Summary
Middle East respiratory syndrome (MERS) is a highly lethal respiratory disease caused by a novel single-stranded, positive-sense RNA betacoronavirus (MERS-CoV). Dromedary camels, hosts for MERS-CoV, are implicated in direct or indirect transmission to human beings, although the exact mode of transmission is unknown. The virus was first isolated from a patient who died from a severe respiratory illness in June, 2012, in Jeddah, Saudi Arabia. As of May 31, 2015, 1180 laboratory-confirmed cases (483 deaths; 40% mortality) have been reported to WHO. Both community-acquired and hospital-acquired cases have been reported with little human-to-human transmission reported in the community. Although most cases of MERS have occurred in Saudi Arabia and the United Arab Emirates, cases have been reported in Europe, the USA, and Asia in people who travelled from the Middle East or their contacts. Clinical features of MERS range from asymptomatic or mild disease to acute respiratory distress syndrome and multiorgan failure resulting in death, especially in individuals with underlying comorbidities. No specific drug treatment exists for MERS and infection prevention and control measures are crucial to prevent spread in health-care facilities. MERS-CoV continues to be an endemic, low-level public health threat. However, the virus could mutate to have increased interhuman transmissibility, increasing its pandemic potential.
Introduction
The first report of Middle East respiratory syndrome (MERS) described a patient who died from a severe respiratory illness in a hospital in Jeddah, Saudi Arabia, in June, 2012. A previously unrecognised coronavirus (MERS-CoV) isolated from this patient1 was similar to severe acute respiratory syndrome coronavirus (SARS-CoV), which caused an epidemic in 2002–03. The virus was initially designated human coronavirus-EMC, but was renamed MERS-CoV with global consensus.2 The genomic structure of MERS-CoV was delineated3 and dipeptidyl-peptidase 4 (DPP4, also known as CD26) was identified as the host-cell receptor for cell entry.4 Reverse genetics enabled the virus's genome to be studied,5, 6 and molecular diagnostic tests were quickly developed. The high mortality rates in family-based and hospital-based outbreaks, especially in patients with comorbidities such as diabetes and renal failure,7, 8, 9, 10 along with the respiratory droplet route of transmission, evoked global concern and intensive discussion in the media. The numbers of reported MERS cases spiked during hospital-based cluster outbreaks in the spring of 2013 and 2014; some cases are still detected throughout the year. MERS-CoV was deemed a serious public health epidemic threat, because millions of pilgrims from 184 countries converge in Saudi Arabia each year for the Hajj and Umrah pilgrimages. Fortunately, no MERS cases were associated with the 2013 and 2014 Hajj pilgrimages.
In this Seminar, we review MERS epidemiology, virology, clinical manifestations, pathogenesis, diagnosis, case management, treatment, and prophylactic interventions, including the likelihood of widespread outbreak or epidemic spread.
Case definitions
Case definitions of suspected, confirmed, and probable MERS were developed by WHO, the US Centers for Disease Control and Prevention,11 and the Ministry of Health of Saudi Arabia (appendix). In addition to fever and pneumonia or acute respiratory distress syndrome, suspected patients must have a history of travel to countries in or near the Arabian peninsula within 14 days before symptom onset or be in contact with a traveller from this region who developed a febrile respiratory illness. Confirmed cases have laboratory evidence of MERS-CoV infection, generally from PCR. The case definition was updated on Dec 8, 2014, by the Saudi Ministry of Health to include management of patients with health-care-associated MERS-CoV pneumonia, those with acute febrile dengue-like illness, and those with an upper respiratory tract infection and exposed to an infected patient.12
Geographical distribution and surveillance
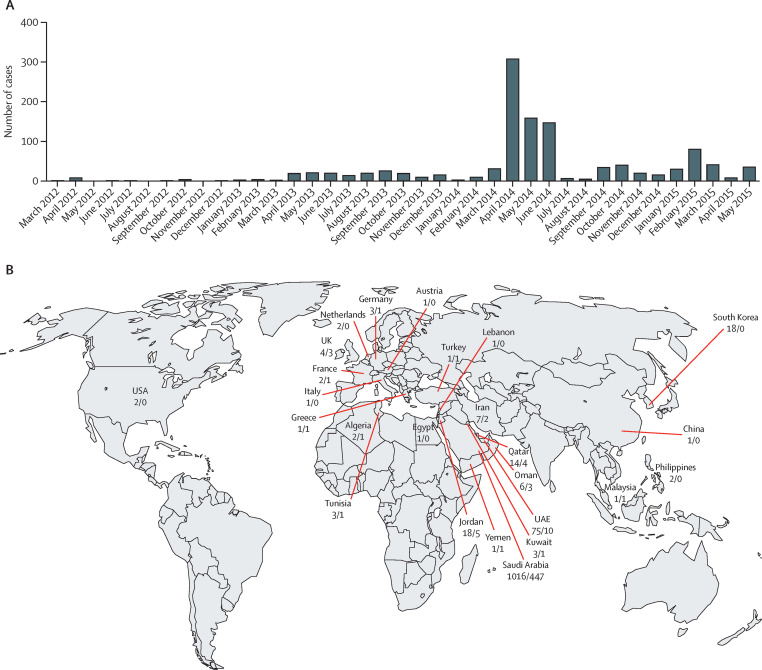
Although the first case of MERS occurred in June, 2012, in Jeddah, Saudi Arabia, it was not reported until September, 2012. Retrospective studies identified an outbreak involving 13 patients in April, 2012, in Zarqa, Jordan.13 Since then, cases have been identified across the Arabian peninsula, in Asia, Europe, Africa, and the USA (figure 1 ). Patients reported outside the Middle East all had a history of recent travel to the Arabian peninsula or had close contact with a primary case. Saudi Arabia has reported the most cases (1016 cases and 447 deaths [44% mortality], as of May 30, 2015). Several proactive global surveillance and information systems are related to MERS and guidelines for prevention, treatment, and infection control are updated regularly by WHO, the US Centers for Disease Control and Prevention, the Saudi Ministry of Health, the European Centre for Disease Prevention and Control, and Public Health England (appendix). Surveillance has intensified over time, especially in health-care and community outbreaks, as it has become clear that infected patients can be asymptomatic or have acute febrile illnesses or upper respiratory tract disease.12
Figure 1.
Global MERS cases
(A) Confirmed cases of MERS as of May 31, 2015, by date (n=1180). (B) Location of MERS deaths/cases, as of May 31, 2015 (n=1180). Shows countries in which patients were identified. Data from WHO14 and Promed Mail.15 MERS=Middle East respiratory syndrome.
Virology
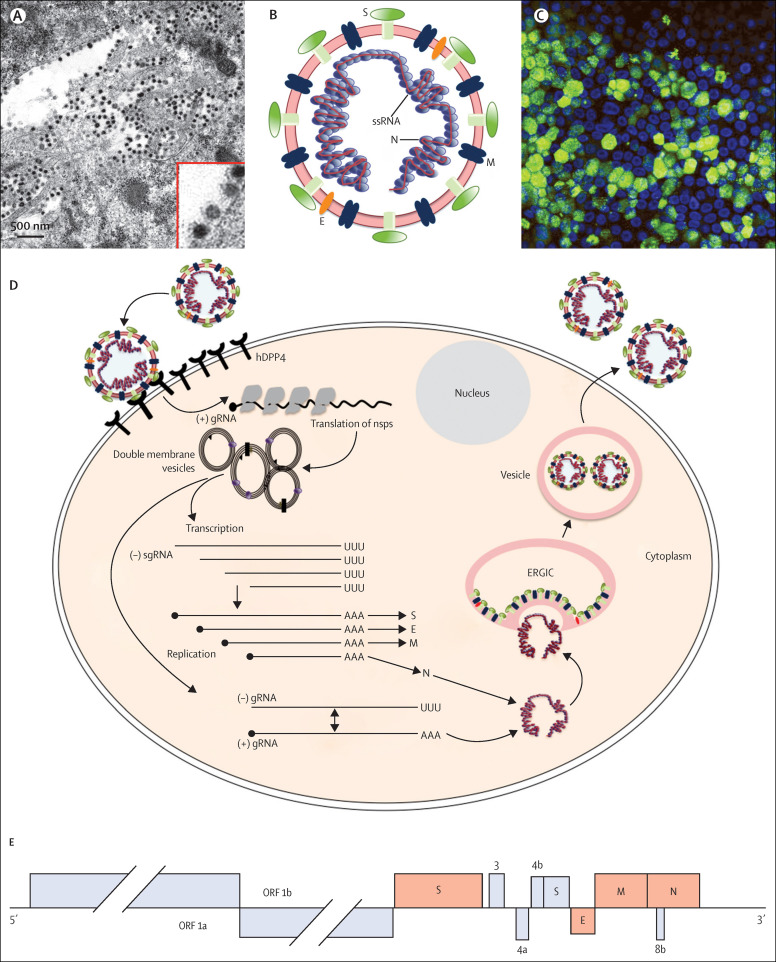
Coronaviruses are large (28–32 kb) single-stranded positive-sense RNA viruses (figure 2 ).18 To enter host cells, MERS-CoV attaches to its receptor, dipeptidyl peptidase 4. Protease cleavage of the S protein is then required for virus–cell fusion and release of genomic RNA into the cytoplasm. Viral RNA transcription and replication occurs on double membrane vesicles and other membranous structures, which are derived from the endoplasmic reticulum. Transcription of the seven subgenomic mRNAs occurs via negative-strand subgenomic RNA intermediates. Subgenomic RNAs are 3′ co-terminally nested and are joined to a common leader encoded at the 5′ end of the genome. Viral RNA is encapsidated in the N protein and transported to the endoplasmic reticulum–Golgi intermediate compartment (ERGIC), the site of assembly. Viral RNA encapsidated in the N protein then buds into vesicles lined with the S, M, and E proteins. Vesicles are then transported to the cell surface before release. Four coronavirus genera have been identified, with human viruses detected in the alphacoronavirus (NL63 and HCoV-229E) and betacoronavirus (HCoV-OC43, HCoV-HKU1, MERS-CoV, and SARS-CoV) genera. MERS-CoV is a lineage c betacoronavirus and SARS-CoV is a lineage b betacoronavirus. The genomic structures of the two viruses are very similar, with proteins involved in virus replication encoded at the 5′ end and structural proteins encoded by genes at the 3′ end of the genome (figure 2).3 Accessory proteins, which are not required for virus viability, are interspersed throughout the structural genes and might interfere with the innate immune response in infected animals. MERS-CoV has five different accessory proteins and SARS-CoV has eight different accessory proteins, which share no sequence similarity. These differences, which presumably have different effects on induction and signalling of type 1 interferon, could explain why MERS-CoV is more sensitive to interferon than is SARS-CoV.19, 20 This difference in interferon sensitivity has implications for treatment because type 1 interferon has been used to treat patients infected with SARS-CoV and MERS-CoV.21, 22
Figure 2.
MERS-CoV virion, replication strategy, and genomic structure
(A) Electron micrograph of MERS-CoV virions in large single membrane vesicles and in the periphery of the cell. Virion spikes are visible as is the electron-dense core consisting of the RNA genome encapsidated in the N protein. Provided by Montserrat Barcena, Ronald Limpens and Eric Snijder (Leiden University Medical Center, Netherlands). (B) MERS-CoV, showing viral RNA structural proteins spike (S), envelope (E), matrix (M), and nucleocapsid (N). (C) Human airway cells infected with MERS-CoV and assessed for viral antigen by indirect immunofluorescence assay with an anti-MERS-CoV N antibody. Green shows MERS-CoV-positive cells, blue shows nuclear stain. A similar assay is used for serological diagnosis of MERS. Provided by Christine Wohlford-Lenane (University of Iowa, Iowa City, IA). (D) Virus life-cycle. (E) The MERS-CoV genome consists of 11 ORFs that code for the virus replication machinery (ORF 1a and ORF 1b) and the major structural proteins: spike (S), envelope (E), matrix (M), and nucleocapsid (N). ORF 1b, produced by a–1 basepair frameshift from ORF 1a, encodes the RNA-dependent RNA polymerase (nsp12), helicase (nsp13), N7-methyltransferase (nsp14), a 3'–5' exonuclease for RNA proofreading (nsp14), 2'-O-methyltransferase (nsp16), and an endonuclease specific for U and found only in nidoviruses (nsp15). MERS-CoV also encodes five accessory proteins in the 3' end of the genome (3a/b, 4, 5, 8b), which share no homology with proteins from host cells or other viruses, including coronaviruses. 4a and 4b are interferon antagonists16, 17 but the functions of the other accessory proteins are unknown.
Coronaviruses have high rates of mutation and recombination and a propensity to cross host species.18 Although this property was most clearly shown by the spread of SARS-CoV from Chinese horseshoe bats to human populations during the 2002–03 epidemic, other coronaviruses such as HCoV-OC43 and bovine coronavirus, or feline coronavirus-II, canine coronavirus-II, and transmissible gastroenteritis virus (swine virus) are very closely related, consistent with cross-species transmission.23 This ability to adapt to new environments raised concerns that MERS-CoV would gain virulence and an enhanced ability to transmit from person to person, but this has not occurred.
Both MERS-CoV and SARS-CoV bind to a large ectopeptidase (DPP4 and angiotensin-converting enzyme 2, respectively) to enter cells.4, 24 Binding to the host-cell receptor is a major determinant of pathogenesis, since no infection occurs in its absence. SARS-CoV probably originated in bats25, 26, 27 and adapted to non-bat variants of angiotensin-converting enzyme 2 as it crossed species to infect human beings.28, 29 Changes in the surface glycoprotein enhanced binding to the human receptor. MERS-CoV has not mutated much during transmission in human populations; only a single mutation at position 1020 of the surface protein has been consistently detected in human isolates.30 This aminoacid is located in a region of the protein involved in fusion to host-cell membranes but not in binding to DPP4. By contrast with SARS-CoV, MERS-CoV can bind to DPP4 from several species.4, 31 Thus, non-human primates, rabbits, goats, sheep, and horses, among other animals, are thought to be susceptible to infection in addition to camels and human beings. This difference in the role of surface glycoprotein mutation in virus adaptation to new populations is probably a result of subtle differences in the mechanism of virus entry: coronavirus entry involves both binding to the receptor and subsequent release of a fusion peptide. The relative importance of each component is different in SARS-CoV and MERS-CoV infections.32 Understanding of the relative roles of receptor binding and protease action will enable prediction of whether specific zoonotic coronaviruses will infect human beings and the likelihood of adaptation.
Pathogenesis, pathology, and immunity
In its most severe form, MERS causes an acute highly lethal pneumonia. Renal dysfunction or failure is common in patients8, 13, 33 and could be a consequence of either hypoxic damage or direct infection of the kidney. The latter is a possibility because DPP4 is expressed at high levels in the kidney.34 Analysis of patient tissue samples is needed for a greater understanding of sites of infection. Unfortunately, no tissue samples are available, largely for cultural and religious reasons, so that much of our understanding of pathogenesis comes from studies of experimentally infected animals. Several animals can be experimentally infected with MERS-CoV, including macaques, marmosets, and camels. Macaques develop mild clinical disease with evidence of inflammatory cell infiltration on radiological examination,35, 36 making them a useful model for non-lethal MERS. By contrast, infected marmosets develop severe interstitial pneumonia.37 Neutrophil and macrophage infiltration and alveolar oedema have been reported in infected lung tissue. Infected marmosets are useful models for severe MERS, but these animals are not readily available, limiting their utility. In another study, three camels that were experimentally infected with MERS-CoV developed mild rhinitis but not systemic disease.38 Virus was shed for several days, suggesting that camel-to-camel and camel-to-person spread contributed to the ongoing MERS outbreak.
A small rodent model would be most useful but mice are not susceptible to MERS-CoV. Mice can be sensitised to MERS-CoV infection with an adenovirus-expressing human DPP4.39 An advantage of this approach is that any strain of mouse can be made susceptible to MERS-CoV. Thus, whereas transduced immunocompetent mice develop only mild or no clinical disease, mice deficient in type 1 interferon signalling develop more clinical disease and more extensive pathological changes in the lungs. Like infected macaques, these mice are most useful for vaccine and antivirus studies. Mice transgenic for human DPP4 develop severe clinical disease after MERS-CoV challenge, but these mice also develop encephalitis, compromising their utility.40
Little is known about what constitutes a protective immune response in patients who recover, but based on studies of other coronaviruses including SARS-CoV, coordinated innate and adaptive immune responses are needed.41, 42 MERS-CoV elicits attenuated innate immune responses with delayed pro-inflammatory cytokine induction in cell culture and in vivo,43, 44, 45, 46, 47 which could contribute to a dysregulated immune response. Similar findings have been reported for patients with SARS:48 ineffectual B-cell and T-cell responses with prolonged cytokine expression have been detected in patients with severe disease, whereas a more rapid shutoff of the innate immune response and a potent anti-SARS-CoV antibody response was reported in recovered patients. The anti-SARS-CoV antibody response waned until it was undetectable by 6 years after infection whereas T-cell responses could still be detected.49 Thus, vaccines that result in only antibody responses will probably be useful in the short term, but might not provide long-term protection against MERS-CoV.
Epidemiology
The exact source and mode of transmission of MERS-CoV to human beings is unknown. Initial investigations suggested that MERS-CoV originated in bats: sequences related to MERS-CoV have been found in several bat species.3, 50, 51, 52 Other studies supporting a bat source showed that a bat coronavirus, Tylonycteris bat coronavirus HKU4,53 and MERS-CoV can each use both human and bat DPP4 as host-cell receptors.54, 55 However, MERS-CoV has never been isolated from bats, so whether direct or indirect transmission from bats to people is important remains unknown.
Several other animal species in the Arabian peninsula have been assessed for serological evidence of MERS-CoV infection. Serological assays have improved and sensitivity and specificity have increased.56, 57, 58 In an early study, 100% of dromedary camels (Camelus dromedarius) in Oman and 14% in the Canary Islands (Spain) were positive for anti-MERS-CoV antibodies.59 Subsequent studies confirmed a high rate of seropositivity in camels in the Arabian peninsula, and also showed no evidence of infection of cows, goats, or sheep.59, 60
One key question is whether MERS-CoV newly emerged into camel or human populations or whether the infection had been present for many years but was not detected. A retrospective analysis of stored human samples obtained in 2012 from blood donors and abattoir workers in Saudi Arabia showed no evidence of MERS-CoV seroreactivity.61 By contrast, anti-MERS-CoV antibodies were detected in archived serum samples from dromedary camels obtained in Saudi Arabia in 1993, and the United Arab Emirates in 2003.57, 62 Furthermore, many camels in Saudi Arabia are imported from east Africa; additional studies53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 showed that serum samples from camels in east, west, and north Africa were positive for MERS-CoV as early as 1992, indicating widespread circulation of MERS-CoV in camel populations for many years.
Transmission from camels to human beings
Transmission from camels has been linked to human MERS-CoV infection (figure 3 ), although few patients have a history of camel exposure and transmission is inefficient.66 However, interpretation of this lack of an obvious exposure might be confounded by less direct exposure; for example, patients might be exposed to infectious virus by consumption of unpasteurised camel milk, which is not uncommon in Saudi Arabia.67 Identical68 or nearly identical69, 70 viruses were isolated from geographically linked infected camels and patients. Interpretation of these types of studies is complicated by the fact that RNA viruses in infected hosts, including MERS-CoV-infected dromedary camels, consist of swarms of closely related RNA molecules (quasispecies).71 On transmission to a new host, only one or a few members of the RNA virus swarm are transmitted, which makes it difficult to isolate the exact same virus from both donors and recipients.
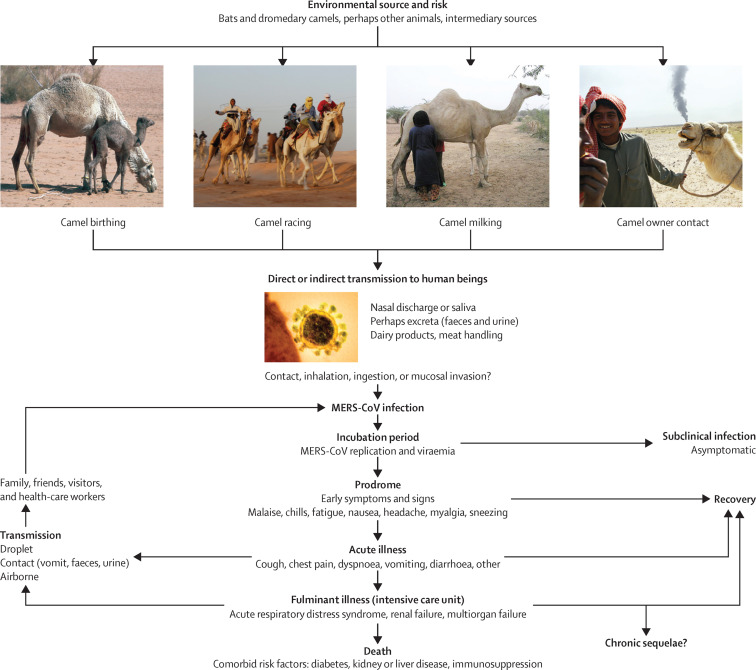
Figure 3.
Ecology and transmission of MERS-CoV
MERS-CoV might have originally spread from bats to camels and other, as yet unidentified, intermediate hosts. The virus has circulated in camel populations in Africa and the Arabian peninsula for at least 20 years. In 2012, MERS-CoV spread to human populations, with camels the most likely source. Several possible routes of spread from camels to humans exist. MERS-CoV is believed to be transmitted among human beings by droplet, contact, and perhaps airborne spread. MERS manifests in people in various ways, ranging from asymptomatic to fulminant infections. Patients with underlying disease such as diabetes or kidney or liver disease or who are immunocompromised develop more severe disease and have a higher mortality rate after infection. Image credits: camel birthing from Noboru Komine/Science Photo Library, camel milking from ACEI Cheung/CC BY-SA 2.0, camel owner contact from Peter Menzel/Science Photo Library, MERS virus from AMI Images/Science Photo Library. MERS-CoV=Middle East respiratory syndrome coronavirus.
The high proportion of adult camels that are anti-MERS-CoV seropositive and the high antibody titres in individual animals suggest that infection occurs at a young age with frequent boosting.57, 58, 59, 62, 67 However, these conclusions have been disputed since the lack of contact with camels in most human cases raises the possibility that transmission is primarily person-to-person or from another intermediate host and that some camel infections result from person-to-camel transmission.72 Additional intermediate hosts might be involved; a coronavirus related to MERS-CoV has been isolated from European hedgehogs (Erinaceus europaeus).73 Together these results suggest that although many details of camel-to-person transmission are unclear, transmission from camels to people is the only confirmed zoonotic source for the human infection. In further support of this hypothesis, although seroprevalence in the general populations is 0·15%, 2·3% of camel shepherds and 3·6% of slaughterhouse workers were MERS-CoV-antibody positive.74 Analyses of human populations in contact with camels in the Horn of Africa, which is a source for many of the camels in Saudi Arabia, might identify seropositive individuals and others who succumbed to infection.
Although MERS cases are reported throughout the year, the disease is seasonal. The first MERS cases identified in April and June, 2012, were followed by more cases in April and May, 2013, and a similar increase in April and May, 2014. A small peak in cases also occurred in September and November, in both 2013, and 2014. The increase in March to May might reflect, in part, transmission from newly infected young camels. The increase in MERS cases in April and May, 2014, in Saudi Arabia was mainly attributed to breaches in infection control and improved reporting and a high degree of awareness for widespread screening.
Human-to-human transmission
Human-to-human transmission of MERS-CoV has been confirmed by epidemiological and genomic studies of cases associated with hospital and household MERS outbreaks.10, 75 In a hospital-based outbreak that occurred in April and May, 2013, in Al-Hasa, an eastern province of Saudi Arabia, 23 patients receiving haemodialysis or in intensive care units were infected with a single clade of virus, with a mortality rate of 65%.10 Spread was assumed largely to occur via large droplets and contact, although the possibility of airborne or fomite transmission was not excluded. Most infections resulted from person-to-person spread, emphasising the importance of appropriate contact and droplet precautions to prevent transmission to other patients, health-care workers, and family members.76 Person-to-person spread in health-care facilities was also implicated in the 2014 MERS-CoV outbreak in Jeddah, Saudi Arabia. Patients that were initially considered primary cases were, on further investigation, found to have been exposed to MERS-CoV-infected patients, generally in health-care facilities.77
MERS-CoVs isolated from single outbreaks are closely related.30 Genomic data are most consistent with human-to-human spread accompanied by periodic reintroduction of the virus into human populations. Ro for MERS-CoV is generally estimated78 to be less than 0·7, substantially less than 1·0 associated with epidemic potential, making sustained transmission of the virus unlikely unless it mutates. By comparison, Ro for SARS-CoV was more than 1, consistent with sustained transmission during the SARS epidemic.79, 80
Serious symptomatic disease occurs most often in patients with comorbidities, such as diabetes, renal failure, and underlying immunosuppression. However, patients without underlying diseases can also be infected, although most develop asymptomatic or mild clinical disease.56, 81, 82 MERS-CoV transmission requires close contact between infected patients and susceptible individuals, but even under these conditions, little transmission has been documented.56, 81, 82, 83 Drosten and colleagues56 showed that the transmission rate from 26 patients to 280 household contacts was roughly 4%. All but one infected contact developed subclinical disease, suggesting that the number of infections in Saudi Arabia and other countries in the Arabian peninsula (and Africa) is much greater than reported and lending support to efforts to increase surveillance in these countries.
Virus evolution
The phylogeny and evolution of MERS-CoV has been studied through whole genome sequencing of samples from several geographic regions (appendix). In one instance, four different MERS-CoV phylogenetic clades were identified in patients in Saudi Arabia from September, 2012, to May, 2013.30 However, by the end of the observation period, three of these clades were no longer circulating, consistent with an Ro of less than 1. Furthermore, the extent of difference between these genetically distinct lineages makes it unlikely that the infections were the result of a single continuous chain of human-to-human transmission.
Clinical features
The clinical manifestations of MERS-CoV infection range from asymptomatic infection to severe pneumonia with acute respiratory distress syndrome, septic shock, and multiorgan failure resulting in death.84 By contrast with SARS (table ), about 75% of patients with MERS had at least one comorbid illness with patients who died more likely to have an underlying condition (86% of patients who died vs 42% of recovered or asymptomatic patients, p<0·001). Index or sporadic cases in the first wave in 2013 were older (median age 59 years vs 43 years, p<0·001), and more likely to have severe disease requiring admission to hospital (94% vs 59%, p<0·001) than were secondary cases. Only secondary cases had mild disease or asymptomatic infection.84
Table.
Comparison of clinical and laboratory features of MERS and SARS
| MERS7, 8, 9, 10, 13, 33, 84, 85 | SARS86, 87, 88, 89, 90, 91 | ||
|---|---|---|---|
| First cases reported | April, 2012 (Zarqa, Jordan), June, 2012 (Jeddah, Saudi Arabia) | November, 2002 (Guangdong, China) | |
| Incubation period | |||
| Mean (95% CI; days) | 5·2 (1·9–14·7) | 4·6 (3·8–5·8) | |
| Range (days) | 2–13 | 2–14 | |
| Serial interval | 7·6 days | 8·4 days | |
| Basic reproduction number | <1 | 2–3 | |
| Patient characteristics | |||
| Adults | 98% | 93% | |
| Children | 2% | 5–7% | |
| Age range (years) | 1–94 | 1–91 | |
| Average age (years) | Median 50 | Mean 39·9 | |
| Sex ratio (male:female) | 64·5%:35·5% | 43%:57% | |
| Mortality | |||
| Overall CFR | 40% | 9·6% | |
| CFR in patients with comorbidities | 60% | 46% | |
| Disease progression | |||
| Time from onset to ventilatory support | Median 7 days | Mean 11 days | |
| Time from onset to death | Median 11·5 days | Mean 23·7 days | |
| Presenting symptoms | |||
| Fever (>38°C) | 98% | 99–100% | |
| Chills or rigors | 87% | 15–73% | |
| Cough | 83% | 62–100% | |
| Dry | 56% | 29–75% | |
| Productive | 44% | 4–29% | |
| Haemoptysis | 17% | 0–1% | |
| Headache | 11% | 20–56% | |
| Myalgia | 32% | 45–61% | |
| Malaise | 38% | 31–45% | |
| Shortness of breath | 72% | 40–42% | |
| Nausea | 21% | 20–35% | |
| Vomiting | 21% | 20–35% | |
| Diarrhoea | 26% | 20–25% | |
| Sore throat | 14% | 13–25% | |
| Rhinorrhoea | 6% | 2–24% | |
| Comorbidities | 76% | 10–30% | |
| Laboratory results | |||
| Chest radiography abnormalities | 90–100% | 94–100% | |
| Leucopenia (<4·0 × 109 cells per L) | 14% | 25–35% | |
| Lymphopenia (<1·5 × 109 cells per L) | 32% | 68–85% | |
| Thrombocytopenia (<140 × 109 platelets per L) | 36% | 40–45% | |
| High lactate dehydrogenase | 48% | 50–71% | |
| High alanine aminotransferase | 11% | 20–30% | |
| High aspartate aminotransferase | 14% | 20–30% | |
| Factors associated with severe disease or death | Being immunocompromised, comorbidity (eg, obesity, diabetes, cardiac disease, lung disease), concomitant infection, low albumin, age ≥65 years | Old age, being male, high initial or peak lactate dehydrogenase, high neutrophil count on presentation, comorbidity, low CD4 and CD8 lymphocyte counts at presentation | |
CFR=case-fatality rate. MERS=Middle East respiratory syndrome. SARS=severe acute respiratory syndrome.
On the basis of data related to human-to-human transmission in several clusters, the incubation period has been estimated as more than 5 days, but could be as long as 2 weeks (table).9 Median time from onset of symptoms to hospital admission is 4·0 days (range 0–16, n=62), from onset of symptoms to admission to an intensive care unit is 5·0 (1–15, n=35), and from onset of symptoms to death is 11·5 days (4–298, n=40).7, 8, 9, 10, 13, 84
MERS typically begins with fever, cough, chills, sore throat, myalgia, and arthralgia, followed by dyspnoea and rapid progression to pneumonia within the first week, often requiring ventilatory and other organ support.7, 8, 9, 10, 13, 85 Although most patients with symptomatic disease present with respiratory illness, immunocompromised patients can present with fever, chills, and diarrhoea and later develop pneumonia.34 Similar to SARS, at least a third of patients with MERS have gastrointestinal symptoms, such as vomiting and diarrhoea.9, 10, 84 Risk factors for development of severe disease, in addition to an immunocompromised state, include comorbidity (eg, obesity, diabetes, cardiac disease, and lung disease).6, 7, 8, 9, 10 Concomitant infections and low albumin concentration are predictors of severe illness, and age older than 65 years was associated with mortality in a case series in Saudi Arabia.85 The few data available about viral dynamics and clinical course suggest that patients with MERS have a shorter time from illness onset to clinical presentation and to a requirement for ventilatory support than do patients with SARS (table), and higher respiratory tract viral loads during the first week of the illness.92
As for SARS and other severe viral illnesses, common laboratory findings of MERS include leucopenia, particularly lymphopenia (table).1, 8, 9, 33, 93 Some patients have a consumptive coagulopathy and high creatinine, lactate dehydrogenase, and liver enzyme concentrations.7, 8 Co-infection with other respiratory viruses (eg, parainfluenza, rhinovirus, influenza A virus[H1N1]pdm09, herpes simplex virus, influenza B virus) has been reported and nosocomial bacterial infections (including Klebsiella pneumoniae, Staphylococcus aureus, Acinetobacter species, Candida species) have occurred in patients receiving invasive mechanical ventilation.1, 33, 84
Chest radiography and tomography findings of MERS are consistent with viral pneumonitis and acute respiratory distress syndrome, with bilateral hilar infiltration, unilateral or bilateral patchy densities or infiltrates, segmented or lobar opacities, ground-glass opacities, and small pleural effusions in some cases. Lower lobes are generally affected more than are upper lobes early in the course of illness with more rapid radiographic progression than in SARS.1, 8, 9, 94
Some patients have viral RNA in blood, urine, and stool but at much lower viral loads than in the respiratory tract.95 MERS-CoV viral loads and genome fractions in upper respiratory tract specimens (eg, nasopharyngeal swabs) are lower than in lower respiratory tract specimens, such as tracheal aspirates and bronchoalveolar lavage fluid,92 which probably accounts for the inefficiency of interhuman transmission. Lower respiratory tract excretion of MERS-CoV RNA can be detected after 1 month of illness in most patients, suggesting that prolonged shedding could be a source for spread in outbreaks.96
Diagnosis
Because lower respiratory tract specimens such as bronchoalveolar lavage fluid, sputum, and tracheal aspirates contain the highest viral loads,33, 92, 95 they should be collected whenever possible. MERS can be confirmed by detection of viral nucleic acid or by serology. The presence of viral nucleic acid can be confirmed either by positive real-time reverse transcription PCR on at least two specific genomic targets or by a single positive target with sequencing of a second positive PCR product.97 Available real-time reverse transcription PCR tests include an assay targeting RNA upstream of the E gene (upE) and assays targeting open reading frames 1b (ORF 1b) and 1a (ORF 1a). The assay for the upE target is highly sensitive and is recommended for screening; the ORF 1a assay is of equal sensitivity. The ORF 1b assay is less sensitive but is useful for confirmation. These assays have not shown cross-reactivity with other respiratory viruses including human coronaviruses. Two target sites on the MERS-CoV genome suitable for sequencing to aid confirmation are in the RNA-dependent RNA polymerase (RdRp; present in ORF 1b) and N genes (figure 2).97
In MERS cases confirmed by PCR, serial sampling for PCR testing from the upper and lower respiratory tracts and other body parts (eg, serum, urine, and stool) are recommended to understand viral replication kinetics and to guide infection control. Respiratory samples should be collected at least every 2–4 days to confirm viral clearance after two consecutive negative results are obtained.
For confirmation of infection by antibody detection, paired serum samples should be collected 14–21 days apart with the first taken during the first week of illness. A positive screening assay (ELISA, immunofluorescence assay) should be followed by a confirmatory (neutralisation) assay. Single samples might also be valuable for identification of probable cases and should be collected at least 14 days after the onset of symptoms.56, 58, 98 Serological results should be carefully interpreted because they might be confounded by cross-reactivity against other coronaviruses.99
Treatment
No specific drug treatment exists for MERS and supportive treatment is the mainstay of management. Evidence-based recommendations for treatment provide the basis for decision making in clinical settings (panel ).100 MERS-CoV is readily inhibited by type 1 interferons (IFN-α and especially IFN-β) in cultured cells,20, 43 and IFN-α2b combined with ribavirin can lessen lung injury and reduce lung titres when administered to rhesus macaques within 8 h of virus inoculation.101 This combination was tested in severely ill patients, showing an improvement in survival at 14 days but not 28 days, possibly a result of administration in the advanced stages of disease.20, 102 Several drugs inhibit MERS-CoV in cell culture, including ciclosporin and mycophenolic acid.103, 104 Other compounds (chloroquine, chlorpromazine, loperamide, and lopinavir) inhibit virus replication (effective concentration50 3–8 μmol/L) in vitro,105, 106 although whether these drugs will be useful in patients is unknown. MERS-CoV-specific peptide fusion inhibitors, which function similarly to the HIV drug enfuvirtide, diminish virus replication in cultured cells, providing a novel approach to MERS treatment.107
Panel. Potentially useful antiviral drugs for Middle East respiratory syndrome coronavirus infection.
Neutralising antibodies * : from convalescent plasma, polyclonal human immunoglobulin from transgenic cows, equine F(ab′)2 antibody fragments, camel antibodies, anti-S monoclonal antibodies.
Interferons * : interferon alfa, interferon β.
Repurposed drugs: ribavirin monotherapy,† ribavirin with interferon, HIV protease inhibitors (lopinavir*, nelfinavir), cyclophilin inhibitors (ciclosporin, alisporivir), chloroquine (active in vitro), mycophenolic acid, nitazoxanide.
Other: Recombinant human mannose-binding lectin. siRNA to key virus genes.
Data from Public Health England.100
Human monoclonal neutralising antibodies and convalescent sera from recovered patients might be useful for treatment if delivered in a timely fashion.100, 108, 109, 110, 111 An exploratory post-hoc meta-analysis of studies of SARS and severe influenza showed a significant reduction of mortality following antibody treatment compared with placebo or no treatment (pooled odds ratio 0·25, 95% CI 0·14–0·45).112
Systemic corticosteroids have been used empirically in some patients to dampen immunopathological host responses, although no survival benefit has been reported.8, 102 Steroids should be used cautiously, if at all, because their use was associated with worsened outcomes in patients infected with SARS-CoV during the 2002–03 epidemic.22 More data are needed from animal studies and carefully done clinical and virological studies of priority treatments such as convalescent plasma and interferons (ideally in randomised clinical trials if sufficient numbers of patients are available). At present, clinical management of patients with severe disease largely relies on meticulous intensive care support and prevention of complications.100
Prevention
Recommendations for prevention of MERS are available from WHO, the US Centers for Disease Control and Prevention, and the Saudi Ministry of Health.113, 114 The main infection prevention and control measures are droplet precautions (wearing a surgical mask within 1 m of patients) and contact precautions (wearing gown and gloves on entering patients' rooms and removing them on leaving). Droplet precautions should be added to the standard precautions when providing care to all patients with signs of acute respiratory infection.113 Eye protection should be used when health-care workers care for probable or confirmed patients. Public Health England,100 US Centers for Disease Control and Prevention,76 and Saudi Ministry of Health113 recommendations for management of known or suspected MERS-CoV infection include the use of personal protective equipment such as gowns, gloves, eye protection (goggles or face shield), and respiratory protection equivalent to a fit-tested National Institute for Occupational Safety and Health-certified disposable N95 filtering facepiece respirator. Patients with MERS should be placed in negative pressure rooms or in rooms in which room exhaust is filtered through high-efficiency particulate air filters. Airborne precautions with at least six air changes per hour should be applied in treatment rooms when performing aerosol-generating procedures.76, 115, 116 These recommendations are evidence-based and have proven to be effective in hospitals in affected countries.
Camels infected with MERS-CoV can develop rhinitis or show no signs of infection and might shed virus through nasal and eye discharge and faeces. The virus can also be found in raw milk from infected camels.67 MERS-CoV is stable in camel breast milk for extended periods of time;117 thus, pasteurisation or cooking is recommended to destroy the virus. Raw urine should not be used for medicinal purposes.114, 118 Because signs of disease are non-specific, it is not possible to know whether an animal in a farm, market, race track, or slaughterhouse is excreting MERS-CoV without virological testing. Camel farm workers, slaughterhouse workers, market workers, veterinarians, and those handling camels at racing facilities should practise good personal hygiene, including frequent handwashing after touching animals, avoiding touching eyes, nose, or mouth with hands, and avoiding contact with sick animals. Consideration should also be given to wearing protective gowns and gloves while handling animals, especially if camels have signs of upper respiratory tract disease.114
The Saudi Government issues updated health guidelines for pilgrims.119 Although MERS-CoV did not cause severe community-acquired pneumonia in any of the 38 hospital-admitted pilgrims investigated during the 2013 Hajj,120 good infectious disease surveillance and control measures are essential to prevent major outbreak of MERS during mass gatherings.115, 121
Unanswered questions and conclusions
Nearly 3 years since the discovery of MERS-CoV, several important questions about its epidemiology, routes of transmission, pathogenesis, and treatment remain unanswered. Although a zoonotic source of the virus is most likely, the route of transmission could be through either direct or indirect contact, or consumption of a contaminated food or food product. Although several studies have shown the possible links between human cases of MERS and transmission from camels, much is unknown of the origins, geographical distribution, exact mode of transmission, and relationships between MERS-CoV and MERS-CoV-like infections in bats, camels, other animals, and human beings. The sporadic nature of many cases of MERS has hindered in-depth case-control studies and investigation of rates of secondary transmission, including establishing the role of subclinical infection in human-to-human transmission. The natural history, pathogenesis, host susceptibility factors, virulence, viral kinetics, period of greatest infectiousness, underlying mechanisms of protective immunity, and factors governing treatment outcome are also unknown.84 This lack of knowledge is hindering the development of drug treatment, adjunctive treatments, specific diagnostic tests, biomarkers, and vaccines.
MERS-CoV is not yet fully adapted to infecting people, and person-to-person transmission is not yet sufficiently efficient for pandemic potential. More information about how long the virus has infected human beings, which could be obtained from analyses of pre-2012 human serum samples (if available), would help assessment of the likelihood of further adaptation to people. Further genomic studies will provide insights into the molecular characteristics, mutation rates, and virus transmission dynamics, defining factors crucial for species specificity and virulence, and enabling the discovery of new drug targets, novel drugs, diagnostic tests, and vaccines. Post-mortem and histological studies have not been available and even limited autopsy or surgical specimens would be useful. The availability of an animal model of severe MERS-CoV infection and disease will be important for understanding pathogenesis, natural history, and immune responses, and for developing effective treatments. These studies are urgently needed because coronaviruses have high genetic mutability and MERS-CoV continues to circulate in countries in the Arabian peninsula. Although interhuman transmission is still inefficient, health authorities, governments, and the research community should be prepared for the emergence of a MERS-CoV with increased capacity for transmission and pandemic potential.
Search strategy and selection criteria
We searched Medline, Embase, and Google Scholar for studies published between Jan 1, 2010, and May 31, 2015. We used the search terms “Middle East Respiratory Syndrome” or “MERS-CoV” in combination with “Coronavirus”, or “Middle East”, or “Epidemiology”, or “Virology” or “Clinical features” or “Aetiology” or “Diagnostic tests”, or “Diagnosis” or “Management” or “Prevention” or “Vaccines” or “nosocomial” or “hospital” or “camels”. We also searched websites of global and national public health agencies such as the US Centers for Disease Control and Prevention, Public Health England, the European Centre for Disease Prevention and Control, and the Saudi Ministry of Health. We selected publications in English, and included references from Center for Infectious Disease Research and Policy, and ProMed (appendix). We also searched the reference lists of articles identified by our search strategy and selected those we judged relevant.
Acknowledgments
Acknowledgments
AZ, DSH, and SP have served MERS-CoV expert advisory groups and participated in the deliberations on MERS-CoV at Global Centre for Mass Gatherings Medicine Conferences, Riyadh, Saudi Arabia. SP was supported in part by grants from the National Institutes of Health (AI091322, AI060699). We thank Dr Yaseen Arabi and Dr Abdullah Assiri for critical review of the report.
Contributors
All authors contributed to development of the draft outline and finalisation of the Seminar.
Declaration of interests
We report no competing interests.
Footnotes
Treatment benefits likely to exceed risks.
Treatment risks likely to exceed benefits.
Supplementary Material
References
- 1.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.de Groot RJ, Baker SC, Baric RS. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Boheemen S, de Graaf M, Lauber C. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012;3:e00473–e00512. doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raj VS, Mou H, Smits SL. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almazán F, DeDiego ML, Sola I. Engineering a replication-competent, propagation-defective Middle East respiratory syndrome coronavirus as a vaccine candidate. MBio. 2013;4:e00650–e00713. doi: 10.1128/mBio.00650-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scobey T, Yount BL, Sims AC. Reverse genetics with a full-length infectious cDNA of the Middle East respiratory syndrome coronavirus. Proc Natl Acad Sci USA. 2013;110:16157–16162. doi: 10.1073/pnas.1311542110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Tawfiq JA, Hinedi K, Ghandour J. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014;59:160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arabi YM, Arifi AA, Balkhy HH. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 9.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assiri A, McGeer A, Perl TM, the KSA MERS-CoV Investigation Team Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC Middle East Respiratory Syndrome (MERS): case definitions. 2014. http://www.cdc.gov/coronavirus/mers/case-def.html (accessed Feb 16, 2015).
- 12.Madani TA. Case definition and management of patients with MERS coronavirus in Saudi Arabia. Lancet Infect Dis. 2014;14:911–913. doi: 10.1016/S1473-3099(14)70918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Abdallat MM, Payne DC, Alqasrawi S, the Jordan MERS-CoV Investigation Team Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Global Alert and Response. Coronavirus infections. http://www.who.int/csr/don/archive/disease/coronavirus_infections/en/ (accessed April 22, 2015).
- 15.ProMED Mail. http://www.promedmail.org/ (accessed April 22, 2015).
- 16.Matthews KL, Coleman CM, van der Meer Y, Snijder EJ, Frieman MB. The ORF4b-encoded accessory proteins of Middle East respiratory syndrome coronavirus and two related bat coronaviruses localize to the nucleus and inhibit innate immune signalling. J Gen Virol. 2014;95:874–882. doi: 10.1099/vir.0.062059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niemeyer D, Zillinger T, Muth D. Middle East respiratory syndrome coronavirus accessory protein 4a is a type I interferon antagonist. J Virol. 2013;87:12489–12495. doi: 10.1128/JVI.01845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masters PS, Perlman S. Coronaviridae. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia, PA: 2013. pp. 825–858. [Google Scholar]
- 19.Kindler E, Jónsdóttir HR, Muth D. Efficient replication of the novel human betacoronavirus EMC on primary human epithelium highlights its zoonotic potential. MBio. 2013;4:e00611–e00612. doi: 10.1128/mBio.00611-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zielecki F, Weber M, Eickmann M. Human cell tropism and innate immune system interactions of human respiratory coronavirus EMC compared to those of severe acute respiratory syndrome coronavirus. J Virol. 2013;87:5300–5304. doi: 10.1128/JVI.03496-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omrani AS, Saad MM, Baig K. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W, Moore MJ, Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge XY, Li JL, Yang XL. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Shi Z, Yu M. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 27.Lau SK, Woo PC, Li KS. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinese SARS Molecular Epidemiology Consortium Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004;303:1666–1669. doi: 10.1126/science.1092002. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Zhang C, Sui J. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cotten M, Watson SJ, Zumla AI. Spread, circulation, and evolution of the Middle East respiratory syndrome coronavirus. MBio. 2014;5:e01062–e01113. doi: 10.1128/mBio.01062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barlan A, Zhao J, Sarkar MK. Receptor variation and susceptibility to Middle East respiratory syndrome coronavirus infection. J Virol. 2014;88:4953–4961. doi: 10.1128/JVI.00161-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millet JK, Whittaker GR. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc Natl Acad Sci USA. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guery B, Poissy J, el Mansouf L, the MERS-CoV study group Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013;381:2265–2272. doi: 10.1016/S0140-6736(13)60982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambeir AM, Durinx C, Scharpé S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 35.de Wit E, Rasmussen AL, Falzarano D. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci USA. 2013;110:16598–16603. doi: 10.1073/pnas.1310744110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao Y, Bao L, Deng W. An animal model of MERS produced by infection of rhesus macaques with MERS coronavirus. J Infect Dis. 2014;209:236–242. doi: 10.1093/infdis/jit590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falzarano D, de Wit E, Feldmann F. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog. 2014;10:e1004250. doi: 10.1371/journal.ppat.1004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adney DR, van Doremalen N, Brown VR. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg Infect Dis. 2014;20:1999–2005. doi: 10.3201/eid2012.141280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao J, Li K, Wohlford-Lenane C. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci USA. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agrawal AS, Garron T, Tao X. Generation of transgenic mouse model of Middle East Respiratory syndrome-coronavirus infection and disease. J Virol. 2015;89:3659–3670. doi: 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Channappanavar R, Zhao J, Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan RW, Chan MC, Agnihothram S. Tropism of and innate immune responses to the novel human betacoronavirus lineage C virus in human ex vivo respiratory organ cultures. J Virol. 2013;87:6604–6614. doi: 10.1128/JVI.00009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu H, Zhou J, Wong BH. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology. 2014;454:197–205. doi: 10.1016/j.virol.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faure E, Poissy J, Goffard A. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One. 2014;9:e88716. doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J, Chu H, Li C. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J Infect Dis. 2014;209:1331–1342. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lau SK, Lau CC, Chan KH. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol. 2013;94:2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 48.Cameron MJ, Ran L, Xu L, the Canadian SARS Research Network Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol. 2007;81:8692–8706. doi: 10.1128/JVI.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang F, Quan Y, Xin ZT. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186:7264–7268. doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
- 50.Ithete NL, Stoffberg S, Corman VM. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis. 2013;19:1697–1699. doi: 10.3201/eid1910.130946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Memish ZA, Mishra N, Olival KJ. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19:1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corman VM, Ithete NL, Richards LR. Rooting the phylogenetic tree of middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. J Virol. 2014;88:11297–11303. doi: 10.1128/JVI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woo PC, Wang M, Lau SK. Comparative analysis of twelve genomes of three novel group 2c and group 2d coronaviruses reveals unique group and subgroup features. J Virol. 2007;81:1574–1585. doi: 10.1128/JVI.02182-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Q, Qi J, Yuan Y. Bat origins of MERS-CoV supported by bat coronavirus HKU4 usage of human receptor CD26. Cell Host Microbe. 2014;16:328–337. doi: 10.1016/j.chom.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y, Du L, Liu C. Receptor usage and cell entry of bat coronavirus HKU4 provide insight into bat-to-human transmission of MERS coronavirus. Proc Natl Acad Sci USA. 2014;111:12516–12521. doi: 10.1073/pnas.1405889111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drosten C, Meyer B, Müller MA. Transmission of MERS-coronavirus in household contacts. N Engl J Med. 2014;371:828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- 57.Hemida MG, Perera RA, Al Jassim RA. Seroepidemiology of Middle East respiratory syndrome (MERS) coronavirus in Saudi Arabia (1993) and Australia (2014) and characterisation of assay specificity. Euro Surveill. 2014;19:20828. doi: 10.2807/1560-7917.es2014.19.23.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perera RA, Wang P, Gomaa MR. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. 2013;18:20574. doi: 10.2807/1560-7917.es2013.18.36.20574. [DOI] [PubMed] [Google Scholar]
- 59.Reusken CB, Haagmans BL, Müller MA. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. 2013;13:859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reusken CB, Ababneh M, Raj VS. Middle East Respiratory Syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Euro Surveill. 2013;18:20662. doi: 10.2807/1560-7917.es2013.18.50.20662. [DOI] [PubMed] [Google Scholar]
- 61.Aburizaiza AS, Mattes FM, Azhar EI. Investigation of anti-middle East respiratory syndrome antibodies in blood donors and slaughterhouse workers in Jeddah and Makkah, Saudi Arabia, fall 2012. J Infect Dis. 2014;209:243–246. doi: 10.1093/infdis/jit589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyer B, Müller MA, Corman VM. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis. 2014;20:552–559. doi: 10.3201/eid2004.131746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corman VM, Jores J, Meyer B. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992–2013. Emerg Infect Dis. 2014;20:1319–1322. doi: 10.3201/eid2008.140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Müller MA, Corman VM, Jores J. MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983–1997. Emerg Infect Dis. 2014;20:2093–2095. doi: 10.3201/eid2012.141026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reusken CB, Messadi L, Feyisa A. Geographic distribution of MERS coronavirus among dromedary camels, Africa. Emerg Infect Dis. 2014;20:1370–1374. doi: 10.3201/eid2008.140590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hemida MG, Al-Naeem A, Perera RA, Chin AW, Poon LL, Peiris M. Lack of Middle East respiratory syndrome coronavirus transmission from infected camels. Emerg Infect Dis. 2015;21:699–701. doi: 10.3201/eid2104.141949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reusken CB, Farag EA, Jonges M. Middle East respiratory syndrome coronavirus (MERS-CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Euro Surveill. 2014;19:20829. doi: 10.2807/1560-7917.es2014.19.23.20829. [DOI] [PubMed] [Google Scholar]
- 68.Azhar EI, El-Kafrawy SA, Farraj SA. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 69.Haagmans BL, Al Dhahiry SH, Reusken CB. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14:140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Memish ZA, Cotten M, Meyer B. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20:1012–1015. doi: 10.3201/eid2006.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Briese T, Mishra N, Jain K. Middle East respiratory syndrome coronavirus quasispecies that include homologues of human isolates revealed through whole-genome analysis and virus cultured from dromedary camels in Saudi Arabia. MBio. 2014;5:e01146–e01214. doi: 10.1128/mBio.01146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samara EM, Abdoun KA. Concerns about misinterpretation of recent scientific data implicating dromedary camels in epidemiology of Middle East respiratory syndrome (MERS) MBio. 2014;5:e01430–e01514. doi: 10.1128/mBio.01430-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corman VM, Kallies R, Philipps H. Characterization of a novel betacoronavirus related to middle East respiratory syndrome coronavirus in European hedgehogs. J Virol. 2014;88:717–724. doi: 10.1128/JVI.01600-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Müller MA, Meyer B, Corman VM. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015;15:559–564. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drosten C, Muth D, Corman VM. An observational, laboratory-based study of outbreaks of Middle East respiratory syndrome coronavirus in Jeddah and Riyadh, kingdom of Saudi Arabia, 2014. Clin Infect Dis. 2015;60:369–377. doi: 10.1093/cid/ciu812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.CDC Interim infection prevention and control recommendations for hospitalized patients with Middle East respiratory syndrome coronavirus (MERS-CoV) May 15, 2014. http://www.cdc.gov/coronavirus/mers/infection-prevention-control.html (accessed Feb 16, 2015).
- 77.Oboho IK, Tomczyk SM, Al-Asmari AM. 2014 MERS-CoV outbreak in Jeddah-A link to health care facilities. N Engl J Med. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chowell G, Blumberg S, Simonsen L, Miller MA, Viboud C. Synthesizing data and models for the spread of MERS-CoV, 2013: key role of index cases and hospital transmission. Epidemics. 2014;9:40–51. doi: 10.1016/j.epidem.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lipsitch M, Cohen T, Cooper B. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Riley S, Fraser C, Donnelly CA. Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science. 2003;300:1961–1966. doi: 10.1126/science.1086478. [DOI] [PubMed] [Google Scholar]
- 81.Memish ZA, Al-Tawfiq JA, Makhdoom HQ. Screening for Middle East respiratory syndrome coronavirus infection in hospital patients and their healthcare worker and family contacts: a prospective descriptive study. Clin Microbiol Infect. 2014;20:469–474. doi: 10.1111/1469-0691.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369:884–886. doi: 10.1056/NEJMc1308698. [DOI] [PubMed] [Google Scholar]
- 83.Abroug F, Slim A, Ouanes-Besbes L, the World Health Organization Global Outbreak Alert and Response Network Middle East Respiratory Syndrome Coronavirus International Investigation Team Family cluster of Middle East respiratory syndrome coronavirus infections, Tunisia, 2013. Emerg Infect Dis. 2014;20:1527–1530. doi: 10.3201/eid2009.140378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.The WHO, the MERS-CoV Research Group State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013 doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saad M, Omrani AS, Baig K. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee N, Hui D, Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 87.Choi KW, Chau TN, Tsang O, the Princess Margaret Hospital SARS Study Group Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med. 2003;139:715–723. doi: 10.7326/0003-4819-139-9-200311040-00005. [DOI] [PubMed] [Google Scholar]
- 88.Christian MD, Poutanen SM, Loutfy MR, Muller MP, Low DE. Severe acute respiratory syndrome. Clin Infect Dis. 2004;38:1420–1427. doi: 10.1086/420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fowler RA, Lapinsky SE, Hallett D, the Toronto SARS Critical Care Group Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 90.Tsang KW, Ho PL, Ooi GC. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 91.Wang JT, Sheng WH, Fang CT. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis. 2004;10:818–824. doi: 10.3201/eid1005.030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Memish ZA, Al-Tawfiq JA, Makhdoom HQ. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis. 2014;210:1590–1594. doi: 10.1093/infdis/jiu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 94.Ajlan AM, Ahyad RA, Jamjoom LG, Alharthy A, Madani TA. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR Am J Roentgenol. 2014;203:782–787. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- 95.Poissy J, Goffard A, Parmentier-Decrucq E, the MERS-CoV Biology Group Kinetics and pattern of viral excretion in biological specimens of two MERS-CoV cases. J Clin Virol. 2014;61:275–278. doi: 10.1016/j.jcv.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Memish ZA, Assiri AM, Al-Tawfiq JA. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis. 2014;29:307–308. doi: 10.1016/j.ijid.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Corman VM, Muller MA, Costabel U. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012;17 doi: 10.2807/ese.17.49.20334-en. pi=20334. [DOI] [PubMed] [Google Scholar]
- 98.WHO Laboratory testing for Middle East Respiratory Syndrome coronavirus. http://www.who.int/csr/disease/coronavirus_infections/MERS_Lab_recos_16_Sept_2013.pdf (accessed Feb 16, 2015).
- 99.Chan KH, Chan JF, Tse H. Cross-reactive antibodies in convalescent SARS patients' sera against the emerging novel human coronavirus EMC (2012) by both immunofluorescent and neutralizing antibody tests. J Infect. 2013;67:130–140. doi: 10.1016/j.jinf.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.ISARIC PHE. Treatment of MERS-CoV: Information for clinicians. Clinical decision-making support for treatment of MERS-CoV. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/360424/MERS_COV_information_for_clinicians_17_July.pdf (accessed April 13, 2015).
- 101.Falzarano D, de Wit E, Rasmussen AL. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chan JF, Chan KH, Kao RY. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013;67:606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Wilde AH, Raj VS, Oudshoorn D. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment. J Gen Virol. 2013;94:1749–1760. doi: 10.1099/vir.0.052910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Wilde AH, Jochmans D, Posthuma CC. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dyall J, Coleman CM, Hart BJ. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu L, Liu Q, Zhu Y. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat Commun. 2014;5:3067. doi: 10.1038/ncomms4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang L, Wang N, Zuo T. Potent neutralization of MERS-CoV by human neutralizing monoclonal antibodies to the viral spike glycoprotein. Sci Transl Med. 2014;6:234ra59. doi: 10.1126/scitranslmed.3008140. [DOI] [PubMed] [Google Scholar]
- 109.Tang XC, Agnihothram SS, Jiao Y. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc Natl Acad Sci USA. 2014;111:2018–2026. doi: 10.1073/pnas.1402074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ying T, Du L, Ju TW. Exceptionally potent neutralization of Middle East respiratory syndrome coronavirus by human monoclonal antibodies. J Virol. 2014;88:7796–7805. doi: 10.1128/JVI.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hui DS, Memish ZA, Zumla A. Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Curr Opin Pulm Med. 2014;20:233–241. doi: 10.1097/MCP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 112.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, the Convalescent Plasma Study Group The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scientific Advisory Council. Ministry of Health. Saudi Arabia Infection prevention/control and management guidelines for patients with Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection 2nd edn. http://www.moh.gov.sa/en/CCC/StaffRegulations/Corona/Documents/GuidelinesforCoronaPatients.pdf (accessed Feb 16, 2015).
- 114.WHO Update on MERS-CoV transmission from animals to humans, and interim recommendations for at-risk groups. 2014. http://www.who.int/csr/disease/coronavirus_infections/MERS_CoV_RA_20140613.pdf?ua=1 (accessed Feb 16, 2015).
- 115.WHO Infection prevention and control of epidemic-and pandemic prone acute respiratory infections in health care. WHO guidelines. 2014. http://apps.who.int/iris/bitstream/10665/112656/1/9789241507134_eng.pdf?ua=1 (accessed Feb 16, 2015). [PubMed]
- 116.Zumla A, Hui DS. Infection control and MERS-CoV in health-care workers. Lancet. 2014;383:1869–1871. doi: 10.1016/S0140-6736(14)60852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Doremalen N, Bushmaker T, Karesh WB, Munster VJ. Stability of Middle East respiratory syndrome coronavirus in milk. Emerg Infect Dis. 2014;20:1263–1264. doi: 10.3201/eid2007.140500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gossner C, Danielson N, Gervelmeyer A. Human-dromedary camel interactions and the risk of acquiring zoonotic Middle East Respiratory Syndrome Coronavirus infection. Zoonoses Public Health. 2014 doi: 10.1111/zph.12171. published online Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Al-Tawfiq JA, Memish ZA. Mass gathering medicine: 2014 Hajj and Umra preparation as a leading example. Int J Infect Dis. 2014;27:26–31. doi: 10.1016/j.ijid.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Memish ZA, Almasri M, Turkestani A, Al-Shangiti AM, Yezli S. Etiology of severe community-acquired pneumonia during the 2013 Hajj-part of the MERS-CoV surveillance program. Int J Infect Dis. 2014;25:186–190. doi: 10.1016/j.ijid.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Memish ZA, Zumla A, Alhakeem RF. Hajj: infectious disease surveillance and control. Lancet. 2014;383:2073–2082. doi: 10.1016/S0140-6736(14)60381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.