Abstract
Human immunodeficiency virus (HIV)–infected persons have higher rates of herpes zoster than HIV-uninfected individuals. We assessed whether twice daily treatment with 400 mg of oral acyclovir reduces the incidence of herpes zoster in a randomized, double-blind, placebo-controlled trial among 3408 persons coinfected with HIV and herpes simplex virus type 2. During 5175 person-years of follow-up, 26 cases of herpes zoster occurred among those assigned acyclovir, compared with 69 cases among those assigned placebo (rates, 1.00 and 2.68/100 person-years, respectively), a relative decrease of 62% (hazard ratio, 0.38; 95% confidence interval, .24–.67; P < .001). Daily acyclovir prophylaxis significantly reduced herpes zoster incidence among HIV-infected persons.
Keywords: herpes zoster, acyclovir, HIV, acyclovir prophylaxis, shingles, VZV
Herpes zoster, or shingles, is a painful, vesicular rash that most often develops in elderly or immunocompromised individuals, including human immunodeficiency virus (HIV)–infected persons [1]. This localized cutaneous eruption results from reactivation of varicella-zoster virus (VZV), which lies dormant in the dorsal root ganglia following primary varicella virus infection (chickenpox) during childhood. Prompt initiation of therapy with high-dose acyclovir, valacyclovir, or famciclovir limits the severity, duration, and complications of the outbreak. Vaccination is effective among HIV-uninfected persons: among adults aged ≥65 years in the United States, the licensed live attenuated zoster vaccine (Zostavax) reduced the incidence of herpes zoster by 48% (95% confidence interval [CI], 39%–56%) [2]. The zoster vaccine is safe and immunogenic for HIV-infected persons with a CD4+ T-cell count of >200 cells/µL [3], but it is recommended for use starting at age 60 years and is contraindicated in persons with CD4+ T-cell counts of <200 cells/µL [4].
Herpes zoster incidence is 12–17-fold higher in HIV-infected persons (2.94 cases/100 person-years), compared with HIV-uninfected individuals (0.20 cases/100 person-years) [5]. Even with antiretroviral therapy (ART) initiation, the incidence of herpes zoster remains 2–3-fold higher among HIV-infected persons and, notably, is higher immediately after ART initiation [6].
High-dose acyclovir (2.4 g daily) reduced zoster recurrence at 12 months by 68% among HIV-infected persons in the pre-ART era [7], but there are limited data and no guidelines for standard, lower-dose antiviral prophylaxis dosing for prevention of zoster [4]. We hypothesized that daily acyclovir prophylaxis would reduce the incidence of herpes zoster among HIV-infected individuals.
METHODS
Study Design and Participants
Between November 2004 and April 2007, we conducted the Partners in Prevention HSV/HIV Transmission Study [8], a randomized, double-blind, placebo-controlled trial of acyclovir (400 mg twice daily), among 3408 HIV-infected women and men from 7 African countries (Botswana, Kenya, Rwanda, South Africa, Tanzania, Uganda, and Zambia) who were coinfected with herpes simplex virus type 2 (HSV-2). The primary aim of the study was to assess the impact of acyclovir prophylaxis on HIV transmission to HIV-uninfected heterosexual partners; as previously reported, no reduction in HIV transmission was seen, although acyclovir prophylaxis did reduce the frequency of genital ulcers due to HSV-2 and resulted in a modest reduction in plasma HIV RNA load (0.25 log10 copies/mL) [8]. A predefined secondary outcome of the trial was incident herpes zoster. VZV serologic assays were not performed. Eligible participants reported no current use of ART, and all had CD4+ T-cell counts of >250 cells/µL and no history of AIDS-defining conditions. The University of Washington Human Subjects Review Committee and ethics review committees at the each of the collaborating organizations approved the study protocol. All participants provided written informed consent.
Procedures
Throughout the study, all participants received individualized, confidential HIV counseling, risk-reduction counseling, couples counseling, free condoms, and treatment of sexually transmitted infections according to World Health Organization guidelines. HIV-infected participants were seen once per month for provision of study medication (acyclovir 400 mg twice daily or matching placebo). CD4+ T-cell counts were assessed every 6 months, and the plasma HIV RNA load was measured at baseline. Herpes zoster detected at a monthly visit was documented during physical examination; once each quarter, a medical history was obtained to record self-reported zoster not observed at a regular visit. During the quarterly medical visits, clinicians asked participants whether they had had any skin rash consistent with zoster and recorded reported cases.
At the quarterly visits, participants were asked whether they had received ART since the last visit, and whether those who initiated ART continued to receive treatment during follow-up. At the time the study was undertaken, national guidelines generally recommended ART initiation for patients with CD4+ T-cell counts of ≤200–250 cells/µL or clinical AIDS. Participants who met national guidelines for initiation of ART during follow-up, as a result of a decline in CD4+ T-cell count or a change in clinical status, were referred to local HIV clinics for ART. HIV-infected women who became pregnant during the study were referred to antenatal clinics for services to prevent mother-to-child transmission.
Statistical Analysis
For the present analysis, the primary outcome was an episode of herpes zoster during follow-up, either observed on examination or reported by the participant. The primary analysis was intention to treat, and survival analysis was used to estimate the effect of acyclovir prophylaxis on herpes zoster incidence in the acyclovir arm, compared with the placebo arm. Multiple visits with an event within the same quarter were rare and were treated as one episode; events in different quarters were treated as separate events. Cox regression with the Anderson–Gill counting method and robust variance was used to include multiple events per participant in the survival analysis. A second survival analysis, using only time to the first zoster event, was used to create cumulative probability curves for zoster incidence and to compare curves by arm, using the log-rank test. To explore the impact of ART on zoster incidence, and because HIV-infected persons receiving ART continue to have an increased risk of zoster as compared to uninfected individuals [6] and because ART initiation is associated with a short-term increase in the risk of herpes zoster [6], data were not censored at ART initiation. Data were analyzed using SAS (version 9.3; Cary, North Carolina).
RESULTS
The Partners in Prevention HSV/HIV Transmission Study enrolled 3381 participant infected with HIV and HSV-2, with 1693 were randomized to acyclovir and 1688 to placebo. As detailed elsewhere [8], two thirds of participants were women, the median age was 32 years, the median CD4+ T-cell count was 462 cells/mL, and randomization was balanced with respect to these characteristics. Four percent in each arm reported a history of herpes zoster in the prior year, and 1% in each arm had zoster on baseline clinical examination.
During 24 months, the study accrued 2605 person-years of follow-up in the acyclovir arm and 2570 person-years of follow-up in the placebo arm. The median follow-up time was 20 months. Acyclovir adherence, assessed by pill count and self-report, was high, with an estimated 96% of doses dispensed consumed and drug consumed on 90% of days overall. A total of 151 participants in the acyclovir arm initiated ART, compared with 180 participants in the placebo arm [9], resulting in 125 and 158 person-years at risk while receiving ART in the acyclovir and placebo groups, respectively.
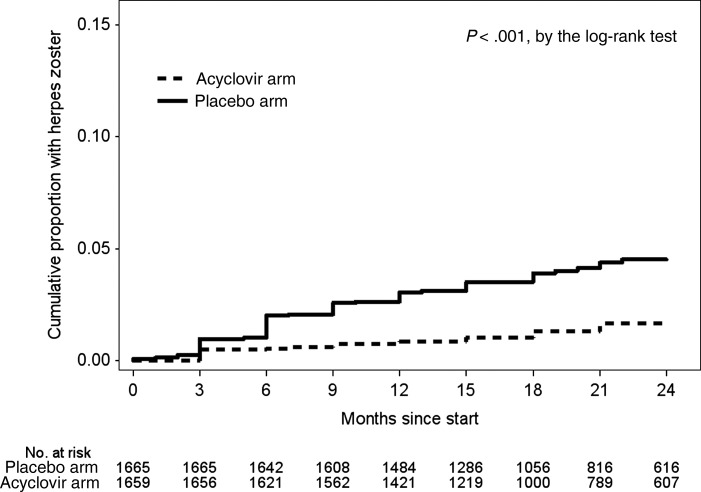
Ninety-five cases of herpes zoster occurred during follow-up, of which 15 were observed by skin examination only, 62 were self-reported as occurring between study visits, and 18 were documented by both examination and self-report. Of these 95 herpes zoster events, 26 occurred in 22 people in the acyclovir arm (incidence, 1.00 cases/100 person-years), compared with 69 in 64 people in the placebo arm (incidence, 2.68 cases/100 person-years; Table 1). The cumulative proportion of participants with herpes zoster during the study was 4.5% (95% CI, 3.5%–5.8%) in the placebo arm and 1.7% (1.1%–2.5%) in the acyclovir arm (P < .001 for the difference in time to first event; Figure 1). Overall, analysis of all events revealed that acyclovir prophylaxis decreased the incidence of herpes zoster by 62% in the acyclovir arm, compared with the placebo arm (hazard ratio [HR], 0.38; 95% CI, .24–.67; P < .001). The protective effect of acyclovir on zoster was not modified by sex, age (<30 years and ≥30 years), CD4+ T-cell count (≤350 and >350 cells/µL; ≤250 and >250 cells/µL), or baseline plasma HIV RNA load (<10 000, 10 000–99 999, and ≥100 000 copies/mL).
Table 1.
Herpes Zoster Incidence, by Treatment Arm
| Variable | Acyclovir Arm |
Placebo Arm |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subjects, No. | Events, No. | PY at Risk | Rate, Cases/100 PY | Subjects, No. | Events, No. | PY at Risk | Rate, Cases/100 PY | HR (95% CI)a | P Value | |
| Overall efficacy | 1693 | 26 | 2605 | 1.00 | 1688 | 69 | 2570 | 2.68 | 0.38 (.24–.67) | <.001 |
| Zoster observed on examination | 1693 | 12 | 2604 | 0.46 | 1688 | 21 | 2570 | 0.82 | 0.57 (.22–1.68) | .33 |
| Report of zoster | 1693 | 16 | 2603 | 0.61 | 1688 | 64 | 2568 | 2.49 | 0.25 (.14–.43) | <.001 |
| Subgroup, based on enrollment characteristics | ||||||||||
| Sex | ||||||||||
| Male | 561 | 10 | 875 | 1.14 | 536 | 28 | 838 | 3.34 | 0.34 (.16–.70) | |
| Female | 1132 | 16 | 1730 | 0.93 | 1152 | 41 | 1732 | 2.37 | 0.41 (.18–.91) | |
| Effect modificationc | .75 | |||||||||
| Age, y | ||||||||||
| <30 | 664 | 13 | 1011 | 1.29 | 665 | 24 | 991 | 2.42 | 0.55 (.28–1.48) | |
| ≥30 | 1028 | 13 | 1593 | 0.82 | 1023 | 45 | 1579 | 2.85 | 0.29 (.16–.54) | |
| Effect modificationc | .24 | |||||||||
| CD4+ T-cell count, cells/µL | ||||||||||
| <350 | 424 | 8 | 648 | 1.23 | 461 | 34 | 703 | 4.84 | 0.27 (.13–.59) | |
| ≥350 | 1269 | 18 | 1957 | 0.92 | 1227 | 35 | 1867 | 1.87 | 0.50 (.23–1.06) | |
| Effect modificationc | .22 | |||||||||
| ≤250 | 4 | 0 | 7 | 0.00 | 6 | 0 | 9 | 0.00 | … | |
| >250 | 1689 | 26 | 2598 | 1.00 | 1682 | 69 | 2561 | 2.69 | 0.38 (.22–.67) | |
| Effect modificationc | >.99 | |||||||||
| Viral load, copies/mL | ||||||||||
| <10 000 | 778 | 6 | 1190 | 0.50 | 800 | 17 | 1214 | 1.40 | 0.37 (.15–.92) | |
| 10 000–99 999 | 645 | 12 | 1004 | 1.20 | 641 | 28 | 986 | 2.84 | 0.44 (.16–1.17) | |
| ≥100 000 | 253 | 8 | 325 | 2.08 | 232 | 24 | 348 | 6.90 | 0.30 (.13–0.68) | |
| Effect modificationc | .85 | |||||||||
| After enrollment | ||||||||||
| ART use in period before clinical visit | ||||||||||
| Yes | … | 0 | 125 | 0.00 | … | 6 | 158 | 3.80 | 0.00 (.00–1.08)b | |
| No | … | 26 | 2480 | 1.05 | … | 63 | 2412 | 2.61 | 0.38 (.24–.60) | |
| Effect modificationd | .41 | |||||||||
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HR, hazard ratio; PY, person-years.
a Unless otherwise indicated, data were determined using Cox regression with the Anderson–Gill counting method, to allow multiple outcomes per participant.
b Data denote the incidence rate ratio and exact 95% CI.
c Evaluated using the likelihood ratio test.
d Evaluated using exact logistic regression, to model discrete survival time.
Figure 1.
Cumulative probability of herpes zoster disease, by time to the first zoster event. Recurrent episodes of zoster are not captured in this figure.
There were 6 cases of herpes zoster among 6 participants who initiated ART after study enrollment; all were in the placebo arm, and all occurred during the first 3 months after ART initiation (range, 3–77 days). Incidence in the placebo arm was 3.80 cases/100 person-years, compared with 0.00 cases/100 person-years in the acyclovir arm (HR, 0.00; 95% CI, .00–1.08). ART did not significantly modify the effect of acyclovir on zoster (P = .41), but the number of participants receiving ART was small. Acyclovir did not prevent zoster recurrences among persons reporting prior zoster episodes at baseline (HR, 1.82; 95% CI, .33–9.96) but did prevent recurrences among participants who had not experienced zoster prior to enrollment (HR, 0.29; 95% CI, .18–.49; P for interaction = .01).
DISCUSSION
In this randomized, double-blind, placebo-controlled trial of acyclovir 400 mg twice daily among African HIV-infected persons, acyclovir prophylaxis substantially reduced herpes zoster events by 62% regardless of sex, age, CD4+ T-cell count, plasma HIV RNA load, or ART use. This reduction in herpes zoster events was consistent with a study from 1999 that found a 68% reduction in herpes zoster with high-dose acyclovir [7]. The incidence of herpes zoster (2.68 cases/100 person-years in the placebo arm) in this population was comparable to previous estimates among HIV-infected individuals (2.90 cases/100 person-years), which is 12–17-fold higher than their age-matched HIV-uninfected controls [5].
Among immunocompromised individuals, including HIV-infected persons, herpes zoster may have a prolonged course and disease recurrence is more common [4]. Acyclovir prophylaxis did not prevent zoster recurrence among persons who reported prior episodes in our study, which might indicate a defect in VZV-specific T-cell immunity [10]; HIV-infected individuals with prior zoster may require a higher acyclovir dose for prophylaxis.
The limitations of our study were the small numbers of participants with low CD4+ T-cell counts, because such persons were excluded from enrollment. Previous studies have found an increased incidence of herpes zoster with declining CD4+ T-cell count [11, 12], which we did not observe, perhaps because of limited power. Some studies have shown an increase in the risk of herpes zoster immediately after ART initiation [6], which is thought to be due to immune reconstitution inflammatory syndrome. We did not observe ART to alter the incidence of herpes zoster events, although the number of individuals and duration of ART in our study were small.
Current Advisory Committee on Immunization Practices guidelines recommend zoster immunization for immunocompetent persons beginning at age 60 years. Because of the risk of vaccine-associated zoster, the zoster vaccine is contraindicated in immunocompromised persons, including HIV-infected persons with CD4+ T-cell count of <200 cells/µL [4, 13]. Immunosuppressed persons, including transplant recipients, who receive antiviral prophylaxis against cytomegalovirus (CMV) or HSV with valganciclovir, ganciclovir, valacyclovir, or acyclovir have a lower risk of herpes zoster [14]. Our data contribute to this evidence by demonstrating prevention of herpes zoster in the setting of acyclovir suppressive therapy for HSV-2.
In summary, in this large placebo-controlled trial of daily acyclovir (400 mg twice daily) for HSV-2 suppression, we found a 68% reduction in zoster events on the acyclovir arm. Acyclovir is well tolerated, safe, and affordable as a generic product, and acyclovir prophylaxis has benefits that include a 73% reduction in HSV-2 genital ulcer disease [8] and a modest (16%) decrease in HIV disease progression [9]. For immunosuppressed patients on antiviral prophylaxis for CMV or HSV, prevention of herpes zoster is an additional benefit. Future research should include a focus on the impact of acyclovir (or related antiviral) prophylaxis on herpes zoster incidence among HIV-infected persons with a low CD4+ T-cell count and among HIV-infected individuals initiating ART, which are the factors associated with greatest frequency of zoster reactivation.
Notes
Acknowledgments. R. V. B. oversaw the analysis and wrote the first draft of the paper, which was revised by all authors. All authors contributed to data analysis, as well as to the interpretation of findings. K. K. T. did the statistical analysis, with input from R. V. B., J. M. B., J. P. H., J. R. L., and C. C. All authors approved the final version of the paper for submission.
The University of Washington human subjects review committee and ethics review committees at the each of the collaborating organizations approved the study protocol. All participants provided written informed consent.
Disclaimer. The work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the Bill and Melinda Gates Foundation (grant 26469) and the National Institute of Allergy and Infectious Diseases, National Cancer Institute, National Institute of Mental Health, National Institute on Drug Abuse, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Heart, Lung, and Blood Institute, National Institute on Aging, National Institute of General Medical Sciences, and National Institute of Diabetes & Digestive & Kidney Diseases of the NIH (grant P30AI027757).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
References
- 1.Gnann JW Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med 2002; 347:340–6. [DOI] [PubMed] [Google Scholar]
- 2.Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med 2013; 10:e1001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson C, Hua L, Anderson JW et al. Zostavax is generally safe and immunogenic in HIV+ adults virologically suppressed on ART: results of a phase 2, randomized, double-blind, placebo-controlled trial. Presented at: 19th Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 2012. [Google Scholar]
- 4.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed 16 May 2014.
- 5.Buchbinder SP, Katz MH, Hessol NA et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis 1992; 166:1153–6. [DOI] [PubMed] [Google Scholar]
- 6.Grabar S, Tattevin P, Selinger-Leneman H et al. Incidence of herpes zoster in HIV-infected adults in the combined antiretroviral therapy era: results from the FHDH-ANRS CO4 cohort. Clin Infect Dis 2015; 60:1269–77. [DOI] [PubMed] [Google Scholar]
- 7.Leautez S, Bani-Sadr F, Billaud E, Raffi F. [Secondary prophylaxis for herpes zoster wi oral acyclovir in HIV patients]. Pathol Biol (Paris) 1999; 47:570–2. [PubMed] [Google Scholar]
- 8.Celum C, Wald A, Lingappa JR et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010; 362:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lingappa JR, Baeten JM, Wald A et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet 2010; 375:824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy O, Orange JS, Hibberd P et al. Disseminated varicella infection due to the vaccine strain of varicella-zoster virus, in a patient with a novel deficiency in natural killer T cells. J Infect Dis 2003; 188:948–53. [DOI] [PubMed] [Google Scholar]
- 11.Shearer K, Maskew M, Ajayi T et al. Incidence and predictors of herpes zoster among antiretroviral therapy-naive patients initiating HIV treatment in Johannesburg, South Africa. Int J Infect Dis 2014; 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebo KA, Kalyani R, Moore RD, Polydefkis MJ. The incidence of, risk factors for, and sequelae of herpes zoster among HIV patients in the highly active antiretroviral therapy era. J Acquir Immune Defic Syndr 2005; 40:169–74. [DOI] [PubMed] [Google Scholar]
- 13.Bhalla P, Forrest GN, Gershon M et al. Disseminated, persistent, and fatal infection due to the vaccine strain of varicella-zoster virus in an adult following stem cell transplantation. Clin Infect Dis 2015; 60:1068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pergam SA, Limaye AP, AST Infectious Diseases Community of Practice. Varicella zoster virus in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]