Abstract
The objective of the present study was to determine whether an in vitro-in vivo correlation (IVIVC) can be established for polymeric microspheres that are equivalent in formulation composition but prepared with different manufacturing processes. Risperidone was chosen as a model therapeutic and poly(lactic-co-glycolic acid) (PLGA) with similar molecular weight as that used in the commercial product Risperdal® Consta® was used to prepare risperidone microspheres. Various manufacturing processes were investigated to produce the risperidone microspheres with similar drug loading (approx. 37%) but distinctly different physicochemical properties (e.g. porosity, particle size and particle size distribution). In vitro release of the risperidone microspheres was investigated using different release testing methods (such as sample-and-separate and USP apparatus 4). In vivo pharmacokinetic profiles of the risperidone microsphere formulations following intramuscular administration were determined using a rabbit model. Furthermore, the obtained pharmacokinetic profiles were deconvoluted using the Loo-Riegelman method and the calculated in vivo release was compared with the in vitro release of these microspheres. Level A IVIVCs were established and validated for the compositionally equivalent risperidone microspheres based on the in vitro release data obtained using USP apparatus 4. The developed IVIVCs demonstrated good predictability and were robust. These results showed that the developed USP apparatus 4 method was capable of discriminating PLGA microspheres that are equivalent in formulation composition but with manufacturing differences and predicting their in vivo performance in the investigated animal model.
Keywords: Poly(lactic-co-glycolic acid) (PLGA) microspheres, Manufacturing differences, In vitro and in vivo correlation, Risperidone, USP apparatus 4, Level A
Graphical abstract
1. Introduction
The U.S. Food and Drug Administration (FDA) has defined an in vitro-in vivo correlation (IVIVC) as “a predictive mathematical model describing the relationship between an in vitro property of a dosage form and a relevant in vivo response” [1]. Generally the in vitro property is the rate or extent of drug dissolution or release, while the in vivo response is the plasma drug concentration or the amount absorbed. Four main levels of IVIVC have been categorized by the U.S. FDA: levels A, B, C, and multiple level C. Level A represents a point-to-point correlation between in vitro and in vivo profiles. A Level B correlation utilizes statistical moment analysis comparing a mean in vitro dissolution time to either a mean in vivo residence or dissolution time. A Level C IVIVC establishes a single point relationship between a dissolution parameter such as time required for 50% dissolution and a pharmacokinetic parameter such as Cmax or AUC. A multiple level C IVIVC relates multiple dissolution time points to one or more pharmacokinetic parameters (e.g. Cmax or AUC). Once an IVIVC is established, it can be used to guide formulation and/or process development changes in various stages of drug product development. In addition, an IVIVC can help set relevant in vitro dissolution specifications to ensure product quality [2]. Most importantly, when a level A IVIVC is established, the in vitro release method may be used as a surrogate for measuring in vivo bioavailability. Thus, the conduct of human studies may be minimized and the regulatory burden could be reduced [3, 4]. To date, the establishment of an IVIVC for complex parenteral drug products (e.g. microspheres) has remained challenging due to their complex characteristics (e.g. multi-phasic release) as well as the lack of standardized, compendial in vitro release testing methods [4].
During the past few decades, poly(lactic-co-glycolic acid) (PLGA)/poly(lactic acid) (PLA)-based microspheres have emerged as one of the most successful complex parenteral drug products on the market owing to their biodegradability, biocompatibility, as well as their capability to deliver drugs in a controlled manner over periods of days to several months [5–8]. Currently, eight parenteral PLGA/PLA microsphere drug products have been approved by the U.S. FDA [9]. These PLGA/PLA based microsphere drug products have been used for a variety of diseases (such as cancer and diabetes) and have brought huge benefits to public health. However, these parenteral products are considered “high-risk” since they normally contain substantial amounts of potent therapeutics, and any unanticipated changes in their in vivo drug release characteristics may lead to severe toxicity [10, 11]. In addition, these products have complex characteristics and processing methodologies. Consequently, minor manufacturing changes (e.g. manufacturing site or instrumentation changes) have the potential to affect their physicochemical characteristics, which in turn may affect their in vivo performance.
In vitro release testing with in vivo relevance is an important quality control tool to assure product performance and safety [10–14]. Over the past decades, the development of IVIVCs for parenteral microspheres has increasingly gained more significance. The same principles as detailed in the FDA IVIVC Guidance on extended release oral dosage forms have been applied in literature to develop IVIVCs for parenteral drug products [15–20]. However, due to their complex release characteristics (e.g. bi- or tri-phasic release profiles), deconvolution of in vivo data and correlation with in vitro release data have been challenging. Until now, there are only a few literature reports on the establishment of IVIVCs for parenteral polymeric microspheres, albeit with different in vitro release testing approaches, such as the USP 4 method [19, 20], dialysis based methods [17, 18], as well as sample-and-separate methods [21–23]. None of these has addressed the importance of developing an IVIVC for compositionally equivalent PLGA microspheres with manufacturing differences.
The objective of the present study was to determine whether an IVIVC can be established for compositionally equivalent PLGA microspheres with manufacturing differences. Risperidone was chosen as an example product, and PLGA with similar molecular weight as that used in the commercial product Risperdal® Consta® was used to prepare the risperidone microspheres via different manufacturing processes. Different in vitro release testing methods (e.g. sample-and-separate, and USP apparatus 4) were used to investigate the in vitro release characteristics of the prepared risperidone microsphere formulations. Furthermore, in vivo release profiles of the prepared microsphere formulations were investigated using a rabbit model and compared with the in vitro release profiles obtained using different release testing methods.
2. Materials and methods
2.1. Materials
PLGA (7525 DLG 6E) was purchased from Evonik (Birmingham, AL). Risperidone was purchased from Jai Radhe, India. Poly(vinyl alcohol) (PVA, MW 30–70 kDa), trifuoroacetic acid (TFA), and reference standards (i.e. risperidone, and risperidone-D4) were purchased from Sigma-Aldrich (St. Louis, MO). Methylene chloride and dimethyl sulfoxide (DMSO, ACS grade) were purchased from Fisher Scientific (Pittsburgh, PA). Formic acid 0.1% water (LC-MS) and methanol (LC-MS) were purchased from VWR (Radnor, PA). Nanopure™ quality water (Barnstead, Dubuque, IA) was used for all studies. All other chemicals were obtained commercially as analytical-grade reagents.
2.2. Preparation of risperidone microspheres
PLGA (7525 DLG 6E) with similar molecular weight as that used in the commercial product Risperdal® Consta® was used to prepare compositionally equivalent risperidone microspheres with manufacturing differences (e.g. homogenization, vortex mixing, and different solvents). Briefly, when methylene chloride (DCM) was used as the solvent, both PLGA and risperidone were dissolved in DCM (polymer/drug, 4/3 (w/w)). The polymer/drug solution was then dispersed into an aqueous PVA solution (1%, w/v) saturated with DCM to form an oil-in-water (o/w) emulsion via homogenization (3,400 rpm, 2 minutes) (IKA® Works, Inc.). The microparticles were hardened via solvent extraction and evaporation at room temperature for 3 hours and then the solvent was further removed under vacuum. The resulting microspheres were collected and washed using distilled water and lyophilized. Different sieving procedures using 25 µm and 212 µm sieves (i.e. wet sieving (pre-lyophilization) and dry sieving (post-lyophilization)) were used. When ethyl acetate (EA) and benzyl alcohol (BA) were used as the solvent system, PLGA was dissolved in EA (16.7%, w/w) and risperidone was dissolved in BA (24%, w/w), respectively. The polymer and the drug solution were then mixed and transferred to the 1% (w/v) PVA solution (saturated with EA) to form oil-in-water (o/w) emulsion via homogenization (3,400 rpm, 30 seconds) or vortex mixing (1,200 rpm, 10 seconds). The resulting emulsions were transferred to a solvent extraction medium (2.5% (v/v) EA in water) and the solvent was extracted overnight at 4°C. Following solvent extraction, residual organic solvents were removed under vacuum at room temperature, following which the microspheres were collected and washed using an aqueous alcoholic solution (25% ethanol, v/v). The resulting microspheres were sieved using 25 µm and 212 µm sieves and lyophilized.
2.3. Characterization of risperidone microspheres
2.3.1. Drug loading
Five mg of the risperidone microspheres were weighed and transferred into a 10 ml volumetric flask. DMSO (2.5 ml) was added into the volumetric flask and the samples were sonicated until all particles were dissolved. Methanol was used to dilute the sample. The solution was filtered (Millex® HV, 0.22 µm PVDF syringe filter) and the risperidone concentration was determined with a validated HPLC assay. Mobile phase: acetonitrile/water/TFA (30/70/0.1, v/v/v); column: Kinetex C18 column (250 × 4.6 mm, 5 µm, 100 Å); detection wavelength: 275 nm; flow rate: 1 ml/min. Drug loading was calculated as: percent drug loading = (weight of drug entrapped/weight of microspheres analyzed) × 100.
2.3.2. Particle size and particle size distribution
Particle size and particle size distribution of the risperidone microspheres were measured using an AccuSizer autodiluter particle sizing system (Nicomp, Santa Barbara, CA). Briefly, microspheres were dispersed in 0.1% (w/v) PVA solution in water to ensure good dispersion and then particle size analysis was conducted.
2.3.3. Differential scanning calorimeter (DSC) analysis
The glass transition temperatures (Tg) of the risperidone microspheres, as well as a physical mixture of the blank microspheres and risperidone were analyzed using a modulated temperature differential scanning calorimeter (MTDSC) (TA Instruments Q2000). Briefly, experiments were performed in hermetically sealed pans using a 2°C/min heating rate and a modulation amplitude of ±0.82°C with an 80 s modulation period. The weight of each sample was ~4 mg. The Tg was determined as the glass transition midpoint in the reversing signal. The crystallinity of risperidone was also investigated.
2.3.4. Morphology
The morphology of the commercial product Risperdal® Consta® and the prepared risperidone microspheres was characterized using scanning electron microscopy (SEM). Briefly, dry microspheres were mounted on carbon taped aluminum stubs and sputter coated with gold. The samples were analyzed using SEM (NanoSEM 450, Nova).
2.3.5. Residual organic solvent content
Residual organic solvent content in the prepared risperidone microspheres was determined using gas chromatography (GC, Agilent 7890A)/mass spectrophotometer (MS, Agilent 5975). Briefly, DCM and EA standards in DMSO (i.e. 600 ppm for DCM and 5,000 ppm for EA according to the Guidance for Industry Q3C-Impurities: Residual Solvents) were injected and peak areas of the standards were recorded as references. Risperidone microspheres (~1.1 mg) were directly injected into GC/MS spectrophotometer through a Syringeless Injector. Peak areas were recorded and compared with references.
2.3.6. Porosity
The porosity of the risperidone microspheres was determined using a Mercury Porosimeter (AutoPore IV 9500, Micromeritics). Briefly, approximately 200 mg of risperidone microspheres were introduced into the porosimeter and tested at a mercury filling pressure of 0.53 psi. Total intrusion volume, total pore area as well as porosity (%) were recorded (porosity (%) = bulk density/apparent (skeletal) density × 100).
2.4. In vitro release studies
In vitro release testing of the risperidone microspheres was investigated using both sample-and-separate and USP apparatus 4 methods. In case of the sample-and-separate method, the microspheres (10 mg) were dispersed in 250 ml of 10 mM phosphate buffered saline (PBS, pH 7.4) and incubated in a shaker water bath at 100 rpm. At pre-determined time intervals, one ml samples were withdrawn and centrifuged at 2,100 g for 3 min. Supernatants (0.9 ml) were filtered through 0.22 µm filters and analyzed via HPLC. Fresh media (0.9 ml) were mixed with pellets (if any) and transferred back to the testing vessels. In case of the USP apparatus 4 method, a previously developed and validated USP apparatus 4 method was used [24]. Briefly, the microspheres (10 mg) were mixed with glass beads (1 mm) and placed in USP apparatus 4 dissolution cells. PBS (10 mM, pH 7.4, 250 ml) with 0.01% (w/v) sodium azide was circulated through the flow through cells at a flow rate of 8 ml/min at 37°C. At pre-determined time intervals, one ml samples were withdrawn and replenished with fresh media. The release samples were analyzed via HPLC.
2.5. In vivo release studies
In vivo release characteristics of the risperidone microspheres were investigated using a rabbit model. Briefly, rabbits were randomly assigned to cages and treated with the prepared risperidone microsphere formulations and the commercial product (n=6). The risperidone microspheres were suspended in the diluent used for dispersion of Risperdal® Consta® prior to injection. The suspended microspheres were injected into the rabbit cranial muscle (dose: 1.92 mg/kg) and blood samples were collected from the marginal ear veins at pre-determined time intervals. The pharmacokinetic profile of the risperidone solution (dose: 0.2 mg/kg) following intravenous administration was also determined (n=6). The collected blood samples were centrifuged for 5 min at 1,500 g and plasma was collected and stored at −20°C until analysis. Risperidone was extracted from the plasma samples and analyzed via LC-MS/MS. The animal study protocol was reviewed and approved by the University of Connecticut’s Institutional Animal Care and Use Committee (IACUC) prior to the beginning of the experiments.
2.6. Plasma sample analysis
Risperidone was extracted from plasma using tert-butyl methyl ether. Risperidone-D4 was used as an internal standard (IS). Briefly, the internal standard solution (100 ng/ml, 20 µl) was added into 200 µl of plasma samples. Then tert-butyl methyl ether (1.1 ml) was added into the plasma samples and vortex-mixed for 10 min followed by centrifugation at 14,000 g (4°C) for 5 min. The supernatants were transferred to the polypropylene centrifuge tubes and dried under nitrogen at 40°C. The dry residues were reconstituted in 100 µl of mobile phase (0.1% formic acid in water /methanol: 80/20 (v/v)). The reconstituted solution (10 µl) was used for HPLC-MS/MS analysis.
The LC-MS/MS system consisted of an Agilent HP-1100 LC system and a TSQ Quantum Ultra Mass Spectrometer (Waters) with an electrospray ionization (ESI) ion source. Chromatographic separations were carried out on a Kinetex Biphenyl column (50 × 2.1 mm, 2.6 µm, 100 Å) through gradient elution at 40°C. Mobile phase A was 0.1% formic acid in water (LC-MS); mobile phase B was methanol (LC-MS). The gradient started at 80% mobile phase A and was decreased to 5% over 8 min, then held constant for an additional 1 min. At 10 min, the column was returned to 80% mobile phase A and re-equilibrated for 8 min. The flow rate was 0.3 ml/mim. The following MS detection parameters were used: 3500 V electrospray voltage, 300°C capillary temperature, and 30 V collision energy. Detection of ions was conducted in the positive-ion selected reaction monitoring mode with the following transitions in a single reaction monitoring (SRM) mode: m/z 411.1→195.1 for risperidone, and m/z 415.1→195.1 for risperidone-D4. The injection volume was 10 µl. The data acquisition was ascertained by Xcalibur software. Calibration curves were established on each day when analysis was conducted. These curves showed good linearity with correlation coefficients of > 0.99. The lowest limit of quantification (LLOQ) for risperidone was 0.517 ng/ml and the mean recovery of plasma samples from low to high concentrations of risperidone was more than 90%. The inter- and intra-day variations of the three different concentrations of risperidone (0.517, 10.34, and 41.38 ng/ml) were less than 15%.
2.7. Development of an IVIVC
The development of an IVIVC for the prepared risperidone PLGA microspheres was performed following the same principles as detailed in the U.S. FDA IVIVC guidance on extended release oral dosage forms [1]. Briefly, the in vivo plasma profiles of the prepared risperidone PLGA microspheres were deconvoluted using the Loo-Riegelman method [19]. Standard errors are not shown in the deconvoluted in vivo absorption profiles because the average plasma concentration values were used. The fraction absorbed in vivo was calculated as below:
Cp, Ct, K10 and AUC are the drug concentration in the central compartment (plasma), apparent tissue compartment concentration, elimination rate constant and area under the plasma vs. time curve, respectively. The distributive and elimination micro rate constants (k12, k21 and k10) that are necessary for calculating Ct, were calculated using GastroPlus™ software (Simulations Plus, Inc., CA) based on the plasma concentrations of risperidone after intravenous administration of the risperidone solution.
2.8. Statistical analysis
Statistical data analysis was performed using a paired student t-test with p < 0.05 as the minimal level of significance.
3. Results and discussion
3.1. Physicochemical properties of risperidone microspheres
The critical physicochemical characteristics of complex parenteral microspheres may be sensitive to even minor manufacturing changes (e.g. manufacturing site or instrumentation changes). In order to understand these critical physicochemical properties of parenteral PGLA microspheres and their relationship with in vitro and in vivo performance, risperidone microspheres were prepared using different manufacturing processes. Four risperidone microsphere formulations were investigated: i) Formulation 1_Homogenization & dry sieving (DCM was used as the solvent); ii) Formulation 2_Homogenization & wet sieving (DCM); iii) Formulation 3_Vortex & wet sieving (EA was used as the solvent); and iv) Formulation 4_Homogenization & wet sieving (EA).
Physicochemical properties (e.g. drug loading, particle size, particle size distribution, morphology, glass transition temperature, organic solvent residual content as well as porosity) of the prepared risperidone microspheres and the commercial product Risperdal® Consta® were investigated. As shown in Table 1, the drug loading of Risperdal® Consta® was around 39% (w/w). Despite the fact that different manufacturing processes and solvent systems were utilized, the prepared risperidone microsphere formulations had similar drug loading (~37%, w/w). GC/MS results showed that the residual organic solvent content (i.e. DCM and EA) in the prepared risperidone microspheres were below the limits stated in the FDA Guidance for Industry Q3CImpurities: Residual Solvents (i.e. 600 ppm for DCM and 5,000 ppm for EA). These results confirmed that the risperidone microsphere formulations prepared with manufacturing differences were equivalent in formulation composition. It was observed that risperidone retained crystallinity in all microsphere formulations investigated (the melting point of risperidone was around 171°C). Moreover, no significant differences in the Tgs of Risperdal® Consta®, the physical mixture of the drug and the PLGA polymer, as well as the prepared risperidone microspheres were observed (ca. 41~42°C).
Table 1.
Physicochemical properties of the risperidone microsphere formulations with manufacturing differences and Risperdal® Consta® (n=3).
| Sample | Solvent | Preparation Method | Drug Loading (%, w/w) |
Tg (°C) |
Porosity (%) |
|---|---|---|---|---|---|
| Risperdal® Consta® | - | - | 39.42±1.92 | 40.97 | 43.97±4.60 |
| Formulation_1 | DCM | Homogenization & dry sieving | 36.77±1.44 | 42.66 | 43.19±4.60 |
| Formulation_2 | DCM | Homogenization & wet sieving | 37.67±0.94 | 42.28 | 46.04±2.90 |
| Formulation_3 | EA | Vortex & wet sieving | 37.33±0.60 | 43.02 | 54.98±1.25 |
| Formulation_4 | EA | Homogenization & wet sieving | 36.45±1.23 | 41.28 | 61.75±1.08 |
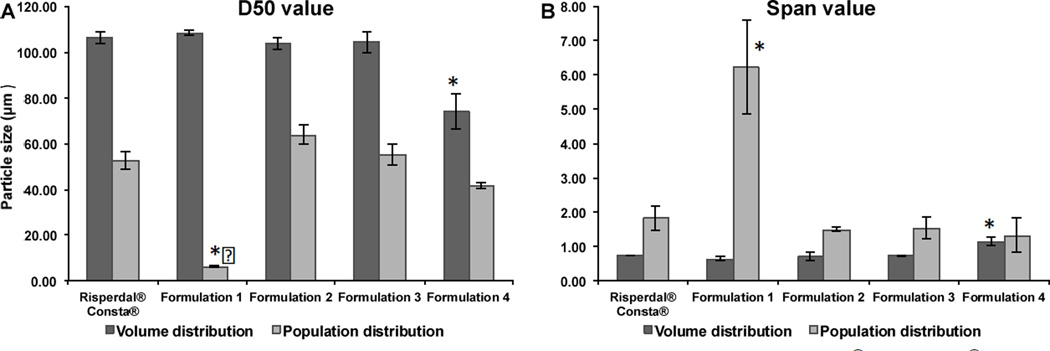
Particle size and particle size distribution results are shown in Figure 1. It was determined that both D50 and Span values of three batches of each formulation were very reproducible. In case of volume distribution, all the prepared risperidone microsphere formulations except for Formulation 4 showed similar D50 (ca. 100 µm) and Span values compared to Risperdal® Consta® (p > 0.05). Although Formulation 4 was prepared using the same solvent system as Formulation 3, the homogenization process provided a stronger emulsification force, thus resulting in significantly smaller microspheres (i.e. Formulation 4) (p < 0.05). In case of population distribution, Formulation 1 showed significantly different D50 and Span values compared with Risperdal® Consta® (p < 0.05). Due to the presence of strong static force during the dry sieving procedure, most small particles were not removed and consequently, Formulation 1 had the smallest D50 value and largest Span value among all microsphere formulations investigated.
Figure 1.
Particle size and particle size distribution of Risperdal® Consta® and the risperidone microspheres prepared using different manufacturing processes. (A) D50 value; and (B) Span value. All values are expressed as mean±SD (n=3 batches) (* indicates difference from the commercial product Risperdal® Consta®, p < 0.05).
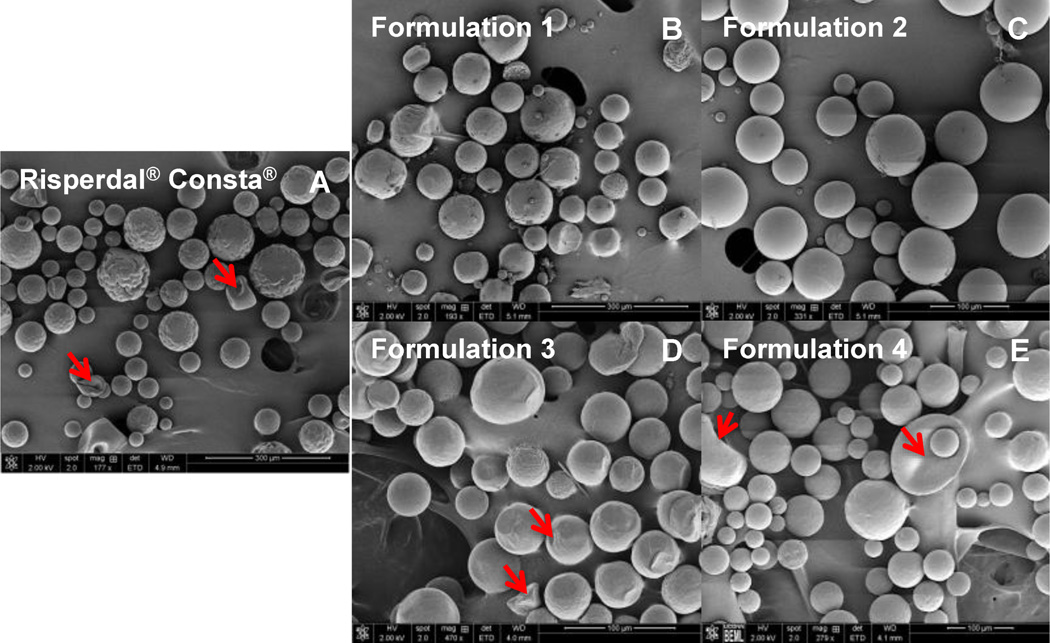
It has been reported that the manufacturing processes (e.g. solvent extraction rate) can affect water inclusion inside PLGA microspheres, resulting in different microsphere inner structures [25, 26]. As shown in Figure 2, the risperidone microspheres prepared using DCM as the solvent (i.e. Formulations 1 and 2) had smooth and spherical characteristics with a less porous structure (Figures 2D and 2E). On the other hand, the risperidone microspheres prepared using the EA solvent system (i.e. Formulations 3 and 4) showed similar morphology to that of Risperdal® Consta®. Some irregular shapes and indentations (shown using red arrows) were observed for these microspheres (Figures 2A, 2B and 2C). Since EA and water are partially miscible (EA solubility in water and water solubility in EA are 8.7% (w/w) and 3.3% (w/w), respectively), dynamic movement of EA and water during the microsphere hardening process often occurs [27]. This dynamic movement can result in water inclusion inside PLGA microspheres, generating irregular shapes and indentation during the drying process. Porosity results are shown in Table 1. Overall, the risperidone microspheres prepared using DCM as the solvent had lower total porosity percentages (~45%), indicating a less porous structure. When EA was used as the solvent (i.e. Formulations 3 and 4), much higher porosity percentages were observed (ca. 55% and 62% for Formulations 3 and 4, respectively). This indicated that the EA solvent extraction rate during the solidification process may be different for Formulations 3 and 4, resulting in different porosities [27]. Interesting, Risperdal® Consta® had a similar porosity (43.97%) as the risperidone microspheres prepared using DCM as the solvent, even though it showed similar morphology to that of the risperidone microspheres prepared using EA as the solvent.
Figure 2.
SEM micrographs of Risperdal® Consta® (A); and risperidone microsphere Formulations 1 to 4 (B, C, D, and E). Red arrows indicate indentations on the surface of the microspheres.
It was confirmed from the above studies that the critical physicochemical properties (e.g. porosity, particle size and particle size distribution) of the risperidone PLGA microspheres were sensitive to manufacturing differences such as solvent systems and microsphere collection procedures. The differences in the critical physicochemical properties of the prepared PLGA microspheres may in turn affect their in vitro and in vivo performance.
3.2. In vitro release studies of risperidone microspheres
Currently, there is a lack of compendial in vitro release methods for complex parenteral microsphere dosage forms. Various methods (e.g. sample-and-separate, membrane dialysis, and continuous flow (USP apparatus 4)) are commonly used for in vitro release testing of parenteral microspheres [24, 28–31]. However, the procedures and apparatus used vary among laboratories, and this makes inter-laboratory comparisons difficult. The U.S. FDA has recommended that a dissolution method using USP apparatus 4 and, if applicable, USP apparatus 2 (Paddle) or any other appropriate method should be developed for risperidone microspheres. In order to develop an IVIVC for the risperidone microspheres that are equivalent in formulation composition to the RLD product, both sample-and-separate and continuous flow methods were used for in vitro release testing of the prepared microspheres.
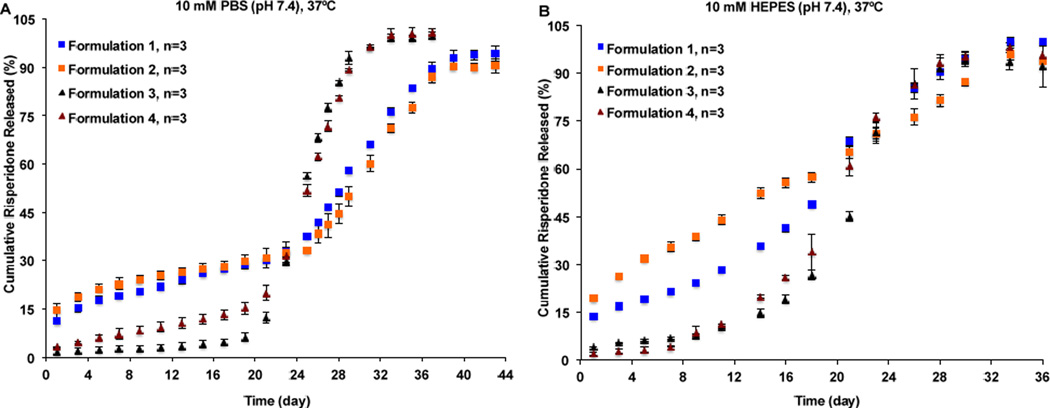
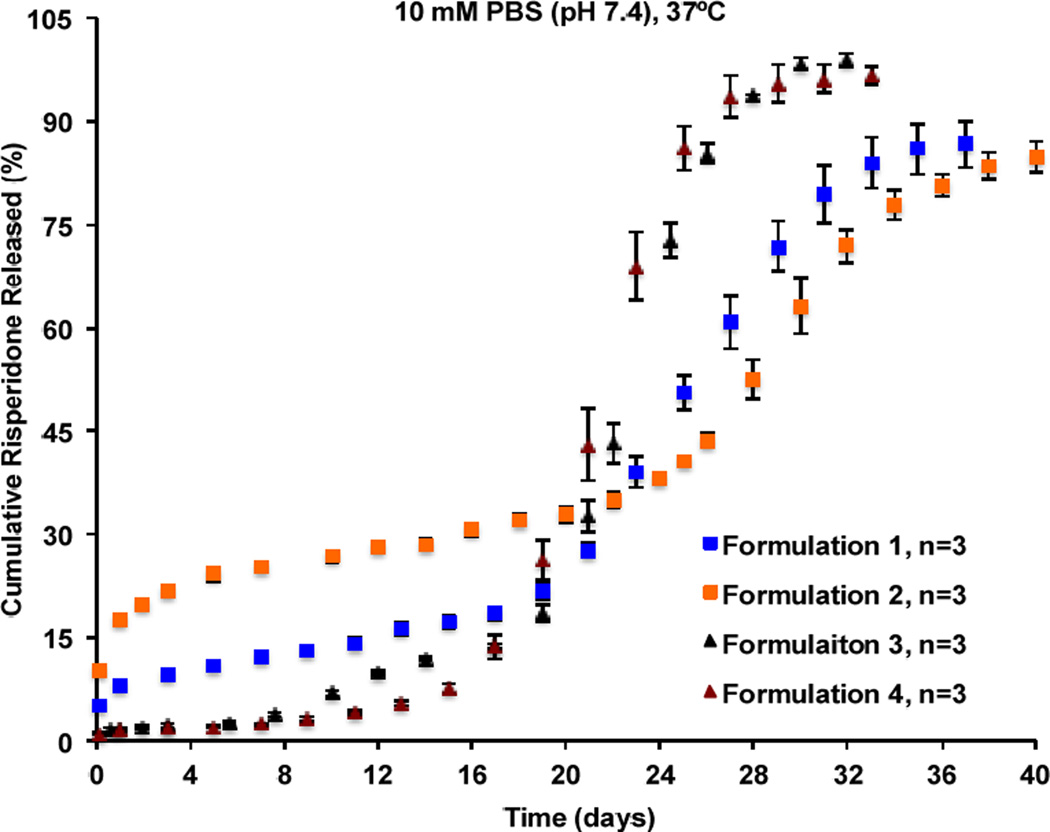
As shown in Figure 3A, the microsphere formulations prepared using DCM as the solvent showed higher burst release percentages (ca. 10–14%) followed by a slightly longer lag phase and reached release plateau around day 40. On the other hand, the microsphere formulations prepared using EA as the solvent showed lower burst release percentages (<3%) followed by a shorter lag phase and reached release plateau around day 33. Water has negligible solubility in DCM (0.2%, w/w) [32] and therefore, water diffusion into the microparticles during the microsphere hardening process was poor, resulting in less porous microspheres. For these microspheres, a longer period of time may be needed for polymer erosion to generate sufficient microsphere porosity to facilitate drug diffusion and subsequent release. Accordingly, these microspheres had longer lag phases and release durations. In addition, since risperidone is soluble in DCM, DCM may carry a certain amount of the drug to the surface and/or outer layer of the microspheres when it is removed from the microparticles resulting in the observed high burst release. In case of the microspheres prepared using EA as the solvent, polymer precipitated fast in the aqueous phase due to the high EA solubility in water (8.7%, w/w). In addition, a co-solvent (BA) was used to help dissolve risperidone. Since the ratio of EA to BA was high (2.75/1, v/v) and the fact that EA evaporates faster than BA at room temperature due to its much lower boiling point, drug diffusion along with BA to the surface of the microspheres during the fast polymer precipitation process may be limited [33]. Consequently, the drug may be mostly entrapped inside the microspheres, which is in agreement with the observed low burst release. Furthermore, due to their highly porous structures, these microspheres degraded faster and thereby, the lag phases and duration to reach release plateau were shorter compared with the microspheres prepared using DCM as the solvent. It can be seen in Figure 3A, that the sample-and-separate method (PBS as the release medium) was able to differentiate microsphere formulations prepared using different solvent systems. However, it failed to discriminate microsphere formulations prepared using the same solvent systems but with particle size differences (e.g. Formulations 1 and 2, as well as Formulations 3 and 4). When PBS was used as the release medium (without the addition of surfactants), microsphere aggregation was observed during long-term release testing, which may have minimized the effect of particle size on drug release from PLGA microspheres, leading to a loss of discriminatory ability of the sample-and-separate method.
Figure 3.
In vitro release profiles of equivalent formulation compositions of risperidone microspheres with manufacturing differences obtained using the sample-and-separate method at 37°C in: (A) 10 mM PBS (pH 7.4); and (B) 10 mM HEPES (pH 7.4) containing 0.02% (w/v) Tween 20 (n=3).
In vitro release testing of the prepared risperidone microspheres was also performed using the sample-and-separate method in a FDA recommended release medium containing surfactant (10 mM HEPES buffer (pH 7.4) containing 0.02% (v/v) Tween 20) (Figure 3B). Overall, the time to reach a plateau in the release profile of the prepared risperidone microspheres was shorter in HEPES buffer than in PBS buffer at 37°C (Figure 3). The presence of surfactant (i.e. Tween 20) in the release medium can facilitate wetting and buffer penetration during release, thus shortening the time needed for polymer erosion and consequent generation of sufficient porosity to facilitate drug diffusion and release. Therefore, a short lag phase and release duration were observed in the presence of surfactant. This was particularly apparent for Formulations 1 and 2, where the release profile appeared to be bi-phasic instead of the typical tri-phasic profile. Furthermore, the addition of surfactant (i.e. Tween 20) into the release medium can minimize microsphere aggregation during long-term release testing. Accordingly, different drug release rates resulting from manufacturing differences (e.g. microspheres prepared with different particle sizes) may be detected and consequently, the discriminability of the sample-and-separate method was improved. However, the sample-and-separate method did not appear to be able to differentiate the more porous microspheres (i.e. Formulations 3 and 4) (Figure 3B).
USP apparatus 4 has been demonstrated to be a more appropriate method for in vitro release testing of PLGA microspheres since it can minimize microsphere aggregation and avoid sample loss during sampling that is often associated with the sample-and-separate method [31]. In addition, USP apparatus 4 is a compendial dissolution apparatus with well-defined geometry and hydrodynamics, and this makes inter-laboratory comparisons feasible. Accordingly, in vitro release characteristics of the prepared risperidone microspheres were investigated using a previously developed USP 4 method (Figure 4). Overall, risperidone release from more porous microspheres (i.e. Formulations 3 and 4) appeared to be faster than that from less porous microspheres (i.e. Formulations 1 and 2). When the same solvent system was used for preparation, microspheres with smaller mean particle size (i.e. Formulations 1 and 4) showed faster risperidone release during the fast release phase compared with the microspheres with larger mean particle size (i.e. Formulations 2 and 3). Smaller microspheres have larger surface area to volume ratios as well as shorter diffusional paths for the dissolved drug to enter into the release media compared to larger microspheres, thus faster release. It was evident that the differences in the critical physicochemical properties (e.g. porosity, and particle size) of the PLGA microspheres that were equivalent in formulation composition can result in different release characteristics. Compared to the sample-and-separate method, the USP apparatus 4 method demonstrated better discrimination against risperidone microspheres that are compositionally equivalent with manufacturing differences. Therefore, the USP apparatus 4 method may be a more appropriate method to detect manufacturing differences for the prepared risperidone microspheres.
Figure 4.
In vitro release profiles of the formulation composition equivalent risperidone microspheres with manufacturing differences obtained using the USP apparatus 4 method at 37°C in 10 mM PBS (pH 7.4) (n=3).
Considering the presence of the in vivo boundary layers as well as the small interstitial fluid volume available for drug release at the intramuscular (and the subcutaneous) local sites, the USP apparatus 4 method may be a comparatively better method to mimic the in vivo environment. In the USP apparatus 4 method, the microspheres are exposed to a limited volume of release media at a time (< 10 ml in the 12 mm dissolution cells) whereas, in the sample-and-separate method, the microspheres have constant access to a large media volume. Following from this, non-sink in vitro conditions may be considered to mimic the local site for s.c and i.m. injectables. However, although the local s.c. and i.m. fluid volumes may be small, there is constant fluid movement and replacement, as well as drug diffusion away from the local site and/or drug metabolism at the site. Accordingly, to capture product performance as well as for quality control purposes, it is important that in vitro release tests are conducted under sink conditions.
In vivo studies of the risperidone microspheres
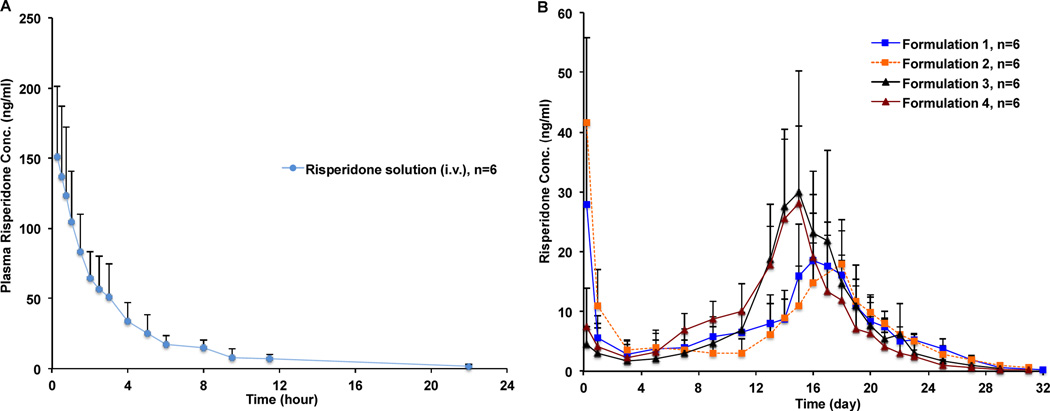
The mean plasma concentration-time profiles of risperidone following intravenous (i.v.) administration of the risperidone solution and intramuscular (i.m.) administration of the risperidone PLGA microspheres in the rabbit model are shown in Figure 5. Overall, the in vivo release profiles of the risperidone microspheres appeared to correlate well with their in vitro release profiles (Figure 4). In case of Formulations 1 and 2, both microsphere formulations had high burst release percentages in vitro and accordingly, high initial plasma concentrations at around 5 hours were observed. In addition, these two formulations had longer lag phases and consequently, their absorption phases peaked later (at around day 16 and day 18, respectively) than Formulations 3 and 4. For Formulations 3 and 4, both formulations had low initial plasma concentrations due to their low burst release percentages in vitro. Moreover, shorter lag phases and faster absorption profiles (ca. absorption phases peaked at around day 15) were observed, correlating well with their in vitro release characteristics.
Figure 5.
Mean plasma concentration-time profiles of risperidone in rabbits following: (A) intravenous administration of the risperidone solution at a single dose of 0.2 mg/kg; and (B) intramuscular administration of the prepared risperidone PLGA microspheres at a single dose of 1.92 mg/kg (mean±SD, n = 6).
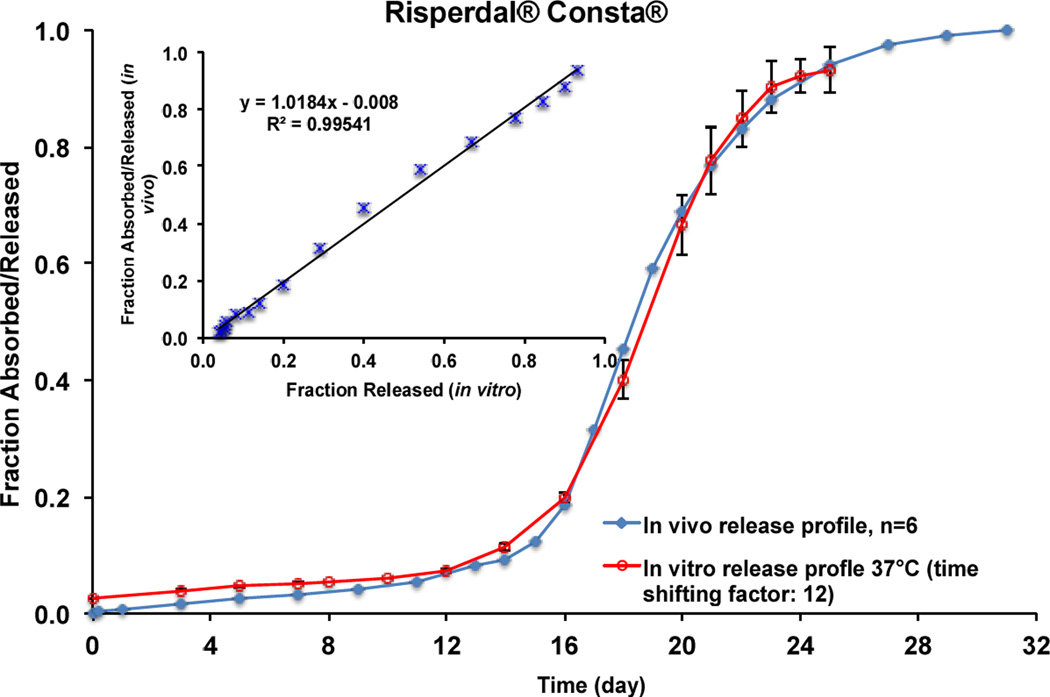
GastroPlus™ software (Simulations Plus, Inc., CA) was used to calculate the pharmacokinetic parameters of the risperidone solution following i.v. administration. Similar to the human data reported in the literature [34], the concentration-time curve of the risperidone solution in rabbits can be fitted using a two-compartment model and the main pharmacokinetic parameters calculated are listed in Table 2. The hybrid rate constants k12, k21 and k10 were determined as 0.181, 0.299 and 0.369 h−1, respectively. The Loo-Riegelman method was used to deconvolute the plasma risperidone profiles of Risperdal® Consta® and the prepared risperidone microspheres [35]. A comparison between the real-time in vitro release profile (37°C) obtained using USP apparatus 4 (time shifting factor: 12) and the deconvoluted plasma profile of Risperdal® Consta® is shown in Figure 6. It was observed that the deconvoluted in vivo profile showed a lag phase of approximately 15 days and around 12% of the drug was absorbed during the 15-day lag phase. The fraction of drug absorbed/released then increased gradually after day 15 and a plateau was reached by day 27, which was faster than the real-time in vitro release profile (plateaued by day 36). Faster in vivo risperidone release may be a result of enhanced PLGA microsphere degradation due to change in the release mechanism from bulk erosion to surface erosion, as a consequence of lower local pH conditions [20, 23] and/or the presence of biological components (e.g. enzymes [36]). However, there is some controversy in the literature with respect to the role of enzymes in PLGA degradation since in vitro assessment cannot be entirely correlated to polymer degradation in vivo [37]. It was also determined that the in vivo risperidone release/absorption in rabbits was faster than that in humans as reported in the literature (plateaued by day 56 [19]). This can be explained by the interspecies differences in the local in vivo environment (such as interstitial fluid volume and components (e.g. enzyme)) between the rabbit cranial muscle and human gluteal or deltoid muscle.
Table 2.
Pharmacokinetic parameters of the risperidone solution following intravenous administration (n = 6).
| A | B | α (h−1) | β (h−1) | K10 (h−1) | K12 (h−1) | K21 (h−1) |
|---|---|---|---|---|---|---|
| 128.33±31.91 | 46.80±34.68 | 0.698±0.17 | 0.152±0.03 | 0.369±0.019 | 0.181±0.075 | 0.299±0.131 |
A and B are empirical constants; α and β are the hybrid rate constants of the distribution and elimination phases, respectively.
Figure 6.
In vivo absorption/release and in vitro release (time shifting factor: 12) profiles in 10 mM PBS (pH 7.4) at 37°C of Risperdal® Consta®. Inserted figure shows linear correlation between fraction released in vitro (37°C) and fraction absorbed/released in vivo.
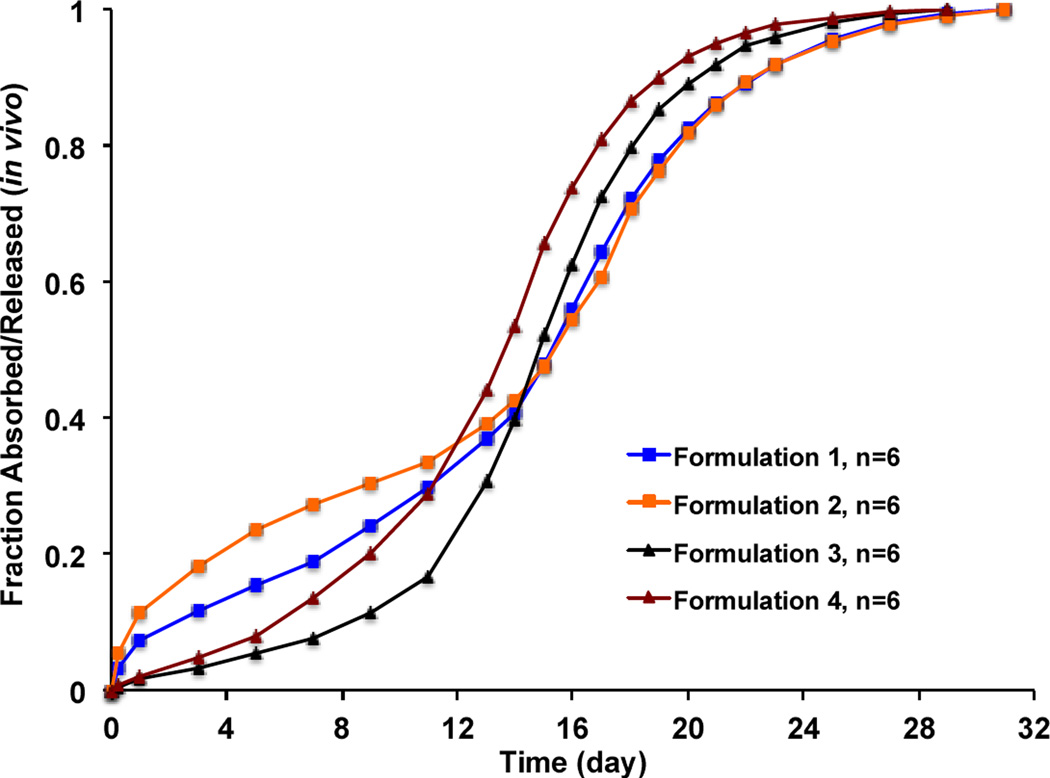
The deconvoluted plasma profiles of the prepared risperidone microspheres are shown in Figure 7. Although the fraction released/absorbed in vivo was faster than the fraction released in vitro, the in vivo release profiles of all formulations in the tested animals still followed a similar rank order as the in vitro release profiles: Formulation 4 > Formulation 3 > Formulation 1 > Formulation 2. Unlike Risperdal® Consta®, the lag phases were not as obvious for the prepared risperidone microspheres, indicating faster degradation. It was noted that even though the prepared risperidone microspheres were equivalent in formulation composition, they showed different in vitro and in vivo release characteristics as a result of manufacturing changes.
Figure 7.
In vivo profiles (fraction absorbed/released) of the prepared risperidone microspheres with manufacturing differences (deconvoluted using the Loo-Riegelman method).
3.3. IVIVC
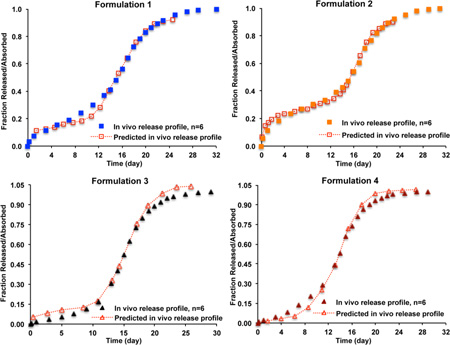
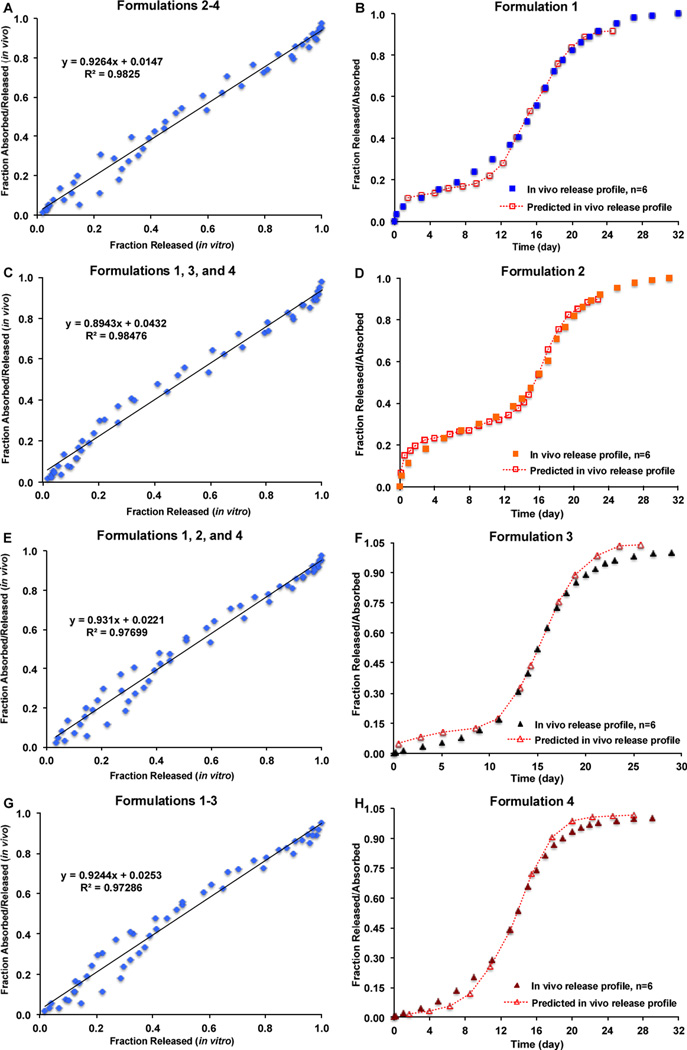
The FDA IVIVC Guidance on extended release oral dosage forms has recommended that a minimum of two, preferably three or more formulations with different release rates can define an IVIVC [1]. All four prepared risperidone microsphere formulations had different release characteristics both in vitro and in vivo and accordingly, all of them were suitable for the use of developing an IVIVC. It has been demonstrated above that the USP apparatus 4 method was capable of detecting manufacturing changes of the risperidone microspheres that are equivalent in composition. Therefore, in vitro release profiles of the risperidone microspheres obtained using USP apparatus 4 were compared with their in vivo release profiles in this animal model and utilized for the establishment of IVIVC. Figures 8A, 8C, 8E, and 8G show IVIVCs developed using any combinations of the three risperidone microsphere formulations. A one-to-one linear relationship (Level A) between the fractions released in vitro and fractions released/absorbed in vivo was observed for all combinations (correlation coefficients greater than 0.97). All the developed IVIVCs were comparable as manifested by similar slopes and intercepts. Furthermore, these developed IVIVCs were used to predict in vivo performance of the microsphere formulations that were not used in developing IVIVCs. As shown in Figures 8B, 8D, 8F, and 8H, the predicted in vivo release/absorption profiles of all risperidone microsphere formulations almost overlapped with their experimental in vivo release profiles except that the predicted initial in vivo release values were slightly different to the experimental values. The combination of the risperidone microsphere formulations had no effect on the development of IVIVC, which demonstrated that the IVIVCs developed were robust.
Figure 8.
Level A IVIVC for risperidone microspheres using the Loo-Reigelman method. (A) IVIVC developed using Formulations 2, 3 and 4. (B) Experimental and predicted in vivo release profiles of Formulation 1. (C) IVIVC developed using Formulations 1, 3, and 4. (D) Experimental and predicted in vivo release profiles of Formulation 2. (E) IVIVC developed using Formulations 1, 2, and 4. (F) Experimental and predicted in vivo release profiles of Formulation 3. (G) IVIVC developed using Formulations 1, 2 and 3. (H) Experimental and predicted in vivo release profiles of Formulation 4.
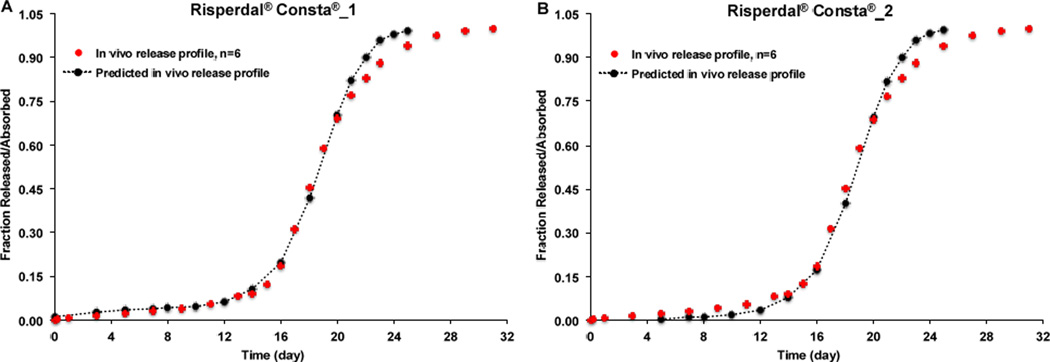
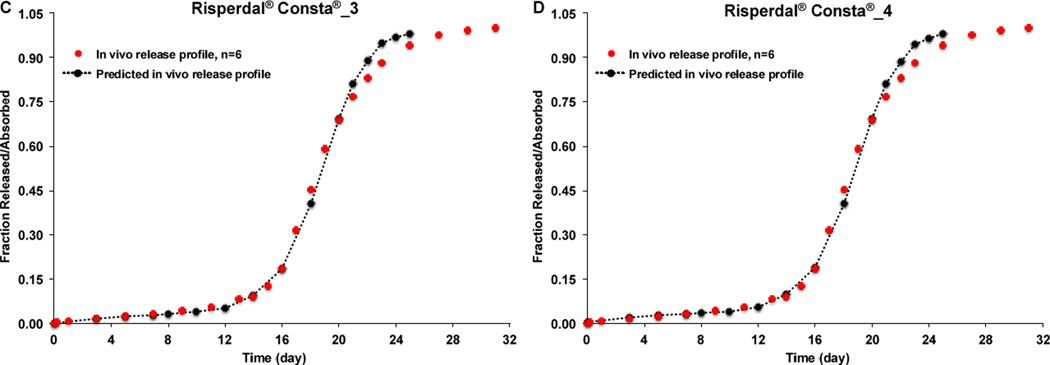
In order to explore the possibility of using the developed IVIVCs to predict in vivo performance of risperidone microspheres that have similar drug loading but may not be equivalent in composition to the prepared microsphere formulations, the developed IVIVCs were used to predict the in vivo release/absorption profile of Risperdal® Consta®. As shown in Figure 9, all the predicted in vivo release profiles of Risperdal® Consta® were almost identical to the experimental in vivo release profiles no matter which developed IVIVC was used. These results confirmed that the developed IVIVCs were sufficiently robust. Most importantly, the developed IVIVCs can be used to predict not only the risperidone microsphere formulations that are equivalent in formulation composition but also those microsphere formulations that are not equivalent in composition but with similar drug loading. PLGA polymers from different vendors may not be exactly the same even though they have similar inherent viscosity and/or molecular weight. In addition, different batches of PLGA polymers often have slightly different inherent viscosity and/or molecular weight. Accordingly, it is important that the developed IVIVCs can be used to predict in vivo release characteristics of microspheres prepared using PLGA polymers with similar molecular weight as the ones used for developing the IVIVCs to guide formulation and/or process development changes in various stages of microsphere drug product development.
Figure 9.
Experimental and predicted (time scaling factor: 12) in vivo release profiles of Risperdal® Consta® using the developed IVIVCs. (A) IVIVC developed using Formulations 2, 3 and 4. (B) IVIVC developed using Formulations 1, 3, and 4. (C) IVIVC developed using Formulations 1, 2, and 4. (D) IVIVC developed using Formulations 1, 2 and 3.
Based on the FDA IVIVC Guidance on extended release oral dosage forms, the predictability (internal and external prediction errors, %PEs) of the IVIVC developed based on microsphere formulations with slow, medium, and fast release rates (i.e. Formulations 2, 3, and 4) was validated. WinNonlin® was used to calculate the %PEs for Cmax and AUC. As shown in Table 3, the average absolute internal %PE for the AUC (6.46%) was within the recommended range of 10% or less. However, the average absolute internal %PE for Cmax (37%) was much beyond 10%, suggesting the internal predictability of the developed IVIVC for Cmax was inconclusive. Accordingly, evaluation of external predictability of the IVIVC was performed. It can be seen in Table 3 that the external %PE for Cmax and AUC were −4.56% and 10.61%, which passed the recommended external predictability evaluation (%PE of 10% or less). Furthermore, the predictability of the developed IVIVC for Risperdal® Consta® was investigated. Both %PEs for Cmax (7.9%) and AUC (0.08%) were below 10%. These results confirmed that the developed IVIVC can be used to predict the in vivo performance of rispierdone microspheres that are equivalent or similar in composition.
Table 3.
Validation and prediction of the developed IVIVC for risperidone microspheres.
| Internal validation | Cmax (µg/L) | AUC (µg/L*day) | ||||
|---|---|---|---|---|---|---|
| Pred. | Obs. | %PE | Pred. | Obs. | %PE | |
| Formulation 2 | 19.64 | 41.62 | −52.81 | 188.26 | 200.41 | −6.06 |
| Formulation 3 | 40.49 | 29.98 | 35.06 | 219.14 | 229.07 | −4.34 |
| Formulation 4 | 35.58 | 28.68 | 24.08 | 201.12 | 220.95 | −8.97 |
| Average absolute %PE | 37.32 | 6.46 | ||||
| External validation | ||||||
| Formulation 1 | 26.71 | 27.99 | −4.56 | 231.51 | 206.92 | 10.61 |
| Prediction | ||||||
| Risperdal® Consta® | 41.32 | 38.29 | 7.90 | 248.69 | 248.50 | 0.08 |
It has been noted that it may be difficult to predict initial in vivo drug release based on the burst release phase in vitro, since the rate-limiting step for the initial in vivo drug availability may actually be drug permeation across the tissue barriers instead of drug release. For example, Formulation 2 had a high initial burst release in vitro (~12% within 2 hours), which subsequently resulted in the first PK sample (taken at 5 hours post-administration) having a high plasma concentration (41.62 µg/l) that exceeded the peak concentration during the absorption phase (17.99 µg/l at around day 18). Therefore, the first point of the concentration profile was detected as the Cmax instead of the peak concentration of the absorption phase. Generally, a predicted Cmax is derived from a predicted absorption peak based on convolution techniques. Therefore, the predicted Cmax for Formulation 2 (19.67 µg/l) was closer to the actual absorption peak concentration (17.99 µg/l, %PE of 9.3%) rather than the concentration at the initial burst (41.62 µg/l, %PE of −52.81%). In addition, the Cmax predictability of the developed IVIVC for Formulations 3 and 4 (with minimal burst release) was also affected (ca. %PE of 35% and 25% for Formulations 3 and 4, respectively) since Formulation 2 was one of the microsphere formulations used in developing the IVIVC.
The in vitro release profiles of the prepared risperidone microspheres obtained via the sample-and-separate method (using PBS and HEPES buffers) were also compared with the in vivo release profiles. Overall, these in vitro release profiles showed poorer in vivo relevance (R2: 0.95~0.96) compared to that obtained using the USP apparatus 4 method. The predictability of the IVIVCs developed based on Formulations 2, 3, and 4 was also evaluated using WinNonlin®. Average absolute internal %PEs for AUC and Cmax were 7.06% and 27.10% (when HEPES buffer was used as the release medium), and 6.80% and 47.50% (when PBS buffer was used as the release medium), respectively. Since the internal predictability of the developed IVIVCs for Cmax was inconclusive, the external %PEs for Cmax and AUC were evaluated. The external %PEs for AUC and Cmax were 10.11% and −39.51% (when HEPES buffer was used as the release medium), and 14.49% and 11.47% (when PBS buffer was used as the release medium), respectively, which were beyond the recommended 10% range.
The USP apparatus 4 method also demonstrated better predictability of the in vivo initial burst release phase. The sample-and-separate method in PBS did not show any difference between Formulations 1 and 2 in the initial burst, and the sample-and-separate method in HEPES did not show an initial burst (but rather a continual constant release). Therefore, the USP apparatus 4 method results were most similar to the in vivo PK profiles obtained from the animal study. A reliable detection of the in vitro initial burst is important, especially in regulatory applications when two formulations are being evaluated for equivalence. These results suggest that the USP apparatus 4 method may be a more suitable in vitro release testing method for the development of IVIVC for the risperidone microsphere formulations that are equivalent in composition. While the USP apparatus 4 method was demonstrated to be superior to the other two methods, it is noted that the IVIVC constructed in this study utilized animal PK data. The results from this IVIVC study cannot be fully extrapolated to humans since there are known differences in drug absorption and drug release between rabbits and humans. A similar study performed with human PK data would be necessary for an IVIVC to be fully applicable to human drug products.
The lack of a standard, compendial in vitro release testing method capable of detecting manufacturing differences and predicting in vivo drug release characteristics has hindered the development of IVIVCs for complex parenteral microsphere drug products. Results from the present study proved that robust Level A IVIVCs can be established for PLGA microspheres, particularly microsphere formulations that have the same composition, with an appropriate in vitro release testing method (e.g. the developed USP apparatus 4 method). The developed USP apparatus 4 method based on standard USP apparatus is capable of discriminating compositionally equivalent microsphere formulations with manufacturing differences and predicting their in vivo drug release characteristics, as was shown in this animal model.
4. Conclusions
This is the first report for the development of a Level A IVIVC based on a compendial dissolution apparatus (USP apparatus 4) for compositionally equivalent risperidone-loaded PLGA microspheres prepared with different manufacturing processes. The critical physicochemical properties of the prepared microspheres were sensitive to minor manufacturing changes, which subsequently resulted in changes in the in vitro and in vivo performance. Compared to the sample-and-separate method, the USP apparatus 4 method showed better capability of differentiating the compositionally equivalent risperidone microspheres with manufacturing differences and most importantly, the USP apparatus 4 method showed better predictability for the in vivo performance of these microspheres. Furthermore, the IVIVCs developed based on the USP apparatus 4 method were robust. These results suggested that the USP apparatus 4 method is an appropriate method to not only ensure product quality, but also assist in product development for complex parenteral microspheres. Furthermore, the established Level A IVIVC based on standard USP apparatus (i.e. USP apparatus 4) can facilitate inter-laboratory comparison, and may be used to support drug product development.
Acknowledgments
The authors would like to acknowledge Janet Trombley and Kimberlie Davenport (Veterinary Technicians) from UConn Animal Care Services for their help with animal care. This work was financially supported by the Office of Research and Standards, Office of Generic Drugs, CDER at the FDA (1U01FD004931-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
References
- 1.FDA Guidance for Industry Extended Release Oral dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations. U.S. Department of Human Health and Human Services, Food and Drug Administration; 1997. [Google Scholar]
- 2.Roudier B, Davit BM, Beyssac E, Cardot JM. In vitro-in vivo correlation's dissolution limits setting. Pharm. Res. 2014;31:2529–2538. doi: 10.1007/s11095-014-1349-8. [DOI] [PubMed] [Google Scholar]
- 3.Emami J. In vitro - in vivo correlation: from theory to applications. J. Pharm. Pharm. Sci. 2006;9:169–189. [PubMed] [Google Scholar]
- 4.Young D. In vitro-in vivo correlation for modified release parenteral drug delivery systems. In: Chilukuri DM, Sunkura G, Young D, editors. Pharmaceutical Product Development: In Vitro-In Vivo Correlation. New York: Informa Healthecare; 2007. pp. 141–151. [Google Scholar]
- 5.Hoffman AS. The origins and evolution of "controlled" drug delivery systems. J. Control. Release. 2008;132:153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Burgess DJ. Microsphere Technologies. In: Wright JC, Burgess DJ, editors. Long Acting Injections and Implants. Springer US; 2012. pp. 167–194. [Google Scholar]
- 7.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao S, Guo C, Shi Y, Li LC. Recent advances in polymeric microspheres for parenteral drug delivery--part 1. Expert Opin. Drug Deliv. 2012;9:1161–1176. doi: 10.1517/17425247.2012.709844. [DOI] [PubMed] [Google Scholar]
- 9.FDA Approved Drug Products. [Accessed July 13, 2015]; http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
- 10.Burgess DJ, Hussain AS, Ingallinera TS, Chen ML. Assuring quality and performance of sustained and controlled release parenterals: AAPS workshop report, co-sponsored by FDA and USP. Pharm. Res. 2002;19:1761–1768. doi: 10.1023/a:1020730102176. [DOI] [PubMed] [Google Scholar]
- 11.Martinez MN, Rathbone MJ, Burgess DJ, Huynh M. In vitro and in vivo considerations associated with parenteral sustained release products: a review based upon information presented and points expressed at the 2007 Controlled Release Society Annual Meeting. J. Control. Release. 2008;129:79–87. doi: 10.1016/j.jconrel.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Martinez MN, Rathbone MJ, Burgess DJ, Huynh M. Breakout session summary from AAPS/CRS joint workshop on critical variables in the in vitro and in vivo performance of parenteral sustained release products. J. Control. Release. 2010;142:2–7. doi: 10.1016/j.jconrel.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Burgess DJ, Crommelin DJ, Hussain AS, Chen ML. Assuring quality and performance of sustained and controlled released parenterals. Eur. J. Pharm. Sci. 2004;21:679–690. doi: 10.1016/j.ejps.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Anand O, Yu LX, Conner DP, Davit BM. Dissolution testing for generic drugs: an FDA perspective. AAPS J. 2011;13:328–335. doi: 10.1208/s12248-011-9272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schliecker G, Schmidt C, Fuchs S, Ehinger A, Sandow J, Kissel T. In vitro and in vivo correlation of buserelin release from biodegradable implants using statistical moment analysis. J. Control. Release. 2004;94:25–37. doi: 10.1016/j.jconrel.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Vlugt-Wensink KD, de Vrueh R, Gresnigt MG, Hoogerbrugge CM, van Buul-Offers SC, de Leede LG, Sterkman LG, Crommelin DJ, Hennink WE, Verrijk R. Preclinical and clinical in vitro in vivo correlation of an hGH dextran microsphere formulation. Pharm. Res. 2007;24:2239–2248. doi: 10.1007/s11095-007-9433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Souza S, Faraj JA, Giovagnoli S, Deluca PP. IVIVC from Long Acting Olanzapine Microspheres. Int. J. Biomater. 2014;2014:407065. doi: 10.1155/2014/407065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Souza S, Faraj JA, Giovagnoli S, Deluca PP. In vitro-in vivo correlation from lactide-co-glycolide polymeric dosage forms. Prog. Biomater. 2014;3:131–142. doi: 10.1007/s40204-014-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawat A, Bhardwaj U, Burgess DJ. Comparison of in vitro-in vivo release of Risperdal((R)) Consta((R)) microspheres. Int. J. Pharm. 2012;434:115–121. doi: 10.1016/j.ijpharm.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Zolnik BS, Burgess DJ. Evaluation of in vivo-in vitro release of dexamethasone from PLGA microspheres. J. Control. Release. 2008;127:137–145. doi: 10.1016/j.jconrel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Negrin CM, Delgado A, Llabres M, Evora C. In vivo-in vitro study of biodegradable methadone delivery systems. Biomaterials. 2001;22:563–570. doi: 10.1016/s0142-9612(00)00214-3. [DOI] [PubMed] [Google Scholar]
- 22.Blanco-Prieto MJ, Campanero MA, Besseghir K, Heimgatner F, Gander B. Importance of single or blended polymer types for controlled in vitro release and plasma levels of a somatostatin analogue entrapped in PLA/PLGA microspheres. J. Control. Release. 2004;96:437–448. doi: 10.1016/j.jconrel.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Chu DF, Fu XQ, Liu WH, Liu K, Li YX. Pharmacokinetics and in vitro and in vivo correlation of huperzine A loaded poly(lactic-co-glycolic acid) microspheres in dogs. Int. J. Pharm. 2006;325:116–123. doi: 10.1016/j.ijpharm.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 24.Rawat A, Stippler E, Shah VP, Burgess DJ. Validation of USP apparatus 4 method for microsphere in vitro release testing using Risperdal Consta. Int. J. Pharm. 2011;420:198–205. doi: 10.1016/j.ijpharm.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Xiao CD, Shen XC, Tao L. Modified emulsion solvent evaporation method for fabricating core-shell microspheres. Int. J. Pharm. 2013;452:227–232. doi: 10.1016/j.ijpharm.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Rouaud O, Poncelet D. Microencapsulation by solvent evaporation: state of the art for process engineering approaches. Int. J. Pharm. 2008;363:26–39. doi: 10.1016/j.ijpharm.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Sah H. Microencapsulation techniques using ethyl acetate as a dispersed solvent: effects of its extraction rate on the characteristics of PLGA microspheres. J. Control. Release. 1997;47:233–245. [Google Scholar]
- 28.Larsen C, Larsen SW, Jensen H, Yaghmur A, Ostergaard J. Role of in vitro release models in formulation development and quality control of parenteral depots. Expert Opin. Drug Deliv. 2009;6:1283–1295. doi: 10.1517/17425240903307431. [DOI] [PubMed] [Google Scholar]
- 29.Shen J, Burgess DJ. Accelerated in-vitro release testing methods for extended-release parenteral dosage forms. J. Pharm. Pharmacol. 2012;64:986–996. doi: 10.1111/j.2042-7158.2012.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Souza S, Faraj JA, Dorati R, DeLuca PP. A short term quality control tool for biodegradable microspheres. AAPS PharmSciTech. 2014;15:530–541. doi: 10.1208/s12249-013-0052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zolnik BS, Raton JL, Burgess DJ. Application of USP apparatus 4 and in situ fiber optic analysis to microsphere release testing. Dissol. Technol. 2005;12:11–14. [Google Scholar]
- 32.Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int. J. Pharm. 2008;364:298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Sturek M, Park K. Microparticles produced by the hydrogel template method for sustained drug delivery. Int. J. Pharm. 2014;461:258–269. doi: 10.1016/j.ijpharm.2013.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang ML, Van Peer A, Woestenborghs R, De Coster R, Heykants J, Jansen AA, Zylicz Z, Visscher HW, Jonkman JH. Pharmacokinetics of the novel antipsychotic agent risperidone and the prolactin response in healthy subjects. Clin. Pharmacol. Ther. 1993;54:257–268. doi: 10.1038/clpt.1993.146. [DOI] [PubMed] [Google Scholar]
- 35.Wagner JG. Application of the Loo-Riegelman absorption method. J. Pharmacokinet. Biopharm. 1975;3:51–67. doi: 10.1007/BF01066595. [DOI] [PubMed] [Google Scholar]
- 36.Clark BC, Dickinson PA, Pyrah IT. Case study: in vitro/in vivo release from injectable microspheres. In: Burgess DJ, editor. Injtecable Dispersed Systems: Formulation Processing and Performance. Boca Raton: Taylor & Francis; 2005. pp. 543–570. [Google Scholar]
- 37.Alexis F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)] Polym. Int. 2005;54:36–46. [Google Scholar]