Abstract
Background & Aims
The UDP-glucuronosyltransferases (UGTs) are a part of the cell machinery that protects the tissues from a toxicant insult by environmental and host cell metabolites. We investigated the mechanism behind tumor growth and UGT repression.
Methods
We initially silenced the Ugt1 locus in human colon cell lines and investigated markers and responses linked to p53 activation. To examine the role of the Ugt1 locus in p53-directed apoptosis and tumorigenesis, experiments were conducted to induce acute colon inflammation and chemically induced colon cancer in mice where we have selectively deleted the Ugt1 locus in the intestinal epithelial cells (Ugt1ΔIEC mice).
Results
Knockdown of the UGT1A proteins by RNAi in human colon cancer cells and knockout of the Ugt1 locus in intestinal crypt stem cells reduces phosphorylated p53 activation and compromises the ability of p53 to control apoptosis. Targeted deletion of intestinal Ugt1 expression in Ugt1ΔIEC mice represses colon inflammation-induced p53 production and proapoptotic protein activation. When we induced colon cancer, the size and number of the tumors were significantly greater in the Ugt1ΔIEC mice when compared with wild-type mice. Furthermore, analysis of endoplasmic reticulum (ER) stress-related markers indicated that lack of UGT1A expression causes higher ER stress in intestinal epithelial cells and tissue, which may account for the lower expression of p53.
Conclusions
Our results demonstrate that UGT1A expression is required to maintain and sustain p53 activation in stress-induced colon epithelial cells and has a significant impact on p53-mediated apoptosis and tumor suppression, thus protecting the colon tissue from neoplastic transformation.
Keywords: Apoptosis, Colon Cancer, ER Stress, UGT1A
Abbreviations used in this paper: AOM, azoxymethane; CRC, colorectal cancer; DSS, dextran sodium sulfate; ER, endoplasmic reticulum; IEC, intestinal epithelial cells; IL, interleukin; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PBS, phosphate-buffered saline; qRT-PCR, quantitative real-time polymerase chain reaction; siRNA, small interfering RNA; TNF, tumor necrosis factor; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; UGT, UDP-glucuronosyltransferase; UGT1, human UGT1 locus; Ugt1, murine Ugt1 locus
Summary.
UGT1A expression is required to maintain and sustain p53 activation in stress-induced colon epithelial cells, and it has a significant impact on p53-mediated apoptosis and tumor suppression.
Colorectal cancer (CRC) ranks as the third most common cancer worldwide and the second leading cause of cancer-related deaths in Western society.1, 2 The majority of colorectal tumors are epithelial tumors, whereas lymphomas, endocrine tumors, and mesenchymal tumors are quite uncommon.3 As an important extrahepatic tissue of xenobiotic metabolism, the colorectum is in direct contact with xenobiotic substances, including potentially toxic or carcinogenic agents, presumably leading to the high incidence rate of CRC.4, 5 By contrast, cancers of the small intestine are rarely seen, even though the small intestine has a larger mucosal surface area than the colorectum.6 One plausible explanation is that expression of biotransformation enzymes, including glutathione S-transferases, UDP-glucuronosyltransferases (UGT), and cytochrome P450, are lower in the colorectum than in the small intestine. These enzymes are responsible for the detoxification of ingested toxins, carcinogens, or tumor-promoting compounds, and their lower expression levels in colorectum are considered to be a contributing factor to the high rate of CRC.7, 8
As an important part of the detoxification process, glucuronidation provides an effective metabolic process leading toward the biological inactivation of potential toxicants and carcinogens. Previous studies have demonstrated that gastrointestinal UGT activity decreases sharply from the small intestines to the colon tissue.7 This decrease in UGT activity contributes to higher colonic DNA damage caused by carcinogens, such as heterocyclic amines and polycyclic aromatic hydrocarbons, which are usually detoxified through UGT glucuronidation.9, 10, 11 It has been speculated that glucuronidation provides a genoprotective defense against the mutagenic actions of chemical carcinogens. Studies have found that UGT expression in colorectal tumor tissues is significantly reduced in comparison to surrounding healthy tissues.12, 13, 14 Indeed, the pattern of UGT down-regulation is also identified in other types of cancer, including liver and biliary cancer,15 breast cancer,16 and bladder cancer.17 These findings indicate that UGT expression is reversely correlated with tissue neoplastic transformation. However, there is no evidence that the UGTs impact the outcome of tumorigenesis, and the underlying mechanism regarding the role of the UGTs in cancer development is largely unexplored.
Recent studies have shown a link between the UGTs and p53, an important regulator of cell cycle, apoptosis, and tumorigenesis. Ariyoshi et al18 observed increased constitutive UGT1A activity in p53+/− mice, and Hu et al19 verified that epirubicin up-regulates UGT2B7 expression via a p53 pathway. In contrast to these studies, our initial discovery uncovered an opposite causal relationship between UGT1A and p53 when we challenged cells with chemical stress. Cell apoptotic death is a well-defined mechanism that is associated with cancer suppression when the body encounters tumor-promoting challenges. P53 and its signaling network are known to play a critical role in the regulation of the cell cycle and apoptosis to conserve gene stability, thus suppressing tumor development.20, 21 Upon occurrence of cellular stress, such as oncogene activation or DNA damage, p53 is activated. When cell damage is minimal, p53 evokes cell cycle arrest by inducing p21 to promote DNA repair and cell survival, whereas sustained p53 activation in response to high damage levels triggers cellular apoptosis, thus preventing the expansion of damaged cells and protecting normal tissue from neoplastic transformation.22, 23 This is evidenced by the fact that the development of certain tumors in p53 null mice has been associated with decreased apoptosis, implying the important role of p53 in promoting cell death during tumor suppression.24
Our study explored the role of the UGT1A proteins in CRC by using colon cancer cell lines and an intestinal conditional knockout animal model deficient in Ugt1 locus expression. By documenting molecular and cellular events that are associated with p53-dependent signaling, this study sheds light on the importance of UGT1A expression on p53-dependent stress responses and tumor suppression.
Materials and Methods
Chemicals and reagents
Actinomycin D, etoposide, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and azoxymethane (AOM) were purchased from Sigma-Aldrich (St. Louis, MO), and dextran sulfate sodium (DSS, molecular weight 36,000–50,000) was obtained from MP Biomedicals (Santa Ana, CA). The quantitative real-time polymerase chain reaction (qRT-PCR) primers were commercially synthesized from Integrated DNA Technologies (San Diego, CA). Antibodies against UGT1A (Abcam, Cambridge, MA), p21 (Chemicon, Temecula, CA), p53 and Bax (Santa Cruz Biotechnology, Dallas, TX), and caspase-9 and caspase-3 (Cell Signaling Technology, Beverly, MA) were used in Western blot analyses.
Cell Culture and UGT1A Silencing
The human colon epithelial cell lines HT29 and LS180 were obtained from the American Type Culture Collection (ATCC, Manassas, VA). For UGT1A gene silencing, two pairs of UGT1A-specific small interfering RNA (siRNA) designed along with nonspecific siRNA as a negative control were used. The siRNA (Invitrogen/Life Technologies, Carlsbad, CA) was mixed with the Lipofectamine RNAiMAX Reagent (Invitrogen) in Opti-MEM I medium (GIBCO/Life Technologies, Grand Island, NY) at a finial concentration of 30 nM and was incubated at room temperature for 10 minutes according to the manufacturer’s instructions. The mixture was added to culture plates, and exponentially growing cells were then seeded in these plates. Cells were incubated for 24–72 hours until further analyses.
Mouse Crypt Cell Preparations and Culture
Crypt cell isolation and culture were performed as previously described elsewhere.25 Intestines from adult Ugt1F/F and Ugt1ΔIEC mice were dissected, opened longitudinally, and gently washed with ice-cold phosphate-buffered saline (PBS) buffer. Intestinal tissue was then incubated in PBS buffer containing 2 mM EDTA at 4°C for 30 minutes. The buffer was removed, and the tissue was shaken vigorously and then filtered through a 70-μm cell strainer. The filtrate was centrifuged at 1000g for 10 minutes to precipitate the crypt cells, followed by a wash with Advanced Dulbecco’s modified Eagle medium/Ham’s F-12 medium (Life Technologies, Carlsbad, CA). The cells were counted, and approximately 1000 crypts were suspended into 50 μL of Matrigel (BD Biosciences, San Jose, CA), and the cells plated into 24-well plates. After 10 minutes, 500 μL of crypt culture medium Advanced Dulbecco’s modified Eagle medium/Ham’s F-12 supplemented with B27 (Life Technologies), N2 (Life Technologies), 1 μM N-acetyl cysteine (Sigma-Aldrich), 100 ng/mL mNoggin and R-spondin 1 (conditioned medium, R-spondin 1 expression 293-HA-Rspol-Fc cell line was a generous gift from Dr. Calvin Kuo, University of Stanford) were added. Growth factors were added every other day, and the medium was changed every 4 days. Cells were passaged every 1 to 2 weeks.
Cytotoxicity Assay
Cells were seeded at 7000 cells per well to a 96-well plate and incubated overnight. The cells were subsequently exposed to the indicated concentrations of actinomycin D or etoposide. After 36 hours, the MTT solution was added to each well at a final concentration of 0.5 mg/mL, and the plate was incubated at 37°C for another 4 hours. The MTT solution was then removed and 150 μL of DMSO per well was added. The absorbance at 595 nm was measured by a microplate reader.
Apoptosis Assay
The HT29 cells were exposed to the indicated concentration of actinomycin D or etoposide for 36 hours and then harvested by 0.25% trypsin without EDTA. The fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit (BioLegend, San Diego, CA) was used to stain the cells. Samples were analyzed using a flow cytometer (BD FACSCalibur; BD Biosciences).
Ugt1 Conditional Knockout Mouse Lines
Mice carrying loxP recombinase sites positioned in Ugt1a1 intron 2 and intron 4 (Ugt1F/F mice) were previously generated.14 When the mice were bred with villin-Cre mice, Cre-mediated recombination resulted in the deletion of exons 3 and 4 in the common region of the Ugt1a locus specifically in intestinal epithelial cells (Ugt1ΔIEC mice), leading to knockout of the entire Ugt1 locus.
Animal Treatment With Dextran Sodium Sulfate or Azoxymethane Plus Dextran Sodium Sulfate
For the acute colitis experiment, 8- to 10-week-old Ugt1F/F or Ugt1ΔIEC mice were treated with 3% DSS in drinking water for 5 days, followed by 5 days of regular water. The mice were sacrificed, and the colons were removed, rinsed with PBS, and cut lengthwise into two segments. One segment was put into liquid nitrogen immediately and then stored at −80°C for subsequent analysis; the other segment was fixed as “Swiss rolls” in 10% formalin at 4°C overnight and stored in 75% alcohol for paraffin embedding.
For the tumorigenesis study, 8- to 10-week-old Ugt1F/F or Ugt1ΔIEC mice were intraperitoneally injected with 10 mg/kg AOM. After 5 days, 2% DSS was provided in the drinking water for 5 days, followed by 16 days of regular water. The cycle of DSS water followed by regular water was repeated twice. Mice were sacrificed 7 days after the last cycle; their colons were removed, analyzed for the presence of tumors (number counted and size measured), and prepared according to the previous description.
TUNEL Analysis
Paraffin-embedded tissues were sectioned (5 μm) and analyzed with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining, a common method for detecting DNA fragmentation resulting from apoptosis, by using the in situ cell death detection kit (Roche Applied Science, Indianapolis, IN). Counterstaining with 4′,6-diamidino-2-phenylindole (DAPI) was performed before the slides were mounted, and they were observed under a Leica fluorescence microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Western Blot Analysis
All Western blots were performed by using NuPAGE BisTris-polyacrylamide gels (Invitrogen) with the protocols described by the manufacturer. Membranes were blocked with 5% skim milk and incubated with specific primary antibodies at 4°C overnight. Subsequently, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. Blots were developed by a Western Lightning Plus-ECL agent (PerkinElmer Life and Analytical Sciences, Waltham, MA) and were visualized under the Bio-Rad gel documentation system.
Total RNA Preparation and Gene Expression Analysis by Q-PCR
The colon sample was homogenized in 1 mL of TRIzol (Invitrogen), and the total RNA was extracted and used to generate cDNA with the iScript cDNA Synthesis Kit (Bio-Rad Laboratories). After cDNA synthesis, RT-PCR was performed with the Soadvanced SYBR Green Supermix (Bio-Rad Laboratories) using a CFX96 Touch Real-Time PCR detection system (Bio-Rad Laboratories). The sequences of the primers used are listed in Supplementary Table 1.
Statistical Analysis
All data were obtained by at least three independent experiments and are presented as mean ± standard deviation. Statistical differences between two treatment groups were evaluated using the Student’s t test. When comparing induction responses between two groups, two-way analysis of variance (ANOVA) was performed followed by Bonferroni post-tests. P < .05 was considered statistically significant, and statistically significant differences are indicated with asterisks (*P < .05; **P < .01; ***P < .001).
Results
UGT1A Knockdown by siRNA Silencing and p53 Expression
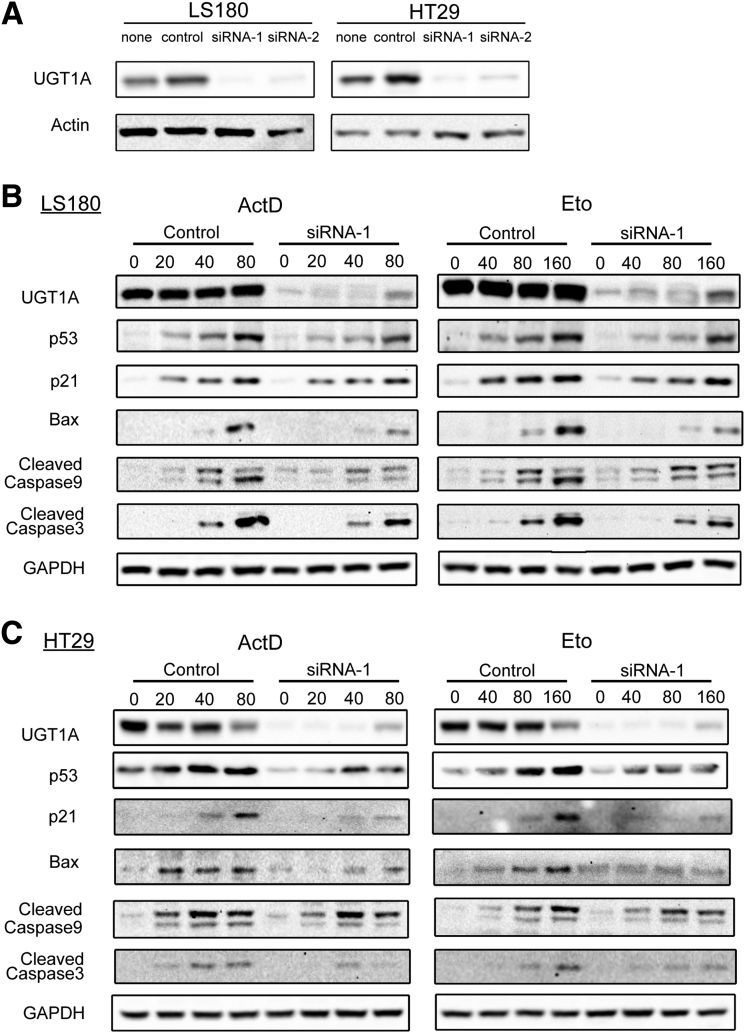
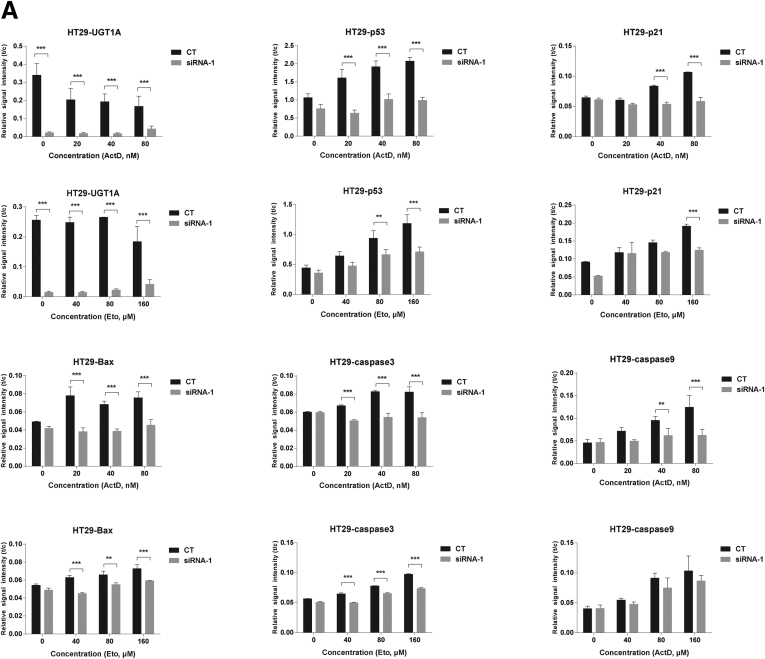
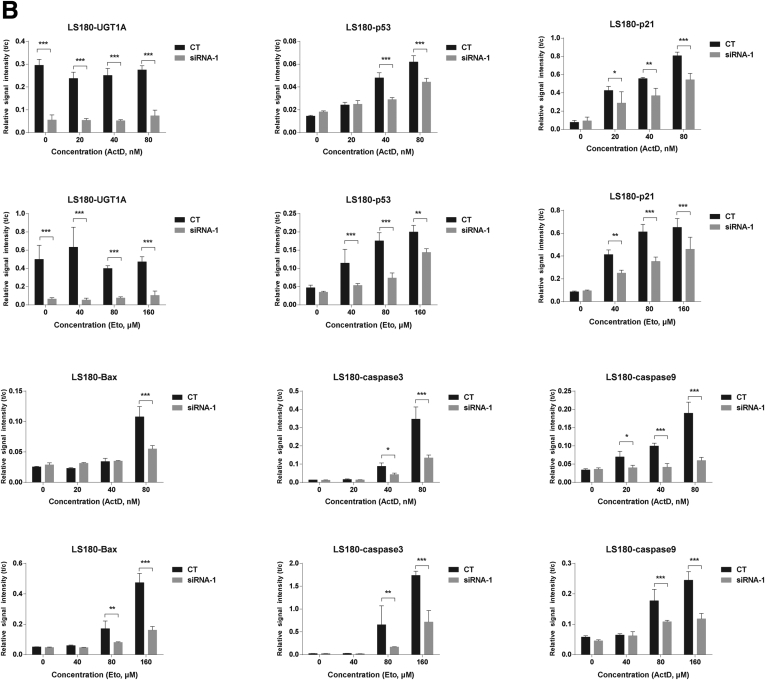
To explore the role of the UGT1A proteins in p53-dependent signaling, the UGT1A genes were silenced by UGT1A siRNA transfections in HT29 and LS180 colon cancer cell lines (Figure 1A and Supplementary Figure 1). When the cells were treated with anticancer drugs actinomycin D and etoposide, which are known to induce p53,26, 27 both actinomycin D and etoposide induced p53 total protein expression in a dose-dependent fashion (see Figure 1B and C, and Supplementary Figure 1). This treatment led to spontaneous increases in p21 and Bax, both of which are well-defined p53 downstream target genes, along with increases in apoptosis markers caspase-9 and caspase-3 (see Figure 1B and C). Loss of UGT1A gene expression led to a statistically significant decrease in actinomycin D- and etoposide-induced p53 expression in both HT29 and LS180 colon epithelial cells. Consequently, actinomycin D- and etoposide-mediated induction of p21 and Bax and apoptosis biomarkers caspase-9 and caspase-3 was also inhibited by UGT1A silencing, indicating the requirement of UGT1A protein expression for p53 elevation and p53-dependent target gene induction.
Figure 1.
UGT1A silencing inhibits actinomycin D and etoposide-induced p53 protein expression and the P53-dependent apoptosis pathway in LS180 and HT29 colon epithelial cells. (A) Assessment of gene-silencing efficiency for the two pairs of UGT1A siRNAs (siRNA1, siRNA2). Cells were treated with UGT1A-specific siRNA or nonspecific siRNA for 48 hours, and protein expression levels were examined by Western blot analysis. The cell sample without any treatment was indicated as “none” and treated with nonspecific siRNA was indicated as “control.” (B) Protein expression of p53 and genes regulated by p53. LS180 cells and HT29 cells (C) were pretreated with UGT1A-specific siRNA or non-specific siRNA for 48 hours and then incubated with actinomycin D (ActD, 20, 40, 80 nM) or etoposide (Eto, 40, 80 160 μM) for 36 hours. Whole cell lysates were prepared and expression of UGT1A, p53, p21, Bax, cleaved caspases-9, and cleaved caspases-3 were examined by Western blots. These represent an example taken from 3 independent experiments, where combined expression levels are quantitated in Supplementary Figures 1A and 1B.
Supplementary Figure 1.
Quantitation of Western blots after UGT1A silencing in LS180 and HT29 colon epithelial cells. Western blots from actinomycin D and etoposide treated cells from LS180 (Figure 1B) and HT29 (Figure 1C) cells were quantitated using imaging software from the BioRad ChemiDoc Touch Imaging System and plotted as Relative Signal Intensity or t/c (target protein/internal reference protein-GAPDH). Statistically significant differences in inducible p53 expression along with p21, Bax, caspase 3 and caspase 9 expressions were demonstrated after knockdown of the Ugt1a locus in these cells.
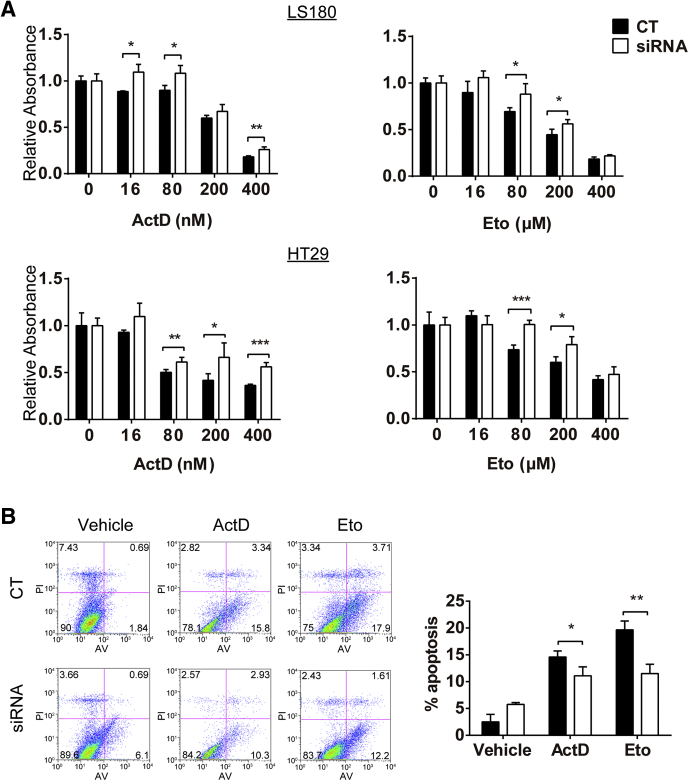
The MTT cell proliferation assay measures total mitochondrial activity, which is a measurement of viable cells to assess antiproliferative effects. The assay detects cell viability but cannot distinguish between apoptosis and proliferation, although proliferation is a common reference for this assay. We observed that actinomycin D and etoposide treatments blocked mitochondrial dehydrogenase activity (relative absorbance) in a dose-dependent manner in both HT29 and LS180 cells, indicating that these agents are apoptotic or antiproliferative. By comparison, knockdown of UGT1A proteins in these cell lines reduced the antiproliferative/apoptotic effects elicited by actinomycin D and etoposide (Figure 2A).
Figure 2.
UGT1A silencing lowers the antiproliferative activity of actinomycin D and etoposide in both HT29 and LS180 cells. Cells were pretreated with UGT1A siRNA1 or nonspecific siRNA for 24 hours and then exposed to gradient concentrations of actinomycin D or etoposide for 36 hours. (A) Cell proliferation was measured by MTT assay in actinomycin- or etoposide-treated LS180 and HT29 cells. The proliferation rate of cells is expressed as Relative Absorbance (mitochondrial dehydrogenase activity) with non-specific siRNA is expressed as 1. (B) Annexin V fluorescein isothiocyanate/propidium iodide staining of HT29 cells was examined by a flow cytometer. Results are presented as mean ± standard deviation of at least four independent experiments (*P < .05, **P < .01, ***P < .001).
We further detected the impact of chemical treatment on apoptosis by using annexin V-fluorescein isothiocyanate/propidium iodide staining, and the results show significantly fewer apoptotic cells in HT29 cells lacking UGT1A protein expression (see Figure 2B). Collectively, these results demonstrate that interruption of UGT1A protein expression influences the regulation of p53 and p53-dependent cell cycle arrest and cell death signaling.
Inhibition of p53 Expression in Ugt1ΔIEC Intestinal Crypt Stem Cells
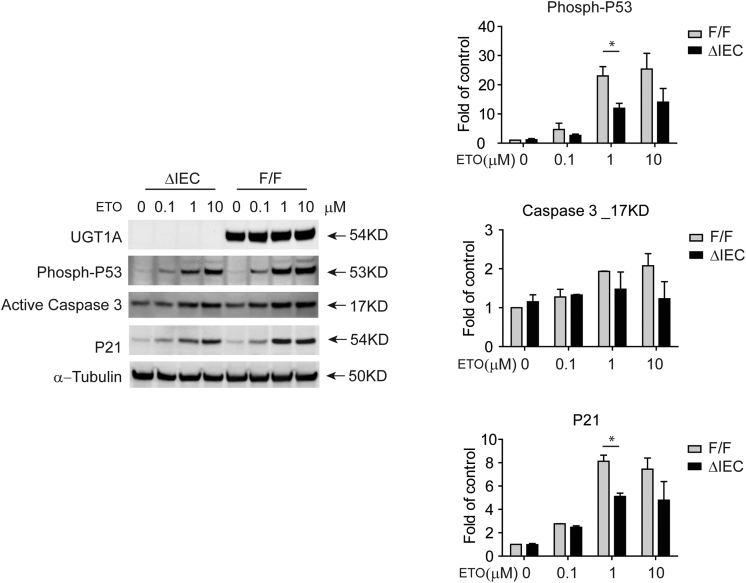
To examine whether UGT1 protein expression has an impact on sustaining p53 activation through phosphorylation and the apoptosis markers in normal epithelial cells, intestinal crypt cells from Ugt1ΔIEC and Ugt1F/F mice were cultured and treated with different concentrations of etoposide (Figure 3). Targeted knockout of the Ugt1 locus in Ugt1ΔIEC crypt cells led to a reduction in etoposide activated phospho-p53 when compared with the same treatments with Ugt1F/F crypt cells. We observed a dose-dependent induction with a maximal response at 1 μM etoposide in Ugt1F/F mice. A similar profile was observed in Ugt1ΔIEC mice, with a statistically significant reduction at 1 μM. Because induction of p53 in both Ugt1F/F and Ugt1ΔIEC mice plateaued at 1 μM etoposide, statistical significance was lost at 10 μM.
Figure 3.
Ugt1 knockout in intestinal crypt stem cells reduces p53 activation by etoposide. Intestinal crypt stem cells were cultured from Ugt1F/F and Ugt1ΔIEC adult mice. After passage, approximately 500–1000 cells per 50 μl of Matrigel per well were cultured in 24-well plates. Four days later, cells were exposed to fresh medium containing vehicle control or different concentrations of etoposide (ETO). Twenty four hours after ETO exposure, cells were collected in RIPA buffer. Whole cell extracts were prepared and protein concentrations were quantitated. Thirty μg of protein were used for gel electrophoresis and Western blot analysis. Primary antibodies directed towards UGT1A proteins (Santa Cruz Biotechnology), phosphorylated P53 (Ser 15, Cell Signaling Technology), p21 (Cell Signaling Technology), and active Caspase 3 (Cell Signaling Technology) were used. Shown is a representation of multiple Western blots. Quantitation of multiple Western blots was performed by density analysis visualized by a ChemiDoc Touch Imaging System (Bio-Rad Laboratories). Each band was normalized to α-tubulin and expressed as “Fold of control”. Since induction patterns were being compared between two groups, the Ugt1F/F and Ugt1ΔIEC mice, statistical comparisons were performed using two-way Anova followed by the Benferroni correction using Graphpad Prism. *P < .05.
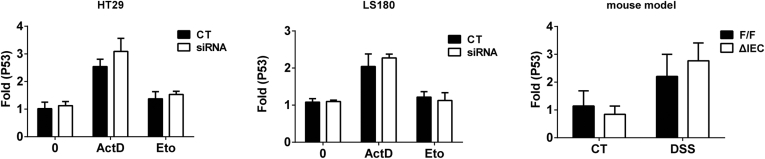
Similar reduction patterns in apoptosis markers caspase 3 and p21 were also evident in Ugt1ΔIEC mice, but there was a lack of statistical significance with caspase 3. The phospho-p53 measured in these experiments is wild type and not mutated p53, indicating that the impact of DNA damage and phosphorylation of p53 activation is linked to expression of the UGT1A proteins in normal epithelial cells. In addition, because induction of p53 protein expression does not result from transcriptional activation of p53 after etoposide treatment (Supplementary Figure 2), expression of the UGT1A proteins may interfere with post-translational modulation of the phosphorylation of p53.
Supplementary Figure 2.
P53 gene expression analysis. Human HT29 and LS180 cells treated with actinomycin D or etoposide after siRNA knockdown of the UGT1 locus were used to quantitate by real time PCR gene expression of the p53 gene. Similar quantitation was performed using intestinal RNA from Ugt1F/F and Ugt1ΔIEC mice after DSS treatment to induce intestinal inflammation.
Apoptosis Is Differentially Induced in Colons of Ugt1ΔIEC and Ugt1F/F Mice That Developed Acute Colitis With Dextran Sodium Sulfate Treatment
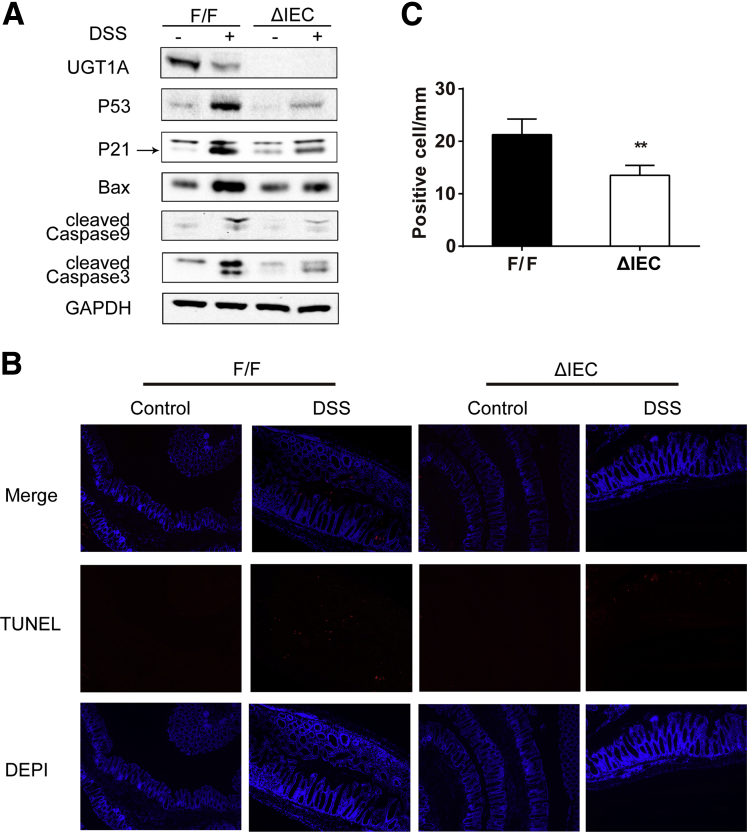
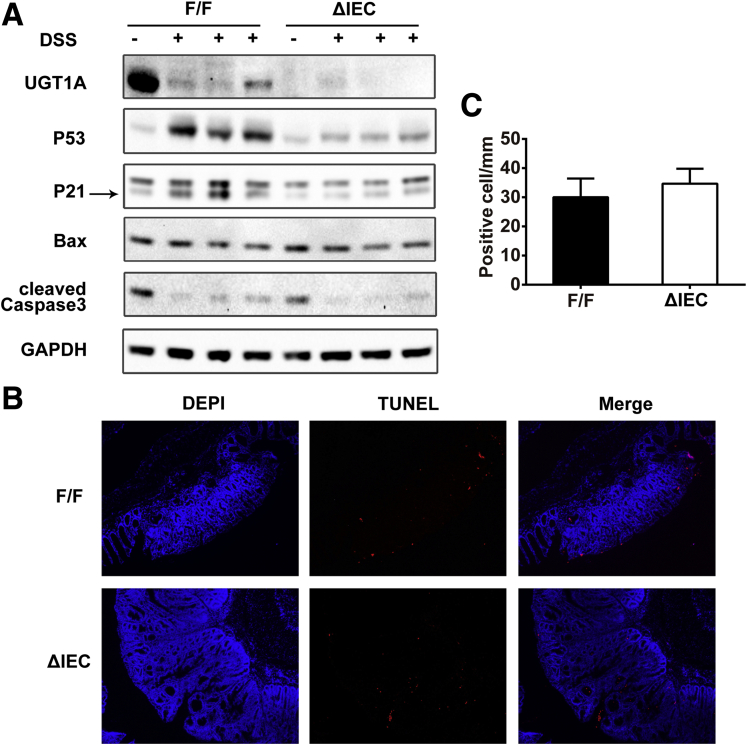
As a well-established acute colitis model in mice, DSS through oral administration induces inflammation and a high level of intestinal epithelial cell (IEC) apoptosis in colorectum.28 It is also appreciated that DSS-induced colonic apoptosis is dependent upon p53.29 With the recent development of a mouse model targeting deletion of the Ugt1 locus in the IECs (Ugt1ΔIEC mice),14 we treated control (Ugt1F/F) and Ugt1ΔIEC mice with 3% DSS for 5 days. The induction of inflammation leads to a decrease in UGT1A expression (Figure 4A), indicating that chemically induced p53 activation is independent of UGT1A1 expression. Compared with mice not given DSS, DSS-treated mice exhibited significant increases in the levels of p53, p21, Bax, caspase-3, and caspase-9, accompanied by abundant apoptotic cells in colon tissues detected by the TUNEL assay (see Figure 4B).
Figure 4.
The impact of DSS induced colitis on p53 and apoptotic cells in Ugt1ΔIECmice.Ugt1ΔIEC and Ugt1F/F mice were treated with 3% DSS in drinking water for 5 days to induce acute colitis and IEC apoptosis. After another 5 days recovery with regular water, colon tissues were collected. (A) Expression of UGT1A, p53, p21, Bax, caspase-9, and caspase-3 detected by Western blot anaylsis. GAPDH was used as a loading control. Quantitation of each Western blot is presented in Supplementary Figure 3. (B) TUNEL staining of colons from untreated mice or DSS-treated mice (100X Magnification). At least four mice were analyzed in each group and the experiment was repeated twice. (C) Quantitation of the number of TUNEL positive cells in DSS-treated mice.
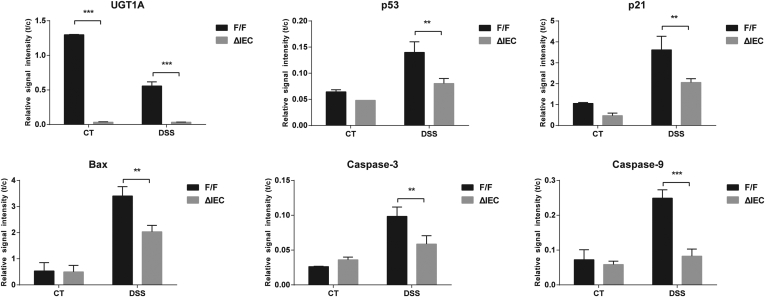
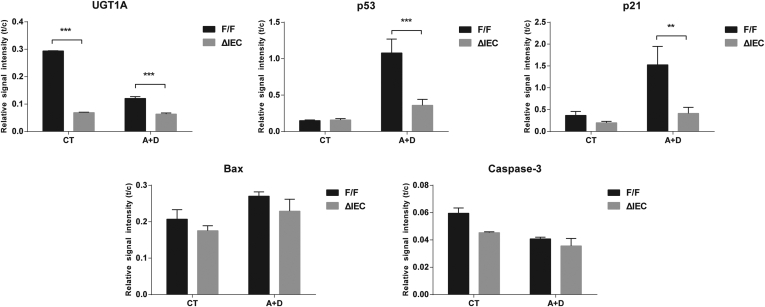
When a comparison was made between Ugt1F/F and Ugt1ΔIEC mice, DSS-induced p53 and p53-dependent target gene products along with apoptotic biomarkers were significantly reduced in colons of Ugt1ΔIEC mice (Supplementary Figure 3). By counting apoptotic epithelial cells in colon, the TUNEL assay showed that apoptosis occurred widely in both differentiated cells on the surface plateau and cells in the crypt proliferative zones in Ugt1F/F mice. However, DSS-treated Ugt1ΔIEC mice displayed a 38% decrease in the number of apoptotic cells (Figure 4B and C), indicating the reduction of p53-dependent apoptosis in the absence of Ugt1a gene expression.
Supplementary Figure 3.
Western blot quantitation after treatment with DSS. After treatment with DSS, colon tissue was collected from Ugt1F/F and Ugt1ΔIEC mice and Western blots performed on total cell extracts (Figure 4). Quantitation of the proteins were conducted and plotted as relative values from three separate experiments.
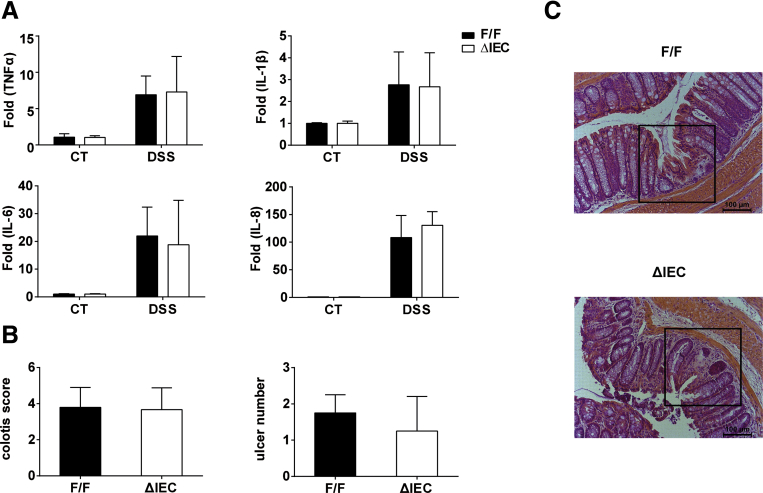
It is interesting to note that although DSS-induced acute colitis is believed to be through inflammatory responses by exposing innate immune cells in the lamina propria to bacteria,30 we found that the extent of colonic inflammation was not correlated to the level of IEC apoptosis between DSS-treated Ugt1ΔIEC and Ugt1F/F mice. When Ugt1F/F mice were treated with DSS, apoptosis in the IEC was accompanied by severe inflammation and immune activation as the colitis scores and the levels of proinflammatory markers, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-8, were increased in colon tissues (Supplementary Figure 4). Yet DSS-treated Ugt1ΔIEC mice, exhibiting lower levels of colonic apoptosis, had similar colitis scores and expression levels of the proinflammatory cytokines TNFα, IL-1β, IL-6, and IL-8 in colon tissues. Colon sections also showed similar morphologic features and ulcer numbers in response to DSS treatment, as evidenced by H&E staining (Supplementary Figure 4C and D), indicating no significant differences in inflammatory responses between Ugt1ΔIEC and Ugt1F/F mice. These data suggest that Ugt1 knockout in the IEC did not influence DSS-induced colon inflammation, and the alteration of apoptosis levels may be largely mediated through p53-dependent signaling.
Supplementary Figure 4.
No marked changes in the severity of ulcer or inflammatory gene expression in colon tissues between Ugt1ΔIECand Ugt1F/Fmice after DSS treatment. Colon tissues from 3% DSS-treated or untreated Ugt1ΔIEC and Ugt1F/F mice were used to prepare total samples of total RNA. (A) Expression of inflammatory genes was quantitated by real-time PCR, and normalized to the level of cyclophilin mRNA. (B) Colitis score quantified by stool occult blood, rectal prolapse, and diarrhea. Results are presented as mean ± standard deviation of at least four mice. (C) Quantification of the number of ulcers by analyzing H&E-stained sections. (D) Colon ulcers are shown by H&E staining (200× magnification).
Increased Colorectal Tumorigenesis in Ugt1ΔIEC Mice With Azoxymethane Plus Dextran Sodium Sulfate Treatment
Inactivation of p53 and decreased levels of apoptosis have been associated with a higher risk of tissue neoplastic transformation. In particular, loss of p53 has been linked to enhancing the incidence and multiplicity of colitis-associated neoplasia.31, 32 Because DSS-induced p53 was diminished in colons of Ugt1ΔIEC mice, we further examined the impact of Ugt1 deletion on CRC formation.
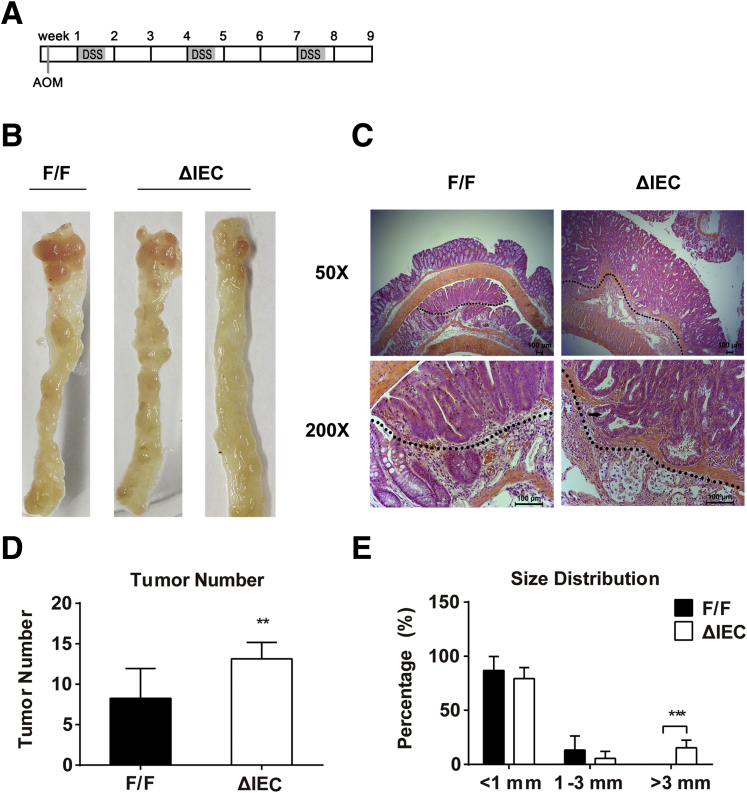
To initiate colon tumorigenesis, Ugt1F/F and Ugt1ΔIEC mice were injected with a single dose of AOM (10 mg/kg), a procarcinogen that induces DNA adduct formation after its metabolic activation by CYP2E1.33 Subsequently, mice were treated with three cycles of 2% DSS administration in drinking water to induce colitis (Figure 5A).34 After 9 weeks of treatment, both groups of mice, regardless of genotype, developed colorectal tumors, which were mostly located in the middle to distal colon sections (see Figure 5B). Strikingly, Ugt1ΔIEC mice had a greater tumor number and larger tumor size than Ugt1F/F mice in response to AOM and DSS treatment. The number of detectable tumors was 62% higher in Ugt1ΔIEC mice than in control Ugt1F/F mice (see Figure 5B and D). In addition, approximately 15% of the tumors in the Ugt1ΔIEC mice were >3 mm in diameter whereas none of the Ugt1F/F mice developed tumors >3 mm (see Figure 5E). When we performed histologic examinations, we found that tumors were adenomas with high-grade dysplasia and inflammatory cell infiltration (see Figure 5C).
Figure 5.
AOM plus DSS treatment causes an increased incidence and increased size of tumors in the colorectum of Ugt1ΔIECmice compared to Ugt1F/Fmice. (A) A schematic schedule is shown as the AOM plus DSS feeding protocol. Each rectangle represents one week. (B) Photos of colon tumors in Ugt1F/F and Ugt1ΔIEC mice. (C) Representative tumor morphologies in colonic sections by H&E staining (50× and 200× magnification). The black arrow indicates the penetrating adenoma. (D) Tumor incidence and (E) size distribution in Ugt1F/F and Ugt1ΔIEC mice. At least seven mice were analyzed in each group, and results are presented as mean ± standard deviation (*P < .05, **P < .01, ***P < .001).
Western blot analysis showed that AOM plus DSS treatment led to a reduction in UGT1A expression in colonic tumor tissues of Ugt1F/F mice, a phenomenon previously observed in tumor tissue. Treatment with DSS after AOM initiation led to induced p53 and p21 in the control Ugt1F/F mice yet was abolished in the Ugt1ΔIEC mice (Figure 6A). These results were similar to what we observed in mice treated with one cycle of DSS alone, although there was no difference in the expression levels of Bax and cleaved caspase-3 between Ugt1F/F and Ugt1ΔIEC mice (see Figure 6A). Quantitation of these Western blots is presented in Supplementary Figure 5.
Figure 6.
Protein expression of p53 and p53-dependent genes and detection of apoptosis in the colorectum of Ugt1F/Fand Ugt1ΔIECmice with colorectal neoplasia after AOM and DSS treatment. Colorectum tissues from AOM plus DSS-treated Ugt1ΔIEC and Ugt1F/F mice were collected and analyzed. (A) Expression levels of UGT1A, p53, p21, Bax, and cleaved caspase-3 were examined by Western blot analysis with GAPDH as a loading control. Quantitation of the Western blots is shown in Supplementary Figure 5. (B) TUNEL staining of colon sections (100× magnification). (C) Quantification of the number of TUNEL positive cells.
Supplementary Figure 5.
Western blot quantitation after AOM (A) and DSS (D) treatment as outlined inFigure 6A.
The TUNEL assay showed that Ugt1F/F and Ugt1ΔIEC mice appeared to exhibit a similar level of apoptosis (see Figure 6B and C). These data suggest that UGT1A proteins are an important factor in determining p53 levels after initial activation and protecting IECs from tumorigenesis. However, the p53-dependent signaling may play a lesser role in controlling apoptosis during the late stage of tumor development.
Endoplasmic Reticulum Stress Is Involved in the Down-Regulation of p53 Caused by UGT1A Knockdown
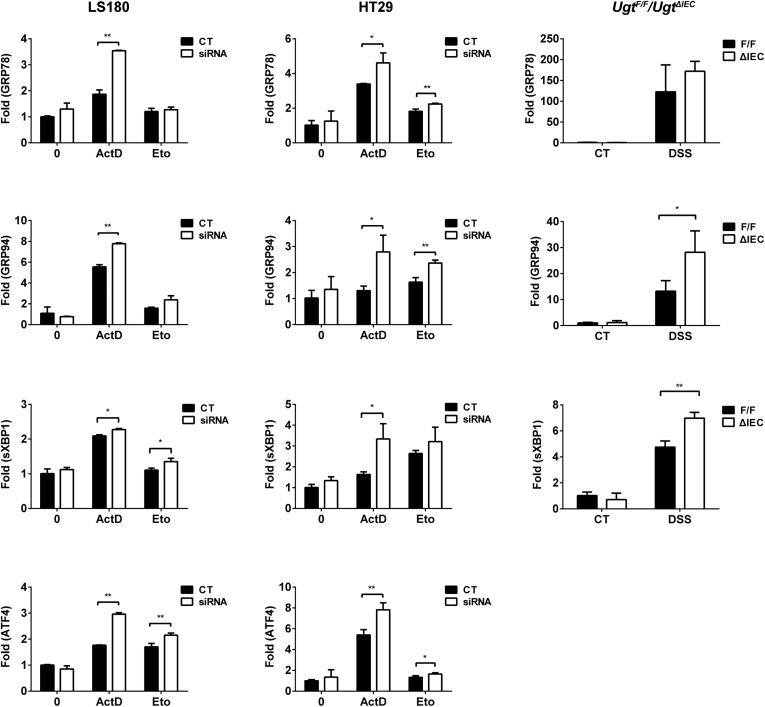
Enzymes localized in the endoplasmic reticulum (ER) play a vital role in maintaining ER homeostasis in response to a variety of challenges. Recent studies have indicated that there may be regulatory links between drug-transforming enzymes and ER stress responding to xenobiotic stimuli.35 It is also known that ER stress plays a role in accelerating p53 degradation and inhibiting cell apoptosis.36, 37 With the known and important association between p53 and ER stress, we elected to explore the involvement of ER stress in UGT1A-dependent p53 regulation by examining ER stress marker genes, including GRP78, GRP94, spliced XBP1, ATF4, by qRT-PCR (Figure 7).
Figure 7.
Loss of UGT1A potentiates ER stress induced by actinomycin D, etoposide, or DSS treatment. LS180 and HT29 cells were pretreated with UGT1A siRNA or nonspecific siRNA for 48 hours and then incubated with actinomycin D (ActD, 40 nM) or etoposide (Eto, 80 μM) for 36 hours. Ugt1ΔIEC and Ugt1F/F mice were treated with 3% DSS in drinking water and colon tissues were collected. Expression of ER stress-responsive genes was quantitated by Q-PCR.
In both intestinal HT29 and LS180 cell lines and the Ugt1ΔIEC mouse model, we found that actinomycin D, etoposide, and DSS, acting as ER stress-inducing agents, induced gene expression of all of the ER stress markers that we examined in addition to activating p53 induction. The knockdown of the Ugt1a genes further potentiated ER stress, resulting in significantly higher expression of ER stress markers (see Figure 7). These results indicate that UGT1A expression may have a role in maintaining ER homeostasis, particularly when the cells are under stress, and that increased levels of ER stress resulting from UGT1A knockdown may have a direct impact on expression and function of p53 after p53 activation has taken place.
Discussion
In healthy cells and organs, low p53 levels are maintained by Mdm2 through a negative-feedback loop.38 When cells sense stress signals, p53 can accumulate and transcriptionally regulate genes to control cell cycle and cell death. For example, p53 accumulation can lead to cell cycle arrest by inducing expression of p21, a cyclin-dependent kinase inhibitor. Accumulation of p53 is also required for the occurrence of apoptosis by inducing the expression of various proapoptotic genes, such as Bax, caspase-3, and caspase-9.39 The maintenance of the growth-inhibitory responses by elevated levels of p53 is essential for cells to adapt to the stress and accounts for p53-dependent antitumorigenesis activity.
In this study, we found that when intestinal cells are under stress, induced by actinomycin D or etoposide, UGT1A expression is required for the maintenance of elevated levels of p53, which determines whether the cell undergoes apoptosis and/or cell arrest. The absence of UGT1A expression, through siRNA silencing, disturbs this adaptive mechanism initiated by p53, leading to inhibition of up-regulation of p53-dependent target genes, such as proapoptotic genes caspase-3 and caspase-9, and to subsequent inhibition of apoptosis.
When an in vivo study was performed using DSS to induce colitis in colon tissue, we observed p53 accumulation accompanied by a higher incidence of apoptosis. However, when colitis is induced in mice deficient in intestinal Ugt1a gene expression, p53 accumulation is reduced and apoptosis is inhibited. More importantly, when challenged with AOM in combination with DSS, Ugt1ΔIEC mice exhibited a large increase in tumor multiplicity and size compared with control mice, indicating that activated p53 requires UGT1A to maintain and exert its function in protecting cells from tumorigenesis.
Down-regulation of UGT1A proteins occurs in solid tumors when compared with the surrounding healthy tissue.13, 14, 15, 16, 17 UGT down-regulation is also observed in inflammation-related diseased organs (e.g., intestine and liver).40, 41 Possible contributing factors that cause a decrease in UGT1A gene expression in tumor and/or diseased tissues have been reported to be epigenetic modulation, inflammatory factor-mediated inhibition, and reduction of xenobiotic nuclear receptor expression.42 These studies emphasize that the down-regulation of UGT1A genes is the result of the disease state in various tissues and its impact on the detoxification capacity of xenobiotics, especially cancer drugs.
By comparison, we report that under stress, UGT1A expression is a key factor that determines p53-dependent cell cycle regulation and cell death and has a direct impact on antitumorigenesis in colon tissue. Our result is of significance because it indicates that UGT1A proteins play a role in cell protection through both chemical detoxification and—reported here for the first time—maintenance of p53 activation in response to cellular stress. Reduced expression of Ugt1a genes in the diseased colon tissue not only leads to lower detoxification capacity for their substrates, it also exacerbates the disease condition by inhibiting p53-dependent proapoptosis and/or cell arrest. This finding may explain that the patients with inflammatory diseases often have a higher risk of developing cancer43 because lower UGT1A expression in the disease condition and subsequent inactivation of the p53-dependent antitumorigenesis pathway may be one of the contributing mechanisms that account for the tumorigenic action. We speculate that UGT1A activity may have a global effect on maintaining p53 activation in various tissues when cells encounter stress.
A central mechanism linking UGT1A expression and sustaining activated p53 is unclear. We explored the involvement of ER stress because it has been shown to induce p53 cytoplasmic localization and degradation, preventing p53-dependent apoptosis.36, 37, 44 ER stress is activated by physiologic conditions such as nutrient deprivation or by xenobiotic and drug toxicities, leading to the accumulation of unfolded proteins.45 The generation of unfolded proteins stimulates an adaptive process, the unfolded protein response that leads to the activation of cellular machinery needed to repair the unfolded proteins. Interestingly, recent studies indicate that there may be a regulatory link between ER stress and biotransformation enzymes: in vivo and in vitro experiments revealed that overexpression or induction of CYPs can cause up-regulation of the unfolded protein response.35 Because the UGTs play a key role in xenobiotic metabolism and cellular protection against toxicants, regulatory links controlling the UGTs, p53, and ER stress may be an important network during tissue neoplastic transformation.
We have observed that when colon cells are under stress, p53 elevation is accompanied by perturbation of ER homeostasis reflected by induction of ER-resident proteins. Loss of UGT1A expression, particularly in DSS-treated Ugt1ΔIEC mice, potentiates ER stress while dramatically down-regulating the expression of p53. Alongside our results, previous studies have shown that ER stress promotes p53 degradation in the cytoplasm by the ER-resident ubiquitin ligase synoviolin.44 We can speculate that since the UGT1A proteins and Synoviolin are located in the ER, further investigation as to the novel role of the UGT1A proteins in down-regulating synoviolin-generated ubiquitin ligase targeting of p53 would shed light on how the absence of UGT1A proteins could potentially lead to ER-associated p53 degradation.
The UGTs exist as dimers in the ER and form both momomeric and heterodimer complexes.46 The UGTs have also been shown to dimerize with other ER-based proteins, such as the cytochrome P450s.47, 48, 49, 50 Because knockout of the UGT1A proteins during stress in colon tissue leads to decreases in p53 abundance, the possibility exists that the UGTs may interact through protein–protein interactions and play an important role in controlling activation of ER-localized synoviolin, which controls the levels of phosphorylated cytoplasmic p53. Our working model of this study is illustrated in Figure 8.
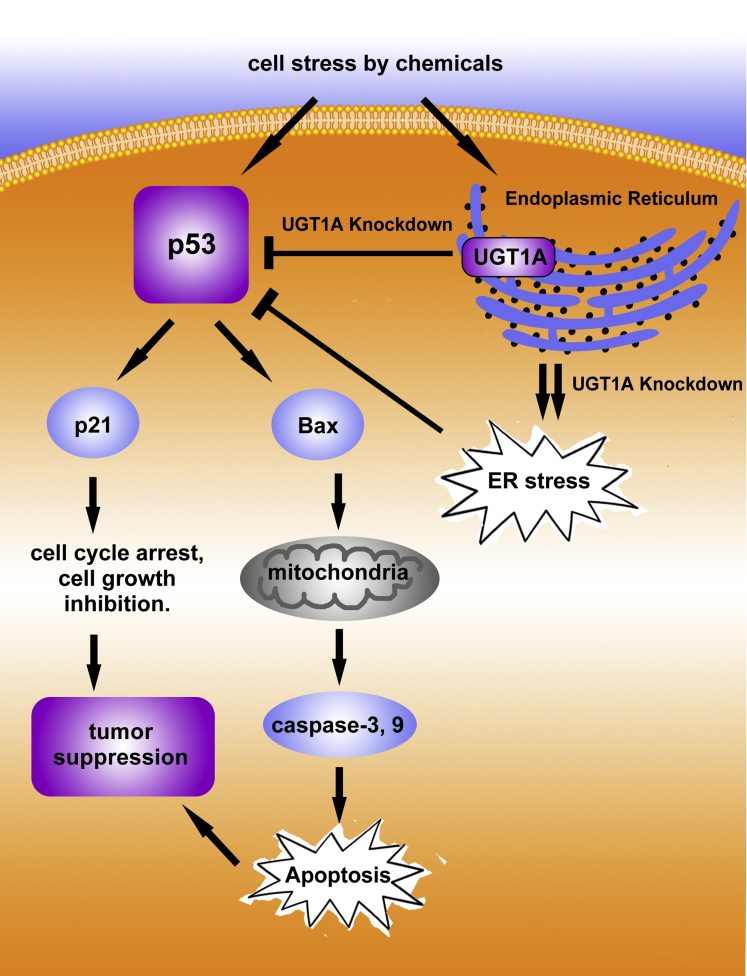
Figure 8.
A working model that demonstrates control of apoptosis and tumorigenesis by UGT1A proteins through regulation of p53. In this model, we are showing that p53 when activated can induce apoptosis and repress tumor formation. After p53 activation by cellular or chemical stress the presence of UGT1A proteins in the endoplasmic reticulum (ER) is required to sustain and maintain p53 levels. Knockdown of the UGT1 locus and the resulting UGT1A proteins increases ER stress leading to inhibition of p53 activation/elevation and subsequent reduction in apoptosis and tumor suppression.
To achieve proper function, p53 is tightly regulated by means of post-translational modifications, cofactor binding, and subcellular localization. For example, a classic p53 regulator Mdm2, an E3 ubiquitin ligase, inactivates p53 by accelerating its nuclear export and degradation when cells are under a basal condition.38 Our findings suggest that UGT1A genes act as an important p53 regulator by maintaining p53 elevation after cellular stress. Studies have shown that when p53 is activated, post-transcriptional modifications such as p53 phosphorylation and acetylation can protect p53 from degradation.38 This statement is consistent with our finding that p53 protein phosphorylation expression was down-regulated with unchanged transcriptional activity (Supplementary Figure 5), as we observed a loss of UGT1A expression after actinomycin D, etoposide, and DSS treatment.
As a tumor suppressor gene, p53 is involved in tumorigenesis through one of three mechanisms: (1) Complete loss of wild-type p53 leading to dysregulation of cell cycle and cell death; (2) suppression of normal function of the wild type p53 by mutant p53; and (3) pro-oncogenic properties exerted by the mutant p53.51 In our study, the tumorigenic action elicited by loss of UGT1A expression is most likely through inhibition of wild-type p53. Our results imply that, at least in certain tissues and conditions, UGT1A levels may be a reliable clinical marker that can predict the tumorigenic potential of these tissues. In summary, this study provides compelling evidence that UGT1A expression is essential to maintain p53 accumulation, promoting apoptosis and preventing cells from undergoing tumorigenesis in the disease state.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was funded part by U.S. Public Health Service Grants ES010337, GM086713, GM100481 (to R.H.T.), and R21CA171008 (to S.C.). In addition, this study was partially funded by Jiangsu Provincial Promotion Foundation for the Key Laboratory of Dug Metabolism and Pharmacokinetics (BM2012012), the Natural Science Foundation of Jiangsu Province for Outstanding Youth Scholars (BK2012026), the National Natural Science Foundation China (No.81430091, 81325025, and 81273586), and a fellowship from China Scholarship Council (to M.L., G.W., and H.H.).
Contributor Information
Haiping Hao, Email: hhp_770505@hotmail.com.
Robert H. Tukey, Email: rtukey@ucsd.edu.
Supplementary Material
Supplementary Table 1.
Sequences of the primers used in the study
| Primer | Forward | Reverse |
|---|---|---|
| TNFα | 5ʹ-CATCTTCTCAAAATTCGAGTGACAA-3ʹ | 5ʹ-TGGGAGTAGACAAGGTACAACCC-3ʹ |
| IL-1β | 5ʹ-GCAACTGTTCCTGAACTCAACT-3ʹ | 5ʹ-ATCTTTTGGGGTCCGTCAACT-3ʹ |
| IL-6 | 5ʹ-GAGGATACCACTCCCAACAGACC-3ʹ | 5ʹ-AAGTGCATCATCGTTGTTCATACA-3ʹ |
| IL-8 | 5ʹ- ATGCCCTCTATTCTGCCAGAT-3ʹ | 5ʹ-GTGCTCCGGTTGTATAAGATGAC-3ʹ |
| Human P53 | 5ʹ-GAGGTTGGCTCTGACTGTACC-3ʹ | 5ʹ-TCCGTCCCAGTAGATTACCAC-3ʹ |
| Human ATF-4 | 5ʹ-TTAAGCCATGGCGCTTCTCA-3ʹ | 5ʹ-GGTCGAAGGGGGACATCAAG-3ʹ |
| Human sxbp-1 | 5ʹ-TTAAGACAGCGCTTGGGGAT-3ʹ | 5ʹ-GCAGAGGTGCACGTAGTCTGA-3ʹ |
| Human GRP-78 | 5ʹ-CCCGTGGCATAAACCCAGAT-3ʹ | 5ʹ-TGGTAGGCACCACTGTGTTC-3ʹ |
| Human GRP-94 | 5ʹ-GCCAGTTTGGTGTCGGTTTC-3ʹ | 5ʹ-GGGTAATTGTCGTTCCCCGT-3ʹ |
| Human actin | 5ʹ-GGCGGCACCACCATGTACCCT-3ʹ | 5ʹ-AGGGGCCGGACTCGTCATACT-3ʹ |
| Mouse p53 | 5ʹ-CCAAACTGCTAGCTCCCATCA-3ʹ | 5ʹ-GGCCCCACTTTCTTGACCAT-3ʹ |
| Mouse ATF-4 | 5ʹ-AATTCGTCAACGAGCGATCC-3ʹ | 5ʹ-CTGCTGCCTCTAATACGCCA-3ʹ |
| Mouse sxbp-1 | 5ʹ-ATCGCAGGGAGGGTCATTTG-3ʹ | 5ʹ-TGGGGTCAAGAGGGTCAGAA-3ʹ |
| Mouse GRP-78 | 5ʹ-CGATACTGGCCGAGACAACA-3ʹ | 5ʹ-GGAGGAGACACGAAGCAGAC-3ʹ |
| Mouse GRP-94 | 5ʹ-ACCGAAAAGGACTTGCGACT-3ʹ | 5ʹ-AGCCTTCTCGGCTTTTACCC-3ʹ |
| Mouse cyclophilin | 5ʹ-CAGACGCCACTGTCGCTTT-3ʹ | 5ʹ-TGTCTTTGGAACTTTGTCTGCAA-3ʹ |
References
- 1.Rim S.H., Seeff L., Ahmed F. Colorectal cancer incidence in the United States, 1999–2004: an updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1967–1976. doi: 10.1002/cncr.24216. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Shin H.R., Bray F. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton S.R., Aaltoned L.A.E. IARC Press; Lyon, France: 2000. World Health Organization classification of tumours. pathology of genetics of tumours of the digestive system. [Google Scholar]
- 4.Butler L.M., Sinha R., Millikan R.C. Heterocyclic amines, meat intake, and association with colon cancer in a population-based study. Am J Epidemiol. 2003;157:434–445. doi: 10.1093/aje/kwf221. [DOI] [PubMed] [Google Scholar]
- 5.Jamin E.L., Riu A., Douki T. Combined genotoxic effects of a polycyclic aromatic hydrocarbon (B(a)P) and an heterocyclic amine (PhIP) in relation to colorectal carcinogenesis. PLoS One. 2013;8:e58591. doi: 10.1371/journal.pone.0058591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overman M.J., Pozadzides J., Kopetz S. Immunophenotype and molecular characterisation of adenocarcinoma of the small intestine. Br J Cancer. 2010;102:144–150. doi: 10.1038/sj.bjc.6605449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters W.H.M., Kock L., Nagengast F.M. Biotransformation enzymes in human intestine: critical low levels in the colon. Gut. 1991;32:408–412. doi: 10.1136/gut.32.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergheim I., Bode C., Parlesak A. Decreased expression of cytochrome P450 protein in non-malignant colonic tissue of patients with colonic adenoma. BMC Gastroenterol. 2005;5:34. doi: 10.1186/1471-230X-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler L.M., Duguay Y., Millikan R.C. Joint effects between UDP-glucuronosyltransferase 1A7 genotype and dietary carcinogen exposure on risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1626–1632. doi: 10.1158/1055-9965.EPI-04-0682. [DOI] [PubMed] [Google Scholar]
- 10.Fang J.L., Beland F.A., Doerge D.R. Characterization of benzo(a)pyrene-trans-7,8-dihydrodiol glucuronidation by human tissue microsomes and overexpressed UDP-glucuronosyltransferase enzymes. Cancer Res. 2002;62:1978–1986. [PubMed] [Google Scholar]
- 11.Bushey R.T., Dluzen D.F., Lazarus P. Importance of UDP-glucuronosyltransferases 2A2 and 2A3 in tobacco carcinogen metabolism. Drug Metab Dispos. 2013;41:170–179. doi: 10.1124/dmd.112.049171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoensch H.P., Roelofs H.M., Edler L. Disparities of conjugating protective enzyme activities in the colon of patients with adenomas and carcinomas. World J Gastroenterol. 2013;19:6020–6025. doi: 10.3748/wjg.v19.i36.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giuliani L., Ciotti M., Stoppacciaro A. UDP-glucuronosyltransferases 1A expression in human urinary bladder and colon cancer by immunohistochemistry. Oncol Rep. 2005;13:185–191. [PubMed] [Google Scholar]
- 14.Chen S., Yueh M.F., Bigo C. Intestinal glucuronidation protects against chemotherapy-induced toxicity by irinotecan (CPT-11) Proc Natl Acad Sci U S A. 2013;110:19143–19148. doi: 10.1073/pnas.1319123110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strassburg C.P., Manns M.P., Tukey R.H. Differential down regulation of the UDP-glucuronosyltransferase 1A locus is an early event in human liver and biliary cancer. Cancer Res. 1997;57:2979–2985. [PubMed] [Google Scholar]
- 16.Starlard-Davenport A., Lyn-Cook B., Radominska-Pandya A. Identification of UDP-glucuronosyltransferase 1A10 in non-malignant and malignant human breast tissues. Steroids. 2008;73:611–620. doi: 10.1016/j.steroids.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izumi K., Li Y., Ishiguro H. Expression of UDP-glucuronosyltransferase 1A in bladder cancer: association with prognosis and regulation by estrogen. Mol Carcinog. 2014;53:314–324. doi: 10.1002/mc.21978. [DOI] [PubMed] [Google Scholar]
- 18.Ariyoshi N., Imaoka S., Nakayama K. Comparison of the levels of enzymes involved in drug metabolism between transgenic or gene-knockout and the parental mice. Toxicol Pathol. 2001;29(Suppl):161–172. doi: 10.1080/019262301753178573. [DOI] [PubMed] [Google Scholar]
- 19.Hu D.G., Rogers A., Mackenzie P.I. Epirubicin upregulates UDP glucuronosyltransferase 2B7 expression in liver cancer cells via the p53 pathway. Mol Pharmacol. 2014;85:887–897. doi: 10.1124/mol.114.091603. [DOI] [PubMed] [Google Scholar]
- 20.Levine A.J., Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vousden K.H., Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X.P., Liu F., Cheng Z. Cell fate decision mediated by p53 pulses. Proc Natl Acad Sci USA. 2009;106:12245–12250. doi: 10.1073/pnas.0813088106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian X.J., Liu F., Zhang X.P. A two-step mechanism for cell fate decision by coordination of nuclear and mitochondrial p53 activities. PLoS One. 2012;7:e38164. doi: 10.1371/journal.pone.0038164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt C.A., Fridman J.S., Yang M. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell. 2002;1:289–298. doi: 10.1016/s1535-6108(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 25.Sato T., Vries R.G., Snippert H.J. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 26.Meley D., Spiller D.G., White M.R. p53-mediated delayed NF-kappaB activity enhances etoposide-induced cell death in medulloblastoma. Cell Death Dis. 2010;1:e41. doi: 10.1038/cddis.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao B., van Leeuwen I.M., Higgins M. Evaluation of an Actinomycin D/VX-680 aurora kinase inhibitor combination in p53-based cyclotherapy. Oncotarget. 2010;1:639–650. doi: 10.18632/oncotarget.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araki Y., Mukaisyo K., Sugihara H. Increased apoptosis and decreased proliferation of colonic epithelium in dextran sulfate sodium-induced colitis in mice. Oncol Rep. 2010;24:869–874. doi: 10.3892/or.2010.869. [DOI] [PubMed] [Google Scholar]
- 29.Dirisina R., Katzman R.B., Goretsky T. p53 and PUMA independently regulate apoptosis of intestinal epithelial cells in patients and mice with colitis. Gastroenterology. 2011;141:1036–1045. doi: 10.1053/j.gastro.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okayasu I., Hatakeyama S., Yamada M. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 31.Fujii S., Fujimori T., Kawamata H. Development of colonic neoplasia in p53 deficient mice with experimental colitis induced by dextran sulphate sodium. Gut. 2004;53:710–716. doi: 10.1136/gut.2003.028779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang W.C., Coudry R.A., Clapper M.L. Loss of p53 enhances the induction of colitis-associated neoplasia by dextran sulfate sodium. Carcinogenesis. 2007;28:2375–2381. doi: 10.1093/carcin/bgm134. [DOI] [PubMed] [Google Scholar]
- 33.Sohn O.S., Fiala E.S., Requeijo S.P. Differential effects of CYP2E1 status on the metabolic activation of the colon carcinogens azoxymethane and methylazoxymethanol. Cancer Res. 2001;61:8435–8440. [PubMed] [Google Scholar]
- 34.Tanaka T. Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int J Inflam. 2012;2012:658786. doi: 10.1155/2012/658786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cribb A.E., Peyrou M., Muruganandan S. The endoplasmic reticulum in xenobiotic toxicity. Drug Metab Rev. 2005;37:405–442. doi: 10.1080/03602530500205135. [DOI] [PubMed] [Google Scholar]
- 36.Qu L., Huang S., Baltzis D., Rivas-Estilla A.M. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3β. Genes Dev. 2004;18:261–277. doi: 10.1101/gad.1165804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pluquet O., Qu L.K., Baltzis D. Endoplasmic reticulum stress accelerates p53 degradation by the cooperative actions of Hdm2 and glycogen synthase kinase 3β. Mol Cell Biol. 2005;25:9392–9405. doi: 10.1128/MCB.25.21.9392-9405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferecatu I., Rincheval V., Mignotte B. Tickets for p53 journey among organelles. Front Biosci (Landmark Ed) 2009;14:4214–4228. doi: 10.2741/3524. [DOI] [PubMed] [Google Scholar]
- 39.He Z., Ma W.Y., Hashimoto T. Induction of apoptosis by caffeine is mediated by the p53, Bax, and caspase 3 pathways. Cancer Res. 2003;63:4396–4401. [PubMed] [Google Scholar]
- 40.Congiu M., Mashford M.L., Slavin J.L. UDP glucuronosyltransferase mRNA levels in human liver disease. Drug Metab Dispos. 2002;30:129–134. doi: 10.1124/dmd.30.2.129. [DOI] [PubMed] [Google Scholar]
- 41.Langmann T., Moehle C., Mauerer R. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127:26–40. doi: 10.1053/j.gastro.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Kacevska M., Downes M.R., Sharma R. Extrahepatic cancer suppresses nuclear receptor-regulated drug metabolism. Clin Cancer Res. 2011;17:3170–3180. doi: 10.1158/1078-0432.CCR-10-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamasaki S., Yagishita N., Nishioka K. The roles of synoviolin in crosstalk between endoplasmic reticulum stress-induced apoptosis and p53 pathway. Cell Cycle. 2007;6:1319–1323. doi: 10.4161/cc.6.11.4277. [DOI] [PubMed] [Google Scholar]
- 45.Harding H.P., Calfon M., Urano F. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 46.Operana T.N., Tukey R.H. Oligomerization of the UDP-glucuronosyltransferase 1A proteins. J Biol Chem. 2007;282:4821–4829. doi: 10.1074/jbc.M609417200. [DOI] [PubMed] [Google Scholar]
- 47.Takeda S., Ishii Y., Mackenzie P.I. Modulation of UDP-glucuronosyltransferase 2B7 function by cytochrome P450s in vitro: differential effects of CYP1A2, CYP2C9 and CYP3A4. Biol Pharm Bull. 2005;28:2026–2027. doi: 10.1248/bpb.28.2026. [DOI] [PubMed] [Google Scholar]
- 48.Ishii Y., Takeda S., Yamada H. Functional protein-protein interaction of drug metabolizing enzymes. Front Biosci. 2005;10:887–895. doi: 10.2741/1583. [DOI] [PubMed] [Google Scholar]
- 49.Ishii Y., Takeda S., Yamada H. Modulation of UDP-glucuronosyltransferase activity by protein-protein association. Drug Metab Rev. 2010;42:145–158. doi: 10.3109/03602530903208579. [DOI] [PubMed] [Google Scholar]
- 50.Konopnicki C.M., Dickmann L.J., Tracy J.M. Evaluation of UGT protein interactions in human hepatocytes: effect of siRNA down regulation of UGT1A9 and UGT2B7 on propofol glucuronidation in human hepatocytes. Arch Biochem Biophys. 2013 doi: 10.1016/j.abb.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim E., Deppert W. Interactions of mutant p53 with DNA: guilt by association. Oncogene. 2007;26:2185–2190. doi: 10.1038/sj.onc.1210312. [DOI] [PubMed] [Google Scholar]