Abstract
Background. The clinical significance of viruses detected in patients with community-acquired pneumonia (CAP) is often unclear.
Methods. We conducted a prospective study to identify the prevalence of 13 viruses in the upper respiratory tract of patients with CAP and concurrently enrolled asymptomatic controls with real-time reverse-transcriptase polymerase chain reaction. We compared age-stratified prevalence of each virus between patients with CAP and controls and used multivariable logistic regression to calculate attributable fractions (AFs).
Results. We enrolled 1024 patients with CAP and 759 controls. Detections of influenza, respiratory syncytial virus, and human metapneumovirus were substantially more common in patients with CAP of all ages than in controls (AFs near 1.0). Parainfluenza and coronaviruses were also more common among patients with CAP (AF, 0.5–0.75). Rhinovirus was associated with CAP among adults (AF, 0.93) but not children (AF, 0.02). Adenovirus was associated with CAP only among children <2 years old (AF, 0.77).
Conclusions. The probability that a virus detected with real-time reverse-transcriptase polymerase chain reaction in patients with CAP contributed to symptomatic disease varied by age group and specific virus. Detections of influenza, respiratory syncytial virus, and human metapneumovirus among patients with CAP of all ages probably indicate an etiologic role, whereas detections of parainfluenza, coronaviruses, rhinovirus, and adenovirus, especially in children, require further scrutiny.
Keywords: pneumonia, etiology, virus, asymptomatic infection, attributable fraction
The recent widespread availability of nucleic acid amplification techniques, such as real-time reverse-transcriptase polymerase chain reaction (rRT-PCR), has facilitated detection of viruses in the upper respiratory tract of patients with acute respiratory illness [1–11]. However, the clinical significance of these viral detections in patients with pneumonia is often unclear [1–3]. When detected in an acutely ill patient with pneumonia, respiratory viruses may represent subclinical infection, persistent shedding after a prior infection, infection restricted to the upper respiratory tract, or infection involving the lower respiratory tract [1–3]. Understanding the significance of viral detections in acute respiratory illness, particularly pneumonia, is essential to inform clinical management decisions and research priorities, especially in the fields of vaccine and antiviral development [12–14].
Few contemporary studies in the United States have systematically assessed the presence of respiratory viruses in asymptomatic persons, particularly in adults. Information on the background prevalence of asymptomatic viral detections is needed to understand the significance of viral detections in patients with pneumonia. Therefore, we performed a prospective study to assess the prevalence of respiratory viruses detected with rRT-PCR in the upper respiratory tract of both asymptomatic children and adults and compared these detections with a concurrent sample of patients hospitalized with community-acquired pneumonia (CAP).
METHODS
We conducted a prospective study in Nashville, Tennessee, and Salt Lake City, Utah, nested within the Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study [15]. Institutional review boards at the enrolling centers and the CDC approved the protocol. Informed consent was obtained for all participants.
Participant Recruitment
Asymptomatic Controls
We recruited a convenience sample of asymptomatic children and adults. Asymptomatic children (aged <18 years) were enrolled from the elective outpatient surgery areas at Monroe Carell Jr. Children's Hospital (Nashville) and Primary Children's Hospital (Salt Lake City). Asymptomatic adults attending outpatient primary care clinics for routine health maintenance were enrolled at Vanderbilt University (Nashville). Eligibility requirements for controls included (1) absence of fever, cough, sore throat, wheeze, shortness of breath, rhinorrhea, ear pain, and vomiting for the past 14 days; and (2) residence in a geographic catchment area surrounding each enrolling center. Children undergoing otolaryngologic surgery were excluded. To avoid inclusion of controls in the incubation period of an existing infection, we excluded patients in whom respiratory symptoms developed within 14 days after enrollment, as determined by a follow-up telephone call [16]. Asymptomatic controls were enrolled during the following time periods: children in Salt Lake City, February 2011 through June 2012; children in Nashville, March 2011 through June 2012; and adults in Nashville, November 2011 through June 2012.
Patients With CAP
Viral detections in the asymptomatic controls were compared with patients with CAP enrolled in the EPIC study [15] during the same time periods and geographic areas as controls. All patients with CAP in the EPIC study were hospitalized with clinical evidence of acute infection, acute respiratory illness, and chest imaging showing consolidation, infiltrate, or pleural effusion. Patients were excluded if they were recently hospitalized or had severe immunosuppression, defined as human immunodeficiency virus infection with a CD4 cell count <200/mm3 or <14%; solid or hematopoietic stem cell transplantation in the past 90 days or with graft-vs-host disease or bronchiolitis obliterans; or cancer with an absolute neutrophil count <500/mm3. Detailed inclusion and exclusion criteria for these patients with CAP have been described elsewhere [15].
Viral Detection Techniques
Nasopharyngeal (NP) and oropharyngeal (OP) samples were obtained with sterile nylon flocked swabs at the time of enrollment from both asymptomatic controls and patients with CAP. The NP/OP swab samples were placed together in viral universal transport media, refrigerated at 4°C, processed within 72 hours, and stored at −70°C. rRT-PCR was conducted on NP/OP samples using CDC protocols and primers [17–22] for detection of the following viruses: human rhinovirus (hRV); respiratory syncytial virus (RSV); human metapneumovirus (hMPV); adenovirus (AdV); influenza A and B (influenza); parainfluenza (PIV) virus types 1, 2, and 3; and coronavirus (CoV) 229E, HKU1, NL63, and OC43. Detection of a virus by rRT-PCR at a cycle threshold (Ct) <40 was considered positive.
Statistical Analysis
The study population was stratified into children (<18 years old) and adults. The prevalence of each virus was calculated for asymptomatic controls and patients with CAP and compared using χ2 or Fisher exact test, as appropriate. Children were then further stratified into 3 age groups (<2, 2–4, and 5–17 years old) for additional comparisons [1, 23–27]. Each virus detected in individual subjects with codetection of multiple viruses was considered separately in prevalence calculations. For example, if an individual had codetection of hRV and RSV, detection of each virus was considered positive in separate hRV and RSV prevalence calculations.
Logistic Regression Models and Attributable Fractions
We constructed multivariable logistic regression models for each virus. The dependent variable in each model was asymptomatic control vs CAP patient status, and the independent variables included viral detection (positive/negative rRT-PCR results), age, enrollment month/year, and enrollment city. Using these models, we calculated an adjusted odds ratio (aOR), which compared the odds of a positive detection for a specific virus between patients with CAP and asymptomatic controls, while adjusting for age, enrollment month, and enrollment city.
We then used aORs from our multivariable logistic regression models to calculate the virus-specific attributable fraction (AF), an estimate of the proportion of patients with CAP positive for a virus who have symptomatic illness due to that virus [23, 28–30]. AF was calculated using the following equation: AF = (aOR – 1)/aOR. For example, an AF equal to 0 indicated that the adjusted odds of detection were the same in asymptomatic controls and patients with CAP. An AF of 0.9 indicated that 90% of detections for that virus in patients with CAP were attributable to symptomatic illness, and 10% were due to asymptomatic shedding [23, 28–30].
Comparison of Ct Values
rRT-PCR Ct represents the first PCR cycle in which fluorescent signal for the target (eg, viral RNA) is greater than the minimal detection level. Ct values are inversely proportional to the quantity of target, offering a semiquantitative assessment of viral load [31]. When viruses were detected in >1% of both asymptomatic controls and patients with CAP, we compared Ct values between these groups using the Wilcoxon rank sum test. Ct values were also used to construct nonparametric receiver operating characteristic (ROC) curves to discriminate between asymptomatic controls and patients with CAP. The area under the curve for each ROC curve was calculated. Sensitivity and specificity were calculated at Ct value cut points that maximized combined sensitivity and specificity [32]. Statistical analyses were performed with Stata 12 software. Differences were considered significant at P < .05 (2 sided).
RESULTS
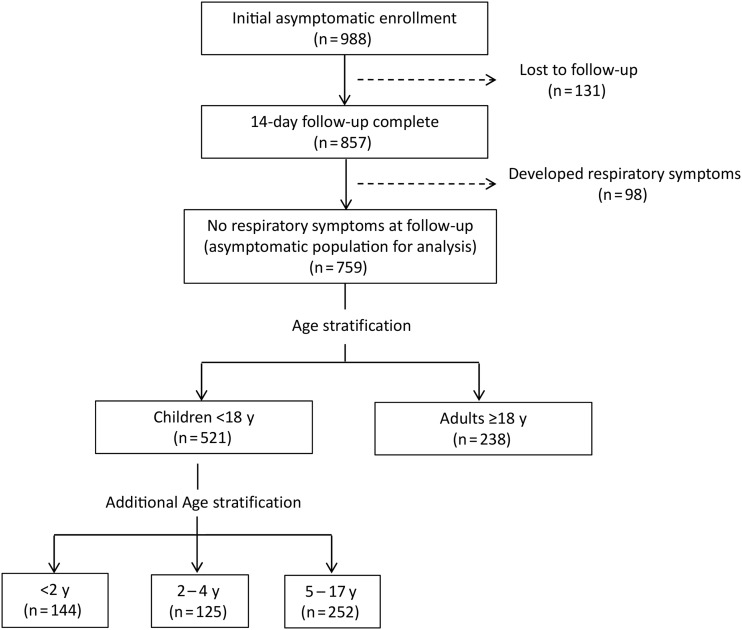
Initially, 988 participants (726 children and 262 adults) were enrolled as potential asymptomatic controls, but 229 (205 children and 24 adults) were later excluded because they were lost to follow-up (125 children and 6 adults) or developed respiratory symptoms during the follow-up period (80 children, 18 adults) (Figure 1; Supplementary Table 1). Therefore, 759 asymptomatic controls (521 children, 238 adults) were included in the final analysis; they controls compared with 1024 concurrently enrolled patients with CAP, including 832 children and 192 adults. Characteristics of the study population are shown in Table 1, and characteristics stratified by age group in children are displayed in Supplementary Table 2. Compared with asymptomatic controls, patients with CAP had a higher prevalence of most comorbid medical conditions. Compared with controls, children with CAP were younger (P < .01), and adults with CAP were older (P < .01).
Figure 1.
Enrollment of asymptomatic controls.
Table 1.
Clinical Characteristics of Asymptomatic Controls and Patients With CAP
| Characteristic | Children, No. (%)a |
Adults, No. (%)a |
||
|---|---|---|---|---|
| Asymptomatic Controls (n = 521) | Patients With CAP (n = 832) | Asymptomatic Controls (n = 238) | Patients With CAP (n = 192) | |
| Age, median (IQR), y | 4 (1–9) | 2 (1–6) | 54 (41–65) | 59 (50–73) |
| Female sex | 184 (35.3) | 374 (45.0) | 129 (54.2) | 114 (59.4) |
| Non-Hispanic, White race | 375 (72.0) | 434 (52.2) | 177 (74.4) | 129 (67.2) |
| Non-Hispanic, Black race | 55 (10.7) | 108 (13.0) | 48 (20.2) | 54 (28.1) |
| Hispanic | 53 (10.2) | 206 (24.8) | 5 (2.1) | 7 (3.7) |
| Site of enrollment | ||||
| Nashville | 298 (57.2) | 408 (49.0) | 238 (100) | 192 (100) |
| Salt Lake City | 223 (42.8) | 424 (51.0) | NA | NA |
| Child care attendanceb | 74 (23.3) | 156 (24.5) | NA | NA |
| Live with child in daycare | NA | NA | 14 (5.9) | 13 (6.8) |
| Current smoker | NA | NA | 22 (9.2) | 44 (23.0) |
| Asthma | 50 (9.6) | 167 (20.1) | 29 (12.2) | 57 (29.7) |
| COPD | NA | NA | 7 (2.9) | 52 (27.1) |
| Congenital heart disease | 6 (1.1) | 46 (5.5) | NA | NA |
| Heart failure | NA | NA | 6 (2.5) | 24 (12.5) |
| Diabetes mellitus, n (%) | 1 (0.2) | 2 (0.2) | 42 (17.7) | 55 (28.6) |
| Chronic kidney disease | 3 (0.6) | 8 (1.0) | 6 (2.5) | 15 (7.8) |
| Chronic liver disease | 1 (0.2) | 3 (0.4) | 8 (3.4) | 10 (5.2) |
| Preterm birthc | 6 (1.2) | 64 (7.7) | NA | NA |
| Cancer | 0 | 3 (0.4) | 19 (8.0) | 35 (18.2) |
Abbreviations: CAP, community-acquired pneumonia; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; NA, not applicable.
a Children were defined as those <18 years old; adults, those ≥18 years old. Unless otherwise specified, data represent No. (%) of patients or controls.
b Child care attendance was evaluated only for children <6 years old.
c Preterm birth was evaluated only for children <2 years old.
Prevalence of Viral Detection
Children
Overall, 127 asymptomatic children (24.4%) had ≥1 virus detected. The most common virus was hRV, detected in 90 (17.3%) of the asymptomatic children (Table 2). Influenza was the only virus not detected in any asymptomatic children. Fourteen asymptomatic children had >1 virus, including the following codetections: hRV-AdV in 5 children, hRV-CoV in 3, hRV-hMPV in 2, and hMPV-PIV, hMPV-CoV, PIV-AdV, and hRV-RSV-AdV in 1 each. Among the 80 children initially enrolled as potential controls but later excluded owing to the development of symptoms, 30 (37.5%) had a virus detected (Supplementary Table 3).
Table 2.
Prevalence of Respiratory Virus Detection With rRT-PCR in Asymptomatic Controls and Patients With CAP <18 Years Old
| Virus | Asymptomatic Children, No. (%) (n = 521) | Children With CAP, No. (%) (n = 832) | P Valuea | aOR (95% CI)b | AF (95% CI) |
|---|---|---|---|---|---|
| Any virusc | 127 (24.4) | 572 (68.8) | <.01 | NCd | NC |
| hRV | 90 (17.3) | 182 (21.9) | .04 | 1.13 (.84–1.51) | 0.12 (−.18–.34) |
| RSV | 10 (1.9) | 221 (26.6) | <.01 | 15.2 (7.92–29.2) | 0.93 (.87–.97) |
| hMPV | 8 (1.5) | 126 (15.1) | <.01 | 10.4 (5.02–21.6) | 0.90 (.80–.95) |
| AdV | 16 (3.1) | 53 (6.4) | <.01 | 1.77 (.99–3.17) | 0.44 (−.01 to .68) |
| Influenza (A and B) | 0 | 28 (3.4) | <.01 | NC | NC |
| PIV (types 1–3) | 10 (1.9) | 39 (4.7) | .01 | 2.29 (1.11–4.69) | 0.56 (.10–.79) |
| CoV (229E, HKU1, NL63, OC43) | 8 (1.5) | 37 (4.5) | <.01 | 3.17 (1.44–6.99) | 0.68 (.31–.86) |
Abbreviations: AdV, adenovirus; AF, attributable fraction; aOR, adjusted odds ratio; CAP, community-acquired pneumonia; CI, confidence interval; CoV, coronavirus; hMPV, human metapneumovirus; hRV, human rhinovirus; NC, not calculated; PIV, parainfluenza virus; rRT-PCR, real-time reverse-transcriptase polymerase chain reaction; RSV, respiratory syncytial virus.
a Univariate comparisons with P values by χ2 (count ≥5 in each cell) or Fisher exact test.
b Logistic regression model adjusted for the following variables: age, enrollment month, and enrollment site.
c The number of subjects with any viral detection does not equal the sum of detections for each virus owing to codetections of multiple viruses in the same subject.
d aOR and AF were not calculated for the “Any virus” group or for viruses with no detections among asymptomatic controls.
At least 1 virus was detected in 572 (68.8%) of the children with CAP; RSV was the most common (26.6%), followed by hRV (21.9%) and hMPV (15.1%). In unadjusted prevalence comparisons, detection of each of the viruses was more common in children with CAP than in asymptomatic children (Table 2).
Adults
Viral detections were rare in asymptomatic adults, with only 5 detections (2.1%) overall, including hRV in 2, CoV in 2, and hMPV in 1. In contrast, 47 (24.5%) of the adults with CAP had a viral detection, with hRV (10.9%) and hMPV (4.2%) the most common. Overall detection of any virus was more common in adults with CAP than in asymptomatic adults (P < .01), and differences were statistically significant for 3 individual viruses: influenza, hRV, and hMPV (Table 3).
Table 3.
Prevalence of Respiratory Virus Detection With rRT-PCR in Asymptomatic Controls and Patients With CAP ≥18 Years Old
| Virus | Asymptomatic Adults, No. (%) (n = 238) | Adults With CAP, n (%) (n = 192) | P Valuea | aOR (95% CI)b | AF (95% CI) |
|---|---|---|---|---|---|
| Any virusc | 5 (2.1) | 47 (24.5) | <.01 | NCd | NCd |
| hRV | 2 (0.8) | 21 (10.9) | <.01 | 13.4 (3.04–59.1) | 0.93 (.67–.98) |
| RSV | 0 | 3 (1.6) | .09 | NCd | NC |
| hMPV | 1 (0.4) | 8 (4.2) | .01 | 13.5 (1.65–110) | 0.93 (.39–.99) |
| AdV | 0 | 3 (1.6) | .09 | NC | NC |
| Influenza (A and B) | 0 | 5 (2.6) | .02 | NC | NC |
| PIV (types 1–3) | 0 | 3 (1.6) | .09 | NC | NC |
| CoV (229E, HKU1, NL63, OC43) | 2 (0.8) | 6 (3.1) | .14 | 3.19 (.59–17.1) | 0.69 (−.69 to .94) |
Abbreviations: AdV, adenovirus; AF, attributable fraction; aOR, adjusted odds ratio; CAP, community-acquired pneumonia; CI, confidence interval; CoV, coronavirus; hMPV, human metapneumovirus; hRV, human rhinovirus; NC, not calculated; PIV, parainfluenza virus; rRT-PCR, real-time reverse-transcriptase polymerase chain reaction; RSV, respiratory syncytial virus.
a Univariate comparisons with P values by χ2 (count ≥5 in each cell) or Fisher exact test.
b Logistic regression model adjusted for the following variables: age, enrollment month, and enrollment site.
c The number of subjects with any viral detection does not equal the sum of detections for each virus owing to codetections of multiple viruses in the same subject.
d aOR and AF were not calculated for the “Any virus” group or for viruses with no detections among asymptomatic controls.
Logistic Regression Models and AFs
Children
In multivariable regression models, detection of RSV and hMPV in children was highly associated with CAP, resulting in AFs of 0.93 (95% confidence interval [CI], .87–.97) and 0.90 (CI, .80–.95), respectively (Table 2). PIV and CoV detection was also significantly associated with CAP, but with lower AFs of 0.56 (CI, .10–.79) and 0.68 (CI, .31–.86), respectively. AdV had an AF of 0.44 (CI, −.01 to .68). hRV was not significantly associated with CAP (AF, 0.02; CI, −.18 to .34). Results were similar after further age stratification of children, except AdV was significantly associated with CAP among the youngest children (<2 years old) (Supplementary Table 4).
Adults
hRV and hMPV were strongly associated with CAP in adults, with AFs of 0.93 (CI, .67–.98) and 0.93 (CI, .39–.99), respectively (Table 3). Although the point estimate for CoV prevalence was higher in patients with CAP than in asymptomatic controls, this difference was not significant in univariate or multivariate comparisons. Models for RSV, AdV, influenza, and PIV were not constructed because there were no asymptomatic detections.
rRT-PCR Ct Values
Ct values were compared between asymptomatic children and children with CAP for all viral groups except influenza, for which there were no detections in controls. None of the viruses were detected in >1% of asymptomatic adults; thus, meaningful Ct comparisons could not be made in adults.
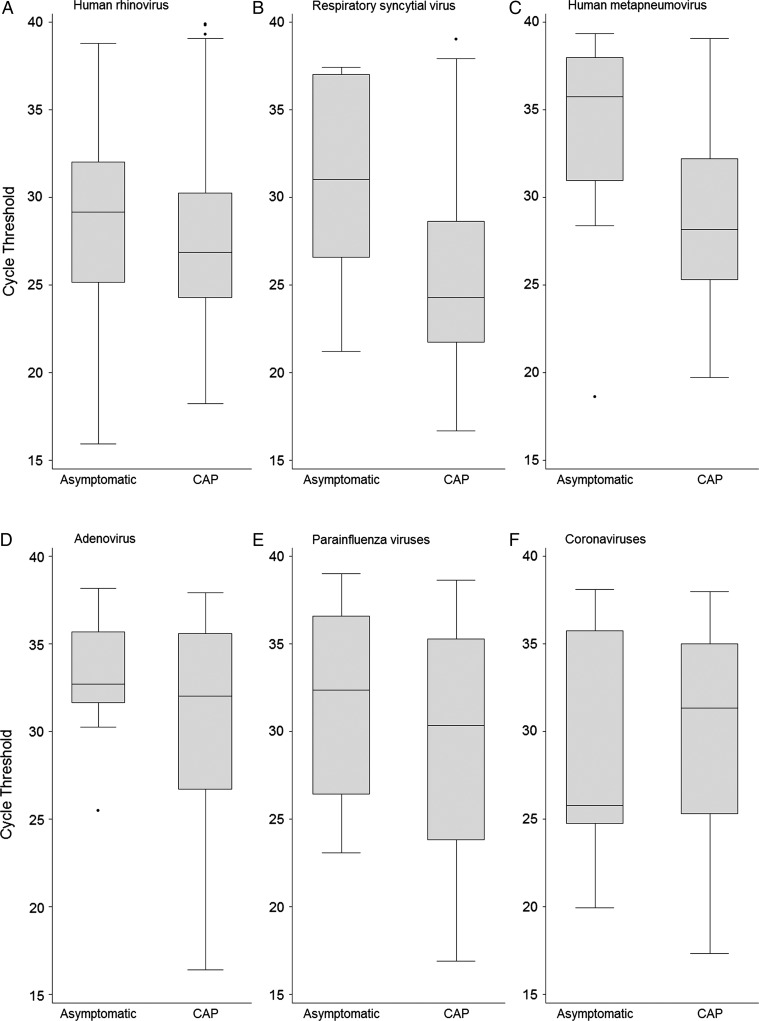
Ct values were significantly higher in asymptomatic children positive for RSV than in patients with CAP (P < .01) (Figure 2; Supplementary Table 5). Using Ct values for all patients and controls positive for RSV, we created an ROC curve to illustrate the performance of these Ct values to discriminate between case patients and controls; the area under this ROC curve was 0.79 (95% CI, .73–.84). Using a Ct value cut point of 26.3 maximized the combination of sensitivity (0.90; 95% CI, .54–.99) and specificity (0.66; CI, .59–.72).
Figure 2.
Box and whisker plots of rRT-PCR cycle thresholds among children (age <18 years) positive for each virus, comparing asymptomatic controls and patients with community-acquired pneumonia (CAP): human rhinovirus (A), respiratory syncytial virus (B), human metapneumovirus (C), adenovirus (D), parainfluenza viruses (E), and coronaviruses (F). The center of each box plot represents the median, with the box denoting the interquartile range (IQR), the upper and lower whiskers representing 1.5 times the IQR above and below the 75% and 25% percentile, respectively, and dots showing outliers beyond the whiskers. Abbreviation: rRT-PCR, reverse transcriptase polymerase chain reaction.
Similarly, hMPV Ct values were also significantly higher in asymptomatic children than in patients with CAP (P = .01) (Figure 2; Supplementary Table 5). The area under the ROC curve for hMPV Ct values to discriminate between case patients and controls was 0.76 (95% CI, .68–.83). For hMPV, a Ct cut point of 33.5 maximized combined sensitivity (0.75; 95% CI, .36–.96) and specificity (0.82; 95% CI, .74–.88). Ct values did not perform well for discriminating between asymptomatic children and those with CAP for the other viruses, including hRV, AdV, PIV, and CoV (Figure 2; Supplementary Table 5).
DISCUSSION
Our study is the largest US study to date to examine the frequency of viral respiratory detections with rRT-PCR in patients with CAP compared with an asymptomatic control population. Our data suggest that accounting for background circulation of respiratory viruses among asymptomatic person is critical when investigating the causes of pneumonia, especially in children, who have more asymptomatic detections than adults. Although it remains challenging to definitively determine the clinical significance of a detected virus in an individual patient with CAP, the virus- and age group–specific AFs presented here provide estimates for the probability that a detected virus contributes to symptomatic illness.
We found that detections of influenza, RSV, and hMPV were all very rare in asymptomatic children and adults compared with detections of CAP in the same communities and during the same time period. Moreover, when these viruses were detected in asymptomatic controls, rRT-PCR Ct values were higher than in patients with CAP, suggesting lower viral loads. These data support a likely etiologic role for influenza, RSV, and hMPV when detected in patients with CAP.
PIV and CoV were detected significantly more commonly in patients with CAP than in asymptomatic controls; however, differences in prevalence and Ct values between case patients and controls were not as marked for PIV and CoV as for influenza, RSV, and hMPV. This suggests that most detections were associated with symptomatic illness, but some caution is needed in interpretation with these viruses.
Associations of hRV and AdV with CAP varied with age. Asymptomatic hRV detection declined with increasing age, with detection in 24.3%, 23.2%, 10.3%, and 0.8% of asymptomatic controls aged <2, 2–4, 5–17, and ≥18 years, respectively. This led to a strong association of hRV detection with CAP in adults, a more modest association in older children (5–17 years old), and no association in younger children. Meanwhile, AdV was strongly associated with CAP in the youngest children (<2 years old) but not in the older age groups. This could be because a person's first lifetime AdV infection in early childhood is more likely to lead to pneumonia, with prolonged AdV shedding from lymphoid tissues later in life [33–36].
Earlier studies comparing viral detections in asymptomatic controls and symptomatic patients focused largely on children and included both lower and upper respiratory infections [2, 23, 28, 37, 38]. By including all age groups in this study, we were able to compare adults and children and examine the relevance of viral detections across age groups. In addition, our symptomatic comparison group was restricted by design to patients with CAP. This enabled us to evaluate the association of each virus specifically with CAP.
Our results are consistent with those of earlier pediatric studies, which suggested that influenza, RSV, and hMPV are rarely found in asymptomatic children [2, 23, 28, 37]. Singleton et al [28] found 1%, 5%, and 7% of 381 asymptomatic Alaskan children <3 years old to have laboratory evidence of influenza, RSV, and hMPV, respectively. In addition, prior work has also shown similar detection of hRV and AdV in asymptomatic and symptomatic children [2, 23, 28]. Similar to our findings, Singleton et al [28] found AFs near 0 for hRV and AdV in young children.
Compared with our estimates, some earlier pediatric studies have suggested that CoV and PIV are more strongly associated with acute respiratory illness [2, 10, 28]. Limiting our patients to children hospitalized with CAP probably led to lower prevalence of CoV and PIV detection in our patients than in prior studies that included outpatients with upper respiratory tract infections [2, 10, 28]. Lower AFs in our study for CoV and PIV may reflect the fact that these viruses are more strongly associated with nonspecific respiratory infection than CAP resulting in hospitalization.
Among prior adult studies, Lieberman et al [1] compared upper respiratory viral detections in 450 asymptomatic adults and 183 patients with CAP in Israel. Similarly to our study, they found rare detections (<1%) of influenza, RSV, and hMPV among asymptomatic controls and significantly higher detection in patients with CAP. In contrast to the relatively low prevalence of CoV among adults with CAP in our study (3.1%), CoVs were the most frequently detected viruses among patients with CAP in the study by Lieberman et al [1] (13.1%) and were significantly more common in patients with CAP than in asymptomatic controls. Differences in geography and season may account some of these differences.
The low prevalence of hRV detection from upper respiratory specimens among asymptomatic adults in our study (0.8%) is consistent with prior work, including that by Lieberman et al [1], who detected hRV in 2% of 450 asymptomatic adults in Israel, and Jennings et al [39], who detected hRV in 2% of 50 asymptomatic adults in New Zealand. Furthermore, Karhu et al [40] recently detected hRV from lower respiratory specimens (tracheal and bronchial aspirates) in 20% of 49 intubated adults with severe CAP in Finland. Combined, these findings of rare hRV detection in upper respiratory specimens of asymptomatic adults and detection of hRV in lower respiratory specimens of intubated adults with CAP suggest that when hRV is detected from upper respiratory swab samples in adults with CAP, it may have an etiologic role. A higher prevalence of asymptomatic hRV detection in children makes it more difficult to interpret a positive rRT-PCR test for hRV from an upper respiratory specimen in children than adults. Further study in children comparing detection of hRV in the upper and lower respiratory tracts would be helpful.
Our study had both strengths and limitations. Strengths include concurrent enrollment of asymptomatic controls and acutely ill patients with CAP in the same geographic regions and time periods and using identical rRT-PCR methods for viral detection. Age stratification and multivariable regression adjustment were used to account for potential confounders. Furthermore, results were presented as AFs, which helps with clinical interpretation. Limitations include pediatric enrollment during only 1 full respiratory season and adult enrollment at only 1 site and for 8 months owing to resource constraints. Inclusion of only 1 respiratory season prevented exploration of how seasonal variation in viral circulation may have affected AFs from year to year. Small numbers of viral detections in adult controls prevented further age stratification. In addition, asymptomatic controls were enrolled as a convenience sample and were not matched with case patients. Although some studies have reported persistent viral detections weeks after acute exacerbations of certain comorbid conditions, such as chronic obstructive pulmonary disease and asthma [41], matching controls to patients with CAP according to the presence of comorbid medical conditions and timing of related exacerbations was found to be impractical and would have severely hampered our enrollment efforts. Although 13% of enrolled subjects were excluded because we could not contact them to ascertain whether they developed respiratory symptoms after enrollment, those excluded were similar to subjects with completed follow-up (Supplementary Table 1). Enrollment of community controls who did not present to a healthcare facility would have provided another comparison group [16] but was not logistically feasible.
In summary, many respiratory viruses detected by rRT-PCR in patients with CAP were also detected in asymptomatic persons from the same time periods and geographic locations. Therefore, it is essential to consider background rates of asymptomatic viral detection when assessing the etiologic contribution of viruses to CAP, especially in children, who had more asymptomatic detections than adults. In our study, influenza, RSV, and hMPV were very rarely detected in asymptomatic persons of all ages compared with patients with CAP, suggesting that when they are detected in patients with CAP, these viruses were associated with disease. Additional research, including evaluation of lower respiratory tract specimens, is needed to further delineate the contribution to CAP of other respiratory viruses, including hRV, AdV, PIV, and CoV.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the following for their dedication in enrolling patients and testing specimens for this study: Adrienne Baughman, Robert Sparks, Kelly Moser, Charity Graves, Rabon Lee Smalling, Karen Miller, Rachel Apple, Rebecca Smith, Shanda Phillips, Markia Ward, Sandy Alvarez, Rendi McHenry, Heather London, Torrance Meyer, Brittany McDowell, Parker Plant, Priscilla Rosen, and Leah Willis.
Disclaimer. The findings and conclusions are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by a cooperative agreement with the CDC (grant U18 IP000299) and a research grant through the Vanderbilt Institute for Clinical and Translational Research (grant UL1TR000445) from the National Institute for Advancing Translational Sciences. W. H. S. was supported in part by career development grants from the National Institute of General Medical Sciences (grant 1K23GM110469) and National Institute for Advancing Translational Sciences (grant KL2TR000446). D. J. W. was supported in part by a career development grant from the National Institute of Allergy and Infectious Diseases (grant K23AI104779). C. G. G. was supported in part by the Agency for HealthCare Research and Quality (grant 1R03HS022342).
Potential conflicts of interest. W. H. S. reports being a scientific advisor for BioFire Diagnostics and Venaxis; receiving research funding from Pfizer, Rapid Pathogen Screening, BRAHMS, and BioMerieux; and holding a pending patent for a blood culture collection device. A. T. P. has been a coinvestigator with BioFire Diagnostics on National Institutes of Health–funded studies and has served as a consultant to BioFire Diagnostics. J. D. C. reports an issued patent for a system to generate viable reovirus from cloned complementary DNA and a pending patent for reovirus vaccine. K. M. E. reports research funding and funding for Data Safety and Monitoring Board participation from Novartis. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lieberman D, Shimoni A, Shemer-Avni Y, Keren-Naos A, Shtainberg R, Lieberman D. Respiratory viruses in adults with community-acquired pneumonia. Chest 2010; 138:811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jansen RR, Wieringa J, Koekkoek SM et al. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol 2011; 49:2631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jartti T, Jartti L, Peltola V, Maris M, Ruuskanen O. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr Infect Dis J 2008; 27:1103–7. [DOI] [PubMed] [Google Scholar]
- 4.Templeton KE, Scheltinga SA, van den Eeden WC, Graffelman AW, van den Broek PJ, Claas ED. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase-chain reaction. Clin Infect Dis 2005; 41:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murdoch DR, Jennings LC, Bhat N, Anderson TP. Emerging advances in rapid diagnostics of respiratory infections. Infect Dis Clin North Am 2010; 24:791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talbot HK, Falsey AR. The diagnosis of viral respiratory disease in older adults. Clin Infect Dis 2010; 50:747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angeles MM, Camps M, Pumarola T et al. The role of viruses in the aetiology of community-acquired pneumonia in adults. Antivir Ther 2006; 11:351–9. [PubMed] [Google Scholar]
- 8.Nolte FS. Molecular diagnostics for detection of bacterial and viral pathogens in community-acquired pneumonia. Clin Infect Dis 2008; 47(suppl 3):S123–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson N, Kalin M, Tiveljung-Lindell A, Giske C, Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010; 50:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Gageldonk-Lafeber AB, Heijnen ML, Bartelds AI, Peters MF, van der Plas SM, Wilbrink B. A case-control study of acute respiratory tract infection in general practice patients in the Netherlands. Clin Infect Dis 2005; 41:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graat JM, Schouten EG, Heijnen ML et al. A prospective, community based study on virologic assessment among elderly people with and without symptoms of acute respiratory infection. J Clin Epidemiol 2003; 56:1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet 2011; 377:1264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niederman MS. Viral community-acquired pneumonia: if we do not diagnose it and do not treat it, can it still hurt us? Chest 2010; 138:767–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain S, Williams DJ, Arnold SR et al. Community-acquired pneumonia requiring hospitalization among US children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deloria-Knoll M, Feiken DR, Scott JA et al. Identification and selection of cases and controls in the Pneumonia Etiology Research for Child Health project. Clin Infect Dis 2012; 54(suppl 2): S117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinberg GA, Erdman DD, Edwards KM et al. Superiority of reverse-transcription polymerase chain reaction to conventional viral culture in the diagnosis of acute respiratory tract infections in children. J Infect Dis 2004; 189:706–10. [DOI] [PubMed] [Google Scholar]
- 18.Mullins JA, Erdman DD, Weinberg GA et al. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis 2004; 10:700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dare RK, Fry AM, Chittaganpitch M, Sawanpanyalert P, Olsen SJ, Erdman DD. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays. J Infect Dis 2007; 196:1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X, Holloway B, Dare RK et al. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol 2008; 46:533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loens K, van Loon AM, Coenjaerts F et al. Performance of different mono- and multiplex nucleic acid amplification tests on a multipathogen external quality assessment panel. J Clin Microbiol 2012; 50:977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace PS, MacKay WG. Quality in the molecular microbiology laboratory. Methods Mol Biol 2013; 943:49–79. [DOI] [PubMed] [Google Scholar]
- 23.Feikin DR, Njenga MK, Bigogo G et al. Etiology and incidence of viral and bacterial acute respiratory illness among older children and adults in rural western Kenya, 2007–2010. PloS One 2012; 7:e43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michelow IC, Olsen K, Lozano J et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics 2004; 113:701–7. [DOI] [PubMed] [Google Scholar]
- 25.Shibli F, Chazan B, Nitzan O et al. Etiology of community-acquired pneumonia in hospitalized patients in northern Israel. Isr Med Assoc J 2010; 12:477–82. [PubMed] [Google Scholar]
- 26.Khetsuriani N, Lu X, Teague WG, Kazerouni N, Anderson LJ, Erdman DD. Novel human rhinoviruses and exacerbation of asthma in children. Emerg Infect Dis 2008; 11:1793–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piotrowska Z, Vazquez M, Shapiro ED et al. Rhinoviruses are a major cause of wheezing and hospitalization in children less than 2 years of age. Pediatr Infect Dis J 2009; 28:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singleton RJ, Bulkow LR, Miernyk K et al. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol 2010; 82:1282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol 1974; 99:325–32. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics 1993; 49:865–72. [PubMed] [Google Scholar]
- 31.Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol 2002; 30:503–12. [DOI] [PubMed] [Google Scholar]
- 32.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using criteria based on the receiver operating characteristic curve. Am J Epidemiol 2006; 163:670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell LS, Taylor B, Reimels W et al. Adenovirus 7a: a community-acquired outbreak in a children's hospital. Pediatr Infect Dis J 2000; 19:996–1000. [DOI] [PubMed] [Google Scholar]
- 34.Tabain I, Ljubin-Sternak S, Cepin-Bogovic J et al. Adenovirus respiratory infections in hospitalized children: clinical findings in relation to species and serotypes. Pediatr Infect Dis J 2012; 31:680–4. [DOI] [PubMed] [Google Scholar]
- 35.Neumann R, Genersch E, Eggers HJ. Detection of adenovirus nucleic acid sequences in human tonsils in the absence of infectious virus. Virus Res 1987; 7:93–7. [DOI] [PubMed] [Google Scholar]
- 36.Garnett CT, Talekar G, Mahr JA et al. Latent species C adenoviruses in human tonsil tissues. J Virol 2009; 83:2417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chonmaitree T, Alvarez-Fernandez P, Jennings K et al. Symptomatic and asymptomatic respiratory viral infections in the first year of life: Association with acute otitis media development. Clin Infect Dis 2015; 60:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prill MM, Iwane MK, Edwards KM et al. Human coronavirus in young children hospitalized for acute respiratory illness and asymptomatic controls. Pediatr Infect Dis J 2012; 31:235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jennings LC, Anderson TP, Beynon KA et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax 2008; 63:42–8. [DOI] [PubMed] [Google Scholar]
- 40.Karhu J, Ala-Kokko TI, Vuorinen T et al. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin Infect Dis 2014. 59:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zlateva KT, de Vries JJ, Coenjaerts FE et al. Prolonged shedding of rhinovirus and re-infection in adults with respiratory tract illness. Eur Respir J 2014; 44:169–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.