Abstract
Highly pathogenic avian influenza (HPAI) H5 viruses derived from A/Goose/Guangdong/1/96 have been continuously circulating globally, severely affecting the public health and poultry industries. The matrix 2 protein ectodomain (M2e) is considered a promising candidate for a universal cross-protective influenza vaccine that provides more effective control over HPAI H5 viruses harboring variant hemagglutinin (HA)-antigens. Here, we evaluated the protective efficacy of a tandem repeat construct of heterologous M2e presented on virus-like particles (M2e5x VLPs) either alone or as a supplement against HPAI H5 viruses in a chicken model. Chickens immunized with M2e5x VLPs alone induced M2e-specific antibodies but were not protected against HPAI H5. The homo- and cross-protective efficacy of M2e5x VLP-supplemented vaccination of chickens was also examined. Importantly, supplementation with M2e5x VLPs induced significantly higher levels of antibodies specific for M2e and different viruses as well as provided improved protection against homologous and heterologous HPAI H5 viruses. Considering the limited efficacy of inactivated vaccines, supplement vaccination with M2e5x VLPs may be an effective measure for preventing outbreaks of HPAI viruses that have the ability to constantly change their antigenic properties in poultry.
Keywords: Highly pathogenic avian influenza, M2e5x virus-like particles, supplemented vaccine, chicken
Introduction
Highly pathogenic avian influenza (HPAI) is a disease that poses a significant threat to public health and can cause severe economic losses to the poultry industry. In 1997, an outbreak of H5N1 HPAI virus on a poultry farm in Hong Kong was caused by the A/Goose/Guangdong/1/96 (Gs/Gd/96) strain, which was isolated from geese in China in 1996 [1]. H5 HPAI viruses have become widely distributed, and remain one of the most important infectious diseases in both poultry and humans in Asia, Africa, Europe, Southeast Asia, and the Middle East [2]. Vaccination, in conjunction with other control methods such as careful surveillance and monitoring strategies, has been used to better control H5 HPAI viruses, particularly in HPAI endemic countries [3, 4].
Most conventional avian influenza vaccines are based on the hemagglutinin (HA) protein. The HA protein is a major antigenic and immunogenic target that enhances humoral immunity and prevents clinical disease. However, HA-based vaccines provide limited cross-protection against novel influenza strains expressing immunodominant surface glycoproteins such as HA and neuraminidase (NA) that have undergone point mutation (antigenic drift) and genetic reassortment (genetic drift) [5]. Therefore, to effectively control an influenza pandemic, continuous selection and updating of vaccine strains is necessary every 2–3 years.
To develop the vaccine providing broadly cross-protection against influenza A viruses, various studies have been conducted to target matrix 2 ectodomain (M2e) consisting of 24-amino acids which are exposed at viral envelope [6–10]. However, although M2e sequence is more conserved when compared to HA, M2e variation between strains can be as high as 25% [11]. In addition, M2e is known as a poor immunogen [12, 13]. Due to the presence of low amounts of M2e on the viral surface and a protein coat comprising large HA and NA proteins, recognition of M2e epitopes on virions by immune cells is inefficient [14, 15]. Therefore, to enhance the immunogenicity to overcome variation between strains of M2e, previous studies of M2e-based vaccines have fused the M2e of different strains of influenza virus to particular immunogenic vehicles [6, 7] or linked M2e to an appropriate carrier to increase its immunogenicity [8–10]. Recent studies generated a novel M2e construct by genetically engineering a tandem repeat comprising M2e epitope sequences (M2e5x) from multiple host origin influenza viruses, and then presenting it with matrix 1 protein (M1) on virus-like particles (M2e5x VLPs) resulting in a significant improvement in cross-protection in mouse models [6, 16, 17]. M1 protein is known as an important component which is essential for VLP formation and virus budding [18, 19]. In addition, recent studies showed that M1 VLP had an adjuvant effect on split vaccine and induced the Th1 type immunity [16].
Here, we aimed to overcome the limitations of HA-based vaccines by evaluating the efficacy of the M2e5x VLP, which is co-expressed with M1, vaccine in a chicken model. This study determined the immunogenicity and protective efficacy of M2e5x VLPs either as a stand-alone vaccine or as a supplement to the inactivated HA-based vaccine. M2e5x VLP-supplemented HA vaccination of chickens induced significantly higher levels of antibodies recognizing different M2e peptide antigens and viruses, and provided good protection without body weight loss after lethal challenge with heterologous H5 HPAI viruses.
Material and methods
Virus strains and cell lines
The HPAI virus strains, A/mandarin duck/Korea/PSC24-24/2010 (H5N1; clade 2.3.2.1; PSC24-24) [20] and A/broiler duck/Korea/Buan2 (H5N8; clade 2.3.4.4; Buan2) [21], were isolated from a wild bird and a poultry farm, respectively, and maintained by the Animal and Plant Quarantine Agency (QIA). The viruses were propagated for 48 h in 10-day-old specific pathogen free (SPF) embryonated chicken eggs. Spodoptera frugiperda 9 (Sf9) insect cells, used to produce M2e5x VLPs, were maintained in SF900-II SFM medium (Invitrogen, Carlsbad, CA, USA) at 27°C in an incubator. 293T cells were obtained from the American Type Culture Collection and cultured for 72 h in MEM (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) in 5% CO2.
Preparation of M2e5x VLPs and recombinant vaccine PSC24-24
M2e5x VLPs were produced in Sf9 insect cells co-infected with recombinant baculovirus expressing influenza M1 matrix protein and M2e5x (comprising a heterologous tandem repeat of M2e peptides derived from human, swine, and avian origin influenza A viruses) [6]. M2e5x VLPs were purified and characterized as previously described [6].
H5N1 reassortant viruses were generated by plasmid-based reverse genetics using the HA and NA genes of PSC24-24 and six internal genes derived from the A/Puerto Rico/8/34 (H1N1) (PR8) virus strain as previously described, with minor modifications [22]. Viral PSC2-24 RNA was isolated from allantoic fluids using a Gene-Spin™ Viral RNA extraction kit (iNtRON Biotechnology, Korea). The HA and NA genes were amplified using segment-specific primers [23] and subsequently cloned into the vector, pHW2000 [22]. To modify the polybasic amino acid cleavage site in the HA genes, site-directed mutagenesis was performed using a commercial kit as described by the manufacturer (Stratagene, USA). The two plasmids containing the HA and NA segments of PSC24-24 were amplified in transformed competent cells (Invitrogen) and individual colonies were randomly picked. The correct plasmid sequences were confirmed by sequence analysis. Virus rescue was performed by culture of 293T cells (1 × 106 cells/well in 6-well plates), followed by co-transfection with 300 ng each of the eight plasmids (two HA and NA genes from PSC24-24 and six internal genes from PR8) using lipofectamine (Invitrogen) in incomplete MEM (final volume, 1 mL). At 24 h post-transfection, the cell culture supernatant was harvested and injected into 10-day-old embryonated SPF chicken eggs. After incubation for 2–3 days at 37°C, the egg allantoic fluid was harvested and viral growth tested in a hemagglutination activity assay. The genome composition of H5N1 reassortants was confirmed by sequencing.
To obtain the recombinant vaccine, PSC24-24 (rvPSC24-24), the harvest from the first passage was injected into additional SPF chicken eggs. An H5N1 reassortant virus obtained from several egg passages showed titer of 106.2 EID50/0.1 mL. Inactivated recombinant vaccine was prepared by treating with 0.1% formalin.
M2e5x VLP vaccination and challenge of chickens
To evaluate the efficacy of M2e5x VLPs, 25 6-week-old SPF chickens were divided into five groups (five chickens per group): four immunized groups and one non-immunized group. The four M2e5x VLP vaccine groups were intramuscularly vaccinated with escalating doses (2 μg, 10 μg, and 50 μg of VLPs per chicken) of the M2e5x VLP vaccine plus the adjuvant Montanide ISA 70 VG (SEPPIC, France) or with 50 μg of M2e5x VLPs without adjuvant at 4 week intervals. The control group was inoculated with PBS and the adjuvant ISA 70 mixture. Serum samples were collected on a weekly basis to determine immune responses to M2e5x VLP vaccination. antibodies in immune sera were analyzed weekly by ELISA based on synthetic human, swine, avian type I, and avian II type M2e peptides (Peptron, Korea). Immune responses to the virus strains PSC24-24, A/chicken/Gimje/2008 (H5N1) (Gimje08), A/chicken/Vietnam/NCVD-A015/2008 (H5N1) (VNA015), A/duck/Korea/BC10/07 (H7N3) (BC10), and A/Korean native chicken/Korea/12AQ005/2012 (H9N2) (12AQ005) were determined by ELISA using the aforementioned strains as a coating antigen. The wells of the ELISA plates (Nunc, Roskilde, Denmark) were coated with 0.3 μg/well of synthetic peptides or inactivated purified virions overnight at 4°C and subsequently blocked with 3% skim milk in PBS for 4 h at 37°C. The coated plates were washed three times with PBS containing 0.05% (v/v) Tween 20 (PBST) and incubated with a 1:200 dilution of immune sera (for synthetic peptides), or with serial dilutions of immune sera (1:200 to 1:26 000) (for purified virions) in 1% skim milk in PBST for 1 h at 37°C. The plates were subsequently washed three times with PBST and incubated with a 1:2000 dilution of HRP-conjugated anti-chicken IgG (KPL, Gaithersburg, ML, USA) for 1 h at room temperature. After washing, wells were incubated with TMB substrate (KPL) for color development and the reaction was stopped with 1 N HCL. Optical densities were read at 450 nm using an ELISA reader (Tecan, Mannedorf, Switzerland). Three weeks following boost immunization, chickens were intranasally challenged with five times of 50% chicken lethal doses (five CLD50) of PSC24-24 virus (105.2 EID50/0.1 mL).
M2e5x VLP supplement vaccination and immunogenicity
To assess the efficacy of M2e5x VLPs as a supplement vaccine, a 10 μg dose of M2e5x VLPs was added to one dose (106.2 EID50/0.1 mL) or 1/4 dose (105.8 EID50/0.1 mL) of rvPSC24-24. Six-week-old SPF chickens were divided into five groups (five chickens per group): four immunized groups and one non-immunized group. Immunized groups were divided according to M2e5x VLPs supplementation and dose of rvPSC24-24 as follows: rvPSC24-24 (one dose) plus M2e5x VLPs, rvPSC24-24 (one dose), rvPSC24-24 (1/4 dose) plus M2e5x VLPs and rvPSC24-24 (1/4 dose). The non-immunized group was inoculated with PBS. All groups were vaccinated using the adjuvant ISA 70. To assess immunogenicity post-vaccination, chickens were bled weekly and a hemagglutination inhibition (HI) assay was used to measure serum antibody levels in each group as previously described [11] using PSC24-24 and Buan2 antigens. Three weeks after vaccination, chickens were intranasally challenged with five CLD50 of homologous H5N1 virus, PSC24-24, or five CLD50 (106.0 EID50/0.1 mL) of the heterologous H5N8 virus, Buan2. All experiments with live H5 virus were performed in biosafety level 3 facilities following a protocol approved by the Animal Ethics Committee of the Animal and Plant Quarantine Agency, Korea (Approval number 2014-229).
Protective efficacy against HPAI virus challenge
The protective efficacy of the vaccine was determined by evaluating clinical signs, mortality, and virus shedding after intranasal challenge with the PSC24-24 and Buan2 viruses. Oropharyngeal (OP) and cloacal (CL) swabs were collected from all groups at 1, 3, 5, 7, 9, 11, and 14 days post-infection (dpi). Samples were inoculated in chicken embryo fibroblast (CEF) cell cultures and virus growth determined based on detection of cytopathic effects (CPE) and HA activity. Virus distribution was determined by collecting various tissue specimens (trachea, lung, liver, spleen, kidney, cecal tonsil, pancreas, heart, and brain) from dead chickens during the challenge experimental period. These tissues were used to prepare 10% (w/v) homogenates in PBS containing antibiotics and the virus titer was measured in CEF cell cultures.
Statistical analysis
Data were analyzed using Prism version 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Comparison of serum titers between the groups was made using one-way analysis of variance (ANOVA). Survival rates among the groups were analyzed using the log-rank test. A two-tailed Student’s t-test was used to compare differences in body weight between two different groups. Statistical significance was determined as a P value < 0.05.
Results
Immunogenicity and protection after injection of M2e5x VLPs as a stand-alone vaccine
To assess the immunogenicity of M2e5x VLPs in chickens, antibody titers in immune sera were determined using M2e peptide antigens from different host species (human, swine, and avian influenza virus) (Table 1). Sera collected from the groups vaccinated with a 10 μg dose of M2e5x VLPs or with a 50 μg dose of M2e5x VLPs using the ISA70 adjuvant had higher levels of antibodies against M2e peptide antigens than immune sera from chickens immunized with 2 μg of M2e5x VLPs and ISA70 or with 50μg of M2e5x VLPs only (Figure 1). In all four immunized groups, very low antibody reactivity to the avian type II M2e peptide was observed when compared with reactivity to peptides derived from other avian, swine, and human virus M2e types (Figure 1D). This result is consistent with that of a previous study in mice, which demonstrated poor antibody responses to avian type II M2e, which were likely due to its internal location within the M2e5x construct on the VLPs [6]. No antibody responses to M2e were detected in sera from non-vaccinated chickens (Figure 1).
Table 1.
List of different M2e peptide amino acid sequences
| Specific M2e type | Amino acid sequence |
|---|---|
| Human | SLLTEVETPIRNEWGSRSN |
| Swine | SLLTEVETPTRSEWESRSS |
| Avian I | SLLTEVETPTRNEWESRSS |
| Avian II | SLLTEVETLTRNGWGSRSS |
M2e, matrix 2 protein ectodomain.
Figure 1. M2e-specific antibody responses after M2e5x VLP vaccination of chickens.
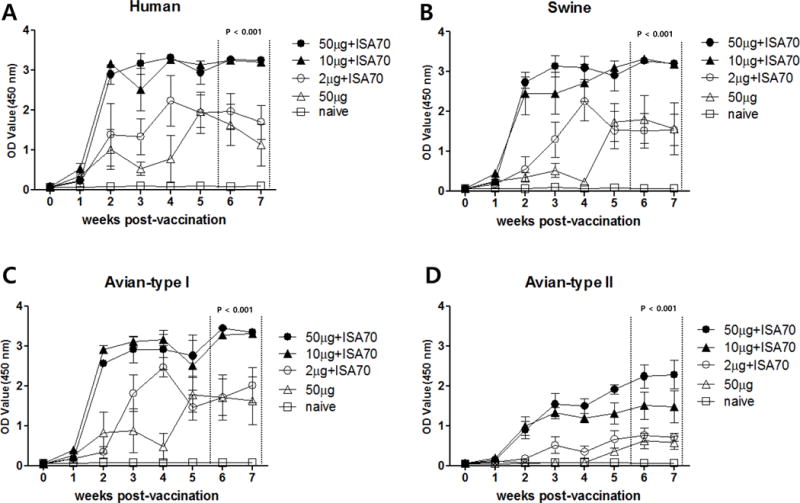
Four immunized groups of chickens (n=5 per group) were intramuscularly immunized (twice) with increasing doses (2 μg, 10 μg, or 50 μg) of M2e5x VLPs plus the adjuvant ISA 70, or with 50 μg of M2e5x VLPs without adjuvant at Weeks 0 and 4. One naïve control group was immunized with PBS plus ISA 70. After vaccination, chicken serum was collected on a weekly basis. Levels of M2e-specific antibodies [human (A), swine (B), avian type I (C), and avian type II (D) were determined by ELISA and expressed as optical density readouts at 450 nm. The results are expressed as the mean titer ± the standard deviation (SD) (sera diluted 1:200). OD, optical density; 50 μg + ISA 70: 50 μg M2e5x VLPs containing ISA 70; 10 μg + ISA 70: 10 μg M2e5x VLPs containing ISA 70; 2 μg + ISA 70: 2 μg M2e5x VLPs containing ISA 70; 50 μg: 50 μg M2e5x VLPs without ISA 70; naïve: control group injected with PBS.
Sera collected from chickens immunized with 10 μg M2e5xVLP plus ISA70 showed high levels of cross-immunogenicity to the various virus strains, although antibody-mediated immune responses to BC10 were lower than those to strains PSC24-24, Gimje08, VN015, and 12AQ005 (Figure 2A).
Figure 2. Vaccination of chickens with M2e5x VLP alone induces virus-specific antibodies but does not confer protection against HPAI virus.
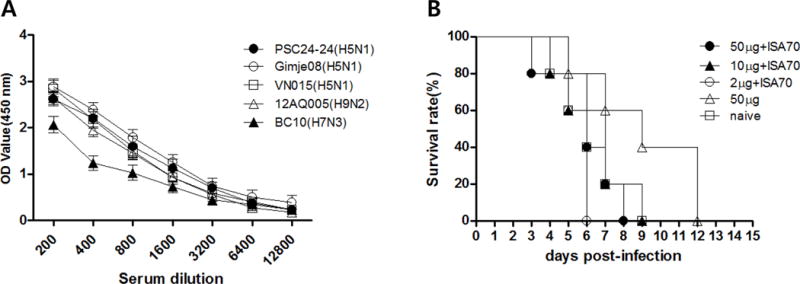
Antibody responses specific for inactivated purified virions were determined by ELISA in vaccinated and naïve chickens at 3 weeks after boost vaccination. (A) Antibody binding to purified virions. Results are expressed as the mean titer ± SD (serial dilutions, 1:200–12800) in sera from five chickens immunized with 10 μg M2e5x VLP containing ISA 70. (B) Survival of chickens after HPAI challenge. Immune and naïve groups were intranasally challenged with a lethal dose (5 × CLD50) of the A/mandarin duck/Korea/PSC24-24/2010 (H5N1) strain 3 weeks after the booster vaccination. Survival was monitored from 0 to 14 days post-infection. OD, optical density; PSC24-24, A/mandarin duck/Korea/PSC24-24/2010 (H5N1) strain; Gimje08, A/chicken/Gimje/2008 (H5N1) strain; VNA015, A/chicken/Vietnam/NCVD-A015/2008 (H5N1) strain; BC10, A/duck/Korea/BC10/07 (H7N3) strain; 12AQ005, A/Korean native chicken/Korea/12AQ005/2012 (H9N2) strain; 50 μg + ISA 70: 50 μg M2e5x VLPs containing ISA 70; 10 μg + ISA 70: 10 μg M2e5x VLPs containing ISA 70; 2 μg + ISA 70: 2 μg M2e5x VLPs containing ISA 70; 50 μg: 50 μg M2e5x VLPs without ISA 70; naïve: control group injected with PBS.
Intranasal challenge with five CLD50 of PSC24-24 at 3 weeks after boost immunization resulted in the death of all chickens in the immunized and non-immunized groups due to infection within the span of 12 days (Figure 2B). These results indicate that M2e-specific antibodies elicited by M2e5x VLPs vaccination alone do not confer protection against lethal HPAI challenge despite the presence of significant levels of antibodies.
M2e5x VLP-supplemented vaccination of chickens induces stronger M2e-specific antibody responses
Immune sera from chickens immunized with rvPSC24-24 containing M2e5x VLPs showed high levels of antibodies reactive with M2e peptide antigens from different host species. Antibody reactivity to M2e peptides increased gradually from 1 week to 3 weeks post-vaccination (Figure 3). By contrast, the rvPSC24-24 vaccine-only groups did not exhibit an M2e-specific antibody response (Figure 3). The antibody response to the avian type II M2e peptide antigen was approximately 2-fold lower than that to human, swine, and avian type I M2e peptides at 3 weeks post-vaccination, and was similar to M2e immune responses after vaccination with M2e5x VLPs alone (Figure 3D). No M2e-specific antibody responses were detected in non-immunized chickens (Figure 3).
Figure 3. M2e-specific antibody responses in chickens receiving M2e5x VLP-supplemented vaccination.
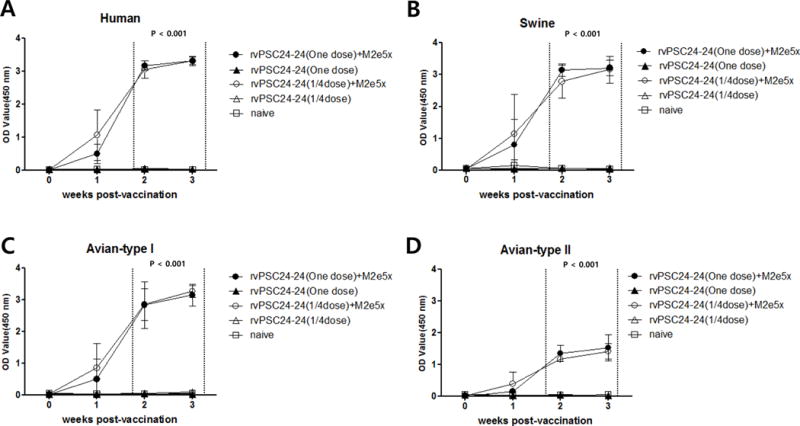
(A) Human influenza virus M2e antibody responses. (B) Swine influenza virus M2e antibody responses. (C) Avian influenza virus major type I M2e antibody responses. (D) Avian influenza virus minor type II M2e antibody responses. Four groups of chickens (n=5 per group) were intramuscularly immunized with the following vaccines: rvPSC24-24 (one dose) + M2e5x VLPs; rvPSC24-24 (one dose); rvPSC24-24 (1/4 dose) + M2e5x VLPs; and rvPSC24-24 (1/4 dose). The naive control group was inoculated with PBS only. Serum from immunized and naïve chickens was taken weekly after vaccination and antibody responses against synthetic peptides analyzed by ELISA. M2e-specific antibody responses are presented as OD values read at 450 nm, and expressed as the mean titer ± SD (sera diluted 1:200). OD, optical density; rvPSC24-24 (one dose) + M2e5x VLPs: one dose of recombinant vaccine PSC24-24 containing 10 μg M2e5x VLPs; rvPSC24-24 (one dose): one dose of recombinant vaccine PSC24-24 alone; rvPSC24-24 (1/4 dose) + M2e5x VLPs: 1/4 dose of recombinant vaccine PSC24-24 containing 10 μg M2e5x VLPs; rvPSC24-24 (1/4 dose): 1/4 dose of recombinant vaccine PSC24-24 alone; naïve: control group injected with PBS.
M2e5x VLP supplemented vaccination significantly enhances antibody responses to various viruses
Antibody levels in chickens immunized with rvPSC24-24 (one dose) and M2e5x VLPs were approximately 10-fold higher than those in chickens immunized with rvPSC24-24 (one dose). The antibody response to influenza virions after immunization with rvPSC24-24 (1/4 dose) and M2e5x VLPs was 5-fold higher than that after immunization with rvPSC24-24 (1/4 dose) and 3-fold higher than after immunization with rvPSC24-24 (one dose) (Figures 4A–E).
Figure 4. M2e5x VLP-supplemented vaccination induces higher levels of antibodies against different virus strains.
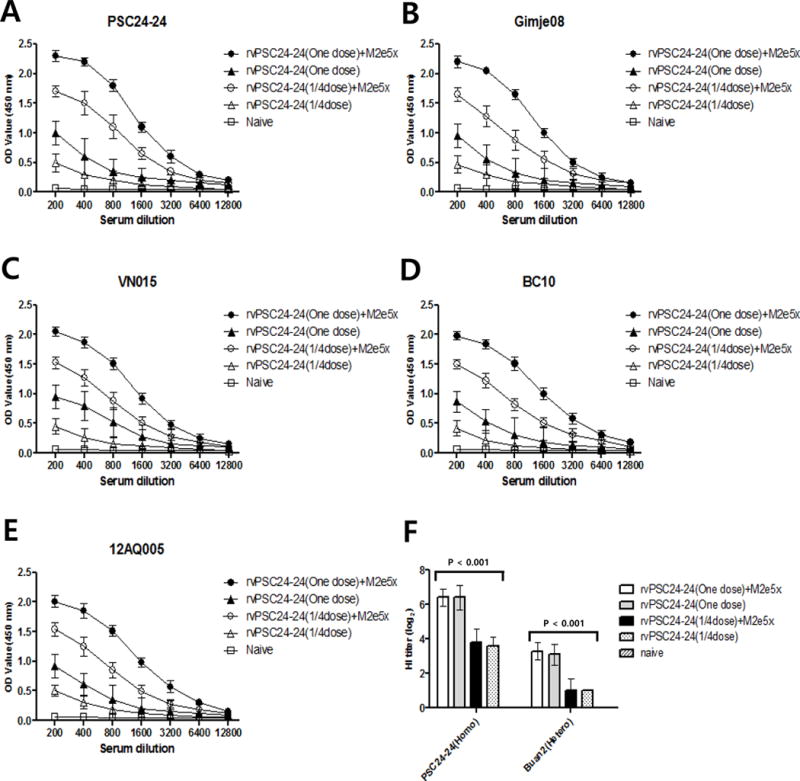
Serum from immunized and naïve chickens (n=5 per group) were obtained 3 weeks after vaccination and inactivated purified virions analyzed by ELISA (A–E). Challenge viruses were examined using the HI method (F). (A) PSC24-24-specific antibody responses. (B) Gimje08-specific antibody responses. (C) VN015-specific antibody responses. (D) BC10-specific antibody responses. (E) 12AQ005-specific antibody responses. OD: optical density; rvPSC24-24 (one dose) + M2e5x VLPs: one dose of recombinant vaccine PSC24-24 containing 10 μg M2e5x VLPs; rvPSC24-24 (one dose): one dose of recombinant vaccine PSC24-24 alone; rvPSC24-24 (1/4 dose) + M2e5x VLPs: 1/4 dose of recombinant vaccine PSC24-24 containing 10 μg M2e5x VLPs; rvPSC24-24 (1/4 dose): 1/4 dose of recombinant vaccine PSC24-24 alone; naïve: control group injected with PBS; homo: homologous; hetero: heterologous.
M2e5x VLP-supplemented vaccination does not affect vaccine-induced HI titers
Serum HI tests using PSC24-24 and Buan2 as challenge viruses were conducted at 3 weeks post-vaccination to compare functional antibody levels according the dose of rvPSC24-24. Two of the groups immunized with one dose of rvPSC24-24 exhibited approximately 26.4 and 23.1–3.3 HI units against homologous virus (PSC24-24) and heterologous virus (Buan2), respectively. The rvPSC24-24 (1/4 dose) groups demonstrated approximately 23.6–3.8 and 21.0 HI units against PSC24-24 and Buan2, respectively (Figure 4F). These results suggest that antibodies elicited by M2e5x VLP-supplemented vaccination do not affect HI titers, although supplemented vaccination does enhance antibody responses to various influenza virus subtypes.
M2e5x VLP-supplemented vaccination enhances protection against homologous and heterologous HPAI H5 viruses
To examine the protective efficacy of M2e5x VLPs as a supplement vaccine, body weight changes, survival rates, and virus shedding were monitored following five CLD50 challenge at 3 weeks post-immunization.
Homologous virus challenge
All chickens immunized with rvPSC24-24 (one dose) plus M2e5x VLPs, rvPSC24-24 (one dose), and rvPSC24-24 (1/4 dose) plus M2e5x VLPs, survived the H5 HPAI lethal challenge. The survival rate in the group immunized with rvPSC24-24 (1/4 dose) was 60% and birds showed significant body weight loss (P < 0.01) at 4–9 days post-challenge when compared with birds immunized with rvPSC24-24 (1/4 dose) plus M2e5x VLPs (Figure 5A). Rapid weight loss occurred in naïve control chickens, with a mortality rate of 100% within 5 dpi (Figures 5A and 5B).
Figure 5. Protection against homologous and hetrologous virus challenge after vaccination with an M2e5x VLP-supplemented vaccine.
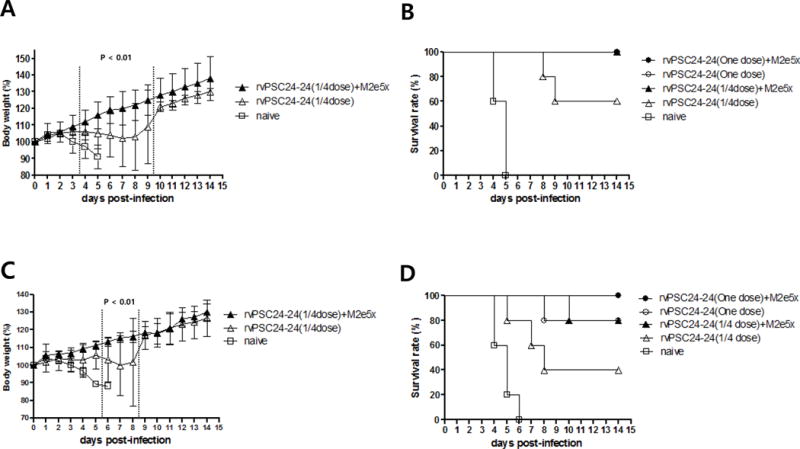
Five immunized chickens were challenged with five chicken lethal doses (CLD50) of the homologous A/mandarin duck/Korea/PSC24-24/2010 (H5N1) (A and B) and the heterologous A/broiler duck/Korea/Buan2 (H5N8) (C and D). (A) Body weight changes after homologous virus challenge. (B) Survival curves after homologous virus challenge. (C) Heterologous virus challenge and body weight changes. (D) Heterologous virus challenge and survival curves. Body weight data are plotted as the percentage of the average initial weight and expressed as the mean data ± SD. OD: optical density; rvPSC24-24 (one dose) + M2e5x VLPs: one dose of recombinant vaccine PSC24-24 containing 10 μg M2e5x VLPs; rvPSC24-24 (one dose): one dose of recombinant vaccine PSC24-24 alone; rvPSC24-24 (1/4 dose) + M2e5x VLPs: 1/4 dose of recombinant vaccine PSC24-24 containing 10 μg M2e5x VLPs; rvPSC24-24 (1/4 dose): 1/4 dose of recombinant vaccine PSC24-24 alone; naïve, control group injected with PBS.
To examine virus shedding, virus replication in OP and CL swabs was determined using the CEF cell line. As shown in Table 2, no virus shedding was observed in the immunized groups, with the exception of chickens immunized with PSC2424 (1/4 dose). Virus shedding was detected in 2/5 chickens immunized with PSC2424 (1/4 dose) from 3–9 dpi (in OP swab samples at 102.6–103.9 TCID50/0.1 mL and in CL swab samples at 100.6–101.6 TCID50/0.1 mL). In the naïve control group, virus shedding was detected within 5 dpi in all chickens (in OP swab samples at 102.8–103.7 TCID50/0.1 mL and in CL swab samples at 100.7– 101.1 TCID50/0.1 mL) (Table 1). These results demonstrate that when M2e5x VLPs was used as a supplement vaccine (a low-dose (1/4) H5-inactivated viral vaccine) it was capable of conferring increased protection against the H5 homologous virus challenge.
Table 2.
Virus shedding in oropharyngeal and cloacal swab samples following virus challenge with homologous PSC24-24 or heterologous Buan2 virus
| Challenge virus | Group | Sample | Virus titer (log10 TCID50/0.1ml, mean±standard deviation)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1dpia | 3dpi | 5dpi | 7dpi | 9dpi | 11dpi | 14dpi | |||||||
| Homologous virus (PSC24-24) | PSC24-24 (One dose) + M2e5x | OPb | − (0/5)c | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | ||||
| CLd | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | ||||||
|
| |||||||||||||
| PSC24-24 (One dose) | OP | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | |||||
| CL | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | ||||||
|
| |||||||||||||
| PSC24-24 (1/4 dose) + M2e5x | OP | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | |||||
| CL | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | ||||||
|
| |||||||||||||
| PSC24-24 (1/4 dose) | OP | − (0/5) | 3.4 ± 2.0 (2/5) | 3.9 ± 0.4 (2/5) | 3.0 ± 0.6 (2/5) |
2.6 (1/4) |
− (0/3) | − (0/3) | |||||
| CL | − (0/5) | 1.6 (1/5) | 0.6 (1/5) | − (0/5) | − (0/4) | − (0/3) | − (0/3) | ||||||
|
| |||||||||||||
| Naive | OP | − (0/5) | 3.7 ± 1.0 (3/5) | 2.8 ± 0.6 (3/3) | nte | nt | nt | nt | |||||
| CL | − (0/5) | 0.7 ± 0.1 (3/5) | 1.1 ± 0.2 (3/3) | nt | nt | nt | nt | ||||||
|
| |||||||||||||
| Heterologous virus (Buan2) | PSC24-24 (One dose) + M2e5x | OP | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | ||||
| CL | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | ||||||
|
| |||||||||||||
| PSC24-24 (One dose) | OP | − (0/5) | − (0/5) | 3.4 (1/5) | 2.8 (1/5) | − (0/4) | − (0/4) | − (0/4) | |||||
| CL | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/4) | − (0/4) | − (0/4) | ||||||
|
| |||||||||||||
| PSC24-24 (1/4 dose) + M2e5x | OP | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/5) | − (0/4) | − (0/4) | |||||
| CL | − (0/5) | − (0/5) | − (0/5) | − (0/5) | 1.7 (1/5) | − (0/4) | − (0/4) | ||||||
|
| |||||||||||||
| PSC24-24 (1/4 dose) | OP | − (0/5) | 3.4 (1/5) | 3.0 ± 0.5 (3/5) | 4.2 ± 0.6 (2/4) |
− (0/2) | − (0/2) | − (0/2) | |||||
| CL | − (0/5) | 4.2 (1/5) | 1.9 ± 0.9 (3/5) | 1.6 (1/4) | − (0/2) | − (0/2) | − (0/2) | ||||||
|
| |||||||||||||
| Naive | OP | − (0/5) | 3.4 ± 0.9 (5/5) | 5.8 ± 1.1 (3/3) | nt | nt | nt | nt | |||||
| CL | − (0/5) | 2.8 ± 0.6 (5/5) | 3.0 ± 0.6 (3/3) | nt | nt | nt | nt | ||||||
Viral titer is expressed as the mean ± SEM of calculable positive samples.
days post-infection;
oropharyngeal swab samples;
number of viruses isolated / number of tested samples;
cloacal swab sample;
not tested.
Heterologous virus challenge
Next, we examined the protective efficacy of a vaccine against the heterologous virus, Buan2. A 100% survival rate was observed in the group that received rvPSC24-24 (one dose) plus M2e5x VLPs, and an 80% survival rate was observed in the group that received rvPSC24-24 (one dose) alone (Figure 5D). An 80% survival rate was also observed in the rvPSC24-24 (1/4dose) plus M2e5x VLPs group, without any signs of weight loss; however, a 40% survival rate and significant (P < 0.01) body weight loss occurred from Days 6–8 following virus challenge in the group immunized with rvPSC24-24 (1/4 dose) alone compared with the group immunized with rvPSC24-24 (1/4 dose) plus M2e5x VLPs (Figures 5C and 5D). Rapid weight loss and 100% mortality were observed in naïve control chickens (Figures 5C and 5D).
After intranasal inoculation with Buan2, OP and CL swabs samples collected from chickens immunized with rvPSC24-24 (one dose) plus M2e5xVLP did not reveal viral shedding from 1–14 dpi. By contrast, virus shedding was detected from 5–7 dpi in 1/5 chickens immunized with rvPSC24-24 (one dose), with a viral titer of 102.8–103.4 TCID50/0.1 mL in the OP swab sample. In the group immunized with rvPSC24-24(1/4 dose) plus M2e5xVLP, virus shedding was detected in 1/5 chickens, with a viral titer of 101.7 TCID50/0.1 mL in the CL swab sample at 9 dpi, whereas three chickens from the group vaccinated with PSC 2424(1/4 dose) alone exhibited viral titers of 103.0–104.2 TCID50/0.1 mL in OP swab samples and 101.6–104.2 TCID50/0.1 mL in CL swab samples. In the naïve control group, viral titers ranged from 103.4–105.8 TCID50/0.1 mL for OP swab samples and from 102.8–103.0 TCID50/0.1 mL for CL swab samples within 5 dpi (Table 2). These results suggest that M2e5x VLP-supplementation plays a significant role in increasing protection against the heterologous HPAI H5 virus, and suppresses viral shedding.
Systemic infection among the immunized and non-immunized groups was analyzed in tissues collected from infected chickens. Chickens vaccinated with rvPSC24-24 (one dose) plus M2e5x VLPs were not analyzed due to no detection of virus replication. In the group immunized with vPSC24-24 (1/4 dose) plus M2e5xVLPs, virus was only recovered from the liver, pancreas, and brain. Viral replication in the brain was lower in the vPSC24-24 (1/4 dose) plus M2e5xVLPs group than in the other groups. By contrast, virus titers of 101.6–107.5 TCID50/0.1 mL were detected in various tissue samples from chickens that were not immunized with M2e5x VLPs (Figure 6). Inter-group differences in virus titers in the various tissue samples were not statistically significant due to variations in the number of infected animals.
Figure 6. Virus re-isolation from infected chickens.
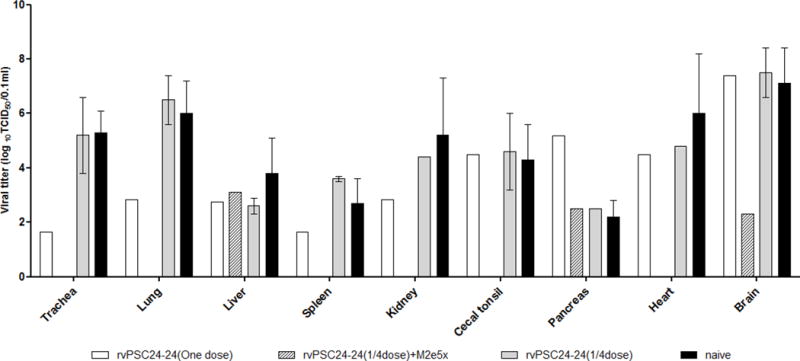
Tissue homogenates (10%) were prepared from the organs of infected chickens and virus titers measured in chicken embryo fibroblast cells. Virus titers were expressed as the mean titer ± SD. rvPSC24-24 (one dose), one dose of recombinant vaccine PSC24-24 alone; rvPSC24-24 (1/4 dose) + M2e5x VLPs: 1/4 dose of recombinant vaccine PSC24-24 containing 10 μg M2e5x VLPs; rvPSC24-24 (1/4 dose): 1/4 dose of recombinant vaccine PSC24-24 alone; naïve: control group injected with PBS.
Discussion
Vaccines based on HA cannot provide complete protection against novel avian influenza viruses that have evolved via antigenic shift and antigenic drift, even within the same subtype influenza virus with vaccine strain. A previous study showed that the reassortant influenza vaccine, Re-1, which harbors the HA and NA genes from the A/goose/Guangdong/1/96 H5N1 strain, provided 80% protection against the A/chicken/Shanxi/2/06 (clade 7.2) H5N1 strain in a chicken model [24]. Furthermore, ferrets immunized with an inactivated split vaccine based on the HA protein of A/Vietnam/1194/04 (clade 1) H5N1 strain showed viral shedding in the upper respiratory tract and viral replication in the lung following intratracheal challenge with the A/Indonesia/5/2005 (clade 2) H5N1 strain [25]. Thus, researchers have attempted to develop a universal influenza vaccine to overcome the limitations of HA-based vaccines.
Previous studies also show that M2e5x VLPs induce a strong antibody response to various subtypes of influenza virions and confer protection against lethal challenge in mice [6, 16, 17]. The aim of the present study was to evaluate the protective efficacy of M2e5x VLPs, either alone or as a supplement to an inactivated viral vaccine, in chickens. To attain optimal immunogenicity in chickens, we used the adjuvant Montanide ISA 70 VG, which improves vaccine efficacy by inducing strong and long lasting humoral and cell-mediated immune responses [26, 27]. After intramuscular administration of M2e5x VLPs alone with or without adjuvant, the serum of chickens immunized with adjuvant demonstrated stronger antibody responses to M2e peptides derived from human-, swine-, and avian-origin influenza viruses and multiple influenza types, compared to those with non-adjuvant chickens.
Despite mounting an immune response, chickens immunized with M2e5x VLPs alone did not survive HPAI influenza virus challenge. Previous studies of M2e-based vaccines demonstrated substantially different results depending on the experimental animal model used. The use of M2e-based vaccines alone in mouse models confers protection against HPAI and low pathogenic avian influenza (LPAI) virus challenge, making them candidate universal influenza A vaccines [6, 16, 28, 29]. However, challenge studies in chickens have demonstrated protection against LPAI but not HPAI, although antibodies elicited by M2e-based vaccines were induced in immunized chickens [7, 30, 31]. The findings of the present study are consistent with those of the aforementioned studies and demonstrate that vaccination with M2e5x VLPs alone does not induce protection against H5 HPAI challenge, despite inducing M2e antibody responses. Although there is no evidence, the different result between mice and chickens might be due to difference of type and distribution for receptor, which is affinity for influenza virus, between species. To better understand about that, it seems necessary to further study on immune mechanism in avian.
We hypothesized that an inactivated viral vaccine supplemented with M2e5x VLPs may provide significantly improved cross-protective efficacy. A recent study vaccinated chickens with a modified M2e-supplemented vaccine harboring three tandem copies of M2e and then challenged them with H9N2 LPAI [32]. The results showed that as a supplement, the M2e-based vaccine was able to reduce viral shedding in the oropharynx and cloaca and reduced viral replication in the trachea and cecal tonsils against the LPAI virus. In the present study, supplementation of the H5-inactivated viral vaccine with M2e5x VLPs also resulted in improved M2e-specific antibody responses. A low-dose of H5-inactivated viral vaccine improved protection following HPAI H5 virus challenge, and better cross-protection was demonstrated against heterologous HPAI H5 virus.
We observed that M2e5x VLPs play an important supplementary role in improving protection. Chickens immunized with identical doses of PSC24-24 demonstrated similar HI titers, regardless of the addition of M2e5x VLPs (Figure 4F), but PSC24-24 plus M2e5x VLPs provided better protection against HPAI H5 viruses (Figure 5). When the M2e5x VLPs as supplement to inactivated viral vaccine are added, immune response is induced by HA as well as M2e-based vaccine. HA-specific antibody prevents infection by neutralizing virus and M2e5x VLPs confer protection via antibody-mediated phagocytosis by macrophage and natural killer (NK) cell-dependent ADCC [33]. Perhaps, M2e5x VLPs as supplement to inactivated viral vaccine may be show better protection than alone inactivated viral vaccine through correlation of different immune mechanism. Most immune-based mechanistic studies on M2e-based vaccines have been performed in mouse models. Based on the results of the present study, further studies are required to gain a better understanding of the mechanisms underlying the enhanced protection provided by M2e5x VLP-supplemented vaccination of chickens.
Human influenza vaccines are updated annually, but do not provide optimal protection against strains that have been undergoing continuous evolution. In case of poultry influenza vaccines, vaccine strain is not only updated not as frequent as that of human influenza, but also changed after being severely damaged by outbreak of influenza virus. To overcome the problems associated with HA-based vaccines, we examined the immunogenicity and protective efficacy of an M2e-based vaccine when used either alone or as a supplement vaccine; the M2e-based vaccine is considered a universal vaccine candidate against HPAI H5 viruses in chickens. Although M2e5x VLPs alone did not prevent viral infection, M2e5x VLPs as a supplement significantly improved protection against homologous and heterologous HPAI by increasing immunogenicity. The results of the present study suggest that the use of broadly cross-protective influenza vaccine (M2e5x VLPs) to supplement a HA-based inactivated vaccine, in combination with eradication policy, may play an important role in effectively controlling unpredictable emerging novel of H5 HPAI influenza virus in endemic countries, although increase of vaccine manufacturing cost is inevitable.
Highlights.
HA-based vaccines provide limited cross-protection against novel influenza virus.
The M2e-based vaccine is considered universal candidate against influenza virus.
M2e5x VLPs induce M2e-specific antibodies, but not provide protection
Supplementation with M2e5x VLPs provide good protection against HPAI H5 viruses.
Acknowledgments
We thank the Animal and Plant Quarantine Agency (QIA) and Ministry of Agriculture, Food, and Rural Affairs (MAFRA) for supporting the ABSL3 facility for the handling of HPAI. This research was supported by a grant from the QIA (No. I-1541781-2012-15-01) of the Republic of Korea and NIH/NIAID grants to S.M.K (AI105170, AI093772, and AI119366).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
All authors have approved the final article.
Conflicts of interest
There are no conflicts of interest to declare.
References
- 1.Xu X, Subbarao, Cox NJ, Guo Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology. 1999;261:15–9. doi: 10.1006/viro.1999.9820. [DOI] [PubMed] [Google Scholar]
- 2.Food and Agriculture Organization of the United Nation. FAO approaches to controlling, preventing and eliminating H5N1 highly pathogenic avian influenza in endemic countries. http://www.fao.org/docrep/014/i2150e/i2150e.pdf.
- 3.Capua I, Alexander DJ. Avian influenza vaccines and vaccination in birds. Vaccine. 2008;26(Suppl 4):D70–3. doi: 10.1016/j.vaccine.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 4.Swayne DE. The role of vaccines and vaccination in high pathogenicity avian influenza control and eradication. Expert Rev Vaccines. 2012;11:877–80. doi: 10.1586/erv.12.60. [DOI] [PubMed] [Google Scholar]
- 5.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–79. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim MC, Song JMOE, Kwon YM, Lee YJ, Compans RW, et al. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther. 2013;21:485–92. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabaghian M, Latify AM, Tebianian M, Nili H, Ranjbar AR, Mirjalili A, et al. Vaccination with recombinant 4 × M2e.HSP70c fusion protein as a universal vaccine candidate enhances both humoral and cell-mediated immune responses and decreases viral shedding against experimental challenge of H9N2 influenza in chickens. Vet Microbiol. 2014;174:116–26. doi: 10.1016/j.vetmic.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 8.De Filette M, Martens W, Roose K, Deroo T, Vervalle F, Bentahir M, et al. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J Biol Chem. 2008;283:11382–7. doi: 10.1074/jbc.M800650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Filette M, Martens W, Smet A, Schotsaert M, Birkett A, Londono-Arcila P, et al. Universal influenza A M2e-HBc vaccine protects against disease even in the presence of pre-existing anti-HBc antibodies. Vaccine. 2008;26:6503–7. doi: 10.1016/j.vaccine.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 10.Ebrahimi SM, Tebianian M. Heterologous expression, purification and characterization of the influenza A virus M2e gene fused to Mycobacterium tuberculosis HSP70(359–610) in prokaryotic system as a fusion protein. Mol Biol Rep. 2010;37:2877–83. doi: 10.1007/s11033-009-9846-2. [DOI] [PubMed] [Google Scholar]
- 11.Wibowo N, Hughes FK, Fairmaid EJ, Lua LH, Brown LE, Middelberg AP. Protective efficacy of a bacterially produced modular capsomere presenting M2e from influenza: extending the potential of broadly cross-protecting epitopes. Vaccine. 2014;32:3651–5. doi: 10.1016/j.vaccine.2014.04.062. [DOI] [PubMed] [Google Scholar]
- 12.Feng J, Zhang M, Mozdzanowska K, Zharikova D, Hoff H, Wunner W, et al. Influenza A virus infection engenders a poor antibody response against the ectodomain of matrix protein 2. Virol J. 2006;3:102. doi: 10.1186/1743-422X-3-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black RA, Rota PA, Gorodkova N, Klenk HD, Kendal AP. Antibody response to the M2 protein of influenza A virus expressed in insect cells. J Gen Virol. 1993;74(Pt 1):143–6. doi: 10.1099/0022-1317-74-1-143. [DOI] [PubMed] [Google Scholar]
- 14.Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40:627–33. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- 15.Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol. 2004;172:5598–605. doi: 10.4049/jimmunol.172.9.5598. [DOI] [PubMed] [Google Scholar]
- 16.Kim MC, Lee YN, Ko EJ, Lee JS, Kwon YM, Hwang HS, et al. Supplementation of influenza split vaccines with conserved M2 ectodomains overcomes strain specificity and provides long-term cross protection. Mol Ther. 2014;22:1364–74. doi: 10.1038/mt.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MC, Lee JS, Kwon YMOE, Lee YJ, Choi JG, et al. Multiple heterologous M2 extracellular domains presented on virus-like particles confer broader and stronger M2 immunity than live influenza A virus infection. Antiviral Res. 2013;99:328–35. doi: 10.1016/j.antiviral.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali A, Avalos RT, Ponimaskin E, Nayak DP. Influenza virus assembly: effect of influenza virus glycoproteins on the membrane association of M1 protein. J Virol. 2000;74:8709–19. doi: 10.1128/jvi.74.18.8709-8719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Puertas P, Albo C, Perez-Pastrana E, Vivo A, Portela A. Influenza virus matrix protein is the major driving force in virus budding. J Virol. 2000;74:11538–47. doi: 10.1128/jvi.74.24.11538-11547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi JG, Kang HM, Jeon WJ, Choi KS, Kim KI, Song BM, et al. Characterization of clade 2.3.2.1 H5N1 highly pathogenic avian influenza viruses isolated from wild birds (mandarin duck and Eurasian eagle owl) in 2010 in Korea. Viruses. 2013;5:1153–74. doi: 10.3390/v5041153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YJ, Kang HM, Lee EK, Song BM, Jeong J, Kwon YK, et al. Novel reassortant influenza A(H5N8) viruses, South Korea, 2014. Emerg Infect Dis. 2014;20:1087–9. doi: 10.3201/eid2006.140233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann E, Krauss S, Perez D, Webby R, Webster RG. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine. 2002;20:3165–70. doi: 10.1016/s0264-410x(02)00268-2. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol. 2001;146:2275–89. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- 24.Chen H. Avian influenza vaccination: the experience in China. Rev Sci Tech. 2009;28:267–74. doi: 10.20506/rst.28.1.1860. [DOI] [PubMed] [Google Scholar]
- 25.Baras B, Stittelaar KJ, Simon JH, Thoolen RJ, Mossman SP, Pistoor FH, et al. Cross-protection against lethal H5N1 challenge in ferrets with an adjuvanted pandemic influenza vaccine. PLoS One. 2008;3:e1401. doi: 10.1371/journal.pone.0001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi JG, Lee YJ, Kim YJ, Lee EK, Jeong OM, Sung HW, et al. An inactivated vaccine to control the current H9N2 low pathogenic avian influenza in Korea. J Vet Sci. 2008;9:67–74. doi: 10.4142/jvs.2008.9.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song JM, Lee YJ, Jeong OM, Kang HM, Kim HR, Kwon JH, et al. Generation and evaluation of reassortant influenza vaccines made by reverse genetics for H9N2 avian influenza in Korea. Vet Microbiol. 2008;130:268–76. doi: 10.1016/j.vetmic.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Tompkins SM, Zhao ZS, Lo CY, Misplon JA, Liu T, Ye Z, et al. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg Infect Dis. 2007;13:426–35. doi: 10.3201/eid1303.061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mardanova ES, Kotlyarov RY, Kuprianov VV, Stepanova LA, Tsybalova LM, Lomonosoff GP, et al. Rapid high-yield expression of a candidate influenza vaccine based on the ectodomain of M2 protein linked to flagellin in plants using viral vectors. BMC Biotechnol. 2015;15:42. doi: 10.1186/s12896-015-0164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Layton SL, Kapczynski DR, Higgins S, Higgins J, Wolfenden AD, Liljebjelke KA, et al. Vaccination of chickens with recombinant Salmonella expressing M2e and CD154 epitopes increases protection and decreases viral shedding after low pathogenic avian influenza challenge. Poult Sci. 2009;88:2244–52. doi: 10.3382/ps.2009-00251. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Liu M, Liu C, Du J, Shi W, Sun E, et al. Vaccination with different M2e epitope densities confers partial protection against H5N1 influenza A virus challenge in chickens. Intervirology. 2011;54:290–9. doi: 10.1159/000319440. [DOI] [PubMed] [Google Scholar]
- 32.Park JK, Lee DH, Cho CH, Yuk SS, To EO, Kwon JH, et al. Supplementation of oil-based inactivated H9N2 vaccine with M2e antigen enhances resistance against heterologous H9N2 avian influenza virus infection. Vet Microbiol. 2014;169:211–7. doi: 10.1016/j.vetmic.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Song JM, Wang BZ, Park KM, Van Rooijen N, Quan FS, Kim MC, et al. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS One. 2011;6:e14538. doi: 10.1371/journal.pone.0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]


