Abstract
There is growing promise in using engineered cells as therapeutic agents. For example, synthetic Chimeric Antigen Receptors (CARs) can redirect T cells to recognize and eliminate tumor cells expressing specific antigens. Despite promising clinical results, excessive activity and poor control over such engineered T cells can cause severe toxicities. We present the design of “ON-switch” CARs that enable small molecule-control over T cell therapeutic functions, while still retaining antigen specificity. In these split receptors, antigen binding and intracellular signaling components only assemble in the presence of a heterodimerizing small molecule. This titratable pharmacologic regulation could allow physicians to precisely control the timing, location, and dosage of T cell activity, thereby mitigating toxicity. This work illustrates the potential of combining cellular engineering with orthogonal chemical tools to yield safer therapeutic cells that tightly integrate both cell autonomous recognition and user control.
Introduction
Cell-based therapies have emerged as promising treatments for a range of disorders including cancer, autoimmunity, and injury or degeneration (1–6). In contrast to small molecules and macromolecules, cellular therapeutic agents have the potential to sense inputs, make decisions, and execute highly complex tasks (7–9). A recent example is the use of engineered T cells for adoptive immunotherapy of cancer. Primary T cells can be isolated and engineered to express synthetic Chimeric Antigen Receptors (CARs) – receptors that combine an extracellular, single chain antibody domain that recognizes a specific tumor associated antigen, with intracellular signaling domains from the T cell receptor and co-stimulatory receptors (2–4, 10). In clinical trials, CAR T cells directed against the B cell antigen CD19 have proven effective against chemotherapy resistant forms of B cell cancers (11–15). Upon antigen ligand engagement, CAR T cells execute multiple key therapeutic functions, including production of anti-tumor cytokines and killing of target tumor cells (Fig. 1A). Antigen binding also stimulates exponential proliferation of the therapeutic T cells in vivo. Infused CAR T cells can expand > 1000-fold, resulting in a highly amplified response, and eradication of a large number of tumor cells within weeks (11).
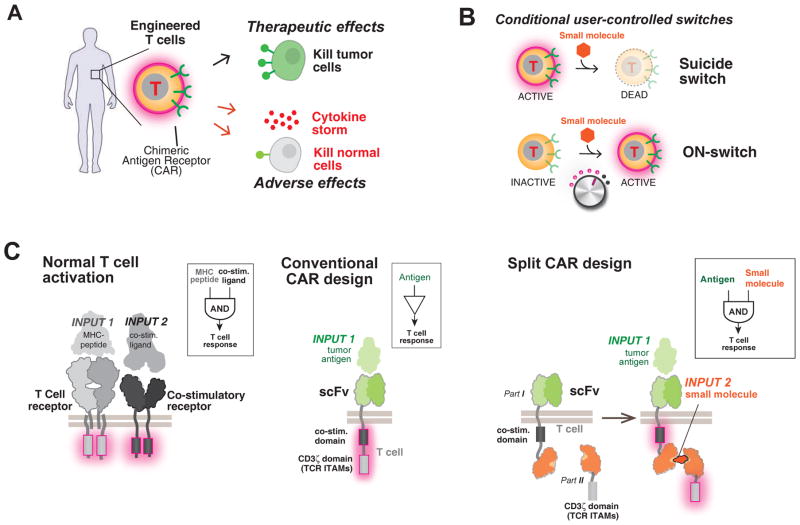
Fig. 1. Strategy for design of a combinatorially activated chimeric antigen receptor (CAR) with re-arranged key signaling modules.
(A) Engineered T cells expressing CARs can have both therapeutic and adverse effects.
(B) User-controlled switches. A suicide switch triggers apoptosis of the engineered cells. An alternative, complementary approach is keeping the cells inactive until addition of an activating small molecule drug signal. Such an ON-switch could allow for titratable control (dialing up or down) of T cell activity.
(C) Molecular strategies to control T cell activation. The normal T cell activation pathway (left) entails dual activation of the T Cell Receptor (TCR) and a co-stimulatory receptor to trigger key cellular responses such as cytokine production and proliferation. The conventional CAR (middle) combines an antigen recognition domain (single chain variable fragment; scFv) with main signaling motifs (such as ITAMs from TCR subunit CD3zeta) and co-stimulatory motifs constitutively linked in a single molecule. A strategy for constructing an ON-switch CAR utilizing a split construct (right). The split CAR design distributes key components from the conventional CAR into two physically separate polypeptides that can be conditionally re-assembled when a heterodimerizing small molecule agent is present. The design resembles an AND logic gate that requires “antigen + small molecule” combinatorial inputs for T cell activation.
Such cell-based therapies, however, can also be associated with severe toxicities (Fig. 1A). Off-tumor cross-reaction of engineered T cells can lead to killing of non-tumor cells. If such cross-reaction occurs to cells in the heart, lung or liver, then the high doses of CAR T cells that these tissues are exposed to upon initial cell injection can lead to rapid death (16). Even with successful tumor targeting, the rapid rise in the overall T cell activity fueled by CAR signaling during treatment can also lead to systemic life-threatening side effects such as those caused by release of excessive cytokines. Rapid elimination of large numbers of tumor cells in a short time frame also can result in tumor lysis syndrome. Both conditions can trigger multi-organ failure and require urgent medical intervention. Patient-to-patient variations in T cell responses and in risks for toxicities make it challenging to predict the optimal number of T cells to infuse (2). Thus the engineering of regulatory systems that allow for control over the dose and timing of T cell function is an important priority.
One approach is to engineer suicide switches to eliminate the infused T cells if their toxic effects begin to get out of control (17, 18). Examples include a small molecule-regulated caspase that triggers apoptosis of the T cells (Fig. 1B). Another approach is to engineer negative regulatory co-receptors that can override killing responses when a specific “do not kill” ligand is recognized (19). Although these strategies are important elements in the toolbox for engineering therapeutic T cells, they have several drawbacks. The suicide switches irreversibly abort the complex and expensive treatment, and may not act fast enough to prevent cross-reaction during initial cell transfer. Overriding inhibitory co-receptors can prevent killing of particular cells that express a specific ligand, but they cannot control the timing and intensity of T cell activity more generally.
We therefore aimed to develop a complementary strategy for controlling CAR T cells that focused instead on positive regulation in which an exogenous, user-provided signal such as a small molecule is required for activation (Fig. 1B). This kind of “ON-switch” would complement the other classes of control systems and provide important advantages. Positive regulation could allow for gradual titration of activity to appropriate therapeutic levels, as well as control of the timing of activation, thus preventing first-pass toxicities that could occur immediately upon cell transplantation. Coupled to technologies for localized and sustained delivery of small molecules (20), an ON-switch could impart spatial control over the therapeutic effects to mitigate off-tumor toxicities. Several features would be important for an ON-switch CAR design. First, the receptor would still need to be dependent on specific tumor antigen recognition for T cell activation – small molecule alone or antigen alone should not activate. Second, therapeutic activity of the T cell population should be titratable by varying concentration of the small molecule, and at high enough levels, should show an activity comparable to that of conventional CAR T cells. Finally, the timing of CAR T cell response should be reversibly controllable by addition or removal of the small molecule.
The engineering goal for an ON-switch CAR embodies a general problem that evolution has repeatedly faced – how to convert a signaling molecule that is gated by a single input into a molecule that is combinatorially gated by two inputs (in this case, a receptor that requires both antigen and small molecule for T cell activation). Natural signaling systems often achieve tight control over critical processes through this type of combinatorial regulation. The general ability to engineer synthetic receptors that function as Boolean AND-gates responding to two inputs – one an autonomously recognized disease signal (i.e. tumor antigen) and the other a user-controlled signal (i.e. small molecule) – would be broadly useful for engineering any kind of safe, cell therapeutic agents. Thus engineering this type of ON-switch CAR has important implications for fundamental questions of the evolvability of multi-input signaling molecules, and addresses a general problem in engineered therapeutic cells.
One can imagine highly complex regulatory mechanisms involving precise conformational allostery that could yield a signaling receptor that functions as a Boolean AND gate. However, very often, controls that have evolved in living systems use simpler strategies such as controlled assembly: an active molecular system can be split into multiple parts, such that it is dependent on multiple inputs that promote assembly of the intact molecular system (21). Thus we focused on constructing split synthetic receptor systems in which the assembly of an activated complex was dependent both on binding of a small molecule and antigen engagement (Fig. 1C). The concept of splitting key components from the CAR is itself inspired by the natural process of T cell activation, which normally requires the co-engagement of the T cell receptor (by peptide-MHC) and co-stimulatory receptors (e.g. 4-1BB or CD28) (Fig. 1C). The conventional CAR represents a construct in which components from the TCR and co-stimulatory receptors are artificially co-localized. Thus our strategy represents re-splitting key signaling modules in a different, now small molecule-controlled configuration.
We used this strategy to engineer a robust ON-switch CAR design that fulfills the criteria of yielding titratable, reversible and temporarily controllable activity in CAR T cell populations. This work provides a general strategy for how to engineer dual-input synthetic receptors that require a small molecule as a co-activation signal. The ON-switch CAR framework also provides an important tool for developing the next generation of precision-controlled therapeutic T cells.
RESULTS
Design of ON-switch CARs
To construct a CAR that required both an antigen AND a small molecule for activation, we used a split-receptor design that structurally resembles natural immune receptors such as the B and T cell receptors (BCRs and TCRs), whose antigen binding and intracellular signaling domains are found on separately expressed polypeptides. In these natural cases, heterodimerization of these distinct polypeptides is required to assemble a functioning receptor complex (22). This type of multi-polypeptide assembly presents the opportunity to engineer receptor control by enforcing small-molecule dependence on this assembly. The envisioned ON-switch CAR (Fig. 1C) consists of two parts that assemble in a small molecule-dependent manner. Part I of the receptor features an extracellular antigen binding domain (scFv; single-chain variable fragment). Part II has a key downstream signaling element, the immunoreceptor tyrosine-based activation motifs (ITAMs) from the T cell receptor CD3ζ subunit (22). The ITAM motifs are phosphorylated upon T cell receptor activation, resulting in the recruitment of SH2 domain effectors such as the kinase ZAP70 and triggering the cascade of T cell activation. The two parts of the split receptor contain heterodimerization domains that conditionally interact upon binding of a heterodimerizing small molecule. Particular cellular responses, including T cell activation, have been engineered to be triggered solely by small molecule-induced dimerization (23, 24). Our goal, in contrast, was to design a new class of CAR whose small molecule-induced assembly is necessary but not sufficient for cellular activation. The small molecule thus acts as a priming or licensing factor that is a precondition for antigen-triggered activation.
We explored multiple ways of splitting components of the conventional CAR molecule to find a configuration that would strongly impair its activity but still allow for strong antigen-induced signaling when the receptor components were assembled in the presence of the small molecule (Fig. 2A). To facilitate the design of a prototype, we used a set of structurally well-defined heterodimerizing components: the FK506 Binding Protein (FKBP) domain and the T2089L mutant of FKBP-rapamycin binding domain (FRB*) that heterodimerize in the presence of the rapamycin analog AP21967, which has less immunosuppressive activity than does rapamycin (25–27). We refer to this modified rapamycin as the rapalog. We screened candidate receptors for rapalog-dependent activation in the human CD4+ Jurkat T cell line with two assays. We assayed the activity of a synthetic promoter composed of multiple copies of Nuclear Factor of Activated T-cells (NFAT) response elements (28), a highly sensitive readout for T cell receptor activation. We also measured Interleukin-2 (IL-2) cytokine secretion, which represents a more stringent, integrated cellular response. The simplest split ON-switch design examined, constructs I.a + II.a (Fig. 2A), comprised a cytoplasmic ITAM fragment that could be recruited to an antigen-binding membrane receptor component upon addition of rapalog. This initial receptor design failed to signal strongly in either assay despite abundant expression of both receptor parts in Jurkat cells (Fig. S1–S3).
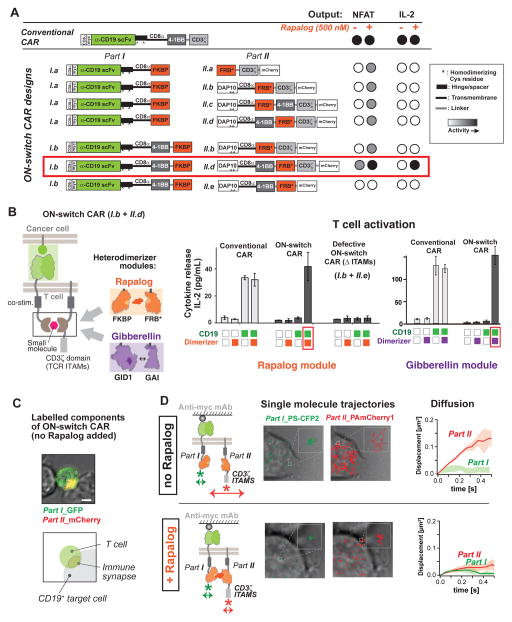
Fig. 2. Construction and screening of ON-switch chimeric antigen receptor (CAR) that is dependent on presence of small molecule dimerizer.
(A) ON-switch CAR candidate constructs and their functional behavior. Candidate construct pairs were expressed in Jurkat T cells. Cells were incubated with K562 target cells expressing the cognate antigen CD19+ in the presence or absence of 500 nM rapalog. Activation was quantified via expression of an NFAT-dependent GFP reporter gene and production of the cytokine IL-2. The part I constructs of the ON-switch CAR share many features with the conventional CAR: the CD8α signal sequence, a Myc epitope, the anti-CD19 scFv, the CD8α hinge and transmembrane domain, in addition to the FKBP domain for heterodimerization. The part II constructs consisted of the T cell receptor CD3ζ signaling chain that is critical for T cell activation, the FRB* domain for heterodimerization, and the mCherry tag. More advanced part II variants contained the additional DAP10 ectodomain for homodimerization and the CD8α transmembrane domain for membrane anchoring. The 4-1BB costimulatory motif was inserted in various locations, depending on the construct. The best ON-switch construct (I.b + II.d) is outlined in red.
(B) Response of ON-switch CAR (I.b + II.d) to rapalog and antigen stimulation. Jurkat cells expressing the specified CARs were incubated with K562 target cells expressing either the cognate antigen (CD19; green squares) or a non-cognate antigen (mesothelin; white squares). Presence of 500 nM rapalog in the sample was indicated by orange squares. Production of IL-2 after an over-night incubation was quantified by ELISA. n = 3, error bar = standard deviation. Similar results were observed for ON-switch CARs in which the rapalog heterodimerization module was replaced by an alternative module, the gibberellic acid heterodimerization module (utilizing Arabidopsis GID1 and GAI domains). The ON-switch CAR with gibberellic acid (GA) dimerizing domains requires both cognate antigen and GA (purple squares) to trigger cytokine production.
(C) The ON-switch CAR components co-localize in the absence of dimerizing rapalog. Parts I and II of the receptor are labeled with GFP and mCherry, respectively. The confocal microscopy images are pseudo-colored to indicate localization of both parts. Image shows a primary human CD8+ T cell expressing the anti-CD19 ON-switch CAR engaged with a CD19+ K562 target cell in the absence of rapalog. Scale bar = 5μm.
(D) Two-color single-molecule tracking shows independent movement of ON-switch CAR components in the absence of rapalog. Left panels: Jurkat T cells were adhered to a cover slip coated with an antibody to the Myc epitope in order to immobilize the receptors (extracellular region of part I CAR is tagged with Myc). Individual parts of the ON-switch CAR were each tagged with photoactivatable fluorescent proteins PS-CFP2 and PAmCherry1. Middle panels: The single molecule trajectories of part I (green) and part II (red) are superimposed on transmitted light images of the cells (gray). Right panels: The average mean-square displacement of trajectories quantifies the diffusive behavior (Solid lines: average from multiple cells. Colored band: standard deviation to represent cell-cell variability). Part I molecules are immobile due to antibody tethering, whereas in the absence of rapalog (top) part II molecules exhibit fast diffusion. In the presence of 500 nM rapalog (bottom), however, the part II molecules became immobile, confirming rapalog-induced assembly of the two-component receptor.
We suspected that the entropic cost for driving a cytoplasmic fragment to bind its membrane-associated partner might be too high for this small molecule-induced interaction. Thus we explored localization of both receptor parts at the plasma membrane. We designed constructs that targeted both fragments to the plasma membrane, but varied the domain composition and order within both parts of the CAR (Fig. 2A). We targeted part II to the plasma membrane by appending the same CD8α transmembrane domain used in part I. We also appended the ectodomain of DNAX-activating protein 10 (DAP10) to part II. The DAP10 ectodomain mediates homo-dimerization (29), effectively doubling the copy number of ITAMs per part II molecule. This modification was expected to increase receptor output activity, as the copy number of ITAMs correlates positively with receptor signaling strength (30, 31). We also varied the positioning of the 4-1BB co-stimulatory domain, which promotes T cell proliferation and survival, in both parts I and II. Previous work suggests that the 4-1BB co-stimulatory domain functions best when placed adjacent to the plasma membrane (32). We also varied the position of the FKBP and FRB* heterodimerizing domains. These changes led to the design of constructs I.b, II.b, II.c, and II.d (Fig. 2A). Molecule II.e, which lacks the CD3ζ sequence, was constructed as a corresponding negative control (defective ON-switch CAR).
Identification of a dual input gated ON-switch CAR construct
The most promising design from this set of components comprised the components I.b and II.d (Fig. 2A). When stimulated by target cells expressing the cognate antigen (CD19) in the presence of 500 nM rapalog, this combination led to strong cytokine production, comparable to that stimulated by the conventional single component CAR. The response was highly small molecule-dependent. This was the only split receptor design within this subset that signaled as strongly as the conventional CAR (Fig. S3). Further evaluation confirmed that neither the CD19 cognate antigen nor the small molecule alone was sufficient to trigger IL-2 production (Fig. 2B). These results in the Jurkat T cell line indicate the successful construction of an AND-gate receptor requiring dual inputs.
ON-switch CAR architecture is compatible with alternative heterodimerization modules and alternative antigen binding domains
We tested whether this ON-switch CAR architecture would show similar dual-input regulation with different small molecule heterodimerization domains, or different antigen recognition domains. We used the gibberellin-induced dimerization system (GID1-GAI) (Fig. 2B) (33). Derived from plants and structurally unrelated to FKBP-FRB*(27, 34), this alternative small molecule input system yielded an ON-switch CAR population that was inactive in the absence of gibberellin, but became activated only when stimulated by both antigen (CD19) and gibberellin. This alternative system worked equally well to gate T cell activation (Fig. 2B). Thus the ON-switch CAR design appears to work with alternative heterodimerization systems.
To test whether the design would function with alternative antigen binding domains, we reconstructed the system with a single chain antibody that recognizes the antigen mesothelin, instead of the antigen CD19. Jurkat T cells expressing this version of the receptor maintained dual input control, requiring both stimulation with mesothelin-expressing target cells and addition of rapalog (Fig. S4).
Single molecule imaging shows that two components of the ON-switch CAR only assemble in the presence of small molecule dimerizer
The ON-switch CAR was designed to conditionally assemble only in the presence of rapalog. To determine the localization of the two parts and how this changed with the addition of rapalog, we labeled part I with Green Fluorescent Protein (GFP) and part II with mCherry. When we mixed the ON-switch CAR T cells with cognate antigen-expressing cells, in the absence of the rapalog, the T cells formed stable cell-cell junctions (immune synapses) with the target cells. Both part I and II of the ON-switch CAR localized at the synapse, even in the absence of rapalog. Fluorescence microscopy of these components revealed overlapping localization (Fig. 2C).
To better understand how the interaction of the two molecular parts changes with addition of rapalog, we performed super-resolution, single molecule imaging using photoactivated localization microscopy (PALM) (35–37). This type of imaging can yield critical information about the location and dynamics of the individual molecules. We expressed the ON-switch CAR in Jurkat cells, with each component labeled with a distinct photoactivatable fluorescent protein [part I was labeled with photoswitchable cyan fluorescent protein 2 (PS-CFP2), and part II labeled with photoactivatable mCherry1 (PAmCherry1)]. Cells were placed on glass slides coated with an antibody that binds to a Myc epitope tag placed at the N-terminus (extracellular region) of part I of the receptor (Fig. 2D, left panel). Interaction with the antibody effectively immobilized the cells and anchored the part I molecules to the slide surface (Fig. S5). The distribution of individual part I and II molecules at the slide interface was then traced over time by tracking the photoactivatable fluorescent protein tags.
We observed small, constrained dynamic trajectories for part I molecules, confirming the immobility of this component upon antibody tethering. In contrast, the large, unconstrained dynamic trajectories of the part II molecules indicated fast diffusion of this component, in the absence of rapalog (Fig. 2D, top panel, Movie S1). The measured average diffusion coefficient of about 0.1 μm2/s, observed for the part II molecules, is well within the range expected for unconstrained transmembrane proteins (38). The major change that we observed upon addition of rapalog was that the part II molecules also became immobile - their trajectories decreased significantly to match those of the antibody tethered part I molecules (Fig. 2D, bottom panel, Movie S2).
Thus although both components of the ON-switch CAR appeared to be co-localized at a macroscopic level in the absence of dimerizing small molecule, at the single molecule level the two components were not appreciably physically associated. Without the dimerizing small molecule, the part II molecules diffused within the membrane in an unconstrained manner, even though the part I molecules were firmly anchored to an extracellular antibody. However, upon addition of the small molecule, the diffusion of part II became equally constrained, consistent with a model in which the two parts only tightly associate in the presence of the dimerizing small molecule. These findings are consistent with a model in which a critical part of the switch design is the localization of both parts of the receptor to the plasma membrane. The interaction mediated by small molecule addition is sufficient to drive a large change in the molecular association of the two parts.
ON-switch CAR requires small molecule AND antigen to activate primary human CD4+ T cells
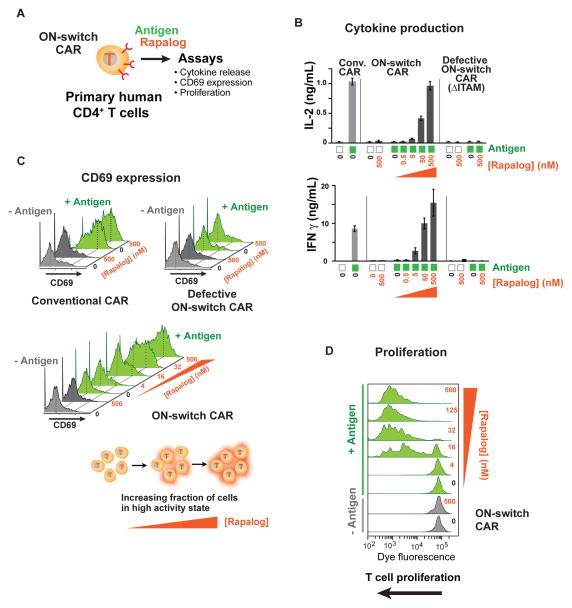
We tested the effectiveness of this ON-switch CAR (I.b + II.d; the rapalog controlled version) in therapeutically relevant T cells. We expressed the components in primary human CD4+ helper T cells and examined multiple T cell responses (Fig. 3A). Expression of the ON-switch CAR was similar to that of the conventional CAR in primary T cells (Fig. S6). CAR T cells were incubated with antigen-expressing target cells in varying concentrations of rapalog. All of the tested CD4+ T cell responses showed the dual requirement for stimulation with cognate antigen (CD19+ target cells) and small molecule dimerizer. These responses included Interleukin-2 (IL-2) production (a general readout for effector T cell activation) and Interferon-γ (IFN-γ) production (an indicator for Th1 anti-tumor response). Production of IL-2 and IFN-γ was minimal when cells were stimulated only by either the small molecule dimerizer or cognate antigen individually. Dual-stimulation with the cognate antigen and increasing amounts of small molecule led to a dose-dependent increase in cytokine secretion by the ON-switch CAR T cells. Notably, cytokine production at high concentrations of dimerizing molecule rivaled that obtained with T cells expressing the conventional CAR (Fig. 3B).
Fig. 3. Small molecule-titratable activation of primary human helper T cell populations engineered with ON-switch CAR.
(A) CD4+ T cells were purified from the peripheral blood of anonymous healthy donors, expanded, engineered with lentivirus to express CARs, and evaluated by functional assays. T cells with comparable CAR expression levels were used. The cognate antigen CD19 was presented to T cells as a cell surface protein on K562 target cells. Various concentrations of the dimerizer rapalog were added to reaction mixtures to examine effects of rapalog titration.
(B) Production of the cytokines IL-2 and IFN-γ quantified by ELISA after an overnight incubation, as described in methods. n = 3, error bar = standard deviation.
(C) Monitoring T cell activation in single cells by quantifying expression of the cell surface protein CD69, whose up-regulation occurs early during T cell activation. T cells in overnight assay mixtures were stained with a fluorophore-conjugated anti-CD69 antibody and analyzed by flow cytometry. Green histograms denote T cells stimulated with CD19+ target cells (+ antigen). Grey peaks denote T cells treated with target cells lacking the CD19 antigen (− antigen). T cell population shows bimodal response, and addition of rapalog increases the fraction of cells in the high response population.
(D) Dimerizer small molecule and antigen dependent T cell proliferation. T cells expressing the ON-switch CAR were pre-labeled with the intracellular dye CellTrace Violet, whose fluorescence intensity per cell progressively decreases with increasing rounds of cell division. Cells were processed in a flow cytometer after 5 days of incubation. Leftward shift of peaks in the histogram indicates T cell proliferation.
Similarly, dual-gated and titratable responses were seen for expression of the induced cell surface marker, CD69. Increased cell surface expression of the CD69 protein occurs within hours after T cell activation and provides a standard method to monitor the activation status of T cells at the single-cell level by flow cytometry (39). As was observed with the conventional CAR T cells, cells expressing the ON-switch CAR displayed a bimodal CD69 expression pattern upon activation. However, unlike the conventional CAR that only required the cognate CD19 antigen to activate T cells, the ON-switch CAR also required the dimerizer molecule to be present (Fig. 3C). Increasing dimerizer concentrations did not appear to titrate the activity level of individual cells, but rather increased the fraction of cells in the CD69high activated state.
ON-switch CAR T cells require both antigen and small molecule dimerizer to drive cell proliferation
Antigen induced proliferation of CAR T cells is a critical facet of therapeutic responses (31, 39–41). Proliferation allows for amplification of T cell action, but excessive proliferation allows for systemic and severe toxicities. We thus tested whether the ON-switch CAR T cells displayed combinatorially gated cell proliferation (Fig. 3D). In a flow cytometry experiment, we labeled human primary CD4+ T cells with an intracellular fluorescent dye that is progressively diluted with increasing rounds of cell division to monitor cell proliferation. Proliferation of these cells indeed required both the cognate antigen and rapalog. The observed small molecule-gated control over T cell proliferation is important; controlling the degree of cell expansion in vivo may be a highly effective way to tune and optimize the strength of the therapeutic response.
In summary, the results with primary human CD4+ helper T cells confirmed that with the ON-switch CAR, the key therapeutic behaviors of CAR T cells can be gated and regulated by a small molecule drug. At the single-cell level, our flow cytometry data (for CD69 surface expression and proliferation) indicate a small molecule-dependent bi-stable switch alters the number of cells in the ON vs OFF states in the presence of cognate antigen. On the population level, the ON-switch design functions additionally as a dial that yields titratable dosage of key anti-tumor responses. The ON-switch CAR T cell populations displayed a high dynamic range of signaling, with a maximum response (at saturating concentrations of small molecule dimerizer) comparable to that of the conventional CAR T cells.
Small molecule-controlled cancer cell killing by ON-switch CAR CD8+ T cells
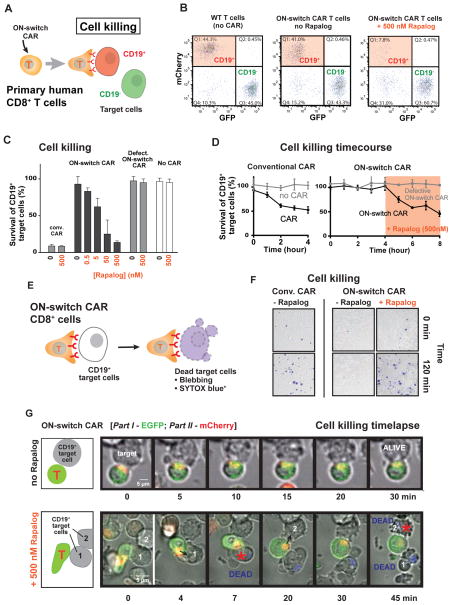
We tested the ON-switch CAR’s ability to control tumor cell killing by primary human CD8+ cytotoxic T cells. Targeted apoptosis of tumor cells is one of the hallmarks of anti-tumor immunity and CAR T cell action. A major purpose of CARs is to re-direct CD8+ T cell cytotoxicity selectively towards cancer cells expressing antigens of interest. T cell-mediated cell killing occurs on a faster time scale (minutes) and with different thresholds than cytokine production and proliferation (42, 43). Thus, we examined whether primary human CD8+ T cells expressing the ON-switch CAR could mount a cytotoxic response that was still antigen specific but gated by the dimerizing small molecule. Control over cytotoxic activity is one of the most important needs for CAR T cell regulation.
To test cell killing, target cells expressing either the cognate antigen (CD19+) or a non-cognate antigen (mesothelin+) were labeled by the selective expression of distinct fluorescent proteins respectively, so that in a mixed population the two target cell types could be independently identified and tracked by flow cytometry (Fig. 4A; Fig. S7a). The cytotoxic activity of CD8+ T cells expressing the CD19 directed ON-switch CAR was then quantified based on reduction in the fraction of cognate target cells among all viable target cells (Fig. 4B). After an overnight incubation (22 hrs), no significant cell-mediated cytotoxicity was observed in the absence of rapalog. When 500 nM rapalog was added, efficient killing of the cognate target cells was observed. Killing of non-cognate target cells was not observed, and the degree of cognate target cell killing could be titrated by changing the concentration of rapalog (Fig. 4C, Fig. S7a). The degree of targeted cell killing with saturating rapalog matched the level observed with the conventional CAR.
Fig. 4. ON-switch CAR yields antigen-specific and titratable killing of target cell population by engineered primary cytotoxic (CD8+) T cells.
(A) Schematic of a flow cytometry-based cell killing assay. Primary human CD8+ T cells were isolated, expanded and engineered to express CARs by transduction with lentivirus. T cells with comparable CAR expression levels were used. T cells were incubated with a mixture of cognate target cells (CD19+, mCherry+) and non-cognate target cells (CD19−, GFP+). Rapalog was added to specified concentrations. After incubation for a designated period of time, the abundance of both types of K562 target cells within the overall surviving target cell population was quantified by flow cytometry.
(B) Representative flow cytometry data. Surviving target cells in sample mixtures at the end of an overnight assay were segregated into cognate (mCherry+) and non-cognate (GFP+) sub-populations. The percentage of CD19+ cells (quadrant 1) was divided by that of CD19− cells (quadrant 3) to calculate the normalized percentage of survival of cognate target cells in each sample.
(C) Cytotoxicity mediated by CARs in an overnight (22hr) endpoint experiment. A low percentage for survival of cognate target cells indicates a high degree of specific target cell killing by CAR T cells.
(D) Cytotoxicity mediated by CARs in a kinetic experiment. Target cell killing by conventional CAR was quantified hourly during a four-hour incubation period. Cytotoxic activities of the ON-switch CAR were first monitored in the absence of dimerizing small molecule hourly for four hours, followed by four more hourly time points in the presence of small molecule (500 nM rapalog).
(E) A schematic of the experimental setup of the time-lapse imaging experiments is shown.
(F) Representative DIC images of primary human CD8+ T cells expressing the conventional CAR or the ON-switch CAR (± rapalog) incubated with CD19+ K562 target cells, overlaid with SYTOX blue dead stain fluorescent images, to assay target cell death after 0 and 2 hours of interaction (n=3).
(G) A time-lapse montage of DIC and fluorescence image overlays of primary human CD8+ T cells expressing ON-switch CAR (part I tagged with EGFP, part II tagged with mCherry) and their interaction with CD19+ K562 targets. The top montage is in the absence of rapalog, and shows T cell binding, but no killing of target cell over the course of the 30 min experiment (Movie S3). The bottom montage is in the presence of 500 nM rapalog, and shows killing of tumor cells, indicated by blebbing and Sytox blue dye uptake, within 45 min (Movie S4).
In an 8 hour time course experiment, T cells expressing the conventional CAR immediately induced specific cytotoxicity in cognate target cells. T cells expressing the ON-switch CAR did not commence killing until addition of rapalog (Fig. 4D). These results confirmed that the ON-switch CAR T cells allow for titratable control over the magnitude and timing of apoptosis in the target cell population. We next tested whether the ON-switch CAR T cells would stop cell killing after rapalog removal and then resume killing upon re-introduction of rapalog. This co-culturing experiment consisted of 3 stages to implement the “on – off – on” sequence of exposure to rapalog, and each stage lasted 36 hours (Fig. S8a). Target cell survival was quantified at the beginning and the end of each stage. Target cell killing was only detectable in the first and last 36 hour periods when the rapalog was present (Fig. S8b and S8c). Our proof-of-principle experiments with CD8+ T cells illustrate that the ON-switch design provides a flexible platform for temporal control over CAR T cell action. In therapeutic settings, the kinetics of on-off and off-on transitions could in principle be tuned with small molecules with different pharmacodynamics, or with competitive antagonist molecules (non-dimerizing).
To confirm the relevance of our observations made with engineered K562 target cells, we investigated rapalog-gated killing of natural CD19+ cancer cells by ON-switch CAR T cells. The Raji and Daudi human B cell lines both naturally express CD19 in amounts greater than that in the CD19+ K562 cell line we had used (Fig. S7b). Cytotoxicity experiments showed that both B cell lines are subject to killing by CD8+ ON-switch CAR T cells in a manner dependent on the dose of the rapalog (Fig. S7c). In summary, we observed titratable killing of multiple types of CD19+ target cell populations.
Imaging of ON-switch CAR T cell killing
To better characterize the spatiotemporal aspects of the ON-switch CAR mediated killing, we performed microscopy-based assays. We quantitatively followed cell killing by CD8+ T cells with the Sytox nucleic acid binding dye that only permeates dead cells (Fig. 4E). Images taken after two hours confirmed killing of CD19+ target cells by the ON-switch CAR expressing T cells, but only in the presence of the small molecule (Fig. 4F).
We directly followed the dynamics of cell death with live cell time-lapse confocal microscopy. In primary human CD8+ T cells, we expressed the ON-switch CAR components labeled with distinct fluorescent proteins (part I comprising the extracellular scFv domain was labeled with enhanced Green Fluorescent Protein at the C-terminus; part II comprising the CD3ζ domain was labeled with mCherry at the C-terminus). We separately tracked both the physical interaction between T cells and cognate target cells, as well as the killing of the cognate target cells. In the absence of rapalog, T cells and target cells associated and formed an immune synapse-like structure, but target cells remained alive for the duration of the 30-minute experiment (Movie S3; Fig. 4G, top panel). Quantitative analysis of time-lapse images revealed that, even in the absence of rapalog, ON-switch CAR T cells formed conjugates with cognate target cells as frequently as did conventional CAR T cells (Fig. S9). However, the physical association alone did not induce target cell death. In contrast, in the presence of rapalog, multiple target cells were rapidly killed within the 45 minute time window (Movie S4; Fig. 4G, bottom panel).
In vivo small molecule-regulation of cognate tumor cell killing
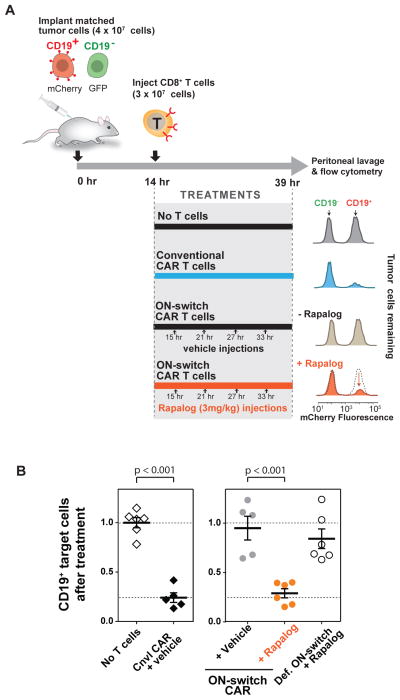
We tested whether the tumor cell killing by primary ON-switch CAR T cells could be regulated in vivo in a mouse xenograft model. In preliminary pharmacokinetic studies, rapalog had a plasma half-life in mice of approximately 4 hours. This property necessitated multiple injections of rapalog per day (3 injections at 3mg/Kg to maintain 50ng/mL in plasma), which, combined with the high cost of the compound, precluded the performance of extended multi-week studies with statistically sufficient numbers of mice. Thus we used a shorter protocol of in vivo tumor killing (Fig. 5A). We implanted a mixture of cognate (CD19+ mCherry+) and non-cognate (CD19− GFPhigh) K562 cells into the peritoneal cavity. Fourteen hours later, CAR-expressing CD8+ T cells and the first dose of rapalog (or vehicle control) were injected i.p.. Three additional doses of the rapalog/vehicle were injected at 6 hour intervals. Mice were euthanized at the experimental end point (after 39 hours) for collection of peritoneal lavage (44), from which recovered cells were analyzed by flow cytometry.
Fig. 5. In vivo control of ON-switch CAR target cell killing by small molecule.
(A) A schematic of the mouse model used and the representative results of tumor cell survival. Matched CD19+/−target cells distinctly labeled with fluorescent proteins were injected into the intraperitoneal space of NOD scid gamma (NSG) immune-deficient mice. T cells, rapalog or vehicle control was injected i.p. at the indicated times. At the end of the experiment, target cells recovered from peritoneal lavage were quantified using flow cytometry to measure ratio of surviving CD19+ vs CD19− target cells.
(B) Quantified flow cytometry results from all experimental groups showing rapalog-dependent killing of cognate (CD19+) target cells by ON-switch CAR T cells. Ratios of surviving CD19+:CD19− target cells were calculated. Averages and standard deviations are plotted. n >=5. p values to compare pairs of experimental groups were calculated with student’s t test.
Mice injected with conventional CAR T cells showed selective depletion of the CD19+ K562 cell population as compared to results obtained with mice that did not receive any T cells (Fig. 5A). ON-switch CAR T cells produced a similar result, but only in mice treated with rapalog. The normalized number of remaining CD19+ target cells (relative to the number of remaining CD19− target cells) was plotted (Fig. 5B). These data show that the ON-switch CAR T cells, in the absence of rapalog, led to the same outcome as in conditions where no T cells were given or defective ON-switch T cells & rapalog were given. However, when the mice injected the ON-switch CAR T cells were also treated with rapalog, the decrease in CD19+ cells matched that observed with the conventional CAR T cells.
In summary, we confirmed that the ON-switch CAR T cells can be effectively controlled with a small molecule in vivo. Both in vitro and in vivo the engineered cells demonstrate no constitutive killing of target cells, but show strong and selective killing of cognate target cells when exposed to the dimerization-inducing molecule. The rapalog we used in vivo is not ideal given its short half-life in plasma. However, we demonstrated that unrelated dimerization systems can be used to control the ON-switch CAR architecture (Fig. 2B). Thus, it is likely that heterodimerization systems optimized for in vivo pharmacokinetic properties and safety could be used to tightly control ON-switch CAR T activity in patients.
DISCUSSION
Safer therapeutic immune cells by integrating autonomous and user control
We engineered a class of synthetic T cell receptors that allows for effective exogenous control over T cell anti-tumor activity, including cytokine production, proliferation and cytotoxicity. This receptor design is modular, in that customizable small molecule dimerization systems can be used to gate signaling. Like conventional CARs, various extracellular domains for recognition of distinct ligand antigens can be used. Further analysis shows that the ON-switch design is also compatible with further customization through a mutated 4-1BB signaling domain in part I or a monomeric form of part II, if it is preferred that these individual components not associate with the endogenous TRAF or DAP10 molecules (Figures S10, S11)(29, 45, 46).
The ON-switch receptor system depends upon two combined inputs to trigger T cell activation – a disease specific ligand and a small molecule drug. This type of antigen ligand + small molecule combinatorial control made possible by the ON-switch design might make adoptive T cell therapy safer, as the activities of infused T cell population could be selectively regulated in a temporal and titratable manner to minimize both off-target and on-target toxicities (7). T cells engineered with an anti-HER2 CAR to treat metastatic colon cancer have caused severe and rapid cross-reaction with normal cells expressing low amounts of HER2 in the lung; CAR T cells first concentrate and transit through the lung immediately after infusion (16). This “first pass” toxicity involving lung, heart and liver, which occurs immediately after T cell infusion, could potentially be limited by delaying activation of the T cells until after they have distributed throughout the body. Using locally administered small molecules, ON-switch CAR T cell could allow for titratable control over T cell activity, as well as the location of therapeutic action when combined with technologies that can locally deliver small molecules into targeted tissues (20).
Need to develop modules for orthogonal chemical control of engineered cells
More generally, this and related work highlights the value of orthogonal chemical control as an interface for any kind of cellular therapeutic agent. Toxicity concerns of cell-based therapies primarily stem from a lack of efficient methods to specifically communicate with and regulate the cells once they are in the patient (aside from systemic treatments such as immunosuppression). A small molecule drug-inducible “suicide” switch that induce apoptosis in engineered T cells can be used to abort cellular therapeutics (18, 24). A modular, RNA-based system has also been developed for small molecule dependent cytokine production and proliferation of engineered T cells in vivo (47). Orthogonal ligands of G-Protein Coupled Receptors can be used to guide T cell migration in vivo (48). The ON-switch CAR described in this study can be implemented along with these other synthetic control devices to produce “smart T cells” whose key therapeutic behaviors are individually under exogenous control.
This work also emphasizes the need for the development of additional orthogonal channels for molecular control. Reagents such as the rapalog molecule used here were primarily developed as tools for chemical biology studies, and they do not have ideal pharmacokinetic properties for clinical use. Advancement in cellular therapeutics may thus require the development of new classes of controller drugs that are optimized for clinical use in combination with engineered therapeutic cells. Such drugs should be safe, bio-inert, have good pharmacokinetic properties, and have cognate response modules that can be flexibly incorporated into the molecular machinery of the engineered cells (48–50). Other modalities of control, such as light — which can be detected by optogenetic modules (51–55) or other physical signals, could in principle also be useful as additional channels to control therapeutic cells. In summary, combining the tools and strategies of chemical biology with genetic engineering may produce more controllable cellular therapeutic agents with improved therapeutic profiles.
Strategies for engineering new layers of combinatorial control in synthetic receptors
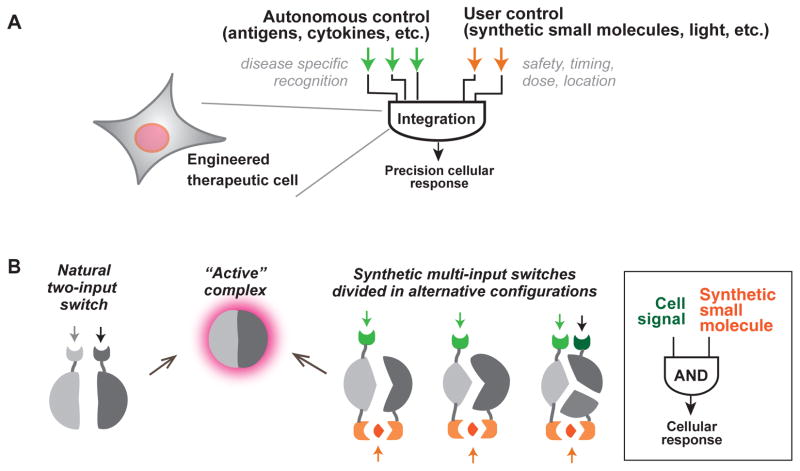
The type of cellular control engineered here represents the general principle of integrating autonomous control (e.g. targeting of disease ligands) with user control (e.g. small molecules) (Fig. 6A). As we begin to engineer more cellular therapies, integration of these two modes of cellular regulation is likely to become increasingly important. Regulation by user inputs allows the physician more precise control over the timing, dose and location of a cellular action, and thus more flexible safety control.
Fig. 6. General strategies for engineering therapeutic cells that integrate autonomous and user control.
(A) Ideal therapeutic cells are expected to (i) produce potent therapeutic effects upon recognizing disease-specific signals and (ii) act in a temporally and spatially regulated manner. As illustrated in this work, cell-autonomous signaling in response to disease-specific inputs can be integrated with exogenous, user-supplied inputs to produce more precisely regulated therapeutic responses.
B) Regulated assembly into conditionally active complexes is commonly observed in natural regulatory systems. This strategy can be exploited to generate synthetic multi-input control by generating alternative split configurations of the active state that are conditionally assembled only with the proper combination of input molecules.
This work also demonstrates that it is possible to engineer additional layers of positive control into an already complex synthetic receptor. The engineering of the CAR performed here is analogous to changes in regulatory function that occur in signaling proteins over the course of evolution, when new regulatory inputs are layered and integrated with one another. We used the simple strategy of harnessing controlled molecular complex assembly as a way to achieve combinatorial multi-input regulation (Fig. 6B). The active signaling complex at the T cell plasma membrane is normally conditionally assembled when the TCR and co-receptors are stimulated, whereas the CAR preassembles this complex. Thus to achieve multi-input control, we have re-split this assembly in a different configuration, and in a manner such that its reassembly is dependent on small molecule binding. This kind of synthetic division and reassembly strategy provides a robust strategy for integrating and layering different control modules over cellular responses in order to develop synthetic combinatorial control systems that mimic the precision of natural cellular signaling responses (56, 57).
Supplementary Material
Acknowledgments
We thank the Pre-clinical Therapeutics Core at UCSF for helping with in vivo studies; K McNally, S Johnston, D Ng, D Nunez, H Yan, and S Zorn for technical assistance; B Huang for providing a custom-built microscope for PALM imaging; Y Huang for mass spectroscopy-based pharmacokinetic analysis. We acknowledge M Milone and C June (U Penn), D Campana (formerly St. Jude), I Pastan (NIH/NCI), T Meyer (Stanford) and T Inoue (Johns Hopkins) for providing materials. Thanks to A Weiss, J Bluestone, Q Tang, A Marson, L Lanier, members of the Lim Lab, Cell Propulsion Lab, and Weiss Lab for advice and feedback. This work was supported by grants from the NIH (PN2 EY016546, P50 GM081879, R01 GM055040, R01 CA196277 - WAL; F32 GM101782 – CW), Jane Coffin Childs Memorial Fund (A121505 – KR) and HHMI (WAL).
References
- 1.Bluestone JA, Bour-Jordan H. Cold Spring Harb Perspect Biol. 2012 Nov;4 doi: 10.1101/cshperspect.a007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadelain M, Brentjens R, Riviere I. Cancer Discov. 2013 Apr;3:388. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalos M, June CH. Immunity. 2013 Jul 25;39:49. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochenderfer JN, Rosenberg SA. Nat Rev Clin Oncol. 2013 May;10:267. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta N, et al. Sci Transl Med. 2012 Oct 10;4:155ra137. doi: 10.1126/scitranslmed.3004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu P, et al. Cell. 2012 Sep 14;150:1264. [Google Scholar]
- 7.Fischbach MA, Bluestone JA, Lim WA. Sci Transl Med. 2013 Apr 3;5:179ps7. doi: 10.1126/scitranslmed.3005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason C, Brindley DA, Culme-Seymour EJ, Davie NL. Regen Med. 2011 May;6:265. doi: 10.2217/rme.11.28. [DOI] [PubMed] [Google Scholar]
- 9.Ye H, Aubel D, Fussenegger M. Curr Opin Chem Biol. 2013 Dec;17:910. doi: 10.1016/j.cbpa.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Roberts MR, et al. Blood. 1994 Nov 1;84:2878. [PubMed] [Google Scholar]
- 11.Kalos M, et al. Sci Transl Med. 2011 Aug 10;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochenderfer JN, et al. Blood. 2012 Mar 22;119:2709. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grupp SA, et al. N Engl J Med. 2013 Apr 18;368:1509. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davila ML, et al. Sci Transl Med. 2014 Feb 19;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maus MV, Grupp SA, Porter DL, June CH. Blood. 2014 Feb 27; doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan RA, et al. Mol Ther. 2010 Apr;18:843. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciceri F, et al. Lancet Oncol. 2009 May;10:489. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 18.Sato T, et al. Mol Ther. 2007 May;15:962. doi: 10.1038/mt.sj.6300122. [DOI] [PubMed] [Google Scholar]
- 19.Fedorov VD, Themeli M, Sadelain M. Sci Transl Med. 2013 Dec 11;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne JD, et al. Sci Transl Med. 2015 Feb 4;7:273ra14. doi: 10.1126/scitranslmed.3009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim W, Mayer B, Pawson T. Garland Science. 2014 [Google Scholar]
- 22.Weiss A, Littman DR. Cell. 1994 Jan 28;76:263. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 23.Graef IA, Holsinger LJ, Diver S, Schreiber SL, Crabtree GR. EMBO J. 1997 Sep 15;16:5618. doi: 10.1093/emboj/16.18.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Stasi A, et al. N Engl J Med. 2011 Nov 3;365:1673. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayle JH, et al. Chem Biol. 2006 Jan;13:99. doi: 10.1016/j.chembiol.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Liberles SD, Diver ST, Austin DJ, Schreiber SL. Proc Natl Acad Sci U S A. 1997 Jul 22;94:7825. doi: 10.1073/pnas.94.15.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi J, Chen J, Schreiber SL, Clardy J. Science. 1996 Jul 12;273:239. doi: 10.1126/science.273.5272.239. [DOI] [PubMed] [Google Scholar]
- 28.Lin J, Weiss A. J Cell Biol. 2003 Aug 18;162:673. doi: 10.1083/jcb.200303040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, et al. Science. 1999 Jul 30;285:730. [Google Scholar]
- 30.Irving BA, Chan AC, Weiss A. J Exp Med. 1993 Apr 1;177:1093. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guy CS, et al. Nat Immunol. 2013 Mar;14:262. doi: 10.1038/ni.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milone MC, et al. Mol Ther. 2009 Aug;17:1453. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyamoto T, et al. Nat Chem Biol. 2012 May;8:465. doi: 10.1038/nchembio.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murase K, Hirano Y, Sun TP, Hakoshima T. Nature. 2008 Nov 27;456:459. doi: 10.1038/nature07519. [DOI] [PubMed] [Google Scholar]
- 35.Manley S, et al. Nat Methods. 2008 Feb;5:155. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- 36.Puchner EM, Walter JM, Kasper R, Huang B, Lim WA. Proc Natl Acad Sci U S A. 2013 Oct 1;110:16015. doi: 10.1073/pnas.1309676110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betzig E, et al. Science. 2006 Sep 15;313:1642. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 38.Douglass AD, Vale RD. Cell. 2005 Jun 17;121:937. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simms PE, Ellis TM. Clin Diagn Lab Immunol. 1996 May;3:301. doi: 10.1128/cdli.3.3.301-304.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyman O, Sprent J. Nat Rev Immunol. 2012 Mar;12:180. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda H, Old LJ, Schreiber RD. Cytokine Growth Factor Rev. 2002 Apr;13:95. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 42.Huppa JB, Gleimer M, Sumen C, Davis MM. Nat Immunol. 2003 Aug;4:749. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 43.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. Nat Immunol. 2004 May;5:524. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 44.Ray A, Dittel B. the Journal of Visualized Experiments. 2010 doi: 10.3791/1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye H, Park YC, Kreishman M, Kieff E, Wu H. Mol Cell. 1999 Sep;4:321. doi: 10.1016/s1097-2765(00)80334-2. [DOI] [PubMed] [Google Scholar]
- 46.Jang IK, Lee ZH, Kim YJ, Kim SH, Kwon BS. Biochem Biophys Res Commun. 1998 Jan 26;242:613. doi: 10.1006/bbrc.1997.8016. [DOI] [PubMed] [Google Scholar]
- 47.Chen YY, Jensen MC, Smolke CD. Proc Natl Acad Sci U S A. 2010 May 11;107:8531. doi: 10.1073/pnas.1001721107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park JS, et al. Proc Natl Acad Sci U S A. 2014 Apr 22;111:5896. doi: 10.1073/pnas.1402087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bishop A, et al. Annu Rev Biophys Biomol Struct. 2000;29:577. doi: 10.1146/annurev.biophys.29.1.577. [DOI] [PubMed] [Google Scholar]
- 50.Banaszynski LA, Wandless TJ. Chem Biol. 2006 Jan;13:11. doi: 10.1016/j.chembiol.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Bacchus W, Fussenegger M. Curr Opin Biotechnol. 2012 Oct;23:695. doi: 10.1016/j.copbio.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Toettcher JE, Gong D, Lim WA, Weiner OD. Nat Methods. 2011;8:837. doi: 10.1038/nmeth.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levskaya A, Weiner OD, Lim WA, Voigt CA. Nature. 2009 Oct 15;461:997. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kennedy MJ, et al. Nat Methods. 2010 Dec;7:973. doi: 10.1038/nmeth.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu YI, et al. Nature. 2009 Sep 3;461:104. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prehoda KE, Scott JA, Mullins RD, Lim WA. Science. 2000 Oct 27;290:801. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- 57.Dueber JE, Yeh BJ, Bhattacharyya RP, Lim WA. Curr Opin Struct Biol. 2004 Dec;14:690. doi: 10.1016/j.sbi.2004.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.