Abstract
IMPORTANCE
Choroidal neovascularization (CNV) is a major cause of vision loss in chronic central serous chorioretinopathy (CSCR). Detecting CNV using fluorescein angiography (FA) may be challenging owing to the coexistence of features related to the primary diagnosis of CSCR. Optical coherence tomography angiography (OCTA) allows noninvasive visualization of retinal and choroidal vasculature via motion contrast and may contribute to the unequivocal diagnosis of CNV in this population.
OBJECTIVE
To evaluate the sensitivity of spectral-domain OCTA in detecting CNV associated with chronic CSCR.
DESIGN, SETTING, AND PARTICIPANTS
Observational cross-sectional study including 23 patients (27 eyes) who presented at the New England Eye Center between August 1, 2014, and November 30, 2014, with suspected CNV complicating chronic CSCR and underwent standard assessment for CNV diagnosis, including FA imaging. Participants were prospectively recruited to receive imaging tests using prototype OCTA software on a commercially available spectral-domain OCT. Orthogonal registration and the merging of 2 consecutive image sets were used to obtain 3 × 3-mm and 6 × 6-mm OCT angiograms centered at the macula. Two independent readers masked to other imaging findings performed a qualitative analysis on OCTA depictions of vascular flow representing CNV and the morphologic appearance of CNV.
MAIN OUTCOMES AND MEASURES
Choroidal neovascularization location as well as retinal pigment epithelial detachment internal reflectivity and the presence of subretinal and intraretinal fluid. Sensitivity and specificity of OCTA in detecting CNV were estimated using FA as the standard examination reference.
RESULTS
Choroidal neovascularization was diagnosed in 8 of 27 eyes (30%) based on FA imaging analysis. Optical coherence tomography angiography and corresponding OCT B-scans detected 100% (8 of 8) of these CNV lesions and correctly excluded 100% (19 of 19) of eyes with CSCR without CNV. Sensitivity was 100% (95% CI, 0.62–1) and specificity was 100% (95% CI, 0.82–1). Morphologic appearance, location, and position of the CNV relative to the retinal pigment epithelium and Bruch membrane were described using OCTA that combined flow and structural information.
CONCLUSIONS AND RELEVANCE
This study suggests that OCT alone (OCTA and coregistered OCT B-scans) features sensitivity and specificity comparable with FA for the detection of CNV in eyes with chronic CSCR.
Choroidal neovascularization (CNV) is a relatively uncommon sequela of chronic central serous chorioretinopathy (CSCR), with incidence estimates ranging from 2% to 9%.1–3 Moreover, CNV can be a major cause of reduced visual acuity in long-standing CSCR.2 It is well known that CNV is more common in certain eyes with CSCR, such as those who have undergone laser photocoagulation,4,5 older patients with CSCR, and eyes that feature diffuse retinal pigment epithelium (RPE) loss.3,6
Because of overlap in presentation and findings with active or chronic CSCR, the definitive diagnosis of CNV in CSCR is often challenging. Clinical features suggestive of CNV in CSCR include subretinal and/or sub-RPE hemorrhage, lipid, sub-RPE, subretinal or intraretinal fluid, subretinal hyper-reflective material on OCT, and interruptions in the RPE on cross-sectional OCT.7,8 However, some of these features are prominently noted in chronic CSCR as well, thereby accounting for possible misdiagnosis.3 Features that can be seen in both chronic CSCR with and without CNV include retinal pigment epithelial detachment (RPED), subretinal fluid, intraretinal fluid, cystoid macular degeneration,3,9 retinal atrophy,10 and diffuse irregular hyperfluorescence on fluorescein angiography (FA) or indocyanine green angiography.6,8,10 Therefore, an imaging modality that would contribute to the unequivocal diagnosis of CNV in this population would be invaluable.
Optical coherence tomography angiography (OCTA) enables distinct depth-resolved 3-dimensional visualization of the choriocapillaris and retinal microvasculature. The concept underlying OCTA is that in a static eye, the only moving structure in the fundus is blood flowing in the vessels. Optical coherence tomography angiography generates contrast in a full depth-resolved data set by differentiating between moving cells in the vasculature and static surrounding tissue without requiring dye injection. As seen in real-time OCT structural images, the amplitude of the signal returning from nonstatic features varies rapidly across time. By calculating the decorrelation of signal amplitude from repeated consecutive B-scans at the same cross-section, a contrast between static and nonstatic tissue is created and generates a vascular decor-relation signal that enables visualization of 3-dimensional retinal and choroidal vasculature.11 The use of motion contrast differentiates OCTA from FA, which requires administration of intravenous markers, such as fluorescein or indocyanine-green angiography, and confers risks ranging from discomfort and nausea to anaphylaxis in rare cases.12
Detection of CNV in CSCR commonly alters clinical management. While CSCR may be treated conservatively, the development of CNV may warrant prompt antivascular endothelial growth factor therapy. The aim of this study was to evaluate the function of OCTA in detecting CNV associated with chronic CSCR and the associated clinical features.
Methods
Consecutive patients examined at the New England Eye Center between August 1, 2014, and November 30, 2014, who had the clinical diagnosis of chronic CSCR underwent standard imaging assessment for CNV diagnosis, which encompassed FA and structural OCT, were prospectively offered enrollment in the study. Exclusion criteria included the absence of RPED on standard Cirrus HD-OCT software, version 4.5 (Carl Zeiss Meditec), spectral-domain (SD) OCT, and any associated, previous, or concomitant ophthalmological condition that could confound the interpretation of clinical and imaging findings for the diagnosis of CSCR, such as age-related macular degeneration. The study protocol was approved by the Tufts MEdical Center Institutional Review Board. The research adhered to the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act of 1996. Written informed consent was obtained before OCTA examination in accordance with the Tufts Medical Center Institutional Review Board.
At a Glance.
Optical coherence tomography angiography successfully distinguished choroidal neovascularization from the surrounding outer retinal tissue and nonflow material under the retinal pigment epithelial detachment in 8 of 8 eyes of patients with the diagnosis of choroidal neovascularization complicating chronic central serous chorioretinopathy.
Optical coherence tomography angiography provided choroidal neovascularization structural information, morphologic appearance, and location in eyes with retinal pigment epithelial detachment and chronic central serous chorioretinopathy.
Manual adjustment of the segmentation level at the outer boundary improved the delineation of choroidal neovascularization boundaries on optical coherence tomography angiography.
Chronic CSCR was defined as at least 6 months of visual acuity symptoms with documented clinical features of CSCR, including subretinal fluid and RPE changes located at the macular area on dye angiography and SD-OCT imaging. Clinical examination of both eyes and standardized imaging assessment were used to diagnose chronic CSCR and evaluate for associated CNV. Mean subfoveal choroidal thickness was measured on cross-sectional B-scans using standardized SD-OCT.
Eyes were divided into 2 groups based on clinical evaluation and FA. Group 1 eyes (neovascular) demonstrated CNV associated with chronic CSCR diagnosed clinically and by FA while group 2 eyes (non-neovascular) demonstrated chronic CSCR with focal or multiple PEDs without CNV.
Consenting patients were enrolled for imaging using a prototype Angio Vue SD-OCT OCTA software (Optovue Inc) within a commercially available Avanti SD-OCT device (Optovue Inc) that operated at 70 000 A-scans per second to acquire OCTA volumes consisting of 304 × 304 A-scans in approximately 2.6 seconds. The split-spectrum amplitude decorrelation angiography software algorithm within this device generated 3-dimensional 3 × 3-mmand6 × 6-mm OCT en face images (OCT angiograms). These were generated for each eye by orthogonal registration and the merging of 2 consecutive volumes centered at the macula. Three-dimensional OCT angiograms were coregistered with the cross-sectional OCT B-scans, allowing visualization of both retinal flow and structure in tandem. The OCTA images of eyes with an RPED secondary to chronic CSCR were evaluated for possible CNV. Three-dimensional OCT angiograms were coregistered with the cross-sectional OCT B-scans, allowing visualization of both retinal flow and structure in tandem. The OCTA images of eyes with RPED secondary to chronic CSCR were evaluated for possible CNV. The OCTA software was used to manually alter the automated segmentation and its relative depth in the retina and choroid. Two automated segmentation lines referencing the outer retina on coregistered OCT B-scans were manually fine-tuned to be located at the outer border of the outer plexiform layer (inner boundary) and the level of the Bruch membrane (outer boundary). In healthy eyes, this segmentation involving the outer retina is not expected to have any vascular structures and, thus, OCTA is not expected to identify blood flow. Therefore, CNV was defined as evidence of a decorrelation signal at the outer-retina level on OCTA consistent with the vascular component of the lesion.
Choroidal neovascularization subtypes were determined on coregistered OCT B-scans as type 1 CNV if domed elevation of the RPE layer with sub-RPE material of mixed reflectivity was present8,13 and as a mixed variant of type 1 and type 2 CNV if a hyperreflective structure anterior to an elevated disrupted RPE band was identified.13,14 The inner and outer boundaries of OCT angiograms included all apparent CNV vessels. The prototype software did not allow the segmentation curvature of the inner or outer boundaries lines to be corrected, only allowing movements up or down on the OCT B-scan using the original automated segmentation line course. An artifact removal toggle function in the software was used to subtract the retinal vessel shadowing from the en face flow image.
Two independent and trained readers from the Boston Image Reading Center (M.A.B.F. and T.E.D.C.) who were masked to other imaging findings evaluated the en face sectioning OCTA images and coregistered OCT B-scans for the following features: evidence of vascular flow above the Bruch membrane at the level of the outer retina representing CNV, CNV appearance, RPED internal reflectivity, subretinal fluid, and intraretinal fluid. The appearance of CNV on OCTA was characterized as either well-circumscribed (lacy wheel or sea fan–shaped vessels) or poorly circumscribed (long filamentous vessels). The RPED internal reflectivity was determined to be either hyperreflective or hyporeflective, with internal reflectivity that could be either heterogeneous or homogeneous. Subretinal fluid and intraretinal fluid was determined to be present or absent using the correlating OCT B-scans. The FA and OCTA examinations were evaluated to estimate the sensitivity and specificity of OCTA in detecting CNV, using FA as the reference standard. For sensitivity and specificity, 95% CIs were calculated by normal approximation to the binomial distribution.
Results
Twenty-seven eyes of 23 patients with consecutive CSCR demonstrated as chronic CSCR and associated RPED were enrolled in this study. Three patients (3 eyes) that presented with subretinal fluid but no findings of RPED via coregistered B-scans were excluded from the study. The baseline characteristics were as follows: 15 of 23 patients were male (65.2%), 21 of 23 were white (91.3%), and 2 of 23 patients were Asian (8.7%), with a mean (SD) age of 50.9 (13.9) years. Mean (SD) sub-foveal choroidal thickness measured on standard OCT B-scans was 414.9 (71.1) μm, which was thicker than the mean choroidal thickness determined by SD-OCT previously reported for healthy participants between their fourth and fifth decades of life (287 [76] μm15 and 272 [81] μm16).
Eight of 27 eyes (30%) demonstrated definite CNV on clinical examination and FA and were included as group 1 (neo-vascular chronic CSCR). In this group, 4 eyes had FA and indocyanine green angiography performed, 2 eyes had only FA, and 2 eyes received only standard SD-OCT on the same day as the OCTA but FA at 12 weeks preceding the OCTA in 1 eye and 3 weeks in the other. Dye-based angiography showed clear evidence of CNV in all group 1 eyes. Of these, 3 eyes received no treatment prior to enrollment, 1 eye was treated with a single session of full-time photodynamic therapy (PDT), 1 eye was treated with 2 sessions of full-time PDT for CSCR, 1 eye was treated with laser photocoagulation and a single session of full-time PDT for CSCR, 1 eye was treated with intravitreal anti-vascular endothelial growth factor therapy for CNV, and 1 eye received focal laser, 1 session of half-time PDT and 2 sessions of full-time PDT, and micropulse laser for CSCR and intravitreal antivascular endothelial growth factor therapy for CNV 2 months to 12 years prior to enrollment in the study.
Nineteen of 27 eyes did not demonstrate CNV on clinical examination or FA and were classified as group 2 (non-neovascular chronic CSCR). Ten eyes had both FA and indocyanine green angiography, 4 eyes had only FA, and 5 eyes had standard SD-OCT on the same day as OCTA but FA within 24 weeks preceding the OCTA. In this group, all eyes imaged with dye-based angiography showed no evidence of CNV. Fourteen eyes received no treatment previous to enrollment, 2 eyes were treated with a single session of full-time PDT, 1 eye received a single session of half-time PDT, 1 eye was treated with a single session of full-time PDT session and intravitreal anti-vascular endothelial growth factor therapy, and 1 eye received laser photocoagulation and a single session of full-time PDT for CSCR 2 months to 4 years prior to enrollment.
Two trained readers at the Boston Image Reading Center who were masked to other imaging findings determined that 8 of 8 eyes (100%) from group 1 and 0 of 19 eyes from group 2 had CNV on OCTA using the split-spectrum amplitude decor-relation angiography algorithm on the prototype OCTA software. Of all confirmed CNV cases, 4 of 8 eyes (50%) had type 1 CNV and 4 of 8 eyes (50%) had type 1 and type 2 mixed-variant CNV detected by both standard OCT and corresponding OCTA B-scans. Thus, there was complete agreement between the results obtained by OCTA and multimodal evaluation for all eyes in both groups. The specificity for the detection of CNV using OCTA was 100% (95% CI, 0.82–1) with a sensitivity of 100% (95% CI, 0.62–1). Positive and negative predictive values were 100%.
From the 8 eyes with confirmed CNV (group 1), 5 eyes had well-circumscribed vessels in the CNV area and 3 eyes showed poorly circumscribed vessels on OCTA (Figure 1). Manual displacement of the outer boundary segmentation line down to the choriocapillaris level on correlating OCT B-scans was performed (Figure 2) and allowed delineation of CNV boundaries in 6 of 8 eyes (75%) and the feeder vessel in 2 of 8 eyes (25%) with CNV (Figure 2 and Figure 3). In 2 eyes that were previously treated with 2 or more PDT sessions and presented with poorly circumscribed vessels on OCTA, manual adjustment of segmentation level to the choriocapillaris was unable to correctly delineate the boundaries of the CNV complex (Figure 4). Six of 8 eyes (75%) with CNV presented with subretinal fluid and 2 of 8 eyes (25%) presented with both intraretinal and sub-retinal fluid on corresponding OCT B-scans. All eyes with CNV presented with domed elevation of the RPE and heterogenous hyperreflective content under the RPE line.
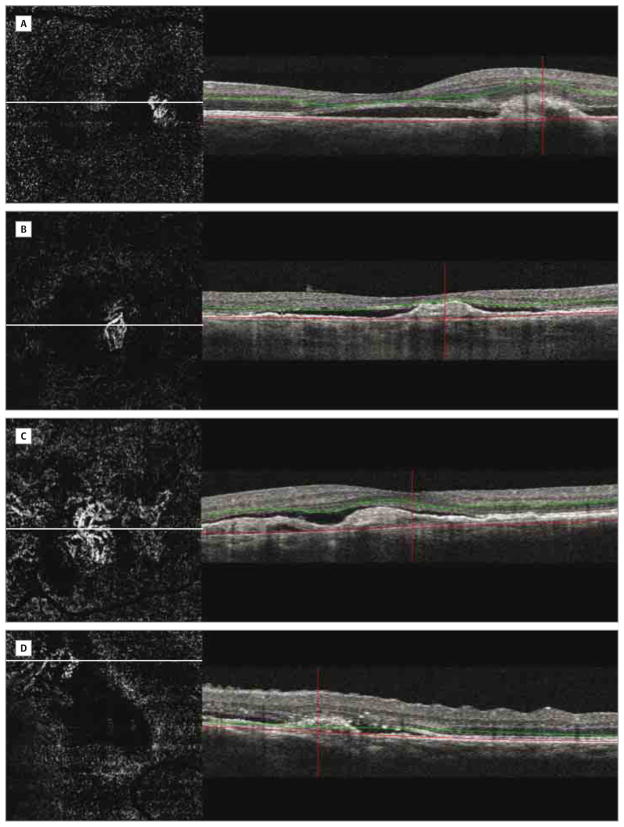
Figure 1. Choroidal Neovascularization on 3×3-mm Optical Coherence Tomography Angiography in 4 Eyes.
B-scans show segmentation boundaries representing inner (green) and outer (red) lines. Artifact removal subtracted the retinal vessel shadowing from the en face flow image and enabled visualization of choroidal neovascularization. A and B, Choroidal neovascularization shows well-circumscribed vessels. C and D, Choroidal neovascularization consists of poorly circumscribed vessels.
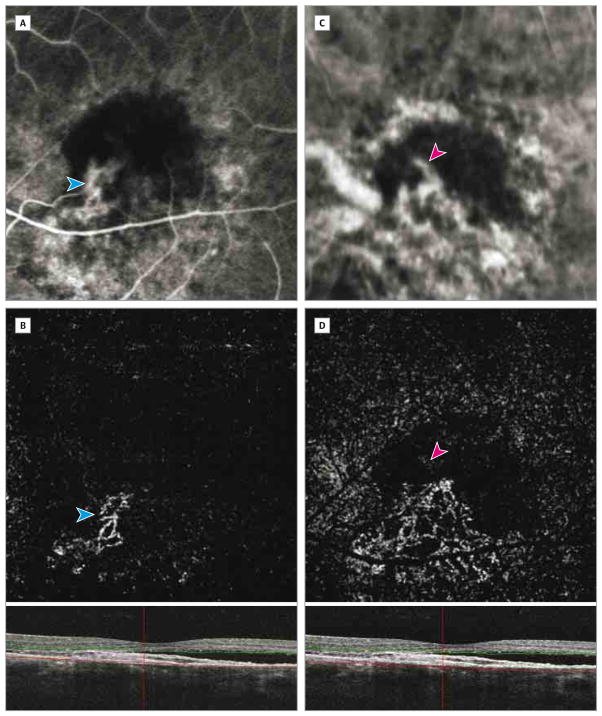
Figure 2. Choroidal Neovascularization Appearance After Manual Depth Adjustment of the Outer Boundary on a Coregistered B-Scan.
Choroidal neovascularization (blue arrowheads) partially identified on fluorescein angiography (A) and optical coherence tomography angiography (B) using automated depth segmentation. The manual adjustment of segmentation allowed for entire choroidal neovascularization visualization. The feeder vessels (pink arrowheads) are identified on indocyanine green angiography (C) and optical coherence tomography angiography (D).
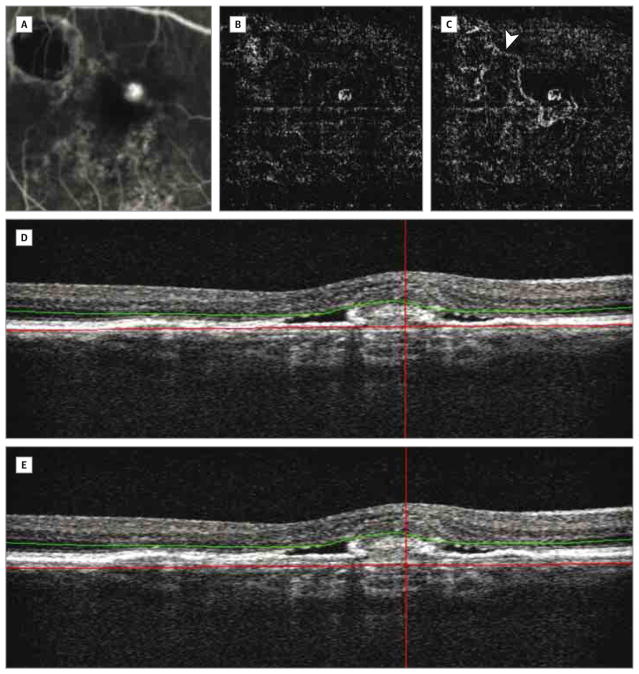
Figure 3. Feeder Vessel and Choroidal Neovascularization After Manual Adjustment of the Outer Boundary on a Coregistered B-Scan.
Fluorescein angiography (A) and an optical coherence tomography angiogram (B) show choroidal neovascularization. Optical coherence tomography angiography with manual adjustment of the outer red segmentation line shows larger choroidal neovascularization and the feeder vessel (arrowhead) originating from the scar area (C). B-scans for panels B (D) and C (E).
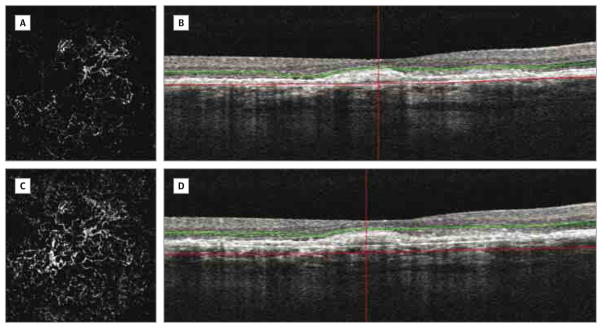
Figure 4. Inability in Delineating Margins of Choroidal Neovascularization on Optical Coherence Tomography Angiography.
Optical coherence tomography angiography of an eye previously treated with photodynamic therapy for choroidal neovascularization. A and B, Automated segmentation at the outer retinal level shows large and poorly circumscribed choroidal neovascularization. C and D, Manual adjustment of the segmentation lines was unable to accurately identify margins of the choroidal neovascularization.
All 19 eyes without CNV (group 2) had RPED identified on coregistered OCT B-scans. In this group, 15 of 19 eyes (78.9%) had subretinal fluid, 1 of 19 eyes (5.3%) had intraretinal fluid, and 3 of 19 eyes (15.8%) had no findings of subretinal or intra-retinal fluid on corresponding B-scans. Nine of 19 eyes (47.4%) had domed elevations of the RPE layer with heterogeneous and hyperreflective content and 10 of 19 eyes (52.6%) had homogeneous and hyporeflective content under the RPE line (Figure 5).
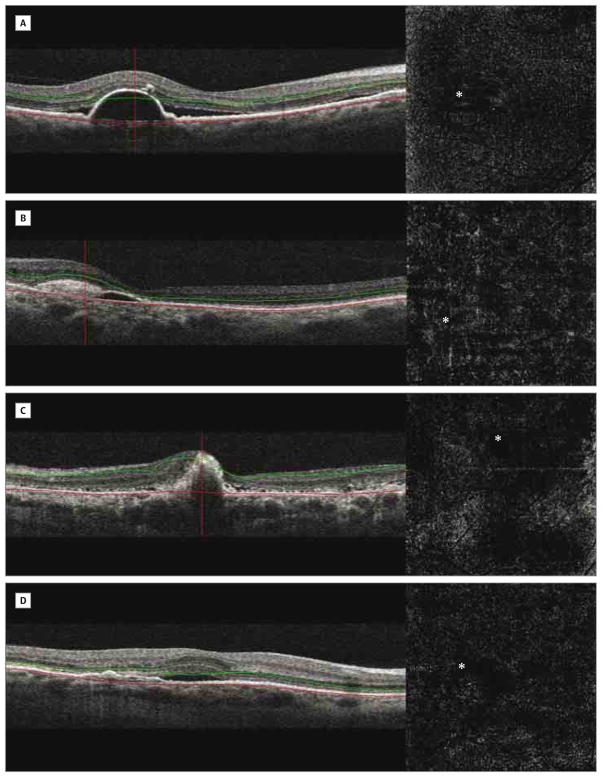
Figure 5. Eyes Without Evidence of Choroidal Neovascularization on Optical Coherence Tomography Angiography.
Absent abnormal vascular flow and the presence of well-circumscribed areas without an optical coherence tomography signal suggest shadowing artifacts in the presence of retinal pigment epithelial detachment. Coregistered B-scans show different retinal pigment epithelial detachment reflectivities. A, Retinal pigment epithelial detachment with homogeneous hyporeflectivity. B–D, Retinal pigment epithelial detachment with heterogenous hyperreflectivity. Asterisks indicate the locations of pigment epithelial detachment on the respective optical coherence tomography angiograms.
Discussion
Dye-based angiography is the current standard to diagnose CNV.17 However, detecting secondary CNV in patients with CSCR using these imaging modalities may be challenging owing to the coexistence of clinical, OCT, and angiographic findings related to the primary diagnosis of CSCR, including subretinal fluid, intraretinal cystic changes, diffuse RPE loss resulting in widespread window defects on FA, scattered points of RPE leakage, RPEDs, and multifocal patchy hyperfluorescence of the inner choroid seen on indocyanine green angiography.3,6
Ferrara et al18 reported OCT findings in 2 eyes with CNV secondary to CSCR using structural en face swept-source OCT images. They reported focal and diffuse vascular dilation at the level of the choriocapillaris. However, corresponding multimodal analysis and 3-dimensional OCT visualization suggested that some of the features may be related to pronounced choroidal vascular dilatation or remodeling or extracellular fluid accumulation associated with CSCR rather than specific to CNV.
Yamamoto19 measured blood flow velocity in feeder vessels and demonstrated that blood flow resistance increases and blood flow velocity decreases in CNV microcirculation, particularly in smaller CNVs. Jia et al11 obtained quantitative information regarding CNV in patients with age-related macular degeneration, showing CNV flow and area in 5 eyes using a swept-source OCT angiography system with a split-spectrum amplitude decorrelation angiography algorithm and detected a higher flow index of CNV in eyes with larger CNVs, those that were type 2 compared with type 1, and combined CNVs. Spectral-domain OCT has lower imaging speeds compared with swept-source OCT, with a higher sensitivity roll-off with imaging depth. However, in our study, limitations of SD-OCT technology did not affect successful visualization of blood flow inside the CNV network in all eyes, regardless of the type and size of the CNV, as OCTA successfully distinguished CNV from the surrounding outer retinal tissue and nonflow material with RPED.
We identified CNV and determined the position of the CNV relative to the RPE and Bruch membrane by using OCTA that combined flow (OCT angiograms) and structural information (coregistered OCT B-scans). This supports previous findings using the split-spectrum amplitude decorrelation angiography OCTA technique in evaluating CNV and monitoring morphologic features for treatment and follow-up.11 Furthermore, because the OCTA image is depth-resolved, manual adjustment of the segmentation level at the outer boundary of the outer retina slightly posterior to the Bruch membrane allowed tomographic visualization of feeder vessels between the choroid and the CNV in 2 eyes, which is rarely possible to identify using angiography.
Except for 2 eyes that were previously treated with PDT sessions, delineation of the CNV boundaries was achieved by performing a manual adjustment of the outer segmentation line. Long-term effects of PDT, such as choroidal vascular remodeling, RPE disturbance, enlargement of CNV membranes, recanalization of occluded vessels, and choriocapillary occlusion,20–22 may limit accurate determination of CNV size in such eyes. However, it did not affect the identification of CNV in this study.
This study was limited by a relatively small number of patients and the inability to manually correct automatically detected segmentation curves, which limited the suppression of choriocapillaris artifact in the en face sectioning of the outer retina. However, high sensitivity and specificity of detection of CNV in this small case series was achieved. There were several reasons for high sensitivity and specificity. Because CNV in CSCR is rarely associated with the massive subretinal hemorrhage that limits penetration of OCT signal, CSCR-associated CNV may be particularly suited to being identified on OCTA. In a similar case series performed on a mixed group of patients with CNV that included CNV secondary to wet age-related macular degeneration, the sensitivity of OCTA in detecting CNV was much lower, at approximately 50% (T. E. de Carlo, BS, unpublished data, 2015). Moreover, readers evaluating OCTA in this study were highly trained and performed a dynamic evaluation of OCTA volume, altering the segmentation lines when needed. Such an evaluation maximizes the sensitivity and specificity of detection of CNV on OCTA because accurate automated segmentation is often difficult in eyes with disrupted outer retinal anatomy. The large interval found between lower and upper limits along with 95% CIs for positive and negative predictive rates, especially for sensitivity, indicate that these results must be interpreted with caution.
Conclusions
We demonstrated an association between noninvasive OCTA and dye-based angiography in detecting CNV associated with chronic CSCR. This study suggests that OCTA alone (OCTA and coregistered OCT B-scans) features sensitivity and specificity comparable with FA imaging for the detection of CNV in eyes with chronic CSCR and may be a viable alternative to dye-based angiography in the diagnosis of CNV in these patients. Because OCTA is a completely noninvasive test, it could be considered a first step in identifying CNV in CSCR. Future studies with larger sample sizes are needed to improve our understanding of this diagnostic method and provide further information to validate this imaging technique in clinical practice.
Acknowledgments
Funding/Support: This work was supported in part by an unrestricted grant from Research to Prevent Blindness received by the New England Eye Center/Department of Ophthalmology, Tufts University School of Medicine; grants R01-EY11289, R01-EY013178, and R01-CA075289-16 from the National Institutes of Health; and the Massachusetts Lions Club.
Footnotes
Author Contributions: Dr Waheed had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bonini Filho, Ferrara, Adhi, Witkin, Duker, Waheed.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Bonini Filho, Baumal, Witkin, Reichel.
Critical revision of the manuscript for important intellectual content: Bonini Filho, de Carlo, Ferrara, Adhi, Baumal, Witkin, Duker, Waheed.
Obtained funding: Duker, Waheed.
Administrative, technical, or material support: de Carlo, Witkin, Duker, Waheed.
Study supervision: Ferrara, Baumal, Witkin, Reichel, Duker, Waheed.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Duker is a consultant for and receives research support from Carl Zeiss Meditech Inc and OptoVue Inc. Dr Ferrara is an employee of Genentech Inc. No other disclosures were reported.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Gomolin JE. Choroidal neovascularization and central serous chorioretinopathy. Can J Ophthalmol. 1989;24(1):20–23. [PubMed] [Google Scholar]
- 2.Loo RH, Scott IU, Flynn HW, Jr, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002;22(1):19–24. doi: 10.1097/00006982-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103(12):2070–2079. doi: 10.1016/s0161-6420(96)30386-2. [DOI] [PubMed] [Google Scholar]
- 4.Gass JD. Photocoagulation treatment of idiopathic central serous choroidopathy. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83(3 pt 1):456–467. [PubMed] [Google Scholar]
- 5.Matsunaga H, Nangoh K, Uyama M, Nanbu H, Fujiseki Y, Takahashi K. Occurrence of choroidal neovascularization following photocoagulation treatment for central serous retinopathy [in Japanese] Nihon Ganka Gakkai Zasshi. 1995;99(4):460–468. [PubMed] [Google Scholar]
- 6.Spaide RF, Hall L, Haas A, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996;16(3):203–213. doi: 10.1097/00006982-199616030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984;91(12):1554–1572. doi: 10.1016/s0161-6420(84)34117-3. [DOI] [PubMed] [Google Scholar]
- 8.Fung AT, Yannuzzi LA, Freund KB. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina. 2012;32(9):1829–1837. doi: 10.1097/IAE.0b013e3182680a66. [DOI] [PubMed] [Google Scholar]
- 9.Iida T, Yannuzzi LA, Spaide RF, Borodoker N, Carvalho CA, Negrao S. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003;23(1):1–7. doi: 10.1097/00006982-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Lafaut BA, Salati C, Priem H, De Laey JJ. Indocyanine green angiography is of value for the diagnosis of chronic central serous chorioretinopathy in elderly patients: Graefe’s archive for clinical and experimental ophthalmology. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1998;236(7):513–521. doi: 10.1007/s004170050114. [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, Bailey ST, Wilson DJ, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014;121(7):1435–1444. doi: 10.1016/j.ophtha.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su Z, Ye P, Teng Y, Zhang L, Shu X. Adverse reaction in patients with drug allergy history after simultaneous intravenous fundus fluorescein angiography and indocyanine green angiography. J Ocul Pharmacol Ther. 2012;28(4):410–413. doi: 10.1089/jop.2011.0221. [DOI] [PubMed] [Google Scholar]
- 13.Jung JJ, Chen CY, Mrejen S, et al. The incidence of neovascular subtypes in newly diagnosed neovascular age-related macular degeneration. Am J Ophthalmol. 2014;158(4):769–779.e2. e762. doi: 10.1016/j.ajo.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Sulzbacher F, Kiss C, Munk M, Deak G, Sacu S, Schmidt-Erfurth U. Diagnostic evaluation of type 2 (classic) choroidal neovascularization: optical coherence tomography, indocyanine green angiography, and fluorescein angiography. Am J Ophthalmol. 2011;152(5):799–806.e1. e791. doi: 10.1016/j.ajo.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147(5):811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150(3):325–329.e1. e321. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Do DV. Detection of new-onset choroidal neovascularization. Curr Opin Ophthalmol. 2013;24(3):244–247. doi: 10.1097/ICU.0b013e32835fd7dd. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara D, Mohler KJ, Waheed N, et al. En face enhanced-depth swept-source optical coherence tomography features of chronic central serous chorioretinopathy. Ophthalmology. 2014;121(3):719–726. doi: 10.1016/j.ophtha.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto Y. Measurement of blood flow velocity in feeder vessels of choroidal neovascularization by a scanning laser ophthalmoscope and image analysis system. Jpn J Ophthalmol. 2003;47(1):53–58. doi: 10.1016/s0021-5155(02)00632-9. [DOI] [PubMed] [Google Scholar]
- 20.Schnurrbusch UE, Welt K, Horn LC, Wiedemann P, Wolf S. Histological findings of surgically excised choroidal neovascular membranes after photodynamic therapy. Br J Ophthalmol. 2001;85(9):1086–1091. doi: 10.1136/bjo.85.9.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dewi NA, Yuzawa M, Tochigi K, Kawamura A, Mori R. Effects of photodynamic therapy on the choriocapillaris and retinal pigment epithelium in the irradiated area. Jpn J Ophthalmol. 2008;52(4):277–281. doi: 10.1007/s10384-008-0551-9. [DOI] [PubMed] [Google Scholar]
- 22.Chan WM, Lam DS, Lai TY, Tam BS, Liu DT, Chan CK. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003;87(12):1453–1458. doi: 10.1136/bjo.87.12.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]