Abstract
A growing body of research has revealed that labeling an emotion, or putting one's feelings into words, can help to downregulate that affect, as occurs with intentional forms of emotion regulation, such as reappraisal and distraction. We translated this basic research to a real-world clinical context, in which spider-fearful individuals were repeatedly exposed to a live spider. Using a between-subjects design, we compared the effects of affect labeling, reappraisal, distraction from the feared stimulus, and exposure alone during this brief course of exposure therapy on subsequent fear responding. At a 1-week posttest involving a different spider in another context, the affect-labeling group exhibited reduced skin conductance response relative to the other groups and marginally greater approach behavior than the distraction group; however, the affect-labeling group did not differ from the other groups in self-reported fear. Additionally, greater use of anxiety and fear words during exposure was associated with greater reductions in fear responding. Thus, perhaps surprisingly, affect labeling may help to regulate aspects of emotion in a clinical context.
Keywords: fear, language, affect labeling, reappraisal, distraction, exposure therapy, skin conductance, approach behavior
Humans are distinctively able to think symbolically and to use language to express and regulate emotion. Several types of linguistic processing have been shown to attenuate negative emotional processes on neural, physiological, and subjective levels (e.g., Antony, McCabe, Leeuw, Sano, & Swinson, 2001; Craske, Street, Jayaraman, & Barlow, 1991; Gross, 1998; Hariri, Bookheimer, & Mazziotta, 2000; Lieberman et al., 2007; Ochsner, Bunge, Gross, & Gabrieli, 2002; Pennebaker, 1997; Tabibnia, Lieberman, & Craske, 2008). In particular, the emotion-regulating functions of affect labeling, reappraisal, and distraction have each been examined. Affect labeling refers to verbalization of current emotional experience— putting feelings into words (Lieberman, 2011; Pennebaker, 1997). Reappraisal refers to verbalization with the intent to regulate emotion by construing an evocative stimulus in a way that reduces its emotional significance (Gross, 1998; Gross & John, 2003). Distraction in this context refers to verbalization unrelated to affective material (e.g., Pennebaker, 1997).
Whereas reappraisal (e.g., Eippert et al., 2007; Gross, 1998, 2002; Ochsner et al., 2002) and distraction (e.g., Antony et al., 2001; Craske et al., 1991) are considered intentional and often effective forms of emotion regulation, affect labeling is relatively unintentional (Lieberman, 2011), and individuals may not expect or believe labeling to be useful for downregulating negative affect (Lieberman, Inagaki, Tabibnia, & Crockett, 2011). However, there is increasing support for affect labeling as a viable form of emotion regulation. For example, experimental studies have found that when current emotional experience is verbalized, whether in spoken or written form, distress is reduced relative to conditions in which no verbalization or verbalization of nonaffective material occurs (for reviews, see Frattaroli, 2005; Pennebaker & Chung, 2011).
Of particular relevance to fear and anxiety, results of neuroimaging research have demonstrated that self-reflective cognitive processing, such as affect labeling, can reduce limbic responses to negative emotional stimuli via a neurocognitive feedback mechanism. For instance, Lieberman and his colleagues (2007) demonstrated that the amygdala activity normally present while viewing an evocative image was diminished while participants performed affect labeling (e.g., choosing the word “scared”), whereas right ventrolateral prefrontal cortex (RVLPFC) was selectively activated during affect labeling. RVLPFC was not activated during nonaffective labeling. A subsequent study (Tabibnia et al., 2008) investigated the effects of labeling on skin conductance response (SCR) as a peripheral index of emotional arousal. Participants viewed evocative images with or without a variety of one-word negative-affect labels as SCRs to the images were measured. At a retest conducted 1 week later, the images were shown with no labels as SCRs were again measured. SCR attenuation was significantly greater for participants in the labeling condition than for participants in the no-label condition. Moreover, this effect was replicated in a spider-fearful sample during exposure to spider images.
To date, no studies have compared the effects of affect labeling, reappraisal, and distraction on fear responding. This oversight is conspicuous given that psychological treatments for emotional disorders involve language (i.e., talking) as well as behavioral interventions. We examined these verbalization methods during a brief course of exposure therapy, the most widely used psychological treatment for phobias and other anxiety disorders. Traditional cognitive-behavioral therapy (CBT) for anxiety disorders (Beck & Emery, 1985; Emmelkamp, 1982) emphasizes reappraisal, or the replacement of negative thoughts with neutral thoughts about a feared stimulus, to reduce fearful responding. However, there has been debate about whether cognitive reappraisal adds significant value to exposure without reappraisal (Longmore & Worrell, 2007). On the basis of previous neuroimaging and psychophysiological research (Lieberman et al., 2007; Tabibnia et al., 2008), we hypothesized that affect labeling would lead to greater decreases in SCR and self-reported fear and greater increases in approach behavior relative to the other conditions in our study, with the potential exception of reappraisal.
Method
Design
This study had a 4 (group: affect labeling, reappraisal, distraction, and exposure alone) × 3 (measurement occasion: pretest, immediate posttest, 1-week posttest) mixed design.
Participants
Eighty-eight participants (mean age = 20.5 years, SD = 4.6) who scored in the top quartile on the Spider Phobia Questionnaire (M = 14.38, SD = 3.78; Klorman, Weerts, Hastings, Melamed, & Lang, 1974) completed the study. Participants were drawn from an undergraduate psychology course and the community and were given 1 hour of experiment credit to be used toward filling a course requirement or $10 payment, respectively, for every hour of participation. Participants were 82% female and 18% male. The sample was 24% White, 41% Asian/Pacific Islander, 14% Latino/Hispanic, 5% African American, 7% biracial, and 9% other.
Procedure
On Day 1, participants signed informed consent and completed a pretest outdoors, in which they were instructed to approach a live Chilean rose-haired tarantula (Phrixotrichus spatulata; leg span = 6 in.; see Fig. 1). The tarantula was in a container, and participants were instructed to approach the spider as closely as possible according to a series of 10 standardized steps lasting 30 s each. On the first step, participants were instructed to stand at a 5-ft distance from the spider. On the last step, participants were instructed to touch the spider continuously with the tip of their index finger.
Fig. 1.
Spider and container used in the pretest, immediate posttest, and 1-week posttest.
Participants were then randomly assigned to four groups (affect labeling, reappraisal, distraction, exposure alone) of 22 participants each. Participants were brought indoors, where they sat 2 ft from a screen covering a live tarantula (leg span = 6 in.) different from the one they saw in the pretest. They underwent 10 exposure trials for durations of 38 s each. The pretest and exposure contexts were different in order to maximize sensitivity of the dependent measures and examine generalizability to a naturalistic outdoor setting (e.g., Bouton, 1993). After the first 8 s of each trial, a tone was presented to prompt participants to follow group-specific instructions.
Participants in the affect-labeling group were instructed to create and speak a sentence including a negative word to describe the spider and a negative word or two to describe their emotional response to the spider (e.g., “I feel anxious the disgusting tarantula will jump on me”). In the reappraisal group, participants were instructed to create and speak a sentence including a neutral word to describe the spider and a neutral word or two to describe a way of thinking about the spider in order to feel less negatively about it (e.g., “Looking at the little spider is not dangerous for me”). In the distraction group, participants were instructed to create and speak a sentence including an object or piece of furniture found in their home and a room or location in which the furnishing is found (e.g., “There is a television in front of my couch in the den”). Participants in the affect-labeling, reappraisal, and distraction groups were also instructed to vary their sentences across exposure trials. Participants in the exposure-alone group received no verbalization instructions.
After participants heard the tone, exposure lasted an additional 30 s. Intertrial intervals lasted 60 s, during which the screen was placed in front of the spider.
On Day 2, participants underwent 10 exposure trials for 38 s each, in the same manner as on Day 1. Participants then completed an immediate posttest, which followed the same procedure as the pretest, with instructions to remain silent throughout. On Day 9, participants completed a 1-week posttest following the same procedure as the pretest and immediate posttest.
Measures
SCR
At each test, SCR was utilized as the physiological measure of fearful arousal. SCR was recorded using an ambulatory monitoring device (LifeShirt, VivoMetrics, Ventura, CA; Wilhelm, Roth, & Sackner, 2003). Skin conductance signals were transmitted using two electrodes attached to the ring and middle fingers of the nondominant hand, and amplitude was recorded to the nearest microsiemens. Data were cleaned, inspected, and analyzed using VivoLogic software (Version 3.1; VivoMetrics). Baseline skin conductance for each test was calculated during the 3-min period prior to each test. SCR for each test was scored as the number of peak skin conductance values for the final step completed by each participant. Peak values of less than 2 s were discarded as artifacts (Davis, Barad, Otto, & Southwick, 2006; Ressler et al., 2004).
Behavioral approach
Behavioral approach was measured during the tests as the number of test steps fully completed. The raw number of additional steps completed from each test to the next (negative or positive) was also computed.
Reported fear
Self-reported fear was assessed using a visual analogue scale ranging from 0 (no fear) to 100 (extreme fear) following the final step of each test trial.
Word use
Participants' sentences across all exposure trials were transcribed and analyzed using Pennebaker's Linguistic Inquiry and Word Count (LIWC) software (Pennebaker & Francis, 1999). LIWC includes a text processor and dictionary of words and word stems organized along separate scales. Analyses were limited to the scale of anxiety and fear words (e.g., nervous, afraid, tense).
Results
Baseline group differences
One-way analyses of variance (ANOVAs) indicated no significant group differences in Spider Phobia Questionnaire score or in SCR, behavioral approach, or reported fear at pretest.
SCR
Table 1 shows raw SCR scores. A univariate analysis of covariance (ANCOVA) was conducted on SCR change from the pretest to the immediate posttest, with baseline skin conductance and the number of steps completed for each test entered as covariates. Planned pairwise comparisons indicated no significant differences between the affect-labeling group (M = 0.53 μS) and the reappraisal group (M = 0.22 μS), distraction group (M = 0.23 μS), or exposure-alone group (M = −0.24 μS), ds = −0.63 to −0.25, ps = .06–.41.
Table 1.
Mean Raw Scores for the Three Dependent Measures at the Three Test Occasions
| Group |
||||
|---|---|---|---|---|
| Measure and test | Affect labeling | Reappraisal | Distraction | Exposure alone |
| Skin conductance response (μS) | ||||
| Pretest | 1.76 (1.09) | 1.73 (0.77) | 1.59 (0.85) | 2.00 (0.73) |
| Immediate posttest | 2.18 (0.80) | 1.91 (0.97) | 1.82 (1.01) | 1.83 (0.85) |
| One-week posttest | 1.58 (0.80) | 2.18 (1.01) | 1.95 (1.29) | 1.90 (0.98) |
| Behavioral approach | ||||
| Pretest | 5.18 (2.44) | 5.68 (2.97) | 6.36 (2.46) | 6.14 (2.12) |
| Immediate posttest | 6.36 (2.84) | 6.36 (3.03) | 7.18 (2.46) | 7.00 (2.39) |
| One-week posttest | 7.82 (2.44) | 7.45 (2.84) | 7.68 (2.59) | 8.05 (2.50) |
| Reported fear | ||||
| Pretest | 79.75 (20.77) | 65.49 (20.39) | 74.49 (22.02) | 67.34 (24.89) |
| Immediate posttest | 68.99 (31.34) | 60.26 (22.70) | 63.94 (26.50) | 52.42 (30.15) |
| One-week posttest | 56.52 (29.16) | 44.98 (31.96) | 40.64 (25.33) | 40.22 (30.79) |
Note: Standard deviations are given in parentheses. Behavioral approach was indexed by measuring the number of test steps that participants fully completed. Self-reported fear was assessed using a visual analogue scale ranging from 0 (no fear) to 100 (extreme fear) following the final step of each test trial.
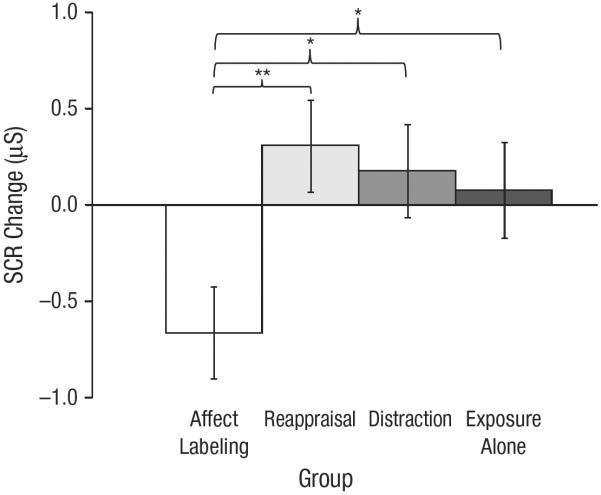
A univariate ANCOVA was conducted on SCR change from the immediate posttest to the 1-week posttest, with baseline skin conductance and the number of steps completed for each test entered as covariates. Planned pairwise comparisons indicated significantly greater SCR decrease for the affect-labeling group (M = −0.66, SE = 0.24) than for the reappraisal group (M = 0.31, SE = 0.24; p = .005, d = 0.85), distraction group (M = 0.18, SE = 0.24; p = .017, d = 0.74), and exposure-alone group (M = 0.08, SE = 0.25, p = .044, d = 0.64). A contrast analysis comparing the affect-labeling group with all other groups combined was significant (p = .004; see Fig. 2).
Fig. 2.
Change in skin conductance response (SCR) from the immediate posttest to the 1-week posttest as a function of group. Error bars indicate ±1 SE. Asterisks indicate that pairwise comparisons between groups were significant (*p < .05, **p < .01).
Behavioral approach
Table 1 shows raw behavioral-approach scores. A univariate ANOVA was conducted on the number of additional steps completed from the pretest to the immediate posttest. Planned pairwise comparisons indicated no significant differences between the affect-labeling group (M = 1.18) and the reappraisal group (M = 0.68), distraction group (M = 0.82), or exposure-alone group (M = 0.86), ds = 0.18–0.28.
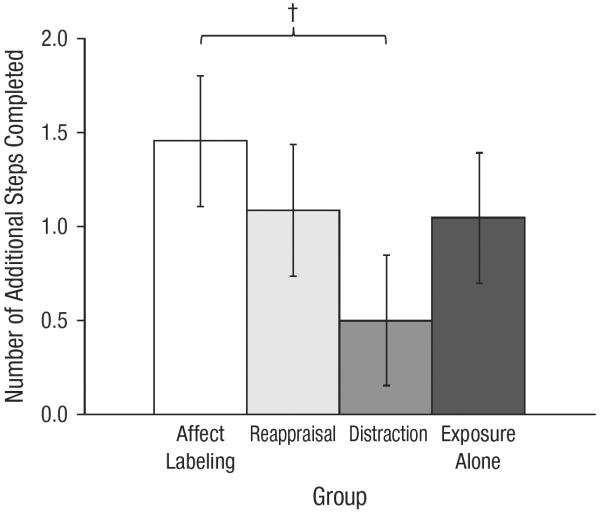
A univariate ANOVA was conducted on the number of additional steps completed from the immediate posttest to the 1-week posttest. Planned pairwise comparisons indicated that a marginally greater number of additional steps were completed by the affect-labeling group (M = 1.46, SE = 0.35) than by the distraction group (M = 0.50, SE = 0.35), p = .054, d = 0.59 (see Fig. 3).
Fig. 3.
Number of additional steps completed from the immediate posttest to the 1-week posttest as a function of group. Error bars indicate ±1 SE. The dagger indicates that a pairwise comparison between groups was marginally significant (†p = .05).
Reported fear
Table 1 shows raw self-reported fear scores. For the analysis, we removed four change scores that were statistical outliers (> 3 SD from the mean). A univariate ANCOVA was conducted on change in reported fear from the pretest to the immediate posttest, with the number of steps completed for each test entered as covariates. Planned pairwise comparisons indicated no significant differences between the affect-labeling group (M = −14.69) and the reappraisal group (M = −5.47), distraction group (M = −10.92), or exposure-alone group (M = −11.13), ds = 0.18–0.48.
A univariate ANCOVA was conducted on change in reported fear from the immediate posttest to the 1-week posttest, with the number of steps completed for each test entered as covariates. Planned pairwise comparisons indicated no significant differences between the affect-labeling group (M = −15.82) and the reappraisal group (M = −16.21), distraction group (M = −19.55), or exposure-alone group (M = −11.78), ds = −0.18–0.20.
Word use
A univariate ANOVA was conducted on the percentage of anxiety and fear words used during exposure by the affect-labeling, reappraisal, and distraction groups. Pairwise comparisons indicated greater use of anxiety and fear words by the affect-labeling group (M = 6.00%, SE = 0.40%) than by the reappraisal group (M = 0.35%, SE = 0.40%), p < .001, d = 3.00, and the distraction group (M = 0.02%, SE = 0.40%), p < .001, d = 3.18. Bivariate correlational analyses across the three groups indicated that a greater percentage of anxiety and fear words was associated with greater reduction in SCR from the immediate posttest to the 1-week posttest, r = −.288, p = .019, and with a marginally greater number of additional test steps completed from the pretest to the immediate posttest, r = .234, p = .059, and from the immediate posttest to the 1-week posttest, r = .219, p = .077.
Discussion
The goal of this study was to extend findings reported in the growing basic-science literature on word use and emotion regulation to a real-world clinical context—in this case, exposure therapy for individuals fearful of spiders. Overall, our results highlight the potential value of affect labeling in psychological treatments for phobias and other anxiety disorders.
Affect labeling was shown to be more effective than reappraisal, distraction, and exposure alone in attenuating SCR from the immediate posttest to the 1-week posttest, and marginally more effective than distraction in increasing behavioral approach from the immediate posttest to the 1-week posttest. Additionally, the greater percentage of anxiety and fear words that participants verbalized during exposure, the essence of affect labeling in this setting, the relatively greater the SCR reduction they exhibited at the 1-week posttest and the marginally closer they approached the spider at both posttests. These results support prior psychophysiological findings (Tabibnia et al., 2008) and appear to be the first to document benefits for affect labeling on a behavioral measure of fear and anxiety.
In contrast, affect labeling was not shown to be more effective than the other conditions in reducing reported fear. Individuals may not expect or believe labeling to be useful for downregulating negative affect (Lieberman et al., 2011), and this may have differentially impacted the self-report measure used in this study, relative to the more objective SCR and behavioral-approach measures. In addition, reported fear was assessed after the participant ended each test, which makes this measure difficult to interpret because multiple affective processes (e.g., residual fear, relief) were likely involved.
It is perhaps counterintuitive that affect labeling was more effective than reappraisal in reducing psychophysiological arousal, as traditional CBT emphasizes reappraisal. Affect labeling recruits RVLPFC (Lieberman, 2011) and may enhance inhibitory learning through mediation by medial prefrontal cortex (Lieberman et al., 2007), which has been shown to downregulate the amygdala in fear-extinction studies (e.g., Phelps, Delgado, Nearing, & LeDoux, 2004). Thus, RVLPFC activation through labeling of affective experience may enhance the normal processes of fear extinction, which is important because anxious individuals exhibit deficits in inhibitory regulation during extinction (e.g., Sehlmeyer et al., 2011). Indeed, affect labeling may enhance outcomes by increasing exposure to one's own fear and anxiety. Such a process may be similar to mindfulness, in which one becomes more aware and accepting of ongoing experience, and for which data support its relationship to reduced amygdala activity (e.g., Creswell, Way, Eisenberger, & Lieberman, 2007) and improved psychiatric outcomes (e.g., Roemer & Orsillo, 2009; Segal, Williams, & Teasdale, 2002). In contrast, the instructions we gave to participants were rather limited relative to the more in-depth guidelines often provided in clinical practice. Therefore, it is likely that the skill-based application of reappraisal was not optimized relative to a full course of CBT.
It should also be noted that participants in the exposure-alone group may have internally verbalized their affect naturally in response to the spider, thus possibly benefiting marginally from the effects of affect labeling. However, the results suggest that instruction in explicit affect labeling produces more pronounced effects than simply allowing for the possibility that individuals may internally label their affect. Another limitation of this study is that the experimenters who attached the psychophysiological equipment and coded behavioral approach were not blind to the study conditions.
Future investigations should examine the effects of affect labeling, reappraisal, and distraction on fear responding in a larger clinical sample over a longer time period. It is possible that word use may have differing effects across various samples and time intervals. Together, the findings reported here suggest that verbalizing fear and anxiety during exposure to a feared stimulus can improve the subsequent ability to effectively manage aspects of one's emotional experience and behavior.
Acknowledgments
Katharina Kircanski is now at Stanford University. The authors wish to acknowledge the contributions of Robert Bjork, John Piacentini, Steven Reise, and Golnaz Tabibnia to the conception and design of this study.
Funding This research was supported by National Institutes of Health Grant No. 1F31MH084380 (to Katharina Kircanski) and by the American Psychological Association.
Footnotes
Declaration of Conflicting Interests The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Antony MM, McCabe RE, Leeuw I, Sano N, Swinson RP. Effect of distraction and coping style on in vivo exposure for specific phobia of spiders. Behaviour Research and Therapy. 2001;39:1137–1150. doi: 10.1016/s0005-7967(00)00089-9. doi:10.1016/S0005-7967(00)00089-9. [DOI] [PubMed] [Google Scholar]
- Beck AT, Emery G. Anxiety disorders and phobias: A cognitive perspective. Basic Books; New York, NY: 1985. [Google Scholar]
- Bouton ME. Context, time and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:90–99. doi: 10.1037/0033-2909.114.1.80. doi:10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Craske MG, Street LL, Jayaraman J, Barlow DH. Attention versus distraction during in vivo exposure: Snake and spider phobias. Journal of Anxiety Disorders. 1991;5:199–211. doi:10.1016/0887-6185(91)90001-A. [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine. 2007;69:560–565. doi: 10.1097/PSY.0b013e3180f6171f. doi:10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Davis M, Barad M, Otto S, Southwick S. Combining pharmacotherapy with cognitive behavioral therapy: Traditional and new approaches. Journal of Traumatic Stress. 2006;19:571–581. doi: 10.1002/jts.20149. doi:10.1002/jts.20149. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28:409–423. doi: 10.1002/hbm.20291. doi:10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmelkamp P. Phobic and obsessive-compulsive disorders: Theory, research and practice. Plenum; New York, NY: 1982. [Google Scholar]
- Frattaroli J. Experimental disclosure and its moderators: A meta-analysis. Psychological Bulletin. 2005;132:823–865. doi: 10.1037/0033-2909.132.6.823. doi:10.1037/0033-2909.132.6.823. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. doi:10.1037/0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. doi:10.1017/S0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. doi:10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional response: Effects of a neocortical network on the limbic system. NeuroReport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. doi:10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Klorman R, Weerts TC, Hastings JE, Melamed BG, Lang PJ. Psychometric description of some specific fear questionnaires. Behaviour Research and Therapy. 1974;5:401–409. doi:10.1016/S0005-7894(74)80008-0. [Google Scholar]
- Lieberman MD. Why symbolic processing of affect can disrupt negative affect: Social cognitive and affective neuroscience investigations. In: Todorov A, Fiske ST, Prentice D, editors. Social neuroscience: Toward understanding the underpinnings of the social mind. Oxford University Press; Oxford, England: 2011. pp. 188–209. [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: Affect labeling disrupts amygdala activity to affective stimuli. Psychological Science. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. doi:10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Inagaki TK, Tabibnia G, Crockett MJ. Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion. 2011;3:468–480. doi: 10.1037/a0023503. doi:10.1037/a0023503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmore RJ, Worrell M. Do we need to challenge thoughts in cognitive behavior therapy? Clinical Psychology Review. 2007;27:173–187. doi: 10.1016/j.cpr.2006.08.001. doi:10.1016/j.cpr.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. doi:10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW. Writing about emotional experiences as a therapeutic process. Psychological Science. 1997;8:162–166. doi:10.1111/j.1467-9280.1997.tb00403.x. [Google Scholar]
- Pennebaker JW, Chung CK. Expressive writing: Connections to physical and mental health. In: Friedman HS, editor. The Oxford handbook of health psychology. Oxford University Press; New York, NY: 2011. pp. 417–437. [Google Scholar]
- Pennebaker JW, Francis ME. Linguistic inquiry and word count (LIWC): A computerized text analysis program. Erlbaum; Mahwah, NJ: 1999. [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. doi:10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Davis M. Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Archives of General Psychiatry. 2004;61:1136. doi: 10.1001/archpsyc.61.11.1136. doi:10.1001/archpsyc.61.11.1136-1144. [DOI] [PubMed] [Google Scholar]
- Roemer L, Orsillo SM. Mindfulness and acceptance-based behavioral therapies in practice. Guilford Press; New York, NY: 2009. [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression. Guilford Press; New York, NY: 2002. [Google Scholar]
- Sehlmeyer C, Dannlowski U, Schöning S, Kugel H, Pyka M, Pfleiderer B, Arolt V. Neural correlates of trait anxiety in fear extinction. Psychological Medicine. 2011;41:789–798. doi: 10.1017/S0033291710001248. doi:10.1017/S0033291710001248. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Lieberman MD, Craske MG. The lasting effect of words on feelings: Words may facilitate exposure effects to threatening images. Emotion. 2008;8:307–317. doi: 10.1037/1528-3542.8.3.307. doi:10.1037/1528-3542.8.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm FH, Roth WT, Sackner MA. The LifeShirt: An advanced system for ambulatory measurement of respiratory and cardiac function. Behavior Modification. 2003;27:671–691. doi: 10.1177/0145445503256321. doi:10.1177/0145445503256321. [DOI] [PubMed] [Google Scholar]