Abstract
Background
Healthcare claims data may provide a cost-efficient approach for studying chronic kidney disease (CKD).
Study Design
Prospective cohort study.
Setting & Participants
We compared characteristics and outcomes for individuals with CKD defined using laboratory measurements versus claims data from 6,982 Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study participants who had Medicare fee-for-service coverage.
Predictors
Presence of CKD as defined by both the REGARDS Study (CKDREGARDS) and Medicare data (CKDMedicare), absence of CKD as defined by both, presence of CKDREGARDS but not CKDMedicare, and presence of CKDMedicare but not CKDREGARDS.
Outcomes
Mortality and incident end-stage renal disease (ESRD).
Measurements
The research study definition of CKD (CKDREGARDS) included estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73m2 or albumin-creatinine ratio (ACR) > 30 mg/g at the REGARDS study visit. CKD in Medicare (CKDMedicare) was identified during the two years before each participant’s REGARDS visit using a claims-based algorithm.
Results
Overall, 32% of participants had CKDREGARDS and 6% had CKDMedicare. The sensitivity, specificity, and positive and negative predictive values of CKDMedicare for identifying CKDREGARDS were 15.5% (95% CI, 14.0%–17.1%), 97.7% (95% CI, 97.2%–98.1%), 75.6% (95% CI, 71.4%–79.5%), and 71.5% (95% CI, 70.4%–72.6%), respectively. Mortality and ESRD incidence rates, expressed per 1,000 person-years, were higher for participants with versus without CKDMedicare (mortality: 72.5 [95% CI, 61.3–83.7] versus 33.3 [95% CI, 31.5–35.2]; ESRD: 16.4 [95% CI, 11.2–21.6] versus 1.3 [95% CI, 0.9–1.6]) and with versus without CKDREGARDS (mortality: 59.9 [95% CI, 55.4–64.4] versus 25.5 [95% CI, 23.6–27.4]; ESRD: 6.8 [95% CI, 5.4–8.3] versus 0.1 [95% CI, 0.0–0.3]). Among participants with CKDREGARDS, those with abdominal obesity, diabetes, anemia, a lower eGFR, more outpatient visits, a hospitalization and a nephrologist visit in the two years before their REGARDS visit were more likely to have CKDMedicare.
Limitations
CKDREGARDS relied on eGFR and albuminuria assessed at a single visit.
Conclusions
CKD, whether defined in claims or through research study measurements, was associated with increased mortality and ESRD. However, individuals with CKD identified in claims may represent a select high-risk population.
Index words: chronic kidney disease (CKD), health care claims data, sensitivity, specificity, predictive value, claims-based algorithm, albuminuria, estimated glomerular filtration rate (eGFR), end-stage renal disease (ESRD)
There has been substantial interest over the past few years in using healthcare claims data for conducting comparative effectiveness and safety research studies, quality improvement projects and public health surveillance.1–4 Several prior studies have evaluated the ability of claims data to identify individuals with chronic kidney disease (CKD). These studies have been summarized in two literature reviews that concluded claims data have low sensitivity and low negative predictive value (NPV) and high specificity for identifying CKD.5, 6 The positive predictive value (PPV) varied widely (range, 29%–100%) in the studies identified for the literature review.
One potential source for studying CKD in claims is the US Medicare program. Medicare provides health insurance for eligible US adults aged 65 years or older, a population with a high prevalence of CKD. Given that over 95% of older US adults have health insurance through Medicare, it provides a large population with high generalizability to the US population of older adults.7 One prior study evaluated the validity of Medicare claims for identifying individuals with CKD.8 The study used data on Medicare beneficiaries presenting to a hospital with a myocardial infarction and several claims algorithms to identify CKD patients, yielding test characteristics ranging from 3% to 27% for sensitivity, 93% to 99% for specificity, 89% to 97% for PPV, and 32% to 37% for NPV. Given the high PPV and high specificity, the authors concluded that patients identified in Medicare as having CKD most likely have it, and cohorts of older adults with CKD can be assembled using Medicare claims.
As claims-based algorithms have generally demonstrated low sensitivity, they do not identify the majority of individuals with CKD. Studies using Medicare claims data to identify populations with CKD could produce biased results if the characteristics of individuals with CKD identified through Medicare claims are systematically different from those who are not identified. To better understand the strengths and limitations of using claims data to study CKD, we determined whether (1) correlates of having CKD, (2) the risk for all-cause mortality and end-stage renal disease (ESRD) associated with having CKD, and (3) risk factors for all-cause mortality and ESRD among individuals with CKD were similar when CKD was defined using Medicare claims versus estimated glomerular filtration rate (eGFR) and albuminuria measured in a research study. Additionally, among participants with CKD defined using eGFR and albuminuria, we determined whether (4) the presence of Medicare claims for CKD differed by participant characteristics and co-morbid conditions and (5) the risk for all-cause mortality and ESRD differed for those with versus without CKD claims in Medicare.
METHODS
Study Participants
We conducted an analysis of participants enrolled in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study linked to Medicare claims data. REGARDS is a population-based cohort study of 30,239 adults aged 45 years or older from across the continental United States.9 Participants were enrolled from January 2003 through October 2007, and baseline data were collected during a telephone interview followed by an in-home study visit.
The REGARDS participants were linked to Medicare enrollment and claims data by social security number, gender, and date of birth. Medicare Part A covers hospital care, skilled nursing facility care, hospice, and home health services. Medicare Part B covers services or supplies that are needed to diagnose or treat medical conditions and preventive services such as to prevent illness or detect it at an early stage. Medicare Advantage plans include health plans offered by private companies that contract with Medicare to provide beneficiaries with coverage. Medicare Advantage plans include health maintenance organizations, preferred provider organizations, private fee-for-service plans, special needs plans, and Medicare medical savings account plans.
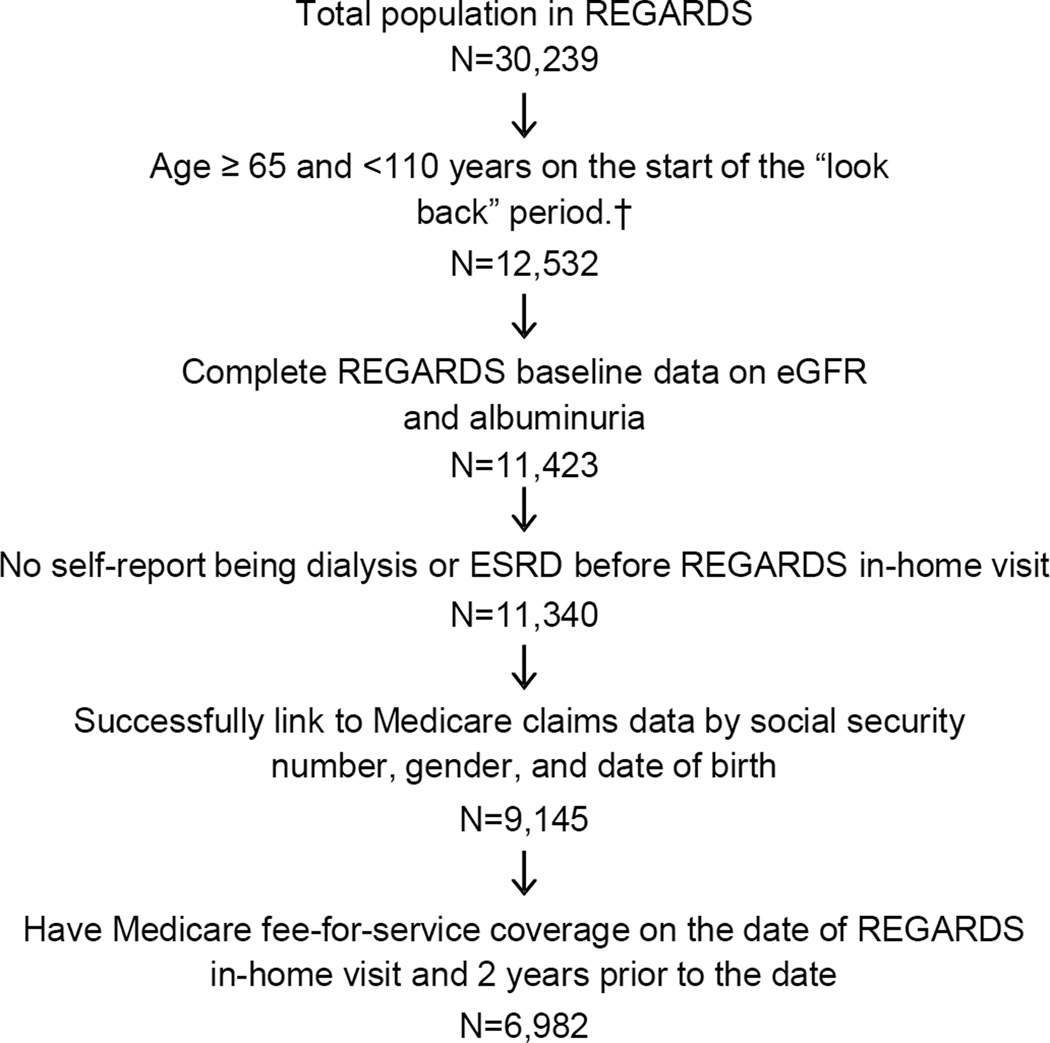
For this analysis, we included REGARDS participants who were aged 65 years or older at the beginning of a two-year look-back period (i.e., two years prior to the baseline REGARDS study visit) and provided a blood and urine sample during their in-home REGARDS study visit, had complete data for calculating eGFR and albumin-creatinine ratio (ACR), and had been living in the United States, continuously enrolled in Medicare parts A and B (fee-for-service hospital and outpatient coverage), but not in a Medicare Advantage plan for the two year look-back period.
We excluded participants enrolled in a Medicare Advantage plan as claims are not complete for these individuals. Additionally, we excluded participants who self-reported a history of ESRD at baseline or who had a record for ESRD in the US Renal Data System (USRDS) prior to their REGARDS baseline study visit.
A CONSORT diagram is provided in Figure 1. Overall, 6,982 participants met all of the inclusion criteria for this analysis. Institutional review boards of the collaborating institutions approved the REGARDS Study protocol and participants gave informed consent.
Figure 1.
Eligible cohort for identifying chronic kidney disease with claims data. †The look back period is the two years prior to each participant’s REGARDS study visit
Determination of CKD in the REGARDS Study (CKDREGARDS)
Serum creatinine assays, calibrated with an isotope dilution mass spectroscopic standard, were performed at the University of Vermont.10 The CKD-EPI (CKD Epidemiology Collaboration) creatinine equation was used to calculate eGFR.11 Results were similar using the Modification of Diet in Renal Disease (MDRD) Study equation and therefore not presented. Urinary albumin and creatinine were measured at the Department of Laboratory Medicine and Pathology at the University of Minnesota, using the BN ProSpec Nephelometer from Dade Behring (Marburg, Germany). The results were expressed for each participant as the ACR. For our primary analysis, CKD in the REGARDS study (CKDREGARDS) was defined as an eGFR < 60 ml/min/1.73 m2 or an ACR > 30 mg/g.12 In secondary analyses, we defined CKDREGARDS as an eGFR < 45 ml/min/1.73 m2 or an ACR > 300 mg/g. This definition of CKDREGARDS was used to capture a cohort of participants with more severe CKD.
Other Variables From the REGARDS Baseline Visit
Self-reported items determined in the REGARDS Study included age, race, gender, region of residence (West, Midwest, Northeast, and South), current smoking, family history of ESRD, history of coronary heart disease (CHD), history of diabetes, and use of antihypertensive, insulin or antidiabetes medications. During the REGARDS baseline study visit, blood pressure and anthropometric variables were measured. Blood pressure was measured two times and averaged for analysis. Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or current antihypertensive medication use. Waist circumference was measured mid-way between the lowest rib and the iliac crest using a tape measure with the participant standing; abdominal obesity was defined as levels > 88 cm for women and > 102 cm for men. Using blood collected during the baseline study visit, serum glucose and hemoglobin were measured. Anemia was defined as a hemoglobin level < 12 g/dL for women and < 13 g/dL for men. Diabetes mellitus was defined as fasting glucose level ≥ 126 mg/dL, non-fasting glucose ≥ 200 mg/dL, or self-report of a prior diagnosis of diabetes mellitus with current use of insulin or antidiabetes medication.
Medicare Claims
Medicare claims for the current analysis include those from the outpatient and inpatient settings. To identify CKD in Medicare claims (CKDMedicare), we abstracted Medicare claims during the two years prior to each participant’s REGARDS study visit (i.e., the look-back period). For the primary analysis, the algorithm for defining CKDMedicare follows that from the Centers for Medicare & Medicaid Services (CMS) chronic conditions warehouse (Item S1, available as online supplementary material).13–15 This algorithm uses ICD-9 discharge diagnosis codes associated with a hospitalization or physician evaluation and management claims associated with outpatient physician visits. Coding in Medicare is a two-step process. Physicians provide diagnostic and procedures that were performed and administrative staff translate these into codes for billing purposes. Modifications to ICD-9 codes are used in Medicare claims. Therefore, some of the codes used in the current study may not be present in other settings. In secondary analyses, we evaluated the sensitivity, specificity, PPV and NPV using a more narrow set of claims to define CKDMedicare (Item S2). The claims used in the secondary analyses were chosen by authors to be more specific in identifying individuals with CKD as opposed to acute or transient kidney problems. For each participant, we also identified the number of outpatient visits, hospitalizations, and the occurrence of a nephrologist visit from Medicare claims during the two-year look-back period.
Outcomes
Participants were followed for all-cause mortality and incident ESRD. Mortality, subsequent to the REGARDS Study in-home examination and through March 2012 was assessed through contact with proxies provided by the participant upon recruitment or during follow-up. If a proxy reported a participant had died, an interview was conducted with their next of kin. The REGARDS Study confirmed dates of death through the Social Security Death Index, death certificates, or the National Death Index. ESRD subsequent to the in-home examination and through September 2011 was assessed via linkage with the USRDS. The USRDS is a registry of ESRD and captures the vast majority of incident cases in the United States.16
Statistical Analysis
The sensitivity, specificity, PPV, and NPV of CKDMedicare during the look-back period was calculated, with CKDREGARDS considered the “gold standard”. Additionally, sensitivity, specificity, PPV and NPV for CKDMedicare was calculated for level of eGFR (<60, <45, and <30 ml/min/1.73 m2 and ACR > 30 and >300 mg/g). In a sensitivity analysis, we also calculated these test characteristics for CKDMedicare using Medicare claims from the one year before to one year after each participant’s REGARDS Study visit. Participant characteristics were calculated by the cross-classification of CKDMedicare and CKDREGARDS with the statistical significance of differences calculated using ANOVA, a Kruskal-Wallis or chi-square test. Next, hazard ratios for all-cause mortality and ESRD were calculated using Cox proportional hazards models comparing individuals with versus without CKDMedicare and, separately, with versus without CKDREGARDS. Hazard ratios for ESRD were calculated accounting for the competing risk of mortality using the method described by Fine and Gray.17 Three levels of adjustment were performed. An initial model included adjustment for age, race, and gender. A second model included additional adjustment for number of outpatient visits, nephrologist visits, hospitalization and Medicaid eligibility during the look-back period as identified in Medicare. A third model also adjusted for smoking, abdominal obesity, hypertension, diabetes, and history of CHD from the REGARDS study. To evaluate whether risk factors for outcomes differ for individuals identified as having CKD in claims versus in a research study, we calculated the age, race, sex adjusted hazard ratios for all-cause mortality and ESRD among individuals with CKDMedicare (n=451) and, separately, among those with CKDREGARDS (n=2,203).
Next, to evaluate the selective coding of CKD in claims data, we restricted the sample to participants with CKDREGARDS and calculated the proportion of participants with CKDMedicare by demographic factors, co-morbid conditions and Medicare variables. The prevalence ratios for having CKDMedicare associated with participant characteristics were also calculated. Also, among those with the CKDREGARDS, rates and hazard ratios for all-cause mortality and, separately, ESRD associated with having versus not having CKDMedicare were calculated. Prevalence ratios and hazard ratios were calculated with three levels of adjustment as described above. Additionally, we calculated the sensitivity, specificity, PPV and NPV for CKDMedicare using the secondary definition of CKDREGARDS (eGFR < 45 ml/min/1.73 m2 or ACR > 300 mg/g). The rates and hazard ratios for all-cause mortality and ESRD associated with having versus not having CKDMedicare among those with CKDREGARDS were also calculated using this secondary definition. Multiple imputation was conducted using chained equations to account for missing data from the REGARDS Study. Data were missing for <1% of all variables except history of CHD (1.7% missing) and anemia (38.0% missing) and family history of ESRD (35.6% missing). The high percentage of participants missing anemia and family history of ESRD occurred because these variables were added to REGARDS data collection in May 2004 (about one third through recruitment of the cohort). Analyses were conducted using SAS V9.3 (SAS Institute Inc, Cary, NC) and Stata/MP 12.1 (Stata Corp, College Station, TX).
RESULTS
Test Characteristics of CKD Claims in Medicare
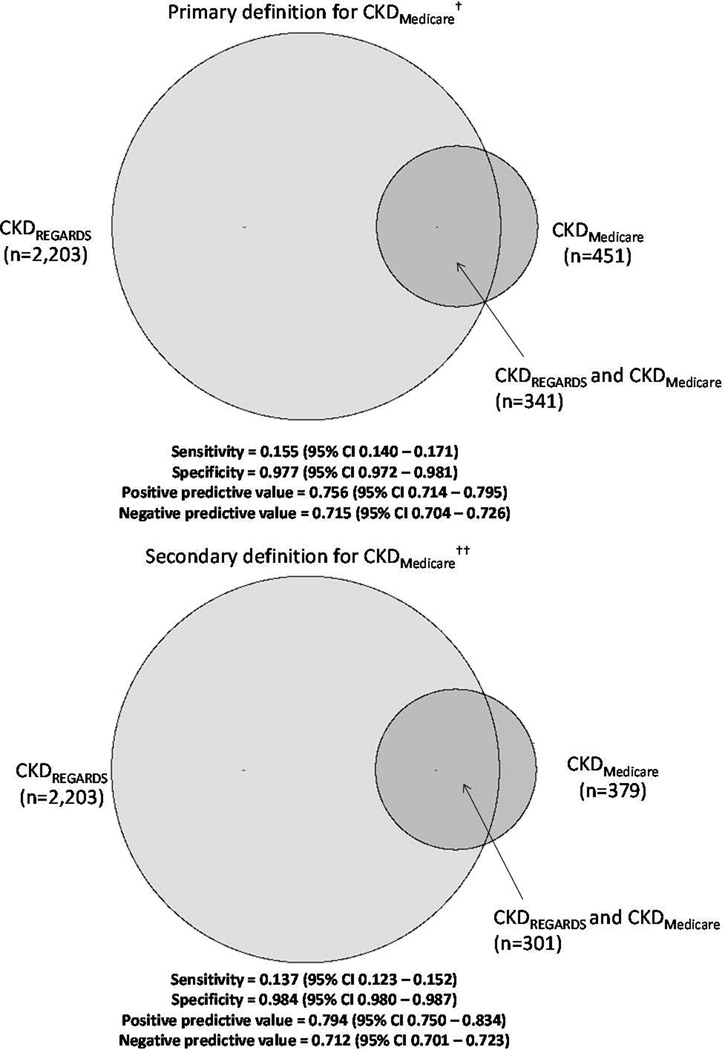
Overall, 2,203 of the 6,982 participants (32%) included in this analysis met the primary definition for CKDREGARDS (eGFR < 60 ml/min/1.73 m2 or ACR > 30 mg/g) and 6% (n=451) met the primary definition for CKDMedicare. Of participants with CKDREGARDS, 15.5% (95% confidence interval [CI], 14.0%–17.1%) had CKDMedicare (sensitivity) and 97.7% (95% CI, 97.2%–98.1%) without CKDREGARDS did not have CKDMedicare (specificity, Figure 2 – left panel). Among participants with CKDMedicare, 75.6% (95% CI, 71.4%–79.5%) had CKDREGARDS (PPV) and among participants without CKDMedicare, 71.5% (95% CI, 70.4%–72.6%) did not have CKDREGARDS (NPV). Sensitivity was higher but specificity and PPV were lower for identifying lower levels of eGFR and higher levels of ACR (Table S1). Using Medicare claims from one year before to one year after the REGARDS study visit, the sensitivity, specificity, PPV, and NPV were 21.2% (95% CI, 19.5%–22.9%), 96.9% (95% CI, 96.3%–97.3%), 75.6% (95% CI, 72.1%–79.0%), and 72.7% (95% CI, 71.6%–73.8%), respectively.
Figure 2.
Sensitivity, specificity, positive and negative predictive values of a Medicare claims-based algorithm (CKDMedicare) for identifying chronic kidney disease with estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2 or albumin-creatinine ratio > 30 mg/g measured in a research study (CKDREGARDS) serving as the gold standard. CKDREGARDS defined as eGFR < 60 mL/min/1.73 m2 or albumin-creatinine ratio > 30 mg/g. †Primary definition for CKDMedicare provided in Item S1. ††Secondary definition for CKDMedicare is provided in Item S2.
Overall, 379 participants met the secondary definition for CKDMedicare (Figure 2 – right panel). The sensitivity was lower and the specificity and PPV were higher for this secondary definition. Sensitivity was higher but specificity and PPV were lower using the secondary definition of CKDMedicare to identify lower levels of eGFR and higher levels of ACR (Table S2). Due to the lower number of cases of CKDMedicare using this secondary definition, it was not investigated further.
Factors and Outcomes Associated With CKDREGARDS and CKDMedicare
Characteristics of participants by the cross-classification of CKDMedicare and CKDREGARDS is provided in Table 1. CKDMedicare and CKDREGARDS were each associated with an increased risk for all-cause mortality and ESRD (Table 2). Although the mortality rate and incidence of ESRD were higher among participants with CKDMedicare compared to their counterparts with CKDREGARDS, the hazard ratio for mortality was similar for CKDMedicare and CKDREGARDS and the hazard ratio for ESRD associated with CKDREGARDS was numerically larger than for CKDMedicare.
Table 1.
Characteristics of REGARDS study participants with and without CKDMedicare and CKDREGARDS.
| No CKDMedicare No CKDREGARDS (n=4669) |
No CKDMedicare Yes CKDREGARDS (n=1862) |
Yes CKDMedicare No CKDREGARDS (n=110) |
Yes CKDMedicare Yes CKDREGARDS (n=341) |
p-value | |
|---|---|---|---|---|---|
| REGARDS Variables, Collected at Baseline | |||||
| Age category | <0.001 | ||||
| <75 y | 2924 (62.6) | 865 (46.5) | 80 (72.7) | 166 (48.7) | |
| 75 – 84 y | 1604 (34.4) | 855 (45.9) | N<11 | 146 (42.8) | |
| ≥ 85 y | 141 (3.0) | 142 (7.6) | N<11 | 29 (8.5) | |
| Black race | 1325 (28.4) | 603 (32.4) | 47 (42.7) | 133 (39.0) | <0.001 |
| Female sex | 2334 (50.0) | 920 (49.4) | 45 (40.9) | 163 (47.8) | 0.3 |
| Region of residence | 0.8 | ||||
| West | 299 (6.4) | 119 (6.4) | N<11 | 18 (5.3) | |
| Midwest | 701 (15.0) | 303 (16.3) | 18 (16.4) | 60 (17.6) | |
| Northeast | 262 (5.6) | 96 (5.2) | N<11 | 21 (6.2) | |
| South | 3407 (73.0) | 1344 (72.2) | 77 (70.0) | 242 (71.0) | |
| Current smoker | 358 (7.7) | 199 (10.7) | N<11 | 24 (7.1) | <0.001 |
| Abdominal obesity | 1986 (42.8) | 910 (49.2) | 55 (50.9) | 206 (60.4) | <0.001 |
| Hypertension | 2774 (59.6) | 1449 (78.0) | 75 (68.2) | 280 (82.8) | <0.001 |
| Diabetes | 780 (16.8) | 540 (29.1) | 35 (31.8) | 158 (46.5) | <0.001 |
| History of CHD | 1037 (22.5) | 578 (31.7) | 42 (40.4) | 149 (44.6) | <0.001 |
| Anemia | 336 (11.4) | 259 (23.8) | 25 (32.1) | 109 (48.0) | <0.001 |
| Family history of ESRD | 262 (8.6) | 131 (11.6) | 11 (13.1) | 31 (13.7) | 0.003 |
| eGFR <60 ml/min/1.73 m2 | 0 (0) | 1108 (59.5) | 0 (0) | 288 (84.5) | <0.001 |
| eGFR (ml/min/1.73 m2) | 82.3 ±11.8 | 62.3 ±19.1 | 79.4 ±13.2 | 45.8 ±17.1 | <0.001 |
| ACR >30 mg/g | 0 (0) | 1056 (56.7) | 0 (0) | 214 (62.8) | <0.001 |
| ACR (mg/g) | 7.2 [4.9–12.0] | 34.8 [9.4–77.4] | 9.4 [5.7–14.3] | 56.1 [13.3–175.9] | <0.001 |
| Antihypertensive medication use | 2351 (52.0) | 1277 (70.5) | 68 (64.2) | 269 (80.8) | <0.001 |
| Statin use | 1652 (35.4) | 792 (42.5) | 48 (43.6) | 165 (48.4) | <0.001 |
| BMI category | <0.001 | ||||
| <25 kg/m2 | 1415 (30.4) | 544 (29.4) | 24 (21.8) | 72 (21.4) | |
| 25-<30 kg/m2 | 1921 (41.3) | 720 (38.9) | 45 (40.9) | 126 (37.4) | |
| ≥30 kg/m2 | 1317 (28.3) | 588 (31.7) | 41 (37.3) | 139 (41.2) | |
| SBP ≥140 mm Hg | 1075 (23.1) | 621 (33.5) | 24 (21.8) | 106 (31.1) | <0.001 |
| Medicare Variables, During Look-Back Period | |||||
| No. of outpatient visits | <0.001 | ||||
| <10 | 1617 (34.6) | 505 (27.1) | 14 (12.7) | 18 (5.3) | |
| 10–19 | 1641 (35.1) | 633 (34.0) | 24 (21.8) | 79 (23.2) | |
| ≥ 20 | 1411 (30.2) | 724 (38.9) | 72 (65.5) | 244 (71.6) | |
| Hospitalization | 1179 (25.3) | 657 (35.3) | 59 (53.6) | 220 (64.5) | <0.001 |
| Nephrology visit | 134 (2.9) | 68 (3.7) | 23 (20.9) | 138 (40.5) | <0.001 |
| Medicaid eligible | 319 (6.8) | 173 (9.3) | 16 (14.5) | 50 (14.7) | <0.001 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables are given as mean ± standard deviation or median [interquartile range].
Abbreviations and definitions: CKDMedicare, chronic kidney disease defined using the claims-based algorithm outlined in Item S1;CKDREGARDS, chronic kidney disease defined as eGFR < 60 ml/min/1.73 m2 or ACR > 30 mg/g at the REGARDS study visit; CKD – chronic kidney disease; CHD – coronary heart disease; ESRD – end-stage renal disease; eGFR – estimated glomerular filtration rate; ACR – albumin-creatinine ratio. BMI, body mass index; SBP, systolic blood pressure; N<11 – cells with less than 11 Medicare beneficiaries are suppressed per the Center for Medicare & Medicaid Services data agreement; REGARDS, Reasons for Geographic and Racial Differences in Stroke
Table 2.
Rates and hazard ratios for mortality and ESRD associated with CKDMedicare and CKDREGARDS
| No CKDMedicare (n=6531) |
CKDMedicare (n=451) |
No CKDREGARDS (n=4779) |
CKDREGARDS (n=2203) |
|
|---|---|---|---|---|
| Mortality | ||||
| No. of cases (%) | 1242 (19.0) | 160 (35.5) | 713 (14.9) | 689 (31.3) |
| Incidence rate (95% CI)† | 33.3 (31.5–35.2) | 72.5 (61.3–83.7) | 25.5 (23.6–27.4) | 59.9 (55.4–64.4) |
| Hazard ratio (95% CI) | ||||
| Model 1 | 1.00 (reference) | 2.10 (1.78–2.48) | 1.00 (reference) | 1.90 (1.71–2.12) |
| Model 2 | 1.00 (reference) | 1.54 (1.29–1.85) | 1.00 (reference) | 1.75 (1.56–1.95) |
| Model 3 | 1.00 (reference) | 1.47 (1.22–1.77) | 1.00 (reference) | 1.63 (1.45–1.83) |
| ESRD | ||||
| No. of cases (%) | 50 (0.8) | 38 (8.4) | N<11 | N<11* |
| Incidence rate (95% CI)† | 1.3 (0.9–1.6) | 16.4 (11.2–21.6) | 0.1 (0.0–0.3) | 6.8 (5.4–8.3) |
| Hazard ratio (95% CI) | ||||
| Model 1 | 1.00 (reference) | 11.6 (7.55–17.7) | 1.00 (reference) | 48.1 (17.6–131) |
| Model 2 | 1.00 (reference) | 5.80 (3.49–9.7) | 1.00 (reference) | 37.8 (13.8–104) |
| Model 3 | 1.00 (reference) | 4.62 (2.72–7.84) | 1.00 (reference) | 30.9 (11.2–85.5) |
Note: Model 1 is adjusted for age, race, and gender; model 2 is adjusted for age, race, gender and Medicare variables during the look-back period (outpatient visits, nephrologist visits, hospitalization during baseline and Medicaid eligible); model 3 is adjusted for variables in model 2 and smoking, abdominal obesity, hypertension, diabetes, and history of coronary heart disease from the REGARDS study.
Abbreviations and definitions: CKDMedicare, chronic kidney disease defined using the claims-based algorithm outlined in Item S1; CKDREGARDS, chronic kidney disease defined as eGFR < 60 ml/min/1.73 m2 or albumin-creatinine ratio > 30 mg/g at the REGARDS study visit. CI – confidence interval; CKD – chronic kidney disease; ESRD, end-stage renal disease; REGARDS, Reasons for Geographic and Racial Differences in Stroke; N<11: cells with less than 11 Medicare beneficiaries are suppressed per the Center for Medicare & Medicaid Services data agreement.
Incidence rate per 1,000 person-years (95% CI).
Although the number of ESRD cases with CKDREGARDS is >11, this cell is suppressed to prevent calculation of the cell size for ESRD cases with no CKDREGARDS.
Risk Factors for All-Cause Mortality and ESRD Among Participants With CKDMedicare and CKDREGARDS
Among participants with CKDMedicare and those with CKDREGARDS, older age, anemia, ACR > 30 mg/g, and being hospitalized during baseline were associated with an increased risk for all-cause mortality (Table 3). Among those with CKDREGARDS, women were less likely to die than men but no association between gender and all-cause mortality was present among those with CKDMedicare. Diabetes and history of CHD were associated with an increased risk for all-cause mortality among those with CKDREGARDS but not for those with CKDMedicare while eGFR<60 ml/min/1.73 m2 was associated with an increased risk for all-cause mortality among those with CKDMedicare but not their counterparts with CKDREGARDS. Older age was associated with a lower risk for ESRD among those with CKDMedicare and CKDREGARDS. Being black versus white, having diabetes, anemia, eGFR<60 ml/min/1.73 m2, ACR > 30 mg/g, and having a nephrologist visit were each associated with an increased risk for ESRD among those with CKDMedicare and CKDREGARDS. A history of CHD was associated with an increased risk for ESRD among those with CKDREGARDS but not those with CKDMedicare.
Table 3.
Hazard ratios for all-cause mortality and ESRD among study participants with CKDMedicare and CKDREGARDS
| All-Cause Mortality | ESRD | |||
|---|---|---|---|---|
| CKDMedicare (n=451) |
CKDREGARDS (n=2,203) |
CKDMedicare (n=451) |
CKDREGARDS (n=2,203) |
|
| Risk Factors From REGARDS Study | ||||
| Age category | ||||
| <75 y | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 75–84 y | 1.75 (1.25 – 2.47) | 1.89 (1.61 – 2.22) | 0.56 (0.28 – 1.11) | 0.57 (0.37 – 0.90) |
| ≥ 85 y | 3.89 (2.37 – 6.37) | 3.18 (2.51 – 4.03) | NA* | 0.11 (0.02 – 0.81) |
| Black vs. white | 1.19 (0.85 – 1.68) | 0.86 (0.73 – 1.01) | 3.03 (1.57 – 5.86) | 3.69 (2.32 – 5.85) |
| Women vs. men | 0.85 (0.61 – 1.18) | 0.60 (0.52 – 0.70) | 0.79 (0.42 – 1.48) | 0.77 (0.50 – 1.18) |
| Hypertension | 0.76 (0.52 – 1.11) | 0.85 (0.72 – 1.00) | NA* | 2.14 (1.01 – 4.52) |
| Diabetes | 1.16 (0.83 – 1.6) | 1.52 (1.31 – 1.77) | 3.06 (1.52 – 6.16) | 1.77 (1.14 – 2.73) |
| History of CHD | 1.09 (0.78 – 1.51) | 1.45 (1.25 – 1.69) | 0.96 (0.52 – 1.79) | 1.60 (1.03 – 2.48) |
| Anemia | 1.53 (0.97 – 2.41) | 1.40 (1.07 – 1.84) | 3.07 (1.25 – 7.55) | 2.51 (1.57 – 4.01) |
| eGFR < 60 ml/min/1.73 m2 | 1.68 (1.14 – 2.47) | 0.94 (0.80 – 1.09) | 5.29 (1.94 – 14.4) | 5.17 (2.65 – 10.1) |
| ACR > 30 mg/g | 1.55 (1.12 – 2.14) | 1.46 (1.25 – 1.69) | 4.11 (1.91 – 8.84) | 3.98 (2.16 – 7.32) |
| Risk Factors From Medicare Claims | ||||
| No. of outpatient visits | ||||
| <10 | 1.00 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 10–19 | 1.50 (0.61 – 3.71) | 0.91 (0.74 – 1.13) | 0.96 (0.22 – 4.22) | 0.82 (0.44 – 1.53) |
| ≥ 20 | 2.03 (0.86 – 4.78) | 1.24 (1.02 – 1.51) | 1.13 (0.28 – 4.47) | 1.07 (0.61 – 1.89) |
| Hospitalization during baseline | 2.08 (1.43 – 3.03) | 1.79 (1.54 – 2.08) | 1.57 (0.83 – 2.96) | 1.19 (0.77 – 1.84) |
| Nephrologist visit | 1.00 (0.72 – 1.40) | 0.83 (0.64 – 1.08) | 5.05 (2.58 – 9.87) | 4.57 (2.91 – 7.18) |
| Medicaid eligible | 0.65 (0.38 – 1.10) | 1.09 (0.84 – 1.41) | 0.81 (0.37 – 1.79) | 1.18 (0.68 – 2.05) |
Note: Values are given as hazard ratio (95% confidence interval). Models include adjustment for age, race, gender.
Abbreviations and definitions: CKDMedicare, chronic kidney disease defined using the claims-based algorithm outlined in Item S1; CKDREGARDS, chronic kidney disease defined as eGFR < 60 ml/min/1.73 m2 or ACR > 30 mg/g at the REGARDS study visit; CI – confidence interval, eGFR – estimated glomerular filtration rate, ESRD, end-stage renal disease; ACR – albumin-creatinine ratio, CHD – coronary heart disease. NA, not available; REGARDS, Reasons for Geographic and Racial Differences in Stroke
All participants with CKDMedicare developing ESRD had hypertension and none were ≥ 85 years of age; therefore, a hazard ratio could not be calculated.
Factors and Outcomes Associated With CKDMedicare Among Individuals With CKDREGARDS
Among participants with CKDREGARDS, blacks, non-smokers, individuals with abdominal obesity, hypertension, diabetes, a history of CHD, anemia, eGFR < 60 ml/min/1.73 m2, ACR > 30 mg/g were more likely to have CKDMedicare (Table S3). Additionally, having more outpatient visits, being hospitalized, having a nephrologist visit, and being Medicaid eligible during the look-back period were each associated with having CKDMedicare. After multivariable adjustment, each of these factors except being black, not smoking, hypertension, history of CHD, ACR > 30 mg/g and being Medicaid eligible remained associated with an increased prevalence of CKDMedicare (Table 4).
Table 4.
Prevalence ratios for CKDMedicare among participants with CKDREGARDS
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| REGARDS Variables, Collected at Baseline | |||
| Age category | |||
| < 75 y | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 75–84 y | 0.92 (0.75 – 1.13) | 0.95 (0.79–1.15) | 0.99 (0.82 – 1.20) |
| ≥ 85 y | 1.07 (0.75 – 1.54) | 1.08 (0.81–1.44) | 1.16 (0.86 – 1.57) |
| Black race | 1.29 (1.05 – 1.57) | 1.12 (0.93–1.34) | 1.09 (0.91 – 1.30) |
| Female sex | 0.91 (0.75 – 1.11) | 0.84 (0.71–1.00) | 0.83 (0.69 – 1.00) |
| Region of residence | |||
| West | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Midwest | 1.18 (0.72 – 1.93) | 1.18 (0.76 – 1.82) | 1.18 (0.69 – 2.01) |
| Northeast | 1.32 (0.74 – 2.36) | 1.38 (0.81 – 2.35) | 1.18 (0.68 – 2.03) |
| South | 1.14 (0.73 – 1.78) | 1.12 (0.76 – 1.65) | 1.18 (0.68 – 2.05) |
| Current smoker | 0.66 (0.45 – 0.98) | 0.83 (0.58 – 1.18) | 0.87 (0.60 – 1.25) |
| Abdominal obesity | 1.50 (1.22 – 1.84) | 1.27 (1.06 – 1.52) | 1.21 (1.01 – 1.46) |
| Hypertension | 1.27 (0.97 – 1.65) | 1.10 (0.87 – 1.39) | 1.06 (0.84 – 1.34) |
| Diabetes | 1.83 (1.50 – 2.23) | 1.31 (1.09 – 1.56) | 1.24 (1.03 – 1.49) |
| History of CHD | 1.64 (1.33 – 2.01) | 1.20 (1.01 – 1.44) | 1.18 (0.99 – 1.41) |
| Anemia | 2.50 (1.93 – 3.24) | 1.61 (1.28 – 2.01) | 1.54 (1.22 – 1.94) |
| Family history of ESRD | 1.09 (0.73 – 1.62) | 0.84 (0.62 – 1.14) | 0.82 (0.60 – 1.12) |
| eGFR < 60 ml/min/1.73 m2 | 3.32 (2.51 – 4.40) | 2.24 (1.71 – 2.95) | 2.29 (1.74 – 3.01) |
| ACR > 30 mg/g | 1.20 (0.98 – 1.48) | 1.15 (0.96 – 1.39) | 1.11 (0.92 – 1.34) |
| Medicare Variables, During Look-Back Period | |||
| No. of outpatient visits | |||
| < 10 | 1.00 (reference) | 1.00 (reference) | 1 (reference) |
| 10–19 | 3.32 (2.02 – 5.48) | 2.56 (1.57 – 4.19) | 2.50 (1.53 – 4.09) |
| ≥ 20 | 7.58 (4.75 – 12.1) | 4.03 (2.49 – 6.52) | 3.71 (2.31 – 5.97) |
| Hospitalization | 2.79 (2.27 – 3.42) | 1.79 (1.47 – 2.17) | 1.74 (1.43 – 2.11) |
| Nephrology visit | 6.59 (5.58 – 7.77) | 4.77 (4.01 – 5.69) | 4.62 (3.88 – 5.51) |
| Medicaid eligible | 1.47 (1.11 – 1.96) | 1.04 (0.83 – 1.32) | 0.99 (0.79 – 1.24) |
Note: Values are given as prevalence ratio (95% confidence interval). Model 1 includes adjustment for age, race, gender; model 2 includes adjustment for age, race, gender, outpatient visits, nephrologist visits, hospitalization during baseline and Medicaid eligible; model 3 is adjusted for variables in model 2 and smoking, abdominal obesity, hypertension, diabetes, and history of CHD from the REGARDS study.
Abbreviations and definitions: CKD – chronic kidney disease, eGFR – estimated glomerular filtration rate, ACR – albumin-creatinine ratio, CHD – coronary heart disease; REGARDS, Reasons for Geographic and Racial Differences in Stroke; CKDMedicare, chronic kidney disease defined using the claims-based algorithm outlined in Item S1; CKDREGARDS, chronic kidney disease defined as eGFR < 60 ml/min/1.73 m2 or ACR > 30 mg/g at the REGARDS study visit.
Among participants with CKDREGARDS, the crude mortality rate and age, race, gender adjusted hazard ratio for mortality was increased for those with versus without CKDMedicare (Table 5). The hazard ratio was 1.23 (95% CI, 0.99–1.53) after full multivariable adjustment. Among participants with CKDREGARDS, the incidence of ESRD was 4.4 (95% CI, 3.2–5.7) and 22.1 (95% CI, 15.0–29.2) per 1,000 person-years among those without and with CKDMedicare, respectively. The increased risk for ESRD associated with CKDMedicare remained present after multivariable adjustment.
Table 5.
Rates and hazard ratios for mortality and ESRD associated with CKDMedicare among participants with CKDREGARDS
| Mortality | ESRD | |||
|---|---|---|---|---|
| No CKDMedicare (n=1862) |
CKDMedicare (n=341) |
No CKDMedicare (n=1862) |
CKDMedicare (n=341) |
|
| No. of cases (%) | 551 (29.6) | 138 (40.5) | 47 (2.5) | 37 (10.9) |
| Incidence rate (95% CI)† | 55.7 (51.0 – 60.3) | 85.9 (71.6 – 100.0) | 4.4 (3.2 – 5.7) | 22.1 (15.0 – 29.2) |
| Hazard ratio (95% CI) | ||||
| Model 1 | 1.00 (reference) | 1.59 (1.32 – 1.91) | 1.00 (reference) | 4.32 (2.80 – 6.67) |
| Model 2 | 1.00 (reference) | 1.27 (1.03 – 1.57) | 1.00 (reference) | 2.49 (1.45 – 4.27) |
| Model 3 | 1.00 (reference) | 1.23 (0.99 – 1.53) | 1.00 (reference) | 2.19 (1.26 – 3.83) |
Incidence rate per 1,000 person-years.
Abbreviations and definitions: CI – confidence interval; CKD – chronic kidney disease; ESRD, end-stage renal disease; CKDMedicare, chronic kidney disease defined using the claims-based algorithm outlined in Item S1; CKDREGARDS, chronic kidney disease defined as estimated glomerular filtration rate < 60 ml/min/1.73 m2 or albumin-creatinine ratio > 30 mg/g at the REGARDS study visit; REGARDS, Reasons for Geographic and Racial Differences in Stroke
Note: Model 1 is adjusted for age, race, and gender; model 2 is adjusted for age, race, gender and Medicare variables during the look-back period (outpatient visits, nephrologist visits, hospitalization during baseline and Medicaid eligible); model 3 is adjusted for variables in model 2 and smoking, abdominal obesity, hypertension, diabetes, and history of coronary heart disease from the REGARDS study.
Secondary Definition of CKDREGARDS
Overall, 603 REGARDS participants (9%) had an eGFR < 45 ml/min/1.73 m2 or ACR > 300 mg/g. Of participants meeting this secondary definition of CKDREGARDS, 32.8% (95% CI, 29.1%–36.7%) had CKDMedicare (Figure S1). In contrast, 96.0% (95% CI, 95.5%–96.5%) of participants not meeting the secondary definition of CKDREGARDS did not have CKDMedicare. Using the secondary definition of CKDREGARDS, the PPV and NPV for CKDMedicare were 43.9% (95% CI, 39.3%–48.6%) and 93.8% (95% CI, 93.2%–94%), respectively. Among participants meeting the secondary definition of CKDREGARDS, the risk for mortality was similar for participants with and without CKDMedicare (Table S4). The ESRD risk was higher for those with versus without CKDMedicare. This association was attenuated and no longer present after adjustment for age, race, gender, and Medicare variables from the look-back period.
DISCUSSION
In this study, the majority of individuals with CKD in the REGARDS Study (CKDREGARDS) as defined by eGFR < 60 ml/min/1.73 m2 or ACR > 30 mg/g did not have Medicare claims for CKD (CKDMedicare). This confirms prior studies showing claims data have low sensitivity for identifying CKD. The current study extends findings from prior studies by demonstrating the similarities and differences that exist when defining CKD using eGFR and ACR versus a claims data definition of CKD. With few exceptions, the same factors were associated with CKDREGARDS and CKDMedicare, and CKDREGARDS and CKDMedicare were each associated with an increased risk for all-cause mortality and ESRD. However, some risk factors for all-cause mortality and ESRD differed for individuals with CKDMedicare and CKDREGARDS, and among individuals with CKDREGARDS, we identified differences among individuals with versus without CKDMedicare. For example, among individuals with CKDREGARDS, those with co-morbidities including diabetes, anemia and more severe kidney disease at baseline were more likely to have CKDMedicare. Individuals with CKDMedicare also had very high risk for ESRD.
The validity of Medicare claims to identify individuals with CKD has been evaluated previously.8 The sensitivity, specificity, PPV and NPV for having CKD associated with Medicare claims was studied in 1,852 low income Medicare beneficiaries presenting to a hospital for myocardial infarction. The eGFR was calculated from the first in-hospital serum creatinine measurement and claims for CKD were identified over the 12 and 24 months prior to hospitalization. The prevalence of CKD was 67% (versus 32% in the present study) and the PPV ranged from 89% to 97% depending on the ICD-9 codes used to define CKD (versus 76% in the present study). Given the high PPV, the authors concluded claims data can be used to identify cohorts with “clinically relevant CKD”. However, PPV is influenced by the prevalence of the outcome (e.g., CKD) with higher values present at higher disease prevalences. The lower prevalence of CKD in the REGARDS Study, when compared with prior studies of hospitalized patients, may explain the lower PPV we observed in the current study. A substantially higher sensitivity was present for more severe CKD (e.g., 36% and 56% for identifying eGFR <45 ml/min/1.73 m2 and < 30 ml/min/1.73 m2, respectively). Due to the low prevalence of these more severe reductions in eGFR, the PPV was substantially lower.
The current study extends prior investigations in several important ways. We included a population-based sample rather than patients presenting to the hospital with myocardial infarction and defined CKD using either reduced eGFR or elevated ACR. A number of recent studies and meta-analyses have demonstrated the prognostic importance of albuminuria for cardiovascular and kidney disease outcomes.18–20 Additionally, the REGARDS Study had data on a broad range of objectively measured co-morbid conditions that allowed us to assess the generalizability of Medicare beneficiaries with CKD claims. Among those with CKDREGARDS, determined by eGFR and ACR levels measured objectively in the REGARDS study, we found those with versus without CKDMedicare to have more co-morbid conditions including abdominal obesity, diabetes and an eGFR < 60 ml/min/1.73 m2.
Traditional epidemiology studies are very expensive and take a long time to conduct. Therefore, claims data may provide an alternative approach to study CKD. In the current study, we found correlates of CKD and the risk for all-cause mortality to be similar when CKD was defined in claims data or in the REGARDS Study. However, the incidence of ESRD was substantially higher among individuals with CKDMedicare versus CKDREGARDS. Additionally, in the current analysis, four risk factors (gender, diabetes, history of CHD and eGFR < 60 ml/min/1.73 m2) for all-cause mortality and one risk factor (history of CHD) for ESRD were different when evaluated in two parallel cohorts of individuals with CKD, one defined using claims data and the other by measured eGFR and ACR. This suggests that assembling a cohort of individuals with CKD claims to study risk factors for outcomes may lead to different findings compared to studies that use measured eGFR and ACR to define CKD.
Although the current study found potential limitations in using claims data to study CKD in Medicare, algorithms for identifying several other diseases including diabetes, heart failure, and myocardial infarction have been validated in claims databases.21–23 In general, these algorithms had substantially higher PPV than observed for CKD in the current study. For our primary analysis we used a broad set of claims to identify CKD. The narrower set of ICD-9 codes we used to define CKD in Medicare in secondary analyses resulted in a higher PPV but the sensitivity remained low. Future studies are needed to investigate whether other algorithms with higher sensitivity and PPV can be developed to identify CKD in Medicare claims.
The current study should be interpreted in the context of potential and known limitations. While the REGARDS Study measured both eGFR and ACR, they were obtained at a single time point. Some REGARDS participants identified as having CKD may not have had it if repeat eGFR or ACR measurements were performed.24 The baseline visit in the REGARDS Study occurred in 2003–2007. ICD-9 diagnosis codes corresponding to CKD stage were introduced into Medicare claims in 2005. Too little look-back time was available in the current study to investigate the correlation of 585 sub-codes with CKD stage. Also, future studies with more contemporary data on CKD are needed to re-evaluate whether the differences between individuals with and without CKD claims in Medicare still remain present. Strengths of the current analysis include the large nationwide reach of the REGARDS Study. Participants were enrolled from across the continental United States. Broad data collection was conducted at baseline using a standardized protocol and participants have been prospectively followed for outcomes. This allowed us to evaluate correlates and outcomes associated with having CKD claims.
In conclusion, the current analysis identified similarities and differences between older US adults with CKD identified in a research study versus in Medicare claims. Our data suggest that most individuals with claims for CKD in Medicare have reduced eGFR or albuminuria. Additionally, CKD, whether identified using a claims-based algorithm or through eGFR and ACR measurements, is associated with an increased risk for all-cause mortality and ESRD. However, regardless of the algorithm applied in the current study, Medicare claims algorithms had low sensitivity and identified a subset of individuals with CKD who had a high mortality and ESRD risk. Future studies are needed to assess whether the generalizability of individuals identified as having CKD in Medicare claims has improved since 2007 and to develop better approaches for identifying CKD in claims. In the interim, the inferences from studies of CKD defined using Medicare claims should be interpreted with caution.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the REGARDS Study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at www.regardsstudy.org.
Support: This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Department of Health and Human Services and R01 HS018517 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIH. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation to Dr Warnock. Amgen played no role in the study design, collection, analysis and interpretation of data for this manuscript.
Additional support was provided through the National Institute on Aging (grant R03AG042336) and the T. Franklin Williams Scholarship Award (funding provided by Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, the American Society of Nephrology and the American Geriatrics Society) to Dr Bowling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: Research idea and study design: PM, OMG, CSF, CBB; data acquisition: JRC, DGW; data analysis and interpretation: PM, OMG, HZ, CSF, NCW, JRC, WM, HW, MK; statistical analysis: HZ; supervision and mentorship: PM, MK, CBB. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. PM takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplementary Material
Table S1: Sensitivity, specificity, PPV, and NPV of claims-based algorithm for identifying progressively lower levels of eGFR and higher ACRs using primary CKD definition in Medicare.
Table S2: Sensitivity, specificity, PPV, and NPV of claims-based algorithm for identifying progressively lower levels of eGFR and higher ACRs using secondary CKD definition in Medicare.
Table S3: Number and percentage of REGARDS participants with CKDMedicare in those with CKDREGARDS at their study visit.
Table S4: Rates and HRs for mortality and ESRD associated with CKDMedicare in participants with eGFR < 45 mL/min/1.73 m2 or ACR >300 mg/g at REGARDS study visit.
Figure S1: Sensitivity, specificity, PPV, and NPV of CKDMedicare for identifying CKD defined as eGFR < 45 ml/min/1.73 m2 or ACR >300 mg/g measured in REGARDS Study.
Item S1: Inpatient and outpatient ICD-9 codes in claims used to define CKDMedicare in primary analyses.
Item S2: Inpatient and outpatient ICD-9 codes in claims (inpatient and outpatient) used to define CKDMedicare in secondary analyses.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
References
- 1.Lauer MS. Time for a creative transformation of epidemiology in the united states. JAMA. 2012;308:1804–1805. doi: 10.1001/jama.2012.14838. [DOI] [PubMed] [Google Scholar]
- 2.Concato J, Lawler EV, Lew RA, Gaziano JM, Aslan M, Huang GD. Observational methods in comparative effectiveness research. Am J Med. 2010;123:e16–e23. doi: 10.1016/j.amjmed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaum HG, Cremieux PY, Greenberg PE, LeLorier J, Ostrander JA, Venditti L. Using healthcare claims data for outcomes research and pharmacoeconomic analyses. PharmacoEconomics. 1999;16:1–8. doi: 10.2165/00019053-199916010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Hicks J. The potential of claims data to support the measurement of health care quality. 2003 [Google Scholar]
- 5.Grams ME, Plantinga LC, Hedgeman E, Saran R, Myers GL, Williams DE, Powe NR, Team CCS. Validation of ckd and related conditions in existing data sets: A systematic review. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57:44–54. doi: 10.1053/j.ajkd.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlasschaert ME, Bejaimal SA, Hackam DG, Quinn R, Cuerden MS, Oliver MJ, Iansavichus A, Sultan N, Mills A, Garg AX. Validity of administrative database coding for kidney disease: A systematic review. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57:29–43. doi: 10.1053/j.ajkd.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Moon M. What medicare has meant to older americans. Health care financing review. 1996;18:49–59. [PMC free article] [PubMed] [Google Scholar]
- 8.Winkelmayer WC, Schneeweiss S, Mogun H, Patrick AR, Avorn J, Solomon DH. Identification of individuals with ckd from medicare claims data: A validation study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2005;46:225–232. doi: 10.1053/j.ajkd.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: Objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 10.Kurella TM, Wadley V, Yaffe K, McClure LA, Howard G, Go R, Allman RM, Warnock DG, McClellan W. Kidney function and cognitive impairment in us adults: The reasons for geographic and racial differences in stroke (regards) study. Am.J.Kidney Dis. 2008;52:227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van LF, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann.Intern.Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidney Disease Improving global Outcomes (KDIGO) CKD Work Group. 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International. 2013;2013:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 13.Chronic condition data warehouse - chronic condition categories. 2011 doi: 10.1111/j.1475-6773.2011.01277.x. PDF available at https://www.ccwdata.org/web/guest/condition-categories. [DOI] [PMC free article] [PubMed]
- 14.Collins AJ, Chen SC, Gilbertson DT, Foley RN. Ckd surveillance using administrative data: Impact on the health care system. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;53:S27–S36. doi: 10.1053/j.ajkd.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 15.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the united states medicare population, 1998 to 1999. J.Am.Soc.Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 16.Foley RN, Collins AJ. The usrds: What you need to know about what it can and can’t tell us about esrd. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:845–851. doi: 10.2215/CJN.06840712. [DOI] [PubMed] [Google Scholar]
- 17.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:14. [Google Scholar]
- 18.Matsushita K, vandervelde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gansevoort RT, Matsushita K, vandervelde M, Astor BC, Woodward M, Levey AS, Jong PE, Coresh J, Gansevoort RT, Matsushita K, van d V, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, MacMahon S, Tonelli M, Hemmelgarn B, Wang Y, Atkins RC, Polkinghorne KR, Chadban SJ, Shankar A, Klein R, Klein BE, Sacks F, Curhan G, Shlipak M, Sarnak MJ, Katz R, Fried LP, Hallan S, Lydersen S, Holmen J, Lee BJ, Ishani A, Neaton J, Svendsen K, Iseki K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Auguste P, Veldhuis K, Camarata L, Thomas B, Manley Ts. Lower estimated gfr and higher albuminuria are associated with adverse kidney outcomes in both general and high-risk populations. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warnock DG, Muntner P, McCullough PA, Zhang X, McClure LA, Zakai N, Cushman M, Newsome BB, Kewalramani R, Steffes MW, Howard G, McClellan WM, Investigators R. Kidney function, albuminuria, and all-cause mortality in the regards (reasons for geographic and racial differences in stroke) study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;56:861–871. doi: 10.1053/j.ajkd.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the department of veterans affairs based on computerized patient data. Diabetes Care. 2004;(27 Suppl 2):B10–B21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- 22.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of medicare claims-based diagnosis of acute myocardial infarction: Estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Saczynski JS, Andrade SE, Harrold LR, Tjia J, Cutrona SL, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiology and drug safety. 2012;(21 Suppl 1):129–140. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am.J.Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.