Abstract
We leveraged data from the Preexposure Prophylaxis Initiative (iPrEx), a global trial of preexposure chemoprophylaxis against human immunodeficiency virus type 1 (HIV-1) infection, to compare T-cell activation between those who remained negative for HIV-1 and those who became infected during the trial. The frequency of CD38+HLA-DR+ CD8+ T cells was greater in those who seroconverted, relative to the frequency in those who remained uninfected (1.30% vs 0.82%, respectively; P = .005). This translated to an odds ratio of 4.26 (95% confidence interval, 1.54–11.78) for the association between CD8+ T-cell activation and infection with HIV-1. T-cell activation may be a biomarker for elevated HIV-1 infection risk.
Keywords: HIV-1, T-cell activation, HESN, infection risk
The effectiveness of antiretroviral preexposure prophylaxis (PrEP) in preventing human immunodeficiency virus type 1 (HIV-1) infection has been clearly established with the use of coformulated emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) [1]. However, the ultimate effectiveness of PrEP may depend on careful attention about who should initiate therapy [2]. Objective clinical measures could assist the clinician and patient in deciding on the most-appropriate preventive interventions. One such measure could be T-cell activation.
T-cell activation during the chronic infection phase has been shown to correlate with the course of HIV-1 disease [3–5]. The source of activation during chronic infection has been linked directly to HIV-1, as well as indirectly through coinfection with other pathogens [6]. Activation prior to infection has also been shown to have a relationship with disease progression after infection [7].
Transmission of HIV-1 may also be related to immune activation, based on studies of subjects who were exposed to but seronegative for HIV-1 (HESN) [8–11]. Immune activation in this population has been associated with causes ranging from sexually transmitted infection [6] to more-direct exposure to HIV-1, even at low virus levels [12]. Despite the compelling links between T-cell activation and transmission, findings differ across studies, likely representing regional, technical, or other confounding variables difficult to control for in cross-sectional studies.
We sought to examine the relationship of T-cell activation to the risk of HIV-1 infection among men who have sex with men in the Preexposure Prophylaxis Initiative (iPrEx), a global trial of preexposure chemoprophylaxis [1]. We used the infrastructure of a controlled clinical trial to test the hypothesis that trial participants who seroconverted had a greater frequency of activated T cells than those who remained seronegative.
METHODS
Cryopreserved peripheral blood mononuclear cells (PBMCs) from 55 iPrEx participants across multiple enrollment sites were analyzed for T-cell activation. Thirty-nine HESN participants and 16 participants who seroconverted during the course of the study but had HIV-1–negative preinfection samples were included in the analysis. PBMCs were selected as a convenience sample from ongoing case-control immunologic studies, based on biological sample availability. Included in the convenience sample were 5 sets of case-control pairings. All PBMC specimens used in the present study were obtained from consenting participants in the iPrEx trial, completed in 2010 (clinical trials registration NCT00458393).
PBMCs were thawed, washed, and then resuspended in R10 medium at a concentration of 5 × 106 cells/mL and were plated at a concentration of 5 × 105 cells/well in a 96-well V-bottomed plate. Two different phenotyping cocktails with the same antibodies for detection of CD38 and HLA-DR were used (Supplementary Table 1). All PBMC samples were stained in the presence of human immunoglobulin G on ice for 30 minutes, washed once with fluorescence-activated cell sorting buffer, and fixed in 2% paraformaldehyde in PBS. Samples were analyzed on a 4-laser LSR II flow cytometer. All collected data were included in the analyses, to avoid potential bias from inclusion or exclusion criteria; there were no duplicates, no missing data, and no repeats permitted. All data were analyzed first by one scientist according to a standardized gating strategy (Supplementary Figure 1) and then reanalyzed in a blinded fashion by a second scientist. It was prospectively planned that if the difference between the scientists’ findings varied by <10%, the first result was reported (Supplementary Methods); all results were within 10%. Six parameters were generated: the percentages of CD38+HLA-DR+ cells in the CD4+ and CD8+ T-cell populations, the percentages of HLA-DR+ cells in the CD4+ and CD8+ T-cell populations, and the percentages of CD38+ cells in the CD4+ and CD8+ T-cell populations.
Analyses were prospectively planned unless otherwise described. Only samples previously matched (in other concurrent studies) were used to query differences in T-cell activation. The Wilcoxon rank sum test was used to analyze differences in activation in a hierarchical fashion, looking first at CD38+HLA-DR+ double-positive cells and then at CD38+ and HLA-DR+ single-positive cells. Associations of known risk factors with activation results were assessed by fitting a generalized linear model for cross-sectional data, both individually (primary analysis) and as a pooled data set irrespective of infection outcome (exploratory analysis). Self-reported risk factors at study visits most proximal to the sample acquisition date were used for the primary analysis. If no association was noted, the use of self-reported risk factors at enrollment was prospectively defined as an exploratory analysis since this correlated most closely with infection outcomes in the parent iPrEx trial. Within-subject longitudinal data were queried by fitting a generalized linear model. To explore the association of the frequency of CD8+CD38+HLA-DR+ T cells independent of the effect of randomization, we used a multivariate logistic linear regression model that controlled for treatment assignment, with infection status as the outcome. The distribution of memory phenotypes was assessed as a post-hoc analysis and analyzed using the Mann–Whitney U test. Based on the hierarchical analysis plan, discrete hypothesis- and mechanism-driven queries, and reinforcing relationships among the variables tested, no adjustments for multiple comparisons were included. All statistical analyses were performed using Stata SE, version 13.1, and a P value of was .05 used as a cutoff for statistical significance.
More-detailed methods can be found online in the Supplementary Methods.
RESULTS
Demographic characteristics of the study population can be found in Supplementary Table 2. Only the difference in age (absolute difference, 2 years) met the threshold for statistical significance. All 55 subjects included in our analysis were determined to be free of HIV-1 infection at all visits, using a combination of 2 different Food and Drug Administration–approved rapid tests to detect HIV-1–specific antibodies and by testing for viral RNA with an assay that has a lower limit of detection of 40 copies/mL. Of the 39 HESN subjects, 14 contributed additional samples for longitudinal analysis. A total of 81 PBMC specimens were analyzed.
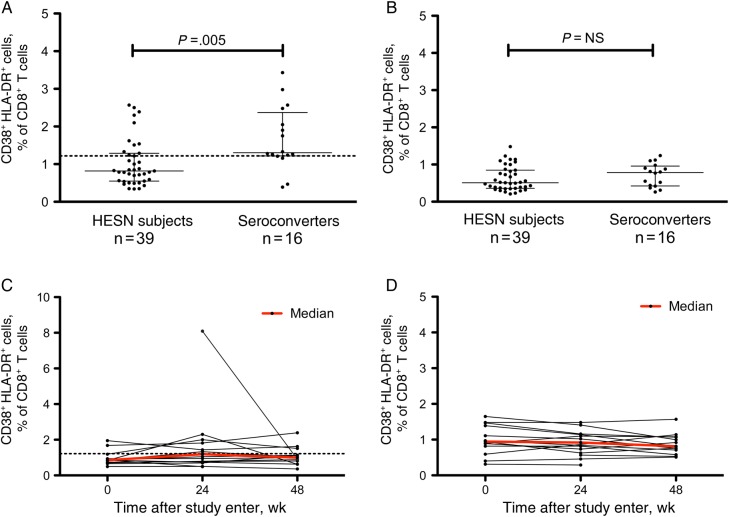
The primary analysis for the study was the percentages of CD4+ and CD8+ T cells that were CD38+HLA-DR+, derived using a standardized gating strategy (Supplementary Figure 1) and analyzed per a prospectively defined statistical analysis plan. We found that individuals who seroconverted during the trial had a 1.59-fold greater frequency of CD38+HLA-DR+ CD8+ T cells than HESN subjects (1.30% vs 0.82%; P = .005; Table 1 and Figure 1A). In contradiction to our hypothesis, there was no statistically significant difference in the frequency of activated CD38+HLA-DR+ CD4+ T cells between groups (Table 1 and Figure 1B). Analyses limited to the 5 complete case-control pairings (5 subjects who seroconverted during the iPrEx trial and 15 HESN subjects) indicated a similar trend, but the difference did not reach statistical significance.
Table 1.
Summary of Activation Markers on CD4+ and CD8+ T Cells Obtained From Subjects Who Were Exposed to but Seronegative for Human Immunodeficiency Virus Type 1 (HIV-1; HESN) and Subjects Who Tested Negative for HIV-1 at Study Entry but Seroconverted After Enrollment
| Activation Marker, by Treatment Group(s) |
HESN Subjects |
Seroconverters |
P Valuea | ||
|---|---|---|---|---|---|
| Mean | Median (IQR) | Mean | Median (IQR) | ||
| All participantsb | |||||
| CD4+ T cells | |||||
| CD38+HLA-DR+ | 0.62 | 0.51 (0.36–0.85) | 0.72 | 0.79 (0.43–0.94) | .228 |
| HLA-DR+ | 1.87 | 1.32 (0.85–2.86) | 2.01 | 1.93 (1.56–2.42) | .247 |
| CD38+ | 42.3 | 44.1 (32.5–53.0) | 35.0 | 37.4 (19.3–52.1) | .176 |
| CD8+ T cells | |||||
| CD38+HLA-DR+ | 1.02 | 0.82 (0.55–1.29) | 1.67 | 1.30 (1.22–2.26) | .005 |
| HLA-DR+ | 1.64 | 1.33 (1.04–2.27) | 1.96 | 1.82 (1.40–2.36) | .223 |
| CD38+ | 43.5 | 44.0 (32.3–55.6) | 34.7 | 25.3 (18.2–51.6) | .085 |
| FTC/TDF recipientsc | |||||
| CD4+ T cells | |||||
| CD38+HLA-DR+ | 0.94 | 1.01 (0.73–1.14) | 0.86 | 0.90 (0.77–0.98) | .578 |
| HLA-DR+ | 3.12 | 3.03 (1.95–4.35) | 2.32 | 2.04 (1.93–2.44) | .267 |
| CD38+ | 35.4 | 34.4 (28.1–42.1) | 32.8 | 29.4 (22.0–37.7) | .711 |
| CD8+ T cells | |||||
| CD38+HLA-DR+ | 1.32 | 1.19 (0.85–1.62) | 2.12 | 2.05 (1.75–2.57) | .042 |
| HLA-DR+ | 2.04 | 1.84 (1.32–2.67) | 2.15 | 1.84 (1.80–2.87) | .643 |
| CD38+ | 37.2 | 34.8 (26.7–50.4) | 22.8 | 22.6 (20.2–24.0) | .064 |
| Placebo recipientsd | |||||
| CD4+ T cells | |||||
| CD38+HLA-DR+ | 0.44 | 0.39 (0.33–0.50) | 0.65 | 0.55 (0.37–0.88) | .078 |
| HLA-DR+ | 1.17 | 1.04 (0.80–1.32) | 1.87 | 1.82 (1.24–2.40) | .027 |
| CD38+ | 46.1 | 49.1 (40.6–53.7) | 36.1 | 40.4 (14.7–52.4) | .135 |
| CD8+ T cells | |||||
| CD38+HLA-DR+ | 0.86 | 0.74 (0.50–0.85) | 1.47 | 1.27 (1.16–1.90) | .017 |
| HLA-DR+ | 1.41 | 1.22 (0.90–1.59) | 1.87 | 1.53 (1.29–2.30) | .140 |
| CD38+ | 47.1 | 46.5 (36.0–57.5) | 40.3 | 50.1 (16.2–66.5) | .548 |
Abbreviations: FTC, emtricitabine; IQR, interquartile range; TDF, tenofovir disoproxil fumarate.
a By the Wilcoxon rank sum test.
b Data are for 39 HESN subjects and 16 seroconverters.
c Data are for 14 HESN subjects and 5 seroconverters.
d Data are for 25 HESN subjects and 11 seroconverters.
Figure 1.
A and B, Median percentages (interquartile ranges) of CD38+HLA-DR+ cells among CD8+ (A) and CD4+ (B) T cells obtained from subjects who were exposed to but seronegative for human immunodeficiency virus type 1 (HIV-1; HESN) and subjects who tested negative for HIV-1 at study entry but seroconverted after enrollment. Data were analyzed using the Wilcoxon rank sum test. C and D, Activation over time of CD8+ (C) and CD4+ (D) T cells obtained from 14 HESN subjects. The dotted line indicates 1.22%, which is the 25th percentile of the frequency distribution of CD38+HLA-DR+ CD8+ T cells in subjects who seroconverted.
We calculated the sensitivity and specificity of the CD38+HLA-DR+ CD8+ T-cell frequency as a means to differentiate individuals on the basis of infection outcomes (Supplementary Table 3). We estimated a 75.0% sensitivity and 69.2% specificity, using a threshold of 1.22% (the 25th percentile of the frequency distribution of CD38+HLA-DR+ CD8+ T cells in subjects who later seroconverted). The positive predictive value was 50%, and the negative predictive value was 87.1%.
T-cell activation was not associated with the number of sex partners or with the frequency of receptive anal intercourse in which a condom was not use. Similarly, no relationships were observed with herpes simplex virus infection, Chlamydia trachomatis infection, or syphilis diagnoses (data not shown). The frequency of gonorrhea diagnoses was too low to assess relationships.
Memory phenotype data in 10 HESN samples and 10 preinfection samples from individuals who later seroconverted showed no statistically significant differences in distribution at a significance threshold of.05, using the Mann–Whitney test (Supplementary Figure 2). Numerically greater median proportions of naive and central memory CD4+ T cells but lower proportions of effector memory and terminally differentiated CD4+ T cells were observed in HESN subjects. Among CD8+ T cells, HESN subjects had greater median proportions of naive and terminally differentiated cells but lower frequencies of effector memory cells.
The naive CD38+HLA-DR+ CD4+ T-cell frequency was lower in HESN subjects, but frequencies of other memory subsets were comparable between the participant groups. Effector memory and terminally differentiated CD38+HLA-DR+ CD8+ CD8+ T-cell frequencies were lower for HESN subjects, with comparable naive and central memory subset frequencies relative to individuals who later seroconverted.
The convenience sample approach led to a similar proportion of individuals assigned to the FTC-TDF treatment arm in our study. Post hoc multivariate logistic regression modeling was used to assess the effect of T-cell activation independent of treatment arm by controlling for treatment assignment. This analysis confirmed the association between CD8+ T-cell activation and infection, with an odds ratio of 4.26 (95% confidence interval, 1.54–11.78); that is, the odds of subsequent HIV infection associated with a 1% increase in the percentage of activated CD8+ T cells was 4.26 times the odds associated with no increase.
The frequency of CD8+ and CD4+ T cells coexpressing CD38 and HLA-DR remained mostly stable over the first year of the study in the 14 HESN subjects analyzed for up to 1 year (Figure 1C and 1D, respectively). One subject had a marked drop in the frequency of CD38+HLA-DR+ cells, but the sample collected on day 0 was not available to analyze stability over a longer period. The decrease in frequency was not associated with factors known to increase the risk of HIV-1 acquisition. Overall, no statistically significant associations were observed for within-subject fluctuations in activation with respect to behavior or sexually transmitted disease diagnosis. Longitudinal analyses in these subjects were not performed owing to the lack of time points analyzed (most infections occurred within 6–12 months of study initiation) and limited preinfection sample inventories.
DISCUSSION
The results of our primary analysis show that the frequency of CD8+ T cells coexpressing CD38 and HLA-DR was substantially elevated prior to HIV-1 infection in participants who seroconverted, compared with those who did not. Although exploratory in nature, the observed odds ratio of 4.26 from the multivariate analysis suggests that interventions to decrease CD8+ T-cell activation (or the mechanism by which it relates to HIV-1 infection) may be beneficial clinically.
HESN participants had a greater median frequency of naive T cells, possibly explaining the increased CD38+ T-cell frequency in this group. A shift toward more effector memory CD8+ T cells and greater frequencies of effector memory and terminally differentiated CD38+HLA-DR+ CD8+ T cells paralleled the greater CD38+HLA-DR+ CD8+ T-cell frequency in the primary analysis. Notably, subjects who later seroconverted had a greater frequency of CD38+HLA-DR+ (or activated) CD4+ naive T cells, compared with HESN subjects. Although the differences among the memory populations were not statistically significant, the patterns were mechanistically consistent with increased immune activation and greater HIV-1 infection risk, reinforcing the findings of this study.
Nonclinical and human studies suggest that T-cell and generalized immune activation are amenable to treatment. Modification of generalized immune activation or related markers with the antiinflammatory compound glycerol monolaurate has been associated with decreased simian immunodeficiency virus transmission [13]. Coadministration of nonsteroidal antiinflammatory drugs in HIV-1–infected virologically suppressed patients resulted in reduced frequencies of CD38+HLA-DR+ CD4+ and CD8+ T cells [14]. Moderating activation may have benefits more broadly, as well, in that T-cell activation has been shown to negatively influence the ability to respond to vaccines [15], which could be important for HIV-1 vaccine trials.
Although our analysis included participants from around the globe, we were not able to analyze samples from all enrollment sites. Thus, the convenience sample approach is a limitation of the study. Additionally, the association of CD8+ T cells and infection risk suggests an indirect or biomarker relationship to mechanisms more directly associated with transmission of HIV-1, since CD8+ T cells are not targets for HIV-1 infection. This may also be explained by the analysis of PBMCs rather than mucosal mononuclear cells. Investigation into the underlying mechanisms relating CD8+ T cells to HIV-1 infection and the relationship of PBMCs to mucosal mononuclear cells was beyond the scope of the present study.
In conclusion, we have shown that individuals who seroconverted in the iPrEx study had a greater proportion of CD38+HLA-DR+ CD8+ T cells (prior to infection), relative to HESN subjects, and that this marker was relatively stable in participants who remained uninfected. Measuring the proportion of CD38+HLA-DR+ CD8+ T cells may be a useful objective clinical tool that can aid the clinician and the patient in deciding the most appropriate means of preventing HIV-1 infection. Further studies are needed to explore the mechanisms relating CD8+ T-cell activation to transmission and to test pharmacologic interventions that alter these mechanisms.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We dedicate this manuscript to the memory of James Jeff McConnell. We thank Claudia Brockmeyer, for carefully reviewing the manuscript; and the participants in the iPrEx trial.
Financial support. This work was supported by the National Institutes of Health (grants AI62333, AI64002, AI087131, and RR024131), the Bill and Melinda Gates Foundation, the J. David Gladstone Institutes, Fundação de Amparo a Pesquisa do Estado de São Paulo (04/15856-9/EGK and 2010/05845-0/EGK/DFN), CNPq/CAPES (grant 056/2012 to D. F. N.), and the Peter and Shelagh Godsoe Family Foundation, through the AIDS Research Institute at University of California–San Francisco (to P. J. K. and D. F. N.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Grant RM, Lama JR, Anderson PL et al. . Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchbinder SP, Glidden DV, Liu AY et al. . HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis 2014; 14:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeks SG, Kitchen CM, Liu L et al. . Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 2004; 104:942–7. [DOI] [PubMed] [Google Scholar]
- 4.Mahnke YD, Song K, Sauer MM et al. . Early immunologic and virologic predictors of clinical HIV-1 disease progression. AIDS 2013; 27:697–706. [DOI] [PubMed] [Google Scholar]
- 5.Lederman MM, Calabrese L, Funderburg NT et al. . Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis 2011; 204:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawn SD, Butera ST, Folks TM. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin Microbio Rev 2001; 14:753–77, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Asten L, Danisman F, Otto SA et al. . Pre-seroconversion immune status predicts the rate of CD4T cell decline following HIV infection. AIDS 2004; 18:1885–93. [DOI] [PubMed] [Google Scholar]
- 8.Begaud E, Chartier L, Marechal V et al. . Reduced CD4T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology 2006; 3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biasin M, Caputo SL, Speciale L et al. . Mucosal and systemic immune activation is present in human immunodeficiency virus-exposed seronegative women. J Infect Dis 2000; 182:1365–74. [DOI] [PubMed] [Google Scholar]
- 10.Jennes W, Sawadogo S, Koblavi-Deme S et al. . Cellular human immunodeficiency virus (HIV)-protective factors: a comparison of HIV-exposed seronegative female sex workers and female blood donors in Abidjan, Cote d'Ivoire. J Infect Dis 2003; 187:206–14. [DOI] [PubMed] [Google Scholar]
- 11.Koning FA, Otto SA, Hazenberg MD et al. . Low-level CD4+ T cell activation is associated with low susceptibility to HIV-1 infection. J Immunol 2005; 175:6117–22. [DOI] [PubMed] [Google Scholar]
- 12.Restrepo C, Rallon NI, del Romero J et al. . Low-level exposure to HIV induces virus-specific T cell responses and immune activation in exposed HIV-seronegative individuals. J Immunol 2010; 185:982–9. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Estes JD, Schlievert PM et al. . Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009; 458:1034–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Brien M, Montenont E, Hu L et al. . Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: a pilot study. J Acquir Immune Defic Syndr 2013; 63:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milagres LG, Costa PR, Santos BA et al. . CD4+ T-cell activation impairs serogroup C Neisseria meningitis vaccine response in HIV-infected children. AIDS 2013; 27:2697–705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.