Abstract
Cancer susceptibility genes have been classified into two groups: gatekeepers and caretakers1. Gatekeepers are genes that control cell proliferation and death, whereas caretakers are DNA repair genes whose inactivation leads to genetic instability. Abrogation of both caretaker and gatekeeper function markedly increases cancer susceptibility. Although the importance of Ku80 in DNA double-strand break repair is well established, neither Ku80 nor other components of the non-homologous end-joining pathway are known to have a caretaker role in maintaining genomic stability. Here we show that mouse cells deficient for Ku80 display a marked increase in chromosomal aberrations, including breakage, translocations and aneuploidy. Despite the observed chromosome instabilities, Ku80−/− mice have only a slightly earlier onset of cancer2,3. Loss of p53 synergizes with Ku80 to promote tumorigenesis such that all Ku80−/−p53−/− mice succumb to disseminated pro-B-cell lymphoma before three months of age. Tumours result from a specific set of chromosomal translocations and gene amplifications involving IgH and c-Myc, reminiscent of Burkitt's lymphoma. We conclude that Ku80 is a caretaker gene that maintains the integrity of the genome by a mechanism involving the suppression of chromosomal rearrangements.
The three subunits of the DNA-dependent protein kinase, Ku70, Ku80 and DNA-PKcs, are, together with XRCC4 and Ligase IV, essential for the non-homologous end-joining (NHEJ) pathway in mammalian cells. Mice that carry the targeted disruption of components of the NHEJ pathway share some common phenotypic features including arrested lymphocyte development and increased sensitivity to ionizing radiation3–9. Deficiencies in Ku80, Ku70, XRCC4 and Ligase IV, but not in DNA-PKcs, result in growth retardation and decreased proliferation in vitro. This senescence has been proposed to result from an inability to repair double-strand breaks (DSBs) in DNA incurred during normal DNA metabolism3.
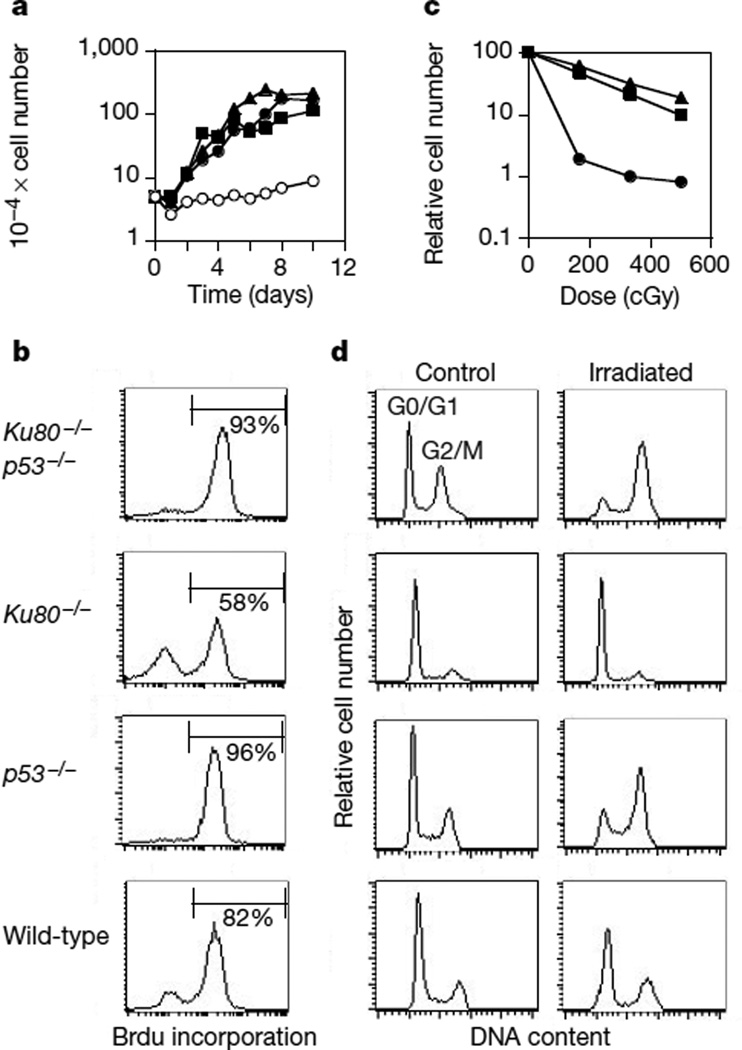
p53 monitors chromosome damage and either arrests cell-cycle progression or triggers apoptosis in cells with unrepaired lesions10. To determine whether p53 is involved in the growth arrest of Ku80−/− mice and mouse embryo fibroblasts (MEFs), we generated Ku80−/−p53−/− double-mutant mice. The absence of p53 did not markedly affect the initial viability of Ku80−/−p53−/− mice, and they were indistinguishable in size from Ku80−/− littermates. By contrast, inactivation of p53 did alleviate the growth arrest of the Ku80 knock-out MEFs (Fig. 1a, b). Ku80−/−p53−/− cells proliferated as rapidly and saturated at a similar density as p53−/− cells (Fig. 1a), and contained a significantly greater cycling population than Ku80−/− MEFs (Fig. 1b). Although the Ku80−/− proliferation defect was abrogated by the concomitant loss of p53, Ku80−/−p53−/− MEFs were more sensitive to DNA damage induced by ionizing radiation than p53−/− or wild-type controls (Fig. 1c). After γ-irradiation, Ku80−/−p53−/− MEFs initially accumulated in the G2/M phase of the cell cycle (Fig. 1d). Within 48–96 hours after irradiation, Ku80−/−p53−/− MEFs lost their adherence to the tissue culture dish, exhibited extensive nuclear fragmentation and showed an increase in the number of cells with a DNA content less than that in G0/G1 (not shown). By contrast, Ku80−/− MEFs were arrested permanently in the G0/G1 and G2/M phases of the cell cycle in response to γ-irradiation3 (Fig. 1d). Thus, p53 is required for early senescence in Ku80−/− fibroblasts, indicating that cell-cycle arrest in these cells is correlated with an accumulation of DNA damage (see below). The fact that a loss of p53 does not rescue the size of Ku80−/− mice indicates either that the dwarfism is unrelated to DNA damage or that Ku80−/−p53−/− cells that sustain irreparable damage are eliminated by p53-independent mechanisms in vivo.
Figure 1.
Growth characteristics of untreated and irradiated mouse embryo fibroblasts. a, Growth kinetics of Ku80−/− (open circles), p53−/− (filled triangles), Ku80−/−p53−/− (filled circles) and wild-type (filled squares) MEFs. b, Incorporation of BrdU in MEF cultures after 16 h of continuous labelling. c, Radiation sensitivity of Ku80−/−p53−/− (circles), p53−/− (triangles) and wild-type (squares) MEFs, plotted as the fraction of surviving cells relative to unirradiated samples of the same genotype. Ku80−/− MEFs exhibited a sevenfold decrease in cell count independent of the dose (83–600 cGy) owing to the premature senescence of unirradiated cultures, and the permanent arrest of the dividing population in response to γ-irradiation. d, DNA content histogram measured in untreated samples (control) and in cultures 24 h after treatment with 10 Gy of γ-radiation (Irradiated).
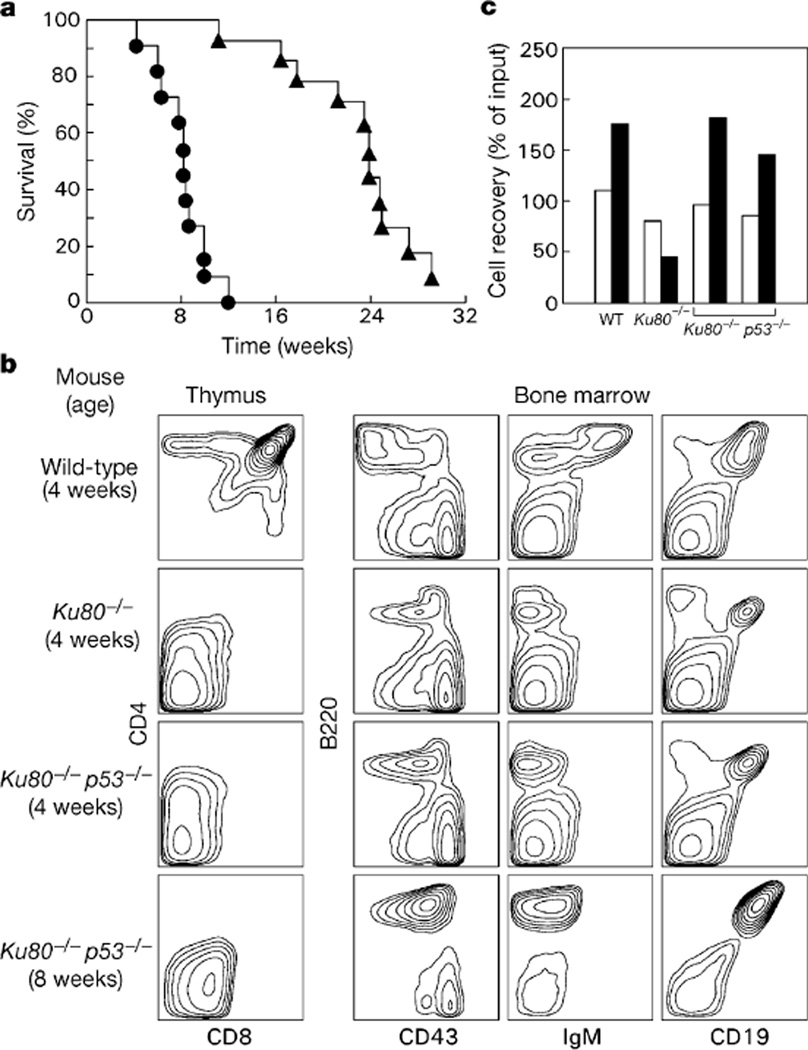
Although Ku80−/−p53−/− mice developed normally, all (n = 11) such mice died within 12 weeks after birth (Fig. 2a) from disseminated lymphoma. Tumour cells expressed B-cell lineage-specific surface markers B220, CD19 and CD43, but were negative for IgM, indicating maturational arrest at the pro-B-cell stage of development (Fig. 2b; right panel, Ku80−/−p53−/− 8 weeks). In contrast, p53−/− mice are predisposed to thymic lymphomas11, which develop at a slower rate than Ku80−/−p53−/− pro-B-cell lymphomas (Fig. 2a), and Ku80−/− mice only occasionally develop lymphoma2 (only 1 out of 11 Ku80−/− mice developed T-cell lymphoma (Thy1.2+, CD4+, CD8+) after seven months). We conclude that the loss of p53 in the Ku80 knockout background invariably results in the rapid onset of B-lineage tumours before the age at which T-lineage tumours occasionally arise in Ku80−/− mice.
Figure 2.
Analysis of lifespan and lymphocyte development. a, Kaplan–Meier analysis comparing mortality of Ku80+/+p53−/− (triangles) and Ku80−/−p53−/− (circles) mice as a function of time. The survival of Ku80−/−p53−/− mice is statistically different from that of the Ku80+/+p53−/− group; P = 0.0001. b, Flow cytometric analysis of thymocyte (left panel) and bone-marrow (right panels) suspensions obtained from wild-type, Ku80−/− and Ku80−/−p53−/− mice. An 8-week-old Ku80−/−p53−/− mouse, which had a B-cell lymphoma, is compared with a 4-week-old non-tumour-bearing Ku80−/−p53−/− mouse, and with 4-week-old Ku80−/− and wild-type littermates. Data are representative of 10 wild-type, 6 Ku80−/−, 5 non-tumour-bearing Ku80−/−p53−/− mice 2–4 weeks old, and 12 Ku80−/−p53−/− animals that had tumours. Average thymus cellularity was 5 × 105 in Ku80−/− mice, 106 in non-tumour-bearing Ku80−/−p53−/− mice and 2 × 108 in wild-type controls. Average number of B220+CD19+ cells in bone marrow was 4.6 × 105 in Ku80−/− mice, 9.6 × 105 in non-tumour-bearing Ku80−/−p53−/− mice and 2.8 × 106 for wild-type controls. The immuno-phenotypes of Ku80+/+p53−/− and wild-type mice were similar (not shown). c, Survival of wild-type (WT) B220+CD43+IgM− pro-B cells, and Ku80−/−p53−/− and Ku80−/− CD19+ B cells after 1 d (open columns) and 3 d (filled columns) in culture, plotted relative to input cell number at day 0. Results from two independent Ku80−/−p53−/− mice are plotted.
The shift from T-cell to B-cell lymphomas in Ku80−/−p53−/− mice was not accompanied by an obvious alteration in T- or B-lineage surface markers. Ku80−/−p53−/− thymocytes resembled Ku80−/− thymocytes in that they developed no further than the CD4−CD8− stage (Fig. 2b; left panel), whereas B cells remained arrested at the B220+, CD19+, CD43+, IgM− pro-B-cell stage (Fig. 2b; right panel). However, the loss of p53 had different effects on the survival of T-cell and B-cell precursors. Whereas both Ku80−/− pro-B and pro-T cells showed high susceptibility to apoptosis (as indicated by annexin staining; data not shown) and poor proliferation, loss of p53 from the double knockouts seemed to rescue Ku80−/− pro-B cells, but not pro-T cells (not shown), allowing normal proliferation in vitro (Fig. 2c). We conclude that p53 is required for the induction of apoptosis in B-lymphocyte but not T-lymphocyte precursors that harbour chromosome abnormalities induced by the loss of Ku80. This difference might in part explain the increased susceptibility of Ku80−/−p53−/− mice to B-lineage malignancies.
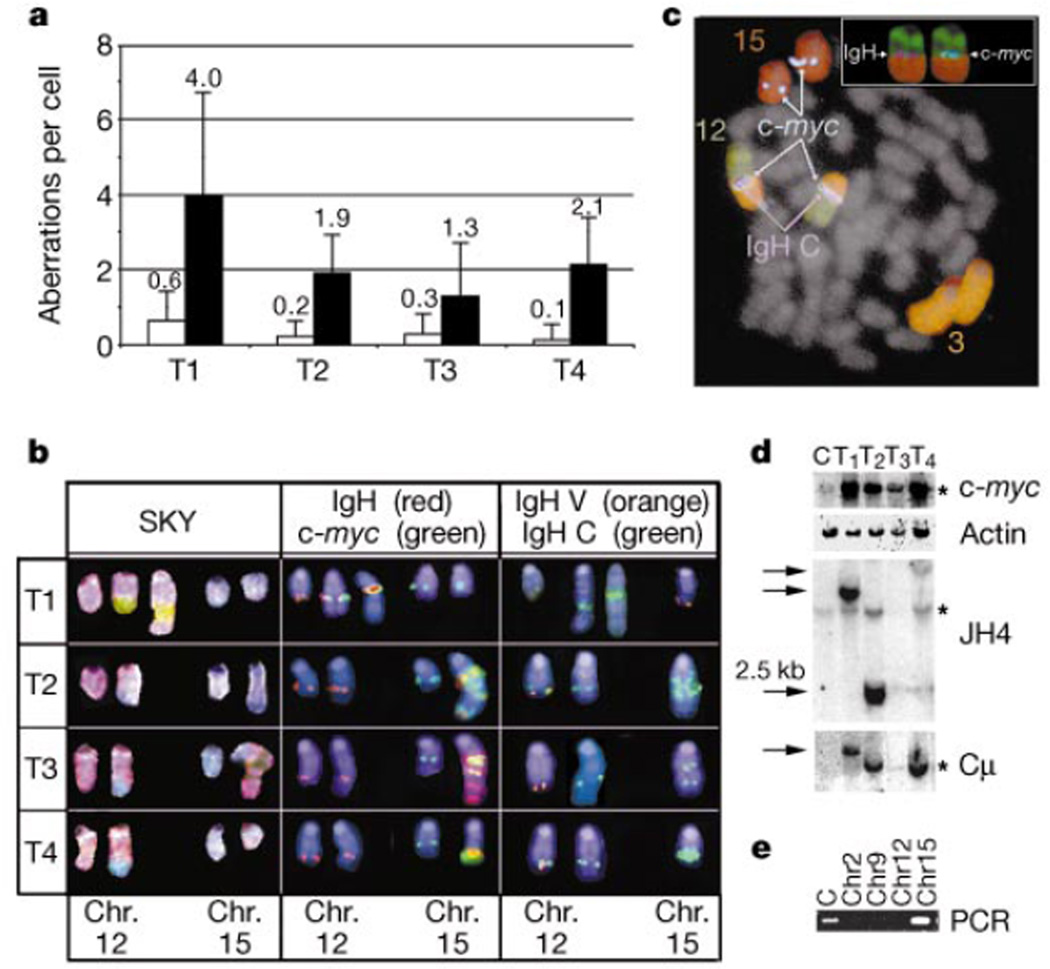
The observation that Ku80−/−p53−/− mice develop lymphomas at an accelerated rate suggests that Ku80 and p53 normally cooperate to limit the oncogenic potential of DSBs during assembly of the antigen receptor gene. Chromosome analysis by spectral karyotyping (SKY)12 revealed karyotype heterogeneity within each Ku80−/−p53−/− tumour (n = 4), exemplified by translocations and apparently random gains or losses of whole chromosomes (Fig. 3a). Metaphases from 20 identically treated Rag2−/− pro-B cells exhibited no detectable aberrations. All tumours contained a translocation of chromosome 12, on which the immunoglobulin heavy chain (IgH) is located (Fig. 3b; SKY). In three tumours (T2, T3 and T4) the partner was chromosome 15, whereas in the remaining tumour (T1) chromosome 3 was the fusion partner (Fig. 3b; SKY). The one Ku80−/− T-cell lymphoma that arose during the 7-month observation period exhibited a translocation that fused chromosomes 3 and 7 (not shown). Thus, both Ku80−/− and Ku80−/−p53−/− mice develop lymphomas that exhibit chromosome translocations.
Figure 3.
c-myc and IgH-associated rearrangements and amplifications in Ku80−/−p53−/− tumours. a, Average number of translocations (other than those juxtaposing chromosomes 12 and 15), unshaded, or gains and loses of chromosomes, shaded, in Ku80−/−p53−/− tumours (T1–T4). b, SKY analysis revealed translocations involving chromosomes (Chr.) 12 (pink) and 15 (blue) in Ku80−/−p53−/− tumours 2–4 (T2–T4) and 12 and 3 (green) in T1. FISH with probes for IgH Cγ1−α (red) and c-myc (green) reveal co-localization and co-amplification of these genes as well as signals on the normal chromosomes. Analysis with probes for IgH variable (orange) and constant (green) regions show co-localization on the normal chromosome 12. The absence of the variable region on the derivative 12 shows that this translocation disrupts the IgH locus. Chromosomes in the middle and right panels were identified by hybridization with painting probes (as in c) and are counterstained with 4,6-diamidino-2-phenylindole (DAPI; blue). c, Five-colour FISH analysis of a metaphase from tumour T1. The inset shows the localization of individual c-myc and IgH signals at the breakpoint between chromosomes 12 and 3. d, Southern blot analysis showing germline bands (stars) as well as rearrangments (arrows) and/or amplifications of c-myc and IgH. e, PCR amplification of tumour 2 breakpoint indicates that the JH1 fusion partner originated from chromosome 15. Abbreviations: C: Liver; T1–T4: Ku80−/−p53−/− tumors 1–4.
Translocations between chromosomes 12 and 15 in Ku80−/−p53−/− lymphomas resemble the translocations in mouse plasmacytomas and Burkitt's lymphomas that juxtapose the c-myc oncogene to the IgH locus13. Translocations involving the IgH locus, but not c-myc, have been reported in B-cell lymphomas arising from crosses between SCID (severe combined immunodeficiency) mice—which are defective in DNA-PKcs—and p53−/− mice14. We therefore performed fluorescence in situ hybridization (FISH) analysis to determine whether c-myc and IgH genes were translocated in Ku80−/−p53−/− lymphomas. All Ku80−/−p53−/− lymphomas showed co-localization of c-myc and IgH with an apparent amplification of both genes (Fig. 3b; IgH, c-myc). In the one Ku80−/−p53−/− tumour that showed a 12:3 translocation, c-myc and IgH co-localized precisely at the breakpoint between chromosomes 12 and 3, indicating a three-way (12:15:3) translocation (Fig. 3c). Amplification of c-myc and IgH was confirmed by Southern blot analysis (Fig. 3d), and IgH locus rearrangements were found in all tumours (Fig. 3d). Furthermore, FISH analysis mapped the breakpoint on chromosome 12 to between the IgH variable cluster and constant region (Fig. 3b; IgH V, IgH C). We conclude that Ku80−/−p53−/− lymphomas result from a specific set of chromosomal translocations and amplifications involving c-myc and IgH, and therefore differ from SCIDxp53−/− B-cell lymphomas14. DNA-PKcs-independent functions of Ku, as established in previous studies, might contribute to the cytogenetic differences in these tumours3,15.
To determine the precise nature of the translocations in Ku80−/−p53−/− lymphomas, we cloned the novel JH-hybridizing EcoRI fragment from one of the four tumours (Fig. 3d; T2). Sequence analysis revealed that the 2.5-kilobase (kb) fragment was a fusion of JH1 to a 173-base pair (bp) segment that bore no significant homologies to previously reported sequences. To determine the chromosomal origin of the 173-bp DNA fragment, FACS-sorted chromosomes were screened by PCR (Fig. 3e). Unfractionated liver DNA and purified chromosome 15 DNA showed a signal, whereas chromosomes 2, 9 and 12 were negative (Fig. 3e). Loss of the variable region on the der(12) chromosome and the proximity of the translocation to JH1 suggest that the translocation resulted from aberrant repair of DNA breaks produced in pro-B cells during V(D)J recombination.
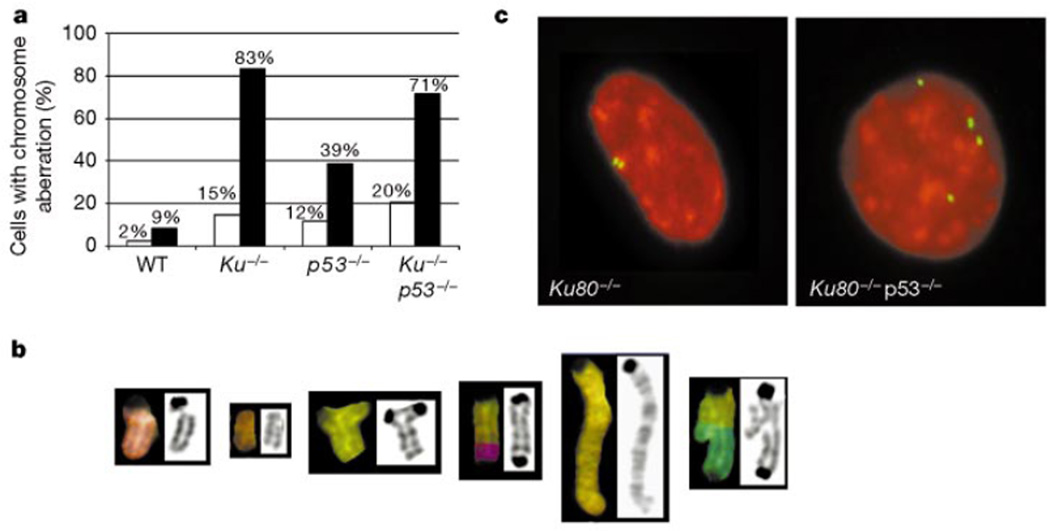
One of the hallmarks of malignant transformation is genomic instability, which promotes a wide range of mutations, including chromosome deletions, gene amplifications, translocations and polyploidy16. To determine whether loss of Ku80 results in chromosome instability, we karyotyped metaphases from passage-1 MEFs (Fig. 4).Whereas only 9% of metaphases from wild-type mice were karyotypically abnormal, we observed profound aberrations in the integrity of the Ku80−/− genome (Fig. 4a); 83% of Ku80−/− metaphases had breaks or translocations (Fig. 4a) and 15% displayed polyploidy (that is, 3n, 4n or 5n chromosome content, where 2n reflects a normal diploid complement). Although chromosome aberrations have been reported in primary dermal fibroblasts from Ku80−/−, Ku80+/− and ligase IV+/− mice17, we found no significant differences between Ku80+/− and wild-type MEFs. The pattern of damage in the Ku80−/− metaphases was further characterized by SKY, which showed chromatid breaks, acentric fragments, triradials, dicentrics, duplications and asymmetric exchanges (Fig. 4b). Ku80−/−p53−/− MEFs were similar to Ku80−/−, whereas p53−/− MEFs had slightly fewer aberrations (Fig. 4a). Thus, the absence of Ku80 precipitates genomic instability, which is not augmented by the additional loss of p53.
Figure 4.
Chromosome aberrations in Ku80−/− and Ku80−/−p53−/− MEFs. a, Percentage of cells with DNA breaks or translocations, shaded (n = 35), and polyploidy, unshaded (n = 400), observed in passage 1 MEFs. WT, wild type. b, Typical chromosome aberrations found in Ku80−/− MEFs. From left to right, chromatid break, acentric fragment, tri-radial, dicentric, duplication and an asymmetric exchange. c, γ-Tubulin staining for centrosomes (green) in Ku80−/− (left) and Ku80−/−p53−/− (right) MEFs counterstained with propidium iodide (red).
An apparently random gain or loss of chromosomes also occurred in 70% of Ku80−/− MEFs (not shown). Because aneuploidy has been associated with centrosome amplification in BRCA1−/− and p53−/− MEFs18,19, we looked at these structures in early-passage MEFs. Our analysis of γ-tubulin structures revealed a similar centrosome amplification in both Ku80−/−p53−/− and p53−/− MEFs. However, despite the numerical aberrations seen in Ku80−/− MEFs, centrosomes in these cells were indistinguishable from normal fibroblast controls (Fig. 4c; not shown). Thus, the acquisition of numerical chromosome aberrations in Ku80−/− MEFs occurs by a mechanism unrelated to centrosome amplification.
Unresolved signal ends in Ku80−/− lymphocytes might contribute to the development of translocations by acting as transposable elements20,21. Alternatively, gross chromosomal rearrangements in Ku80−/− mice might result from the absence of Ku-mediated tethering of broken ends, which normally facilitates their subsequent ligation22,23. Promiscuous chromosome fusions in the absence of Ku80 could either be accomplished by other components of the apparatus of the NHEJ pathway (that is, Ku70, DNA-PKcs, XRCC4 or Ligase IV) or involve alternative DNA repair pathways such as single-strand annealing or break-induced replication24. In either case, the finding that Ku80 suppresses gross genomic rearrangements places Ku80 in the class of caretaker genes, which include ATM, BRCA1 and several proteins that function in homologous recombination1,25,26. p53 provides an additional safeguard against the genomic instability inherent in Ku80-deficient cells by either arresting cell division (as in Ku80−/− MEFs) or triggering apoptosis (as in Ku80−/− B lymphocytes). Consistent with this model is the observation that the abrogation of both Ku80 caretaker and p53 gatekeeper functions markedly increases malignant transformation, especially in lymphocytes that undergo physiological DSBs during the course of V(D)J recombination. Whereas the phenotype of Ku80−/− mice is more severe than that of DNA-PKcs−/− mice, we would expect that other components of the NHEJ machinery (Ku70, XRCC4 and Ligase IV) also have an essential role in maintaining genomic stability. Thus, mutation of the NHEJ pathway components might facilitate the development of malignancy by increasing the frequency of chromosomal aberrations.
Methods
Generation and screening of mice
Ku80 mutant mice were generated from Ku80+/− intercrosses and screened by polymerase chain reaction (PCR) as described3. p53−/− mice were obtained from Taconic Laboratories, and p53 genotyping was performed as described27,28. Mice heterozygous for Ku80 and p53 were crossed to obtain progeny homozygous for both Ku80-null and p53-null alleles. To generate mouse embryo fibroblasts, Ku80+/−p53−/− males were crossed with Ku80+/−p53+/− females and embryos were isolated 13.5 days after plug formation3.
Growth and irradiation assays
Passage-1 MEFs (105) were plated in duplicate six-well dishes and the medium was replaced daily. Individual dishes were treated with trypsin, counted and averaged. For continuous labelling experiments, 105 cells were plated with 50 µM bromodeoxyuridine (BrdU) and grown for 16 h. Cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-BrdU antibodies and propidium iodide (PI) in accordance with the manufacturer's specifications (Becton Dickinson). For radiation survival experiments, cells were exposed to graded doses of γ-rays, counted 4 d after irradiation, and normalized to the number of cells in unirradiated controls of the same genotype. Aliquots fixed at 24–96 h after irradiation were stained with PI for measurements of DNA content.
Spectral karyotyping, FISH and immunocytochemistry
MEFs from wild-type, p53−/−, Ku80−/− and Ku80−/−p53−/− mice were cultured in DMEM containing 10% fetal bovine serum (FBS). Suspensions of tumour cells derived from lymph node were grown in RPMI containing 10% FBS, 50 units ml−1 interleukin-7 (IL-7) (R&D Systems) and 20 units ml−1 IL-2 (Boehringer Mannheim). Tumour T1 was analysed after one passage in culture, whereas T2, T3 and T4 were primary tumours that were processed immediately for SKY, FISH and Southern blot analysis. MEFs and tumour cells were arrested at mitosis by treatment with Colcemid (Gibco/BRL) at 0.1 µg ml−1 for 0.5–3 h. Mitotic chromosome spreads were prepared by following standard procedures, and SKY analysis was performed as described12. For FISH analysis, metaphases were hybridized with BAC probes for the following regions: IgH constant region from Cγ1 to 3′ of Cα, IgH variable region representing the distal end of the V cluster (VJ588), and c-myc. Flow-sorted single chromosomes were used for painting probes. Immunostaining of centrosomes with γ-tubulin antibodies was performed as described29.
Flow cytometry
Single-cell suspensions from thymocytes, bone marrow, lymph node and tumours were stained for various lineage-specific surface markers as described3. The following monoclonal antibodies (PharMingen) were used in various two-colour combinations: phycoerythrin (PE)-conjugated anti-CD4 (clone RM4-5), FITC-conjugated anti-CD8a (clone 53-6.7), FITC-conjugated anti-T-cell antigen receptor (clone H57-597), PE-conjugated anti-B220 (clone RA3-6B2), FITC-conjugated anti-CD19 (clone 1D3), FITC-conjugated anti-IgM (clone II/41), FITC-conjugated anti-CD43 (clone S7) and PE-conjugated Thy1.2 (clone 53-2.1). At least 100,000 data points were collected on a modified immunocytometry systems FACSSTAR Plus using CellQuest software (Becton Dickinson).
Lymphocyte purification and culture
Bone marrow B cells from mutant and wild-type mice were enriched by positive selection with MACS mouse CD19 microbeads (Miltenyi Biotech). Wild-type B cells were further sorted into B220+CD43+CD25−IgM− pro-B cells. Pro-B cells were co-cultured for 3 d with irradiated S17 stromal cells and mouse rIL-7 (10 ng ml−1). Wild-type CD4−CD8− T-cell precursors were enriched by negative selection with magnetic beads coated with anti-mouse CD4 and anti-mouse CD8 (Dynal). Apoptosis and viability of B and T cells were analysed with PE-Annexin (Pharmingen) and staining with PI.
Southern blot analysis and digestion–circularization PCR
Genomic DNA (10 µg) from Ku80−/−p53−/− tumours was digested with EcoRI. Southern blots were sequentially hybridized to 32P-labelled probes for c-myc and mouse actin cDNA; a duplicate blot was prepared and sequentially hybridized with a 1.1-kb JH4 and a 900-bp Cμ genomic fragment. For digestion–circularization PCR30, 2 µg of genomic DNA was digested with EcoRI and ligated under dilute conditions that promote self-ligation30. Primers JH4F (5′-CTTCCCCAAATAGCCTTGCC-3′) and JH4R (5′-CCCACCAAACCG AAAGTCCA-3′) were used to amplify the 2.5-kb product by using the Expand High Fidelity PCR System (Boehringer). The amplified product was cloned into PCR-XL-TOPO (Invitrogen) and individual clones were isolated for sequencing. PCR primers PTK2F (5′-TGAGTTTCTTGGGTGTGGGC-3′) and PTK2R (5′-GCATAGGATTC GGCGTAGA-3′), derived from the 173-bp sequence fused to JH1, were used to screen 10 ng of DNA derived from liver and flow-sorted mouse chromosomes 2, 9, 12 and 15.
Acknowledgments
We thank A. Singer, R. Hodes, A. Bhandoola, E. Besmer and S. Sharrow for comments on the manuscript and helpful discussions; K. Huppi, B. Malynn and R. Riblet for probes; and D. Liewehr, S. Steinberg and T. Brotz for assistance. M.C.N. is an investigator of the Howard Hughes Medical Institute. A.N. was supported in part by an award from the Arthritis Foundation.
References
- 1.Kinzler KW, Vogelstein B. Gatekeepers and caretakers. Nature. 1997;386:761–763. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- 2.Vogel H, Lim DS, Karsenty G, Finegold M, Hasty P. Deletion of Ku86 causes early onset of senescence in mice. Proc. Natl Acad. Sci. USA. 1999;96:10770–10775. doi: 10.1073/pnas.96.19.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nussenzweig A, et al. Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature. 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 4.Zhu C, Bogue MA, Lim DS, Hasty P, Roth DB. Ku86-deficient mice exhibit severe combined immunodeficiency and defective processing of V(D)J recombination intermediates. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 5.Ouyang H, et al. Ku70 is required for DNA repair but not for T cell antigen receptor gene recombination in vivo. J. Exp. Med. 1997;186:921–929. doi: 10.1084/jem.186.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu Y, et al. Growth retardation and leaky SCID phenotype of Ku70-deficient mice. Immunity. 1997;7:367–376. doi: 10.1016/s1074-7613(00)80386-6. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y, et al. A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell. 1998;95:891–902. doi: 10.1016/s0092-8674(00)81714-6. [DOI] [PubMed] [Google Scholar]
- 8.Frank KM, et al. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature. 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- 9.Barnes DE, Stamp G, Rosewell I, Denzel A, Lindahl T. Targeted disruption of the gene encoding DNA ligase IV leads to lethality in embryonic mice. Curr. Biol. 1998;8:1395–1398. doi: 10.1016/s0960-9822(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 10.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 11.Jacks T, et al. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 12.Liyanage M, et al. Multicolour spectral karyotyping of mouse chromosomes. Nature Genet. 1996;14:312–315. doi: 10.1038/ng1196-312. [DOI] [PubMed] [Google Scholar]
- 13.Taub R, et al. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc. Natl Acad. Sci. USA. 1982;79:7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanasse GJ, et al. Genetic pathway to recurrent chromsome translocations in murine lymphoma involves V(D)J recombinase. J. Clin. Invest. 1999;103:1669–1675. doi: 10.1172/JCI6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y, et al. A targeted DNA-PKcs-null mutation reveals DNA-PK-independent functions for KU in V(D)J recombination. Immunity. 1998;9:367–376. doi: 10.1016/s1074-7613(00)80619-6. [DOI] [PubMed] [Google Scholar]
- 16.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 17.Karanjawala ZE, Grawunder U, Hsieh CL, Lieber MR. The nonhomologous DNA end joining pathway is important for chromosome stability in primary fibroblasts. Curr. Biol. 1999;9:1501–1504. doi: 10.1016/s0960-9822(00)80123-2. [DOI] [PubMed] [Google Scholar]
- 18.Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF. Abnormal centrosome amplification in the absence of p53. Science. 1996;271:1744–1747. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, et al. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol. Cell. 1999;3:389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal A, Eastman QM, Schatz DG. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature. 1998;394:744–751. doi: 10.1038/29457. [DOI] [PubMed] [Google Scholar]
- 21.Hiom K, Melek M, Gellert M. DNA transposition by the Rag1 and Rag2 proteins: a possible source of oncogenic translocations. Cell. 1998;94:463–470. doi: 10.1016/s0092-8674(00)81587-1. [DOI] [PubMed] [Google Scholar]
- 22.Ramsden DA, Gellert M. Ku protein stimulates DNA end joining by mammalian DNA ligases: a direct role for Ku in repair of DNA double-strand breaks. EMBO J. 1998;17:609–614. doi: 10.1093/emboj/17.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith GCM, Jackson SP. The DNA-dependent protein kinase. Genes Dev. 1999;13:916–934. doi: 10.1101/gad.13.8.916. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Umezu K, Kolodner RD. Chromosomal rearrangements occur in S. cerevisiae rfa1 mutator mutants due to mutagenic lesions processed by double-strand-break repair. Mol. Cell. 1998;2:9–22. doi: 10.1016/s1097-2765(00)80109-4. [DOI] [PubMed] [Google Scholar]
- 25.Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol. Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RD, Liu N, Jasin M. Mammalian XRCC2 promotes the repair of DNA double-strand breaks by homologous recombination. Nature. 1999;401:397–399. doi: 10.1038/43932. [DOI] [PubMed] [Google Scholar]
- 27.Timme TL, Thompson TC. Rapid allelotype analysis of p53 knockout mice. Biotechniques. 1994;17:462–463. [PubMed] [Google Scholar]
- 28.Donehower LA, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 29.Ghadimi BM, et al. Centrosome amplification and instability occurs exclusively in aneuploid, but not in diploid colorectal cancer cell lines, and correlates with numerical chromosomal aberrations. Genes Chromosomes Cancer. 2000;27:183–190. [PMC free article] [PubMed] [Google Scholar]
- 30.Chu CC, Paul WE, Max EE. Quantitation of immunoglobulin μ-γ1 heavy chain switch region recombination by a digestion–circularization polymerase chain reaction method. Proc. Natl Acad. Sci. USA. 1992;89:6978–6982. doi: 10.1073/pnas.89.15.6978. [DOI] [PMC free article] [PubMed] [Google Scholar]