk4:Cdkn1b – Transgenic Cdk4 mutation knockin: Homozygous Cdkn1b knockout | __ | (Sotillo et al., 2005) | |||
|
Men1:Cdk2 – Heterozygous Men1 knockout: homozygous Cdk2 knockout |
Male and female | (Gillam et al., 2015) | |||
|
Cyclin E:p27 – Tissue-specific transgenic Cyclin E over-expression: Homozygous p27 knockout |
__ Increased adenoma size, proliferation and frequency |
(Roussel-Gervais et al., 2010) | |||
|
Ink4c:Arf – Homozygous Ink4c knockout: Homozygous Arf knockout |
Male and female | (Zindy et al., 2003) | |||
|
Rb – Heterozygous knockout |
__ | (Jacks et al., 1992) | |||
|
Rb – Tissue-specific homozygous knockout |
__ | (Vooijs et al., 1998, Vooijs et al., 2002) | |||
|
Rb:Arf – Heterozygous Rb knockout: Homozygous Arf knockout |
__ Accelerated tumour development |
(Tsai et al., 2002) | |||
|
Rb:p53 – Heterozygous Rb knockout: Heterozygous and homozygous p53 knockout |
__ | (Harvey et al., 1995) |
- = not defined; MEN1 – multiple endocrine neoplasia type 1; MEN4 - multiple endocrine neoplasia type 4; FIPA – familial isolated pituitary adenomas.
2.1. Generation of animal models
Mutant animal models may be generated using: gene deletion (knockouts); over-expression by transgenic expression of wild type or mutant alleles; mutagenesis using chemicals e.g. N-ethyl-N-nitrosourea (ENU), or radiation; drugs e.g. long-term oestrogen treatment; and the breeding of animals with spontaneously arising abnormalities.
2.1.1. Gene deletion models
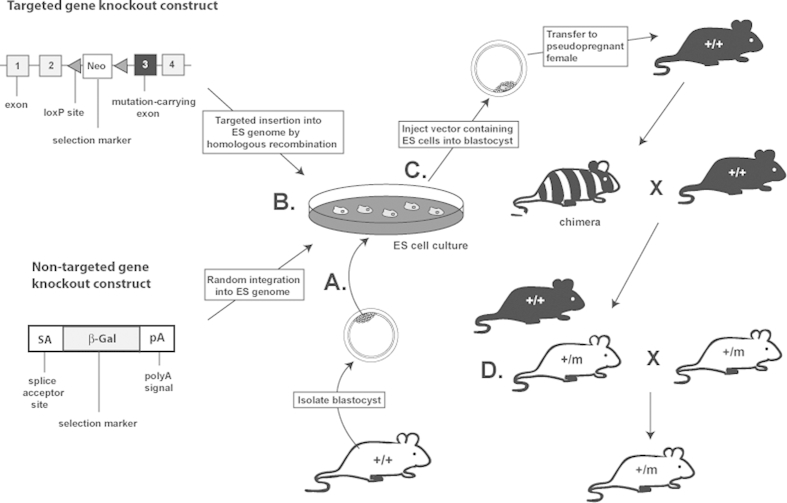
Gene deletion by homologous recombination (also referred to as knockout) is one of the most widely used methods to generate specific mouse models. Methods are based on modifying the gene of interest in embryonic stem (ES) cells (Fig. 1). In conventional knockout models, a vector construct comprising a plasmid or attenuated virus encoding a DNA sequence with homology to the target gene, but carrying a mutated base or bases resulting in loss of the protein, together with a positive selection marker (e.g. Neo), flanked by LoxP sites (allowing subsequent excision from selected cells), is introduced into ES cells where it undergoes homologous recombination resulting in stable integration of the mutated gene into the ES cell genome (Martin, 1981, Hall et al., 2009) (Fig. 1A). These modified ES cells are then injected into a mouse blastocyst to produce chimeric offspring, and ultimately mice heterozygous for the introduced mutation (Fig. 1C). These heterozygous mice may then in turn be interbred to produce homozygous mice, providing the mutation is not embryonic lethal (Fig. 1D). The option of producing either heterozygous or homozygous models can also provide further insights into whether phenotypes are inherited in a dominant or recessive manner. In tumour models this is of particular importance as often patients carry a heterozygous mutation but require a second hit to cause loss of heterozygosity (LOH), and the development of a tumour, for example in patients with MEN1 (Thakker, 2013). New strategies have been developed to generate mouse knockout models on a large scale. For example, non-targeted gene knockout models have been generated using gene trap (Collins et al., 2007a, Collins et al., 2007b) that comprises a plasmid or virus vector containing a promoter-less selectable marker (e.g. β-galactosidase) flanked by a splice acceptor site and a polyA signal, that when introduced into ES cells randomly inserts into the genome (Fig. 1B). If the vector inserts into the intron of a gene then the splice acceptor generates fusion transcripts of the selection marker with the exons upstream in the endogenous gene, thereby leading to truncation and knockout of the protein (Stanford et al., 2001). As the gene trap randomly inserts into the ES cell genome sequencing analysis can be performed to identify the site of gene trap insertion and therefore to confirm the gene knockout. More recently a targeted gene trapping method has been developed in which the vector also contains regions homologous to the gene of interest (Collins et al., 2007a, Collins et al., 2007b, Friedel et al., 2005).
Fig. 1.
Gene knockout methods using embryonic stem (ES) cells. A Totipotent ES cells are isolated from the inner cell mass of a blastocyst from a wild type mouse and cultured. B Targeted or non-targeted vectors are introduced into the genome of the ES cells. ES cells in which homologous recombination or random integration and i.e. gene knockout, has occurred are selected using incorporated markers (e.g. Neo). C Selected ES cells are injected into a blastocyst obtained from a different strain to that in step A, and implanted into a pseudopregnant female, which is the same strain as the injected blastocyst. This results in chimeric offspring containing genetic information from both the manipulated ES cells of the original mouse strain and genetic information of the second blastocyst/pseudopregnant female of a different strain. To generate offspring heterozygous for the gene knockout, chimeric offspring are bred with wild type mice. D Heterozygous offspring can be interbred to generate homozygous knockout mice. m-mutated allele; + – wild type allele.
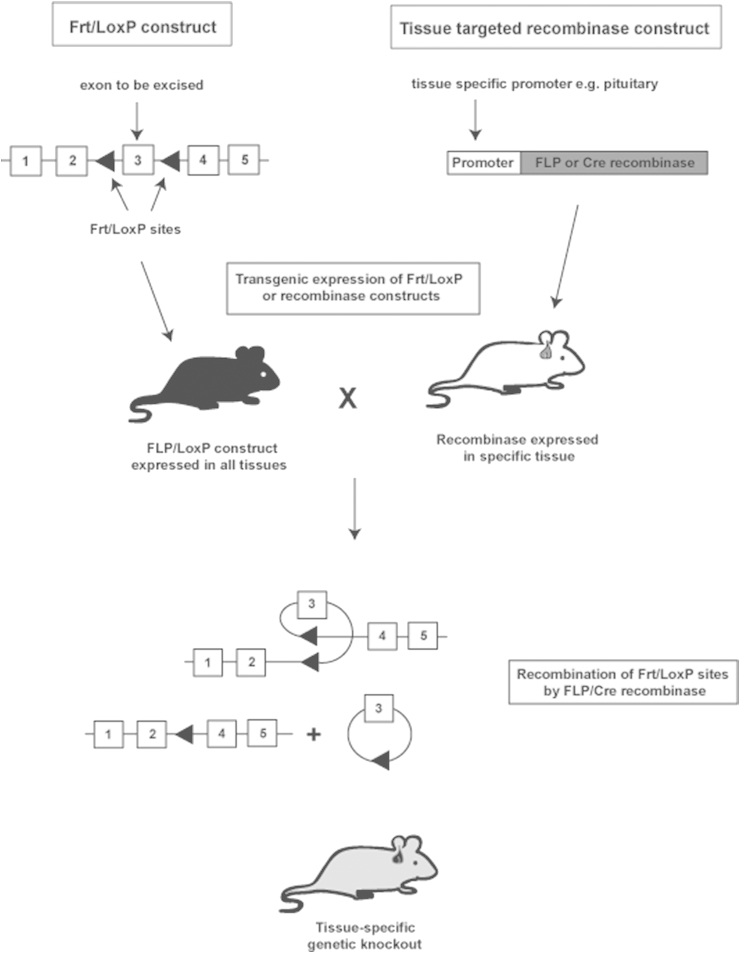
In conventional or gene trap knockout models, generation of homozygous mice may result in embryonic lethality, as illustrated by Men1−/− mice (Crabtree et al., 2001, Bertolino et al., 2003a, Bertolino et al., 2003b, Loffler et al., 2007a, Loffler et al., 2007b, Harding et al., 2009, Fisher et al., 2009, Lemos et al., 2009), indicating that the encoded protein, menin, plays a pivotal role in embryonic development. This embryonic lethality can be overcome by use of conditional knockout models that are tissue or cell type specific. These models can be produced by utilising Cre-LoxP or FLP-Frt systems (Fig. 2), whereby the genomic region of interest is flanked by LoxP or flippase (FLP) recombination target (Frt) sites. These sites are recognised by Cre recombinase or FLP enzymes, respectively, which excise the DNA sequence floxed by the LoxP or Frt sites (Michael et al., 1999). This method of generation requires two mouse lines, one line containing the genomic region of interest flanked by LoxP or Frt and another line expressing the tissue-targeted Cre or FLP, which are generated by transgenic methods (see Section 2.1.2). These mice are then crossed to generate mice expressing both the flanked construct and the recombinase. Tissue targeting of the Cre or FLP is achieved by restricting their expression using a tissue-specific promoter, for example rat growth hormone releasing hormone receptor (Ghrh receptor) to restrict Cre expression to the pituitary (Yin et al., 2008). Inducible models that allow control over the timing of gene knockout can also be generated using fusion proteins. For example, a modified ligand-binding domain of the oestrogen receptor can be fused to Cre, which only upon administration of tamoxifen (which binds the oestrogen receptor), translocates to the nucleus and excises the floxed DNA region, allowing knockout of the gene at a chosen time point during the animals life span (Fisher et al., 2009).
Fig. 2.
Conditional gene knockout. Gene knockout models can be generated using the FLP-Frt or Cre-LoxP systems. This requires the generation of two constructs: 1) a construct containing Frt or LoxP recognition sites inserted into the intron sequences flanking the genomic region to be knocked out; and 2) a construct containing a FLP or Cre recombinase under the control of a tissue-specific promoter. These constructs are introduced into two different mouse strains using knockin/transgenic over-expression methods, to generate one mouse expressing the Frt/LoxP flanked genomic sequence in all tissues, and one mouse expressing FLP/Cre recombinase in a specific organ e.g. pituitary. These two mouse lines are then bred to generate a mouse containing both the Frt/LoxP flanked genomic sequence and the tissue-specific recombinase. In FLP/Cre-expressing tissues e.g. pituitary, the FLP/Cre binds to its target Frt/LoxP sites and catalyses recombination of the DNA, leading to excision of the genetic material contained between the target sites.
2.1.2. Knockin and transgenic models
Knockin and transgenic models can be generated to assess gain-of-function mutations or constitutive over-expression of genes, respectively. The targeted knockin approach is similar to the conventional knockout method described above and in Fig. 1, except that the introduced construct is designed to cause gene over-expression, for example by incorporation of a gain-of-function mutation (Hall et al., 2009, Piret and Thakker, 2011). The transgenic model differs from the knockin model in that a cDNA construct, containing cDNA of either an endogenous gene (i.e. wild type construct) or a gene of interest carrying a desired mutation, and an appropriate promoter and poly(A) sequence, is injected into the pronucleus of fertilised mouse oocyte, which is implanted into a pseudopregnant female (Haruyama et al., 2009). This transgene can randomly insert into the genome, thereby introducing additional genetic information, which upon transcription results in over-expression of the inserted wild type or mutant gene (Piret and Thakker, 2011, Gordon et al., 1980). Transgenic approaches can therefore provide a tool to examine dominant negative effects of mutant proteins, as often mutations are heterozygous and therefore patients express both mutant and wild type proteins.
2.1.3. Gene editing methods
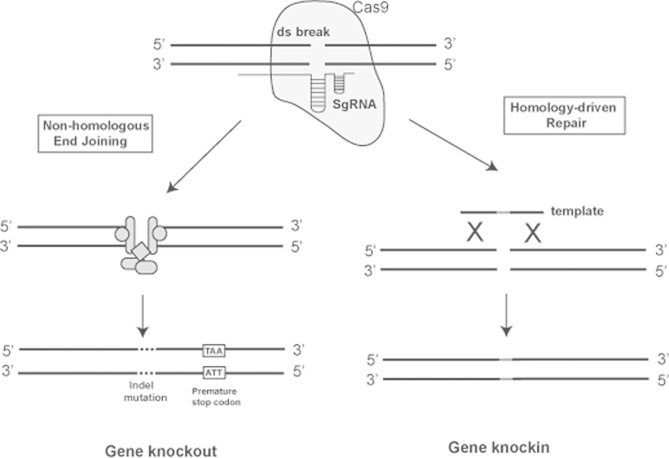
Recent advances in generating animal models involve the technique of genome editing. Gene editing can be achieved by multiple methods including the use of zinc finger nucleases and transcription activator-like effector nucleases (TALENs). The most recently developed method of genome editing, however, is that using the clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR associated (Cas) system (Seruggia and Montoliu, 2014). The CRISPR/Cas system utilises a prokaryotic adaptive immune response mechanism in which RNA transcribed from small segments of plasmids or viral genomes that have been incorporated as CRISPRs during an earlier infection, are used to target Cas9 proteins to new infections where they cleave the DNA and therefore inactivate invading plasmids or viruses (Seruggia and Montoliu, 2014). This system can be used to target Cas9 proteins to specific DNA regions by developing a synthetic targeting RNA template, to cause double strand breaks, which depending on the DNA repair mechanisms employed, allows both the generation of knockout and knockin models (Fig. 4). Mouse models can be produced by either editing an ES cell or by direct microinjection into a zygote, with the advantage that multiple genes can be targeted in parallel, making the generation of, for example double knockout mice more efficient as it negates the need for multiple breeding cohorts (Wang et al., 2013).
Fig. 4.
Gene editing using CRISPR/Cas. The CRIPSR/Cas system requires three components: a CRISPR-associated nuclease, for example Cas9; a single guide RNA (SgRNA) consisting of a guide sequence that binds the target DNA, a scaffold sequence for Cas9 binding and a terminating hairpin; and for gene knockin an oligo template containing the sequence to be inserted. The Cas9 is targeted to a specific genomic site by the SgRNA, where it induces a double strand break. In the absence of a repair template this break is repaired by non-homologous end joining, an error prone mechanism that leads to insertion/deletion (indel) mutations, which in turn cause frameshifts and the occurrence of premature stop codons, thereby leading to gene knockouts. If a DNA repair template is present the double strand break is repaired by a homology-driven repair mechanism, using this template, therefore genetic information can be knocked-in by including, for example, gain-of-function point mutations in the inserted template.
2.1.4. Chemical or radiation induced mutagenised models
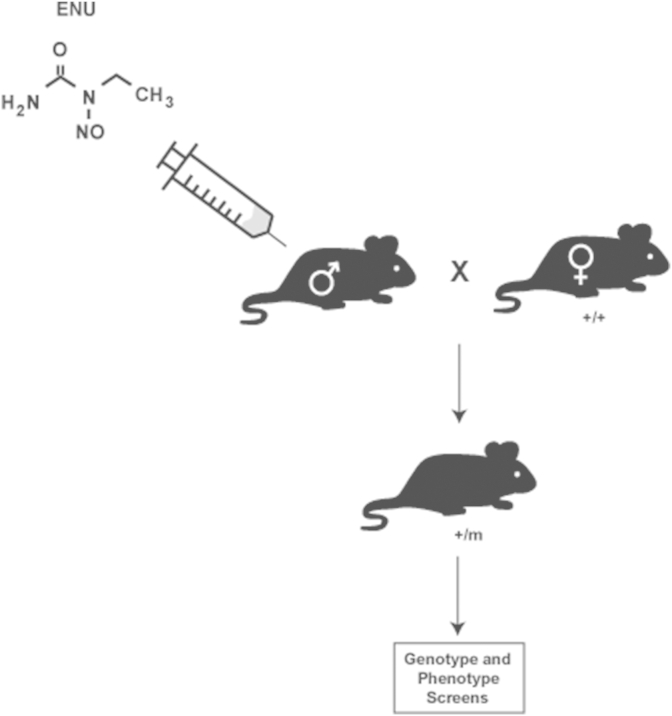
Chemicals and radiation can induce alterations in the DNA sequence, which can lead to either gain-of-function (hypermorphic), loss-of-function (hypomorphic) or haploinsufficiency of inherited mutations. The alkylating agent N-ethyl-N-nitrosourea (ENU) is the most potent mutagen in mice, introducing point mutations via transfer of the ENU alkyl group to DNA bases, and thereby causing mispairing during DNA replication (Piret and Thakker, 2011, Acevedo-Arozena et al., 2008). The ENU is injected into male mice to mutagenise sperm and allow the mutations to be inherited, and offspring assessed for both genetic and phenotypic differences (Fig. 3). For screening genetic differences, archives of DNA can be examined for mutations in a gene of interest (Acevedo-Arozena et al., 2008). For phenotypic screening, the offspring of the mutagenised mice are assessed using biochemical, morphological and behavioural tests and then the DNA sequenced to elucidate novel genes associated with disease (Acevedo-Arozena et al., 2008, Bentley et al., 2014).
Fig. 3.
N-ethyl-N-nitrosourea (ENU) mutagenesis. ENU is a chemical mutagen that induces point mutations via transfer of its ethyl group (CH3) to guanine residues, which in turn causes mispairing during DNA replication. ENU is injected into male mice where it causes mispairing during spermatogenesis, and therefore induces mutations into sperm DNA. Mutagenised mice are mated with female mice of the same strain to generate offspring that have inherited the introduced mutations. Genetic and phenotypic screens are performed to characterise the mutation. m-mutated allele; +–wild type allele.
2.1.5. Spontaneously occurring mutations
Phenotypes can also occur spontaneously in animal models, for example due to either naturally occurring mutations, or viral incorporation. This is especially apparent in neoplasia models, and some strains of mice (e.g. C3H) and rats (e.g. Sprague–Dawley) have been reported to have a much higher tumour incidence, compared to other strains (Dusing-Swartz et al., 1979, Son and Gopinath, 2004, Rosenberg et al., 2009, Son et al., 2010, Mitsui et al., 2013, Carlus et al., 2013). Spontaneous models however are generally less predictable than those specifically generated.
2.1.6. Non-genetic methods
Non-genetic methods have also been used to generate models of pituitary neoplasia. These include long-term hormone administration to induce tumour development, for example oestrogen treatment of ovariectomised rats (Mitsui et al., 2013, Wiklund et al., 1981a, Wiklund et al., 1981b) and injection of carcinogenic agents, for example cadmium (an element able to mimic oestrogen) to induce the development of multiple tumour types (Waalkes, 2003). The use of chemical carcinogens is advantageous as they provide mechanistic insights into environmental factors contributing to tumour development. There are however environmental concerns with their use, and reproducibility.
2.1.7. Animal model repositories
Conventional gene knockout, gene knockin and transgenic methods have been successful in generating models of pituitary neoplasia. Many of these models are available from repositories, as live mice, for example through the European Mouse Mutant Archive (EMMA; Europe; http://strains.emmanet.org/), the Wellcome Trust Sanger Institute (UK; https://www.sanger.ac.uk/resources/mouse/) or Jackson Laboratory (USA; http://jaxmice.jax.org/). These repositories also have available mutagenised ES cell clones, to establish mouse models. Information on the models and availability of ES cells can be found from, for example, the International Mouse Phenotyping Consortium (IMPC; https://www.mousephenotype.org/). In addition, ENU mutagenised sperm and live mice are available from MRC Harwell (UK; http://www.har.mrc.ac.uk/).
2.2. Mouse pituitary neoplasia models
The majority (∼90%) of existing pituitary neoplasia models have been established using mice (Table 2). These models have been predominantly generated using gene knockout and over-expression methods.
2.2.1. Knockout models
To date, homologous deletions of 10 different genes, which are Men1, Cdkn1b, Prkar1a, Rb, Cdkn2b (encoding p19), Drd2, Cdkn2c (encoding p18), Aip, Prl and Prlr, have been reported to yield mouse knockout models for pituitary tumours. Of these 10 genes, 5 (Men1, Cdkn1b, Prkar1a, Cdkn2b and Aip) are tumour suppressors that are associated with human familial disorders with pituitary tumours. Thus, Men1+/− mice develop tumours consistent with MEN1 (Crabtree et al., 2001, Bertolino et al., 2003a, Bertolino et al., 2003b, Biondi et al., 2002, Loffler et al., 2007a, Loffler et al., 2007b, Harding et al., 2009), which is characterised by the occurrence of tumours of the parathyroid glands (in ∼95% of patients), pancreatic islets (in ∼40% of patients) and the anterior pituitary (in ∼30% of patients) (Thakker, 2013). The majority of pituitary adenomas in MEN1 patients are prolactinomas, followed by somatotrophinomas, corticotrophinomas and non-functioning adenomas (Thakker, 2013). Pituitary adenomas occurring in individuals with MEN1 are generally larger and more aggressive than sporadic tumours, and 2–3% of sporadic pituitary adenomas have MEN1 mutations (Thakker, 2013, Verges et al., 2002, Machens et al., 2007). The MEN1 gene is located on human chromosome 11q13 and contains 10 exons, with exon 1 non-coding. More than 1000 different germline mutations have been found in the coding region and splice sites of the MEN1 gene, with the majority (75%) predicted to yield truncated forms and loss of its encoded protein, menin (Lemos and Thakker, 2008). The knowledge of these mutations, and that loss of menin can cause tumour development, has led to the generation of a number of Men1 mouse models. In mice the Men1 gene is located on chromosome 19, but has an exon-intron organisation similar to that of the human gene (i.e. 10 exons), and the mouse menin protein shows 97% homology with the human menin protein (Stewart et al., 1998). Six different mouse models for MEN1 have been generated, consisting of 4 conventional heterozygous and 2 conditional homozygous Men1 knockouts, and 5 of these 6 models develop pituitary adenomas (Crabtree et al., 2001, Bertolino et al., 2003a, Bertolino et al., 2003b, Biondi et al., 2002, Loffler et al., 2007a, Loffler et al., 2007b, Harding et al., 2009, Biondi et al., 2004, Crabtree et al., 2003). The 4 conventional Men1+/− models consist of 1 with deletion of exons 3–8 (Crabtree et al., 2001), 1 with deletion of the transcriptional start site and exon 2 (Biondi et al., 2002, Loffler et al., 2007a, Loffler et al., 2007b), 1 with deletion of exon 3 (Bertolino et al., 2003a, Bertolino et al., 2003b) and 1 with deletion of exons 1 and 2 (Harding et al., 2009). In each of these models pituitary tumours occurred in 26–45% of mice by 18 months of age, with prolactin-expressing tumours being the most common. For each of the conventional models homozygous (Men1−/−) mice were embryonic lethal. To study the effects of complete loss of menin in specific tissues and organs, 2 conditional models have been generated. In one model, mice with Men1 exons 3–8 floxed with LoxP were crossed with mice expressing Cre under the control of the rat insulin promoter, which as well as being expressed in pancreatic islets is weakly expressed in the pituitary. This resulted in the occurrence of pancreatic beta cell tumours, and pituitary adenomas which immunostained for prolactin in up to 58% of mice (Crabtree et al., 2003). In another model exon 3 of Men1 was floxed with LoxP sites and crossing this line with mice expressing Rip-Cre, was reported to result only in the development of pancreatic tumours (Bertolino et al., 2003a).
Mouse models that have also been generated for other pituitary adenoma associated human disorders include: MEN4; Carney Complex; Acromegaly/gigantism and FIPA. Thus, Cdkn1b+/− mice develop tumours consistent with MEN4, which is characterised by the occurrence of pituitary and parathyroid tumours in association with other endocrine tumours (e.g. of gonads and adrenals) (Kiyokawa et al., 1996, Nakayama et al., 1996, Fero et al., 1998). Prkar1a+/− mice develop tumours consistent with Carney Complex syndrome, which is characterised by increased occurrence of different tumour types including myxomas, schwannomas, and endocrine tumours, which include somatotrophinomas, adrenal cortical tumours, sertoli cell tumours, ovarian cysts and thyroid follicular adenomas (Yin et al., 2008). Cdkn2c+/− mice develop tumours consistent with acromegaly/gigantism, due to GH secreting tumours (Franklin et al., 1998). The phenotypic features of Aip+/− mice are similar to that observed in FIPA patients, who have AIP mutations and predominately develop GH secreting adenomas, although some patients may also develop PRL or ACTH secreting and non-functioning adenomas (Leontiou et al., 2008, Vierimaa et al., 2006, Raitila et al., 2010). These models provide valuable resources to study the mechanisms causing pituitary tumours. For example, investigation of the Aip+/− mouse model has revealed that the associated pituitary tumours have activation of a hypoxic response, with induction of HIF-1α expression, and that signalling through the HIF-1α binding partners aryl hydrocarbon receptor nuclear translocator (ARNT) and ARNT2 is a key factor in the development of pituitary tumours, after loss of Aip (Raitila et al., 2010).
Studies of other mouse models deleted for genes that were not previously known to be associated with the development of pituitary tumours in man have revealed roles for such genes, which includes Rb, Cdkn2b, Drd2, Prl and Prlr, in pituitary tumourigenesis. The Rb protein is a tumour suppressor with an integral role in cell cycle progression, controlling the passage from G1 into S phase (Weinberg, 1995) and ∼90% of patients with a heterozygous germline RB mutation develop childhood onset retinoblastoma. However, Rb+/− mice were found not to develop retinoblastoma, but instead to develop pituitary carcinoma, and therefore provide a valuable model for studying the development of pituitary carcinomas (Jacks et al., 1992, Vooijs et al., 1998, Vooijs et al., 2002). Moreover, use of the FLP-frt system to generate conditional Rb knockout mice has revealed that mice null for Rb specifically in the pituitary, rapidly develop melanotroph tumours (Vooijs et al., 1998), and showed that Rb loss is an initiating step in carcinogenesis, leading to inappropriate entry into S phase of the cell cycle (Vooijs et al., 1998). In addition, use of the Cre-LoxP system to knockout Rb in the mouse pituitary proopiomelanocortin (POMC) cell lineages (corticotrophs and melanotrophs), together with expression of firefly luciferase under the control of the POMC promoter has generated a model allowing real time imaging of tumour growth, using bioluminescence, which allows accurate evaluation of the efficacy novel therapies including the chemotherapeutic action of doxorubicin (Vooijs et al., 2002). Finally, the role of p19, encoded by Cdkn2b, as a tumour suppressor in regulating pituitary anterior lobe proliferation has been revealed by a conventional knockout mouse model of Cdkn2b, as Cdkn2b−/− mice developed multiple tumour types including PRL, GH and FSH secreting pituitary adenomas (Bai et al., 2014).
2.2.2. Over-expression and knockin models
Over-expression models have been generated to investigate the roles: of hormones in pituitary tumourigenesis, e.g. over-expression of the growth hormone releasing hormone (Ghrh) gene in mice resulted in pituitary adenomas that led to excessive GH secretion (Asa et al., 1992b); and of oncogenes and tumourigenic viruses. Thus, over-expression of some viral proteins, such as Simian virus 40 (SV40) T antigen, which have been termed ‘oncoviruses’ as infection of tissues can lead to tumour development, generated a mouse model with somatotrophinomas (Stefaneanu et al., 1992), and over-expression of a polyoma early region promoter linked to a cDNA encoding polyoma large T antigen (PyLT), in mice, induced development of ATCH-secreting pituitary tumours, thereby providing a model for Cushing's disease (Helseth et al., 1992). Transgenic over-expression of cortocotropin releasing hormone (Crh) in mice has also generated a model of Cushing's disease (Stenzel-Poore et al., 1992), and an ENU induced mouse mutant with a Crh promoter mutation has been reported to develop Cushing's Syndrome (Bentley et al., 2014). In addition, use of constructs containing promoters of genes expressed in the pituitary has facilitated generation of targeted models. Thus, mice harbouring a transgene with the SV40 early gene encoding large T antigen ligated to the POMC promoter, developed melanotroph tumours (Low et al., 1993), and mice harbouring the SV40 T antigen targeted to gonoadotroph cells using the follicle stimulating hormone β (Fshβ) promoter, generated a model of non-functioning adenomas (Kumar et al., 1998).
The role of the pituitary transforming gene (Pttg) has also been established by the use of tissue-specific transgenic over-expression mouse models (Donangelo et al., 2006, Abbud et al., 2005). Pttg, which was first identified from rat pituitary tumour cells (Pei and Melmed, 1997), and subsequently shown to be over-expressed in human pituitary tumours (Saez et al., 1999), encodes a securin protein that plays a role in cell transformation, aneuploidy, apoptosis and tumour microenvironment communication (Vlotides et al., 2007). Mice with targeted pituitary Pttg over-expression, driven by the pituitary specific alpha subunit glycoprotein promoter have been reported to develop focal pituitary hyperplasia, whilst mice with Pttg inactivation have pituitary hypoplasia (Donangelo et al., 2006), consistent with a pituitary tumourigenic role of Pttg.
The tumourigenic role of the High mobility group A (Hmga) genes has also been investigated by transgenic over-expression in mouse models. HMGA proteins are architectural transcription factors as they regulate the assembly of complexes important for gene transcription (Fedele et al., 2006). The two HMGA genes, HMGA1 and HMGA2, are expressed at high levels during embryogenesis, and in some human carcinomas, but are not expressed in the majority of adult normal tissues (Fedele et al., 2006). The oncogenic potential of the HMGA genes in pituitary tumourigenesis has been evaluated by the generation of global transgenic over-expression mouse models (Fedele et al., 2005, Fedele et al., 2002). Over-expression of either Hmga1 or Hmga2 resulted in the development of mixed somatotroph/lactotroph pituitary adenomas by 16 months of age (Fedele et al., 2005, Fedele et al., 2002), and Hmga2 has been reported to enhance E2F1 activity, which is usually repressed by Rb to prevent progression through the cell cycle (Fedele et al., 2006).
Knockin mouse models of the cyclin dependent kinases (CDKs) and cyclin dependent kinase inhibitors (CKIs) have been reported to develop pituitary tumours (Sotillo et al., 2001, Besson et al., 2006, Roussel-Gervais et al., 2010). The CKIs, p16, p15, p18 and p19, inhibit the activity of the CDKs CDK4 and CDK6, thereby preventing phopsphorylation of Rb, and G1 to S phase transition (Cicenas et al., 2014), and a mouse with a Cdk4 point mutation (Arg24Cys) that resulted in insensitivity to the CKIs (Sotillo et al., 2001), developed tumours of the pituitary, pancreas and testes (Sotillo et al., 2001). In addition, a mouse with a mutation (Ser10Ala) of Cdkn1b, which encodes p27, a member of the p21 family of CKIs that abrogated its ability to bind CDKs (Besson et al., 2006), resulted in the development of pituitary adenomas (Besson et al., 2006). Moreover, one of the target cyclin complexes of p27 is cyclin E-CDK2, and a transgenic over-expression mouse model has indicated that cyclin E expression in the POMC lineage is sufficient to cause cells to re-enter the cell cycle and initiate pituitary adenoma growth (Roussel-Gervais et al., 2010). These mouse models, which highlight the critical role of the G1 checkpoint in pituitary tumourigenesis, provide pre-clinical models for assessing the efficacy of small molecule inhibitors of cyclin dependent kinases that are being developed for the treatment of cancers (Cicenas et al., 2014).
2.2.3. Double mutant models
Studies of double mutant mouse models have provided important in vivo mechanistic insights into cell cycle regulation during pituitary tumour development, and the progression of benign pituitary adenomas to carcinomas. Thus, 12 double mutant mouse models comprising: Pttg/Rb+/−; Rb+/−/Ini1+/−; Rb+/−/Arf−/−; Rb+/−/p53−/−; Cdk4R/R/Cdkn1b−/−; CyclinE/p27−/−; p18−/−/p27−/−; p18−/−/αSU−/−; Ink4c−/−/Arf−/−; Men1+/−/Rb+/−; Men1+/−/Cdk2−/− and Men1+/−/Cdk4−/−, have been generated (Table 2). Five of these double mutant mouse models utilised Rb+/− mice to investigate co-operative pathways in pituitary tumourigenesis, and revealed that: over-expression of the pituitary hyperplasia-promoting gene Pttg in combination with Rb+/− increases the volume and prevalence of anterior pituitary tumours, whereas loss of Pttg expression, as occurring in Pttg−/− mice can protect against tumours initiated by loss of Rb (Donangelo et al., 2006, Chesnokova et al., 2005); Rb is epistatic to a member of the SW1/SNF chromatin remodelling complex, Ini1, in tumour suppression (Guidi et al., 2006); loss of the tumour suppressor proteins p53 or Arf (that stabilises p53 protein), as in occurring in p53−/− or Arf−/− mice, in combination with Rb+/− accelerated pituitary tumour development (Harvey et al., 1995, Tsai et al., 2002); and Men1+/−/Rb+/− mice did not have significant differences in the occurrence or age of onset of pituitary, or other tumours, when compared to Men1+/− or Rb+/− mice (Loffler et al., 2007a, Loffler et al., 2007b), thereby indicating that menin and Rb do not have an additive effect and are therefore likely to function in a common pathway to suppress tumour development. The remaining 7 double mutant models have utilised loss of CKIs from both the INK4 and p21 family to investigate the interaction between different cyclins, CDK, CKIs and their substrates (Rb) in the pituitary (Franklin et al., 1998, Roussel-Gervais et al., 2010, Sotillo et al., 2005, Zindy et al., 2003, Lloyd et al., 2002). Thus, Cdk4R/R/Cdkn1b+/− and Cdk4R/R/Cdkn1b−/− mice, which would be null for p27 and have a mutant of CDK4 that renders it insensitive to INK4 inhibitors, have been reported to develop pituitary tumours with complete penetrance and a short latency, thereby indicating that there is co-operativity between p27 and CDK4 (Sotillo et al., 2005). Such co-operativity was not identified to occur between p18 and CDK4 in similar studies using the Cdk4R/R/Cdkn2c−/− mouse model (Sotillo et al., 2005). These results indicate that p27 may have a wider function that just inhibiting CDK4 activity, which has previously been considered to be the predominant function of p18. Moreover, it has also been reported that up-regulation of cyclin E (using a transgene construct under the control of the POMC promoter, Tg-PCE) and knockout of p27 (Cdkn1b−/−) in the Tg-PCE/Cdkn1b−/−mouse model, leads to increased pituitary adenoma incidence and frequency (Roussel-Gervais et al., 2010) and that p27 and p18 mediate two separate pathways to collaboratively suppress tumourigenesis (Franklin et al., 1998). Men1+/−/Cdk4−/− mice have been demonstrated to not develop any tumours, whereas Men1+/−/Cdk2−/− mice develop pituitary and pancreatic tumours comparable to those in Men1+/− only mice. (Gillam et al., 2015). This indicates that Cdk4 activity is important for Men1-associated tumourigenesis to occur, and that menin may predominantly suppress cell cycle progression through the INK4-Cdk4/6-cyclin D-pRb pathway. Overall, these studies of mutant mouse models with targeted disruption of the cell cycle regulating proteins have provided mechanistic insights in pituitary tumourigenesis.
2.3. Rat pituitary neoplasia models
Some rat strains, including Sprague Dawley rats, are more prone to pituitary tumour development (Son and Gopinath, 2004, Son et al., 2010, Carlus et al., 2013). For example, a variant of the MEN syndromes, termed MENX, was discovered to occur spontaneously in Spague–Dawley rats, which developed parathyroid adenomas, pancreatic islet cell hyperplasia, thyroid c-cell hyperplasia, bilateral phaeochromocytomas and paragangliomas (Marinoni et al., 2013). MENX was inherited as an autosomal recessive disorder and the rats were found to have a germline homozygous frameshifting insertion mutation in the Cdkn1b gene (Fritz et al., 2002). Studies in patients with MEN1-like tumours, but without MEN1 mutations, revealed some to have CDKN1B mutations. To date 10 different MEN4-associated mutations of CDKN1B have been reported, and the MEN4 patients have parathyroid tumours in association with pituitary adenomas and other tumours of the gonads, adrenals, thyroid and kidney (Thakker, 2013, Pardi et al., 2014).
Rat models of pituitary neoplasia have also been generated by implanting tumour cells or using drugs. Thus, a rat model for Cushing's syndrome was generated by implanting a medullary thyroid carcinoma cell line, which stably over-expressed CRH, into WAG/Rij rats (Asa et al., 1992a). Exposure of these rats to elevations in circulating CRH, increased anterior pituitary cell proliferation and circulating ACTH levels (Asa et al., 1992a, Asa et al., 1992b). In addition, a rat model for lactotrophinomas was generated by long-term administration of oestrogens to the F344 and Holtzmon strains (Wiklund et al., 1981a, Wiklund et al., 1981b). Such susceptibility to oestrogen treatment in inducing pituitary tumours in rats varies between strains. For example, oestrogen treatment has been shown to differ between strains, with F344 female rats the most susceptible (Mitsui et al., 2013, Wiklund et al., 1981a, Wiklund et al., 1981b), and with Wistar-Kyoto rats not developing pituitary tumours upon oestrogen treatment due to lack of lactotroph cell proliferation (Mitsui et al., 2013). Oestrogens are known to stimulate expression of IGF-1, which leads to increased proliferation. However in Wistar-Kyoto rats the response to IGF-1 was reduced, with IGF-1 target gene expression (Wnt4, Stc1, Mybl1 and Myc) attenuated or abolished (Mitsui et al., 2013), and this may explain the lack of lactotroph cell proliferation following oestrogen treatment in Wistar-Kyoto rats.
2.4. Zebrafish pituitary neoplasia model
A zebrafish model that develops corticotroph adenomas has been generated by utilising transgenic expression of the Pttg gene that is targeted specifically to the pituitary POMC lineages (Liu et al., 2011). This study illustrates that zebrafish models for pituitary tumours can be generated, and opens the way to utilising additional methods for the study of pituitary tumourigenesis. Thus, zebrafish knockout models for pituitary tumours could be generated by using morpholino oligomers, which are antisense oligomers that bind complementary messenger RNAs thereby blocking gene expression and leading to gene knockdown (Angotzi et al., 2011). In addition, ENU mutagenesis can be used to generate hypermorphic or hypomorphic phenotypes in zebrafish (Solnica-Krezel et al., 1994). Studies in zebrafish offer many advantages, such as a high throughput platform for assessing gene abnormalities and for drug efficacy studies, as demonstrated by a study that evaluated the use of the cell cycle inhibitor R-roscovitine for the treatment of corticotroph tumours, the results of which were subsequently confirmed in a mouse model (Liu et al., 2011).
3. Use of the Men1+/− mouse for evaluating novel therapies
Mouse models for pituitary tumours have provided pre-clinical models for evaluating new therapies for pituitary tumours. This is illustrated by the use of the Men1 mouse model, which has been used to assess the role of Men1 gene replacement therapy and a monoclonal antibody to the vascular endothelial growth factor (VEGF) (Walls et al., 2012, Korsisaari et al., 2008). The tumour suppressor role of menin, encoded by Men1, suggested that restoration of menin expression in tumours would reduce proliferation. Indeed, gene replacement therapy using an adenoviral vector containing Men1 cDNA that was delivered directly to the pituitary tumours of Men1+/− mice, restored menin expression in the pituitary tumours and reduced their proliferation, without significant adverse effects or increased mortality (Walls et al., 2012). These findings indicate that viral delivery of MEN1 may be a potential treatment for pituitary and other neuroendocrine tumours associated with MEN1 mutations. Pituitary tumours, in common with other neoplasms, have increased angiogenesis, and angiogenic pathways that can be targeted by monoclonal antibodies to VEGF. Indeed, the monoclonal antibody G6-31 has been reported to inhibit VEGF-induced pituitary tumour growth in conventional Men1+/− mice (Korsisaari et al., 2008). Such mouse models therefore provide an important resource for the pre-clinical evaluation of novel therapies for pituitary tumours.
4. Conclusion
Studies of animal models of pituitary neoplasias have helped to advance our knowledge about the biological mechanisms of pituitary tumourigenesis. Thus, these models, predominately in mice, which have been generated by a number of approaches including gene knockout and over-expression of genes, have provided novel insights into the regulation of the cell cycle and its dysfunction in pituitary tumourigenesis. In addition, they provide pre-clinical models for the evaluation of novel and emerging therapies.
Acknowledgements
This work was supported by the: United Kingdom Medical Research Council (MRC) programme grants (G9825289 and G1000467 to K.E.L, M.S. and R.V.T.)
References
- Abbud R.A., Takumi I., Barker E.M., Ren S.G., Chen D.Y., Wawrowsky K., Melmed S. Early multipotential pituitary focal hyperplasia in the alpha-subunit of glycoprotein hormone-driven pituitary tumor-transforming gene transgenic mice. Mol. Endocrinol. 2005;19:1383–1391. doi: 10.1210/me.2004-0403. [DOI] [PubMed] [Google Scholar]
- Acevedo-Arozena A., Wells S., Potter P., Kelly M., Cox R.D., Brown S.D. ENU mutagenesis, a way forward to understand gene function. Annu. Rev. Genomics Hum. Genet. 2008;9:49–69. doi: 10.1146/annurev.genom.9.081307.164224. [DOI] [PubMed] [Google Scholar]
- Agarwal S.K., Mateo C.M., Marx S.J. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J. Clin. Endocrinol. Metab. 2009;94:1826–1834. doi: 10.1210/jc.2008-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatzoglou K.S., Andoniadou C.L., Kelberman D., Buchanan C.R., Crolla J., Arriazu M.C., Roubicek M., Moncet D., Martinez-Barbera J.P., Dattani M.T. SOX2 haploinsufficiency is associated with slow progressing hypothalamo-pituitary tumours. Hum. Mutat. 2011;32:1376–1380. doi: 10.1002/humu.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angotzi A.R., Mungpakdee S., Stefansson S., Male R., Chourrout D. Involvement of Prop1 homeobox gene in the early development of fish pituitary gland. Gen. Comp. Endocrinol. 2011;171:332–340. doi: 10.1016/j.ygcen.2011.02.026. [DOI] [PubMed] [Google Scholar]
- Asa S.L., Kovacs K., Hammer G.D., Liu B., Roos B.A., Low M.J. Pituitary corticotroph hyperplasia in rats implanted with a medullary thyroid carcinoma cell line transfected with a corticotropin-releasing hormone complementary deoxyribonucleic acid expression vector. Endocrinology. 1992;131:715–720. doi: 10.1210/endo.131.2.1322279. [DOI] [PubMed] [Google Scholar]
- Asa S.L., Kovacs K., Stefaneanu L., Horvath E., Billestrup N., Gonzalez-Manchon C., Vale W. Pituitary adenomas in mice transgenic for growth hormone-releasing hormone. Endocrinology. 1992;131:2083–2089. doi: 10.1210/endo.131.5.1425411. [DOI] [PubMed] [Google Scholar]
- Asa S.L., Kelly M.A., Grandy D.K., Low M.J. Pituitary lactotroph adenomas develop after prolonged lactotroph hyperplasia in dopamine D2 receptor-deficient mice. Endocrinology. 1999;140:5348–5355. doi: 10.1210/endo.140.11.7118. [DOI] [PubMed] [Google Scholar]
- Bahar A., Simpson D.J., Cutty S.J., Bicknell J.E., Hoban P.R., Holley S., Mourtada-Maarbouni M., Williams G.T., Clayton R.N., Farrell W.E. Isolation and characterization of a novel pituitary tumor apoptosis gene. Mol. Endocrinol. 2004;18:1827–1839. doi: 10.1210/me.2004-0087. [DOI] [PubMed] [Google Scholar]
- Bai F., Chan H.L., Smith M.D., Kiyokawa H., Pei X.H. p19Ink4d is a tumor suppressor and controls pituitary anterior lobe cell proliferation. Mol. Cell Biol. 2014;34:2121–2134. doi: 10.1128/MCB.01363-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers A., Aaltonen L.A., Daly A.F., Karhu A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocr. Rev. 2013;34:239–277. doi: 10.1210/er.2012-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello M.J., De Campos J.M., Isla A., Casartelli C., Rey J.A. Promoter CpG methylation of multiple genes in pituitary adenomas: frequent involvement of caspase-8. Oncol. Rep. 2006;15:443–448. [PubMed] [Google Scholar]
- Bentley L., Esapa C.T., Nesbit M.A., Head R.A., Evans H., Lath D., Scudamore C.L., Hough T.A., Podrini C., Hannan F.M., Fraser W.D., Croucher P.I., Brown M.A., Brown S.D., Cox R.D., Thakker R.V. An N-ethyl-N-nitrosourea induced corticotropin-releasing hormone promoter mutation provides a mouse model for endogenous glucocorticoid excess. Endocrinology. 2014;155:908–922. doi: 10.1210/en.2013-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino P., Tong W.M., Galendo D., Wang Z.Q., Zhang C.X. Heterozygous Men1 mutant mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1. Mol. Endocrinol. 2003;17:1880–1892. doi: 10.1210/me.2003-0154. [DOI] [PubMed] [Google Scholar]
- Bertolino P., Tong W.M., Herrera P.L., Casse H., Zhang C.X., Wang Z.Q. Pancreatic beta-cell-specific ablation of the multiple endocrine neoplasia type 1 (MEN1) gene causes full penetrance of insulinoma development in mice. Cancer Res. 2003;63:4836–4841. [PubMed] [Google Scholar]
- Besson A., Gurian-West M., Chen X., Kelly-Spratt K.S., Kemp C.J., Roberts J.M. A pathway in quiescent cells that controls p27Kip1 stability, subcellular localization, and tumor suppression. Genes Dev. 2006;20:47–64. doi: 10.1101/gad.1384406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi C., Gartside M., Tonks I., Paterson C., Hayward N.K., Kay G.F. Targeting and conditional inactivation of the murine Men1 locus using the Cre recombinase: loxP system. Genesis. 2002;32:150–151. doi: 10.1002/gene.10061. [DOI] [PubMed] [Google Scholar]
- Biondi C.A., Gartside M.G., Waring P., Loffler K.A., Stark M.S., Magnuson M.A., Kay G.F., Hayward N.K. Conditional inactivation of the MEN1 gene leads to pancreatic and pituitary tumorigenesis but does not affect normal development of these tissues. Mol. Cell Biol. 2004;24:3125–3131. doi: 10.1128/MCB.24.8.3125-3131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buslei R., Kreutzer J., Hofmann B., Schmidt V., Siebzehnrubl F., Hahnen E., Eyupoglu I.Y., Fahlbusch R., Blumcke I. Abundant hypermethylation of SOCS-1 in clinically silent pituitary adenomas. Acta Neuropathol. 2006;111:264–271. doi: 10.1007/s00401-005-0009-9. [DOI] [PubMed] [Google Scholar]
- Carlus M., Elies L., Fouque M.C., Maliver P., Schorsch F. Historical control data of neoplastic lesions in the Wistar Hannover Rat among eight 2-year carcinogenicity studies. Exp. Toxicol. Pathol. 2013;65:243–253. doi: 10.1016/j.etp.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Chesnokova V., Kovacs K., Castro A.V., Zonis S., Melmed S. Pituitary hypoplasia in Pttg-/- mice is protective for Rb+/- pituitary tumorigenesis. Mol. Endocrinol. 2005;19:2371–2379. doi: 10.1210/me.2005-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicenas J., Kalyan K., Sorokinas A., Jatulyte A., Valiunas D., Kaupinis A., Valius M. Highlights of the latest advances in research on CDK inhibitors. Cancers (Basel) 2014;6:2224–2242. doi: 10.3390/cancers6042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F.S., Rossant J., Wurst W. A mouse for all reasons. Cell. 2007;128:9–13. doi: 10.1016/j.cell.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Collins F.S., Finnell R.H., Rossant J., Wurst W. A new partner for the international knockout mouse consortium. Cell. 2007;129:235. doi: 10.1016/j.cell.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Crabtree J.S., Scacheri P.C., Ward J.M., Garrett-Beal L., Emmert-Buck M.R., Edgemon K.A., Lorang D., Libutti S.K., Chandrasekharappa S.C., Marx S.J., Spiegel A.M., Collins F.S. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1118–1123. doi: 10.1073/pnas.98.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J.S., Scacheri P.C., Ward J.M., McNally S.R., Swain G.P., Montagna C., Hager J.H., Hanahan D., Edlund H., Magnuson M.A., Garrett-Beal L., Burns A.L., Ried T., Chandrasekharappa S.C., Marx S.J., Spiegel A.M., Collins F.S. Of mice and MEN1: Insulinomas in a conditional mouse knockout. Mol. Cell Biol. 2003;23:6075–6085. doi: 10.1128/MCB.23.17.6075-6085.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Soto M.E., Scheiber M.D., Gregerson K.A., Boivin G.P., Horseman N.D. Pituitary tumorigenesis in prolactin gene-disrupted mice. Endocrinology. 2002;143:4429–4436. doi: 10.1210/en.2002-220173. [DOI] [PubMed] [Google Scholar]
- Cushman L.J., Watkins-Chow D.E., Brinkmeier M.L., Raetzman L.T., Radak A.L., Lloyd R.V., Camper S.A. Persistent Prop1 expression delays gonadotrope differentiation and enhances pituitary tumor susceptibility. Hum. Mol. Genet. 2001;10:1141–1153. doi: 10.1093/hmg/10.11.1141. [DOI] [PubMed] [Google Scholar]
- De Martino I., Visone R., Wierinckx A., Palmieri D., Ferraro A., Cappabianca P., Chiappetta G., Forzati F., Lombardi G., Colao A., Trouillas J., Fedele M., Fusco A. HMGA proteins up-regulate CCNB2 gene in mouse and human pituitary adenomas. Cancer Res. 2009;69:1844–1850. doi: 10.1158/0008-5472.CAN-08-4133. [DOI] [PubMed] [Google Scholar]
- Donangelo I., Gutman S., Horvath E., Kovacs K., Wawrowsky K., Mount M., Melmed S. Pituitary tumor transforming gene overexpression facilitates pituitary tumor development. Endocrinology. 2006;147:4781–4791. doi: 10.1210/en.2006-0544. [DOI] [PubMed] [Google Scholar]
- Dusing-Swartz S., Medina D., Butel J.S., Socher S.H. Mouse mammary tumor virus genome expression in chemical carcinogen-induced mammary tumors in low- and high-tumor-incidence mouse strains. Proc. Natl. Acad. Sci. U. S. A. 1979;76:5360–5364. doi: 10.1073/pnas.76.10.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira N., Minematsu T., Miyai S., Takekoshi S., Camper S.A., Osamura R.Y. Pituitary changes in Prop1 transgenic mice: hormone producing tumors and signet-ring type gonadotropes. Acta Histochem. Cytochem. 2008;41:47–57. doi: 10.1267/ahc.08007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzat S., Zheng L., Zhu X.F., Wu G.E., Asa S.L. Targeted expression of a human pituitary tumor-derived isoform of FGF receptor-4 recapitulates pituitary tumorigenesis. J. Clin. Investig. 2002;109:69–78. doi: 10.1172/JCI14036. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fedele M., Battista S., Kenyon L., Baldassarre G., Fidanza V., Klein-Szanto A.J., Parlow A.F., Visone R., Pierantoni G.M., Outwater E., Santoro M., Croce C.M., Fusco A. Overexpression of the HMGA2 gene in transgenic mice leads to the onset of pituitary adenomas. Oncogene. 2002;21:3190–3198. doi: 10.1038/sj.onc.1205428. [DOI] [PubMed] [Google Scholar]
- Fedele M., Pentimalli F., Baldassarre G., Battista S., Klein-Szanto A.J., Kenyon L., Visone R., De Martino I., Ciarmiello A., Arra C., Viglietto G., Croce C.M., Fusco A. Transgenic mice overexpressing the wild-type form of the HMGA1 gene develop mixed growth hormone/prolactin cell pituitary adenomas and natural killer cell lymphomas. Oncogene. 2005;24:3427–3435. doi: 10.1038/sj.onc.1208501. [DOI] [PubMed] [Google Scholar]
- Fedele M., Visone R., De Martino I., Troncone G., Palmieri D., Battista S., Ciarmiello A., Pallante P., Arra C., Melillo R.M., Helin K., Croce C.M., Fusco A. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell. 2006;9:459–471. doi: 10.1016/j.ccr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Fero M.L., Rivkin M., Tasch M., Porter P., Carow C.E., Firpo E., Polyak K., Tsai L.H., Broudy V., Perlmutter R.M., Kaushansky K., Roberts J.M. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- Fero M.L., Randel E., Gurley K.E., Roberts J.M., Kemp C.J. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E.M., Lana-Elola E., Watson S.D., Vassiliou G., Tybulewicz V.L. New approaches for modelling sporadic genetic disease in the mouse. Dis. Model Mech. 2009;2:446–453. doi: 10.1242/dmm.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin D.S., Godfrey V.L., Lee H., Kovalev G.I., Schoonhoven R., Chen-Kiang S., Su L., Xiong Y. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 1998;12:2899–2911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel R.H., Plump A., Lu X., Spilker K., Jolicoeur C., Wong K., Venkatesh T.R., Yaron A., Hynes M., Chen B., Okada A., McConnell S.K., Rayburn H., Tessier-Lavigne M. Gene targeting using a promoterless gene trap vector (“targeted trapping”) is an efficient method to mutate a large fraction of genes. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13188–13193. doi: 10.1073/pnas.0505474102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz A., Walch A., Piotrowska K., Rosemann M., Schaffer E., Weber K., Timper A., Wildner G., Graw J., Hofler H., Atkinson M.J. Recessive transmission of a multiple endocrine neoplasia syndrome in the rat. Cancer Res. 2002;62:3048–3051. [PubMed] [Google Scholar]
- Gaston-Massuet C., Andoniadou C.L., Signore M., Jayakody S.A., Charolidi N., Kyeyune R., Vernay B., Jacques T.S., Taketo M.M., Le Tissier P., Dattani M.T., Martinez-Barbera J.P. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11482–11487. doi: 10.1073/pnas.1101553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini D., Paez-Pereda M., Theodoropoulou M., Labeur M., Refojo D., Gerez J., Chervin A., Berner S., Losa M., Buchfelder M., Renner U., Stalla G.K., Arzt E. Bone morphogenetic protein-4 inhibits corticotroph tumor cells: involvement in the retinoic acid inhibitory action. Endocrinology. 2006;147:247–256. doi: 10.1210/en.2005-0958. [DOI] [PubMed] [Google Scholar]
- Gillam M.P., Nimbalkar D., Sun L., Christov K., Ray D., Kaldis P., Liu X., Kiyokawa H. MEN1 tumorigenesis in the pituitary and pancreatic islet requires Cdk4 but not Cdk2. Oncogene. 2015;34:932–938. doi: 10.1038/onc.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittleman H., Ostrom Q.T., Farah P.D., Ondracek A., Chen Y., Wolinsky Y., Kruchko C., Singer J., Kshettry V.R., Laws E.R., Sloan A.E., Selman W.R., Barnholtz-Sloan J.S. Descriptive epidemiology of pituitary tumors in the United States, 2004–2009. J. Neurosurg. 2014;121:527–535. doi: 10.3171/2014.5.JNS131819. [DOI] [PubMed] [Google Scholar]
- Gordon J.W., Scangos G.A., Plotkin D.J., Barbosa J.A., Ruddle F.H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc. Natl. Acad. Sci. U. S. A. 1980;77:7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi C.J., Mudhasani R., Hoover K., Koff A., Leav I., Imbalzano A.N., Jones S.N. Functional interaction of the retinoblastoma and Ini1/Snf5 tumor suppressors in cell growth and pituitary tumorigenesis. Cancer Res. 2006;66:8076–8082. doi: 10.1158/0008-5472.CAN-06-1451. [DOI] [PubMed] [Google Scholar]
- Hall B., Limaye A., Kulkarni A.B. Overview: generation of gene knockout mice. Curr. Protoc. Cell Biol. 2009;12:1–17. doi: 10.1002/0471143030.cb1912s44. (Chapter 19), Unit 19 12 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding B., Lemos M.C., Reed A.A., Walls G.V., Jeyabalan J., Bowl M.R., Tateossian H., Sullivan N., Hough T., Fraser W.D., Ansorge O., Cheeseman M.T., Thakker R.V. Multiple endocrine neoplasia type 1 knockout mice develop parathyroid, pancreatic, pituitary and adrenal tumours with hypercalcaemia, hypophosphataemia and hypercorticosteronaemia. Endocr. Relat. Cancer. 2009;16:1313–1327. doi: 10.1677/ERC-09-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruyama N., Cho A., Kulkarni A.B. Overview: engineering transgenic constructs and mice. Curr. Protoc. Cell Biol. 2009;10 doi: 10.1002/0471143030.cb1910s42. (Chapter 19), Unit 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M., Vogel H., Lee E.Y., Bradley A., Donehower L.A. Mice deficient in both p53 and Rb develop tumors primarily of endocrine origin. Cancer Res. 1995;55:1146–1151. [PubMed] [Google Scholar]
- Helseth A., Siegal G.P., Haug E., Bautch V.L. Transgenic mice that develop pituitary tumors. A model for Cushing's disease. Am. J. Pathol. 1992;140:1071–1080. [PMC free article] [PubMed] [Google Scholar]
- Hiyama H., Kubo O., Kawamata T., Ishizaki R., Hori T. Expression of cyclin kinase inhibitor p21/WAF1 protein in pituitary adenomas: correlations with endocrine activity, but not cell proliferation. Acta Neurochir. (Wien) 2002;144:481–488. doi: 10.1007/s007010200069. [DOI] [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E.M., Bronson R.T., Goodell M.A., Weinberg R.A. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Jaffrain-Rea M.L., Rotondi S., Alesse E. New insights in the pathogenesis of pituitary tumours. In: Fedele D.M., editor. Hot Topics in Endocrine and Endocrine-related Diseases. 2013. [Google Scholar]
- Jian F., Cao Y., Bian L., Sun Q. USP8: a novel therapeutic target for Cushing's disease. Endocrine. 2015 doi: 10.1007/s12020-015-0682-y. [DOI] [PubMed] [Google Scholar]
- Jordan S., Lidhar K., Korbonits M., Lowe D.G., Grossman A.B. Cyclin D and cyclin E expression in normal and adenomatous pituitary. Eur. J. Endocrinol. 2000;143:R1–R6. doi: 10.1530/eje.0.143r001. [DOI] [PubMed] [Google Scholar]
- Karga H.J., Alexander J.M., Hedleywhyte E.T., Klibanski A., Jameson J.L. Ras mutations in human pituitary-tumors. J. Clin. Endocrinol. Metab. 1992;74:914–919. doi: 10.1210/jcem.74.4.1312542. [DOI] [PubMed] [Google Scholar]
- Kawashima S.T., Usui T., Sano T., Iogawa H., Hagiwara H., Tamanaha T., Tagami T., Naruse M., Hojo M., Takahashi J.A., Shimatsu A. P53 gene mutation in an atypical corticotroph adenoma with Cushing's disease. Clin. Endocrinol. (Oxf) 2009;70:656–657. doi: 10.1111/j.1365-2265.2008.03404.x. [DOI] [PubMed] [Google Scholar]
- Kelly M.A., Rubinstein M., Asa S.L., Zhang G., Saez C., Bunzow J.R., Allen R.G., Hnasko R., Ben-Jonathan N., Grandy D.K., Low M.J. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron. 1997;19:103–113. doi: 10.1016/s0896-6273(00)80351-7. [DOI] [PubMed] [Google Scholar]
- Kirschner L.S. PRKAR1A and the evolution of pituitary tumors. Mol. Cell. Endocrinol. 2010;326:3–7. doi: 10.1016/j.mce.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa H., Kineman R.D., Manova-Todorova K.O., Soares V.C., Hoffman E.S., Ono M., Khanam D., Hayday A.C., Frohman L.A., Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- Korsisaari N., Ross J., Wu X., Kowanetz M., Pal N., Hall L., Eastham-Anderson J., Forrest W.F., Van Bruggen N., Peale F.V., Ferrara N. Blocking vascular endothelial growth factor-A inhibits the growth of pituitary adenomas and lowers serum prolactin level in a mouse model of multiple endocrine neoplasia type 1. Clin. Cancer Res. 2008;14:249–258. doi: 10.1158/1078-0432.CCR-07-1552. [DOI] [PubMed] [Google Scholar]
- Kumar T.R., Graham K.E., Asa S.L., Low M.J. Simian virus 40 T antigen-induced gonadotroph adenomas: a model of human null cell adenomas. Endocrinology. 1998;139:3342–3351. doi: 10.1210/endo.139.7.6100. [DOI] [PubMed] [Google Scholar]
- Lemos M.C., Thakker R.V. Multiple endocrine neoplaslia type 1 (MEN 1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum. Mutat. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- Lemos M.C., Harding B., Reed A.A., Jeyabalan J., Walls G.V., Bowl M.R., Sharpe J., Wedden S., Moss J.E., Ross A., Davidson D., Thakker R.V. Genetic background influences embryonic lethality and the occurrence of neural tube defects in Men1 null mice: relevance to genetic modifiers. J. Endocrinol. 2009;203:133–142. doi: 10.1677/JOE-09-0124. [DOI] [PubMed] [Google Scholar]
- Leontiou C.A., Gueorguiev M., van der Spuy J., Quinton R., Lolli F., Hassan S., Chahal H.S., Igreja S.C., Jordan S., Rowe J., Stolbrink M., Christian H.C., Wray J., Bishop-Bailey D., Berney D.M., Wass J.A., Popovic V., Ribeiro-Oliveira A., Jr., Gadelha M.R., Monson J.P., Akker S.A., Davis J.R., Clayton R.N., Yoshimoto K., Iwata T., Matsuno A., Eguchi K., Musat M., Flanagan D., Peters G., Bolger G.B., Chapple J.P., Frohman L.A., Grossman A.B., Korbonits M. The role of the aryl hydrocarbon receptor-interacting protein gene in familial and sporadic pituitary adenomas. J. Clin. Endocrinol. Metab. 2008;93:2390–2401. doi: 10.1210/jc.2007-2611. [DOI] [PubMed] [Google Scholar]
- Levy A. Molecular and trophic mechanisms of tumorigenesis. Endocrinol. Metab. Clin. N. Am. 2008;37:23. doi: 10.1016/j.ecl.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Liu N.A., Jiang H., Ben-Shlomo A., Wawrowsky K., Fan X.M., Lin S., Melmed S. Targeting zebrafish and murine pituitary corticotroph tumors with a cyclin-dependent kinase (CDK) inhibitor. Proc. Natl. Acad. Sci. U. S. A. 2011;108:8414–8419. doi: 10.1073/pnas.1018091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R.V., Ruebel K.H., Zhang S., Jin L. Pituitary hyperplasia in glycoprotein hormone alpha subunit-, p18(INK4C)-, and p27(kip-1)-null mice: analysis of proteins influencing p27(kip-1) ubiquitin degradation. Am. J. Pathol. 2002;160:1171–1179. doi: 10.1016/S0002-9440(10)64936-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler K.A., Biondi C.A., Gartside M., Waring P., Stark M., Serewko-Auret M.M., Muller H.K., Hayward N.K., Kay G.F. Broad tumor spectrum in a mouse model of multiple endocrine neoplasia type 1. Int. J. Cancer. 2007;120:259–267. doi: 10.1002/ijc.22288. [DOI] [PubMed] [Google Scholar]
- Loffler K.A., Biondi C.A., Gartside M.G., Serewko-Auret M.M., Duncan R., Tonks I.D., Mould A.W., Waring P., Muller H.K., Kay G.F., Hayward N.K. Lack of augmentation of tumor spectrum or severity in dual heterozygous Men1 and Rb1 knockout mice. Oncogene. 2007;26:4009–4017. doi: 10.1038/sj.onc.1210163. [DOI] [PubMed] [Google Scholar]
- Low M.J., Liu B., Hammer G.D., Rubinstein M., Allen R.G. Post-translational processing of proopiomelanocortin (POMC) in mouse pituitary melanotroph tumors induced by a POMC-simian virus 40 large T antigen transgene. J. Biol. Chem. 1993;268:24967–24975. [PubMed] [Google Scholar]
- Machens A., Schaaf L., Karges W., Frank-Raue K., Bartsch D.K., Rothmund M., Schneyer U., Goretzki P., Raue F., Dralle H. Age-related penetrance of endocrine tumours in multiple endocrine neoplasia type 1 (MEN1): a multicentre study of 258 gene carriers. Clin. Endocrinol. 2007;67:613–622. doi: 10.1111/j.1365-2265.2007.02934.x. [DOI] [PubMed] [Google Scholar]
- Mantovani G., Lania A.G., Spada A. GNAS imprinting and pituitary tumors. Mol. Cell. Endocrinol. 2010;326:15–18. doi: 10.1016/j.mce.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Marinoni I., Lee M., Mountford S., Perren A., Bravi I., Jennen L., Feuchtinger A., Drouin J., Roncaroli F., Pellegata N.S. Characterization of MENX-associated pituitary tumours. Neuropathol. Appl. Neurobiol. 2013;39:256–269. doi: 10.1111/j.1365-2990.2012.01278.x. [DOI] [PubMed] [Google Scholar]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U. S. A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrew J., Paterson A.J., Asa S.L., McCarthy K.J., Kudlow J.E. Targeting of transforming growth factor-alpha expression to pituitary lactotrophs in transgenic mice results in selective lactotroph proliferation and adenomas. Endocrinology. 1995;136:4479–4488. doi: 10.1210/endo.136.10.7664668. [DOI] [PubMed] [Google Scholar]
- Michael S.K., Brennan J., Robertson E.J. Efficient gene-specific expression of cre recombinase in the mouse embryo by targeted insertion of a novel IRES-Cre cassette into endogenous loci. Mech. Dev. 1999;85:35–47. doi: 10.1016/s0925-4773(99)00052-0. [DOI] [PubMed] [Google Scholar]
- Michaelis K.A., Knox A.J., Xu M., Kiseljak-Vassiliades K., Edwards M.G., Geraci M., Kleinschmidt-DeMasters B.K., Lillehei K.O., Wierman M.E. Identification of growth arrest and DNA-damage-inducible gene beta (GADD45 beta) as a novel tumor suppressor in pituitary gonadotrope tumors. Endocrinology. 2011;152:3603–3613. doi: 10.1210/en.2011-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui T., Ishida M., Izawa M., Arita J. Differences between rat strains in the development of PRL-secreting pituitary tumors with long-term estrogen treatment: In vitro insulin-like growth factor-1-induced lactotroph proliferation and gene expression are affected in Wistar-Kyoto rats with low estrogen-susceptibility. Endocr. J. 2013;60:1251–1259. doi: 10.1507/endocrj.ej13-0245. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Ishida N., Shirane M., Inomata A., Inoue T., Shishido N., Horii I., Loh D.Y. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Pagotto U., Arzberger T., Theodoropoulou M., Grubler Y., Pantaloni C., Saeger W., Losa M., Journot L., Stalla G.K., Spengler D. The expression of the antiproliferative gene ZAC is lost or highly reduced in nonfunctioning pituitary adenomas. Cancer Res. 2000;60:6794–6799. [PubMed] [Google Scholar]
- Pardi E., Mariotti S., Pellegata N.S., Benfini K., Borsari S., Saponaro F., Torregrossa L., Cappai A., Satta C., Mastinu M., Marcocci C., Cetani F. Functional characterization of a CDKN1B mutation in a Sardinian kindred with multiple endocrine neoplasia type 4 (MEN4) Endocr. Connect. 2014 doi: 10.1530/EC-14-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L., Melmed S. Isolation and characterization of a pituitary tumor-transforming gene (PTTG) Mol. Endocrinol. 1997;11:433–441. doi: 10.1210/mend.11.4.9911. [DOI] [PubMed] [Google Scholar]
- Pei L., Melmed S., Scheithauer B., Kovacs K., Benedict W.F., Prager D. Frequent loss of heterozygosity at the retinoblastoma susceptibility gene (RB) locus in aggressive pituitary-tumors - evidence for a chromosome-13 tumor-suppressor gene other than RB. Cancer Res. 1995;55:1613–1616. [PubMed] [Google Scholar]
- Pellegata N.S., Quintanilla-Martinez L., Siggelkow H., Samson E., Bink K., Hofler H., Fend F., Graw J., Atkinson M.J. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15558–15563. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H., Liu H., Zhao S., Wu J., Fan J., Liao J. Silencing of RASSF3 by DNA hypermethylation is associated with tumorigenesis in somatotroph adenomas. PLoS One. 2013;8:e59024. doi: 10.1371/journal.pone.0059024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piret S.E., Thakker R.V. Mouse models for inherited endocrine and metabolic disorders. J. Endocrinol. 2011;211:211–230. doi: 10.1530/JOE-11-0193. [DOI] [PubMed] [Google Scholar]
- Qian Z.R., Sano T., Yoshimoto K., Yamada S., Ishizuka A., Mizusawa N., Horiguchi H., Hirokawa M., Asa S.L. Inactivation of RASSF1A tumor suppressor gene by aberrant promoter hypermethylation in human pituitary adenomas. Lab. Invest. 2005;85:464–473. doi: 10.1038/labinvest.3700248. [DOI] [PubMed] [Google Scholar]
- Qian Z.R., Sano T., Yoshimoto K., Asa S.L., Yamada S., Mizusawa N., Kudo E. Tumor-specific downregulation and methylation of the CDH13 (H-cadherin) and CDH1 (E-cadherin) genes correlate with aggressiveness of human pituitary adenomas. Mod. Pathol. 2007;20:1269–1277. doi: 10.1038/modpathol.3800965. [DOI] [PubMed] [Google Scholar]
- Raappana A., Koivukangas J., Ebeling T., Pirila T. Incidence of pituitary adenomas in northern Finland in 1992-2007. J. Clin. Endocrinol. Metab. 2010;95:4268–4275. doi: 10.1210/jc.2010-0537. [DOI] [PubMed] [Google Scholar]
- Raitila A., Lehtonen H.J., Arola J., Heliovaara E., Ahlsten M., Georgitsi M., Jalanko A., Paetau A., Aaltonen L.A., Karhu A. Mice with inactivation of aryl hydrocarbon receptor-interacting protein (Aip) display complete penetrance of pituitary adenomas with aberrant ARNT expression. Am. J. Pathol. 2010;177:1969–1976. doi: 10.2353/ajpath.2010.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reincke M., Sbiera S., Hayakawa A., Theodoropoulou M., Osswald A., Beuschlein F., Meitinger T., Mizuno-Yamasaki E., Kawaguchi K., Saeki Y., Tanaka K., Wieland T., Graf E., Saeger W., Ronchi C.L., Allolio B., Buchfelder M., Strom T.M., Fassnacht M., Komada M. Mutations in the deubiquitinase gene USP8 cause Cushing's disease. Nat. Genet. 2015;47:31–38. doi: 10.1038/ng.3166. [DOI] [PubMed] [Google Scholar]
- Righi A., Jin L., Zhang S., Stilling G., Scheithauer B.W., Kovacs K., Lloyd R.V. Identification and consequences of galectin-3 expression in pituitary tumors. Mol. Cell Endocrinol. 2010;326:8–14. doi: 10.1016/j.mce.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Rosenberg D.W., Giardina C., Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30:183–196. doi: 10.1093/carcin/bgn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel-Gervais A., Bilodeau S., Vallette S., Berthelet F., Lacroix A., Figarella-Branger D., Brue T., Drouin J. Cooperation between cyclin e and p27(Kip1) in pituitary tumorigenesis. Mol. Endocrinol. 2010;24:1835–1845. doi: 10.1210/me.2010-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez C., Japon M.A., Ramos-Morales F., Romero F., Segura D.I., Tortolero M., Pintor-Toro J.A. hpttg is over-expressed in pituitary adenomas and other primary epithelial neoplasias. Oncogene. 1999;18:5473–5476. doi: 10.1038/sj.onc.1202914. [DOI] [PubMed] [Google Scholar]
- Salehi F., Kovacs K., Scheithauer B.W., Lloyd R.V., Cusimano M. Pituitary tumor-transforming gene in endocrine and other neoplasms: a review and update. Endocr Relat. Cancer. 2008;15:721–743. doi: 10.1677/ERC-08-0012. [DOI] [PubMed] [Google Scholar]
- Scheithauer B.W., Gaffey T.A., Lloyd R.V., Sebo T.J., Kovacs K.T., Horvath E., Yapicier O., Young W.F., Meyer F.B., Kuroki T., Riehle D.L., Laws E.R. Pathobiology of pituitary adenomas and carcinomas. Neurosurgery. 2006;59:341–353. doi: 10.1227/01.NEU.0000223437.51435.6E. [DOI] [PubMed] [Google Scholar]
- Schuff K.G., Hentges S.T., Kelly M.A., Binart N., Kelly P.A., Iuvone P.M., Asa S.L., Low M.J. Lack of prolactin receptor signaling in mice results in lactotroph proliferation and prolactinomas by dopamine-dependent and -independent mechanisms. J. Clin. Invest. 2002;110:973–981. doi: 10.1172/JCI15912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seruggia D., Montoliu L. The new CRISPR-Cas system: RNA-guided genome engineering to efficiently produce any desired genetic alteration in animals. Transgen. Res. 2014;23:707–716. doi: 10.1007/s11248-014-9823-y. [DOI] [PubMed] [Google Scholar]
- Simpson D.J., Clayton R.N., Farrell W.E. Preferential loss of death associated protein kinase expression in invasive pituitary tumours is associated with either CpG island methylation or homozygous deletion. Oncogene. 2002;21:1217–1224. doi: 10.1038/sj.onc.1205195. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L., Schier A.F., Driever W. Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics. 1994;136:1401–1420. doi: 10.1093/genetics/136.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son W.C., Gopinath C. Early occurrence of spontaneous tumors in CD-1 mice and Sprague-Dawley rats. Toxicol. Pathol. 2004;32:371–374. doi: 10.1080/01926230490440871. [DOI] [PubMed] [Google Scholar]
- Son W.C., Bell D., Taylor I., Mowat V. Profile of early occurring spontaneous tumors in Han Wistar rats. Toxicol. Pathol. 2010;38:292–296. doi: 10.1177/0192623309359794. [DOI] [PubMed] [Google Scholar]
- Sotillo R., Dubus P., Martin J., de la Cueva E., Ortega S., Malumbres M., Barbacid M. Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors. EMBO J. 2001;20:6637–6647. doi: 10.1093/emboj/20.23.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillo R., Renner O., Dubus P., Ruiz-Cabello J., Martin-Caballero J., Barbacid M., Carnero A., Malumbres M. Cooperation between Cdk4 and p27kip1 in tumor development: a preclinical model to evaluate cell cycle inhibitors with therapeutic activity. Cancer Res. 2005;65:3846–3852. doi: 10.1158/0008-5472.CAN-04-4195. [DOI] [PubMed] [Google Scholar]
- Stanford W.L., Cohn J.B., Cordes S.P. Gene-trap mutagenesis: past, present and beyond. Nat. Rev. Genet. 2001;2:756–768. doi: 10.1038/35093548. [DOI] [PubMed] [Google Scholar]
- Stefaneanu L., Rindi G., Horvath E., Murphy D., Polak J.M., Kovacs K. Morphology of adenohypophysial tumors in mice transgenic for vasopressin-SV40 hybrid oncogene. Endocrinology. 1992;130:1789–1795. doi: 10.1210/endo.130.4.1312426. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore M.P., Cameron V.A., Vaughan J., Sawchenko P.E., Vale W. Development of Cushing's syndrome in corticotropin-releasing factor transgenic mice. Endocrinology. 1992;130:3378–3386. doi: 10.1210/endo.130.6.1597149. [DOI] [PubMed] [Google Scholar]
- Stewart C., Parente F., Piehl F., Farnebo F., Quincey D., Silins G., Bergman L., Carle G.F., Lemmens I., Grimmond S., Xian C.Z., Khodei S., Teh B.T., Lagercrantz J., Siggers P., Calender A., Van de Vem V., Kas K., Weber G., Hayward N., Gaudray P., Larsson C. Characterization of the mouse Men1 gene and its expression during development. Oncogene. 1998;17:2485–2493. doi: 10.1038/sj.onc.1202164. [DOI] [PubMed] [Google Scholar]
- Tanizaki Y., Jin L., Scheithauer B.W., Kovacs K., Roncaroli F., Lloyd R.V. P53 gene mutations in pituitary carcinomas. Endocr. Pathol. 2007;18:217–222. doi: 10.1007/s12022-007-9006-y. [DOI] [PubMed] [Google Scholar]
- Thakker R.V. Multiple endocrine neoplasia type 1 (MEN1) Best. Pract. Res. Clin. Endocrinol. Metab. 2010;24:355–370. doi: 10.1016/j.beem.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Thakker R.V. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4) Mol. Cell. Endocrinol. 2013 doi: 10.1016/j.mce.2013.08.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjornstrand A., Gunnarsson K., Evert M., Holmberg E., Ragnarsson O., Rosen T., Filipsson Nystrom H. The incidence rate of pituitary adenomas in western Sweden for the period 2001–2011. Eur. J. Endocrinol. 2014;171:519–526. doi: 10.1530/EJE-14-0144. [DOI] [PubMed] [Google Scholar]
- Tsai K.Y., MacPherson D., Rubinson D.A., Nikitin A.Y., Bronson R., Mercer K.L., Crowley D., Jacks T. ARF mutation accelerates pituitary tumor development in Rb+/- mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16865–16870. doi: 10.1073/pnas.262499599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verges B., Boureille F., Goudet P., Murat A., Beckers A., Sassolas G., Cougard P., Chambe B., Montvernay C., Calender A., Grp Etud Neoplasies E. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J. Clin. Endocrinol. Metab. 2002;87:457–465. doi: 10.1210/jcem.87.2.8145. [DOI] [PubMed] [Google Scholar]
- Vierimaa O., Georgitsi M., Lehtonen R., Vahteristo P., Kokko A., Raitila A., Tuppurainen K., Ebeling T.M., Salmela P.I., Paschke R., Gundogdu S., De Menis E., Makinen M.J., Launonen V., Karhu A., Aaltonen L.A. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312:1228–1230. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]
- Vlotides G., Eigler T., Melmed S. Pituitary tumor-transforming gene: physiology and implications for tumorigenesis. Endocr. Rev. 2007;28:165–186. doi: 10.1210/er.2006-0042. [DOI] [PubMed] [Google Scholar]
- Vooijs M., van der Valk M., te Riele H., Berns A. Flp-mediated tissue-specific inactivation of the retinoblastoma tumor suppressor gene in the mouse. Oncogene. 1998;17:1–12. doi: 10.1038/sj.onc.1202169. [DOI] [PubMed] [Google Scholar]
- Vooijs M., Jonkers J., Lyons S., Berns A. Noninvasive imaging of spontaneous retinoblastoma pathway-dependent tumors in mice. Cancer Res. 2002;62:1862–1867. [PubMed] [Google Scholar]
- Waalkes M.P. Cadmium carcinogenesis. Mutat. Res. 2003;533:107–120. doi: 10.1016/j.mrfmmm.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Walls G.V., Lemos M.C., Javid M., Bazan-Peregrino M., Jeyabalan J., Reed A.A.C., Harding B., Tyler D.J., Stuckey D.J., Piret S., Christie P.T., Ansorge O., Clarke K., Seymour L., Thakker R.V. MEN1 gene replacement therapy reduces proliferation rates in a mouse model of pituitary adenomas. Cancer Res. 2012;72:5060–5068. doi: 10.1158/0008-5472.CAN-12-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yang H., Shivalila C.S., Dawlaty M.M., Cheng A.W., Zhang F., Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R.A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Westerman B.A., Blom M., Tanger E., van der Valk M., Song J.Y., van Santen M., Gadiot J., Cornelissen-Steijger P., Zevenhoven J., Prosser H.M., Uren A., Aronica E., van Lohuizen M. GFAP-Cre-mediated transgenic activation of Bmi1 results in pituitary tumors. PLoS One. 2012;7:e35943. doi: 10.1371/journal.pone.0035943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund J., Wertz N., Gorski J. A comparison of estrogen effects on uterine and pituitary growth and prolactin synthesis in F344 and Holtzman rats. Endocrinology. 1981;109:1700–1707. doi: 10.1210/endo-109-5-1700. [DOI] [PubMed] [Google Scholar]
- Wiklund J., Rutledge J., Gorski J. A genetic model for the inheritance of pituitary tumor susceptibility in F344 rats. Endocrinology. 1981;109:1708–1714. doi: 10.1210/endo-109-5-1708. [DOI] [PubMed] [Google Scholar]
- Yates C.J., Lines K.E., Thakker R.V. Molecular genetic advances in pituitary tumor development. Expert Rev. Endocrinol. Metab. 2014;10:35–53. doi: 10.1586/17446651.2015.955795. [DOI] [PubMed] [Google Scholar]
- Yin Z., Williams-Simons L., Parlow A.F., Asa S., Kirschner L.S. Pituitary-specific knockout of the Carney complex gene Prkar1a leads to pituitary tumorigenesis. Mol. Endocrinol. 2008;22:380–387. doi: 10.1210/me.2006-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Rice K., Wang Y., Chen W., Zhong Y., Nakayama Y., Zhou Y., Klibanski A. Maternally expressed gene 3 (MEG3) noncoding Ribonucleic Acid: Isoform structure, expression, and functions. Endocrinology. 2010;151:939–947. doi: 10.1210/en.2009-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhang X., Klibanski A. Genetic and epigenetic mutations of tumor suppressive genes in sporadic pituitary adenoma. Mol. Cell. Endocrinol. 2013 doi: 10.1016/j.mce.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Lee K., Asa S.L., Ezzat S. Epigenetic silencing through DNA and histone methylation of fibroblast growth factor receptor 2 in neoplastic pituitary cells. Am. J. Pathol. 2007;170:1618–1628. doi: 10.2353/ajpath.2007.061111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F., Nilsson L.M., Nguyen L., Meunier C., Smeyne R.J., Rehg J.E., Eberhart C., Sherr C.J., Roussel M.F. Hemangiosarcomas, medulloblastomas, and other tumors in Ink4c/p53-null mice. Cancer Res. 2003;63:5420–5427. [PubMed] [Google Scholar]