Abstract
Release of neutrophil extracellular traps (NETs) is a significant antimicrobial host defense mechanism in adults. In neonates, fungal sepsis is a frequent cause of morbidity and mortality and may be a consequence of inadequate neutrophil defense functions. Like neutrophils from adult donors, we found that neutrophils from neonates formed robust cellular aggregates and released NETs in response to fungal β-glucan and Candida albicans hyphae when presented with extracellular matrix. Therefore, in response to fungal stimulation, neonatal neutrophils are capable of NETosis. Neonate susceptibility to fungal infections may not be due to an inability of their neutrophils to produce NETs.
Keywords: neonates, neutrophils, NETs, glucan, fungi
Neutrophils dominate the rapid innate immune response to infection [1]. Neutrophil mechanisms to destroy microbial pathogens include phagocytosis, degranulation, and generation of reactive oxygen and nitrogen species. Neutrophil extracellular traps (NETs) are now recognized as another important antimicrobial effector mechanism in host defense [2, 3].
NETs are composed of DNA strands associated with antibacterial granular proteins, including citrullinated histones, neutrophil elastase, myeloperoxidase, lactoferrin, and defensins [4]. NET release is finely regulated, which may serve to limit collateral tissue damage adjacent to the infectious focus. Elucidating the cues for NETosis is essential for understanding when this mode of host defense functions in antimicrobial protection or when it may contribute to hyperinflammatory pathology [5].
The antimicrobial response of neutrophils to tissue infection necessitates contact of the extravasated neutrophil with extracellular matrix (ECM) components such as fibronectin (Fn) [6, 7]. Our laboratory recently demonstrated that, in response to purified fungal β-glucan or the β-glucan expressed in intact Candida albicans hyphae, neutrophils from adult donors aggregate and release NETs rapidly (ie, <60 minutes after exposure) by a Fn-dependent, reactive oxygen species (ROS)–independent mechanism [8]. Requirement of the β2 integrin CR3 (also known as “CD11b/CD18,” “αMβ2,” and “Mac-1”) for ECM-dependent antifungal NETosis was also shown.
Fungal infections are problematic in neonates owing to deficiencies in neutrophil phagocytosis, respiratory burst, and intracellular microbial killing as compared to full-term infants and adults [9, 10]. Yost et al reported that neonatal neutrophils are deficient in the production of NETs in response to the proinflammatory mediators platelet activating factor (PAF), lipopolysaccharide (LPS), and phorbol myristate acetate (PMA) and to Escherichia coli and Staphylococcus aureus challenge [11]. Whereas these findings are consistent with a deficiency in antibacterial defenses, whether neonatal neutrophils are inherently unable to mount a NETotic response to all pathogens or pathogen-associated molecular patterns (PAMPs) is not known. The response of neutrophils from neonates to fungal β-glucan in the presence of ECM was determined in this report. Neonatal neutrophils, like adult cells, formed homotypic aggregates and released NETs when exposed to Fn together with either purified β-glucan or C. albicans hyphae. These findings suggest that neonatal neutrophils are not incapable of producing NETs and that NETosis is stimulus dependent.
MATERIALS AND METHODS
Reagents
Dulbecco's phosphate-buffered saline (dPBS), Lebovitz's L15 medium (L-15), and Sytox green were obtained from Invitrogen (Carlsbad, California). Medium 199 with Earle's balanced salt solution, L-glutamine, and 25 mM HEPES were obtained from Lonza (Walkersville, Maryland). Purified, endotoxin-free, soluble yeast β-glucan (ImPrime PGG) was provided by Biothera (Eagan, Minnesota). Endotoxin-free human fibronectin was obtained from BD Biosciences (Bedford, Massachusetts).
Neutrophil Isolation
Blood specimens were obtained with the approval of Rhode Island Hospital Institutional Review Board from healthy human volunteers. Neonatal blood specimens were obtained from umbilical cords of healthy term infants immediately following placenta delivery, with the approval of Women and Infants Hospital of Rhode Island Institutional Review Board. A total of 15 mL of blood was collected in Vacutainer tubes (BD Biosciences) containing ethylenediaminetetraacetic acid and used within 15 minutes of collection. Histopaque-1077 cell separation was followed by 3% dextran (molecular weight, 400–500 kDa ) sedimentation. Erythrocytes were removed by hypotonic lysis, yielding a >95% pure neutrophil preparation with a viability of >90%, as determined by trypan dye exclusion. Neutrophils were suspended in Hanks balanced salt solution (without Ca+2/Mg+2) on ice until use.
Neutrophil Adhesion
Six-well tissue culture plates (Falcon Labware, Becton Dickinson) were coated overnight with 6 µg/mL Fn in Tris-buffered saline (TBS; 25 mM Tris and 150 mM NaCl; pH 9.0) or 1 mg/mL β-glucan and were washed with PBS. Neutrophils were resuspended to 3.5 × 106 cells/mL in L-15 plus 2 mg/mL glucose, with 2 mL added per well. Where indicated, cells were preincubated with 6.25 µM diphenyliodonium on ice for 20 minutes. Cells were pretreated on ice with the bacterial formyl-peptide fMLF (10−9 M) for 20 minutes. A total of 1 mM Mn+2 was added to cells before plating and incubating at 37°C for 30 minutes. NETs were visualized by addition of 5 µM Sytox green.
Neutrophil Adhesion to Fungal Hyphae
C. albicans (SC5314, ATCC) was cultured overnight at 37°C with agitation in 1% yeast extract, 2% Bacto Peptone (both from Difco), and 2% dextrose. Six-well culture dishes were coated with 10 µg/mL Fn in TBS (pH 9.0) overnight at room temperature. Approximately 2 × 106 of C. albicans was added to Fn-coated dishes in Medium 199 supplemented with Earle's balanced salt solution, L-glutamine, and 25 mM HEPES to induce hyphal differentiation at 37°C for 4–6 hours. Filamentous phenotype was confirmed by microscopy. Wells were washed 3 times with dPBS, and neutrophils were prepared and added as described above for the adhesion assay. NETs were visualized using 5 µM Sytox green.
Microscopy
Images were captured using a Nikon TE-2000U inverted microscope (Nikon, Melville, New York) coupled to an iXonEM + 897E back illuminated EMCCD camera (Andor, Belfast, United Kingdom) and a 20x Nikon Plan Apochromat objective. Bright-field images were captured using the Elements program (Nikon). For fluorescence microscopy, a xenon lamp illuminated cells through a 33-mm ND4 filter, using a Nikon B2-A long-pass emission filter set cube to visualize Sytox green staining of extracellular DNA.
Scanning Electron Microscopy
Samples were fixed by gently layering 2.5% glutaraldehyde in 0.15 M sodium cacodylate buffer, rinsed with buffer, and postfixed with 1% osmium tetroxide. Slides were rinsed, dehydrated, and covered with resin and placed over Epox 812 filled slide-duplicating molds (Electron Microscopy Sciences, Hatfield, Pennsylvania) overnight. Following dehydration in ethanol, samples were dried in a critical point dryer. Samples were then coated with 20 nm of gold palladium (60:40) in an Emitech K550 sputter coater (Emitech, Ashford, United Kingdom). Cells were imaged with an Hitachi S-2700 scanning electronic microscope, and images were collected with Quartz PCI software (Quartz Imaging Corporation, Vancouver, British Columbia).
NET Quantification
Neutrophils were prepared as described above for adhesion assays. NETs were visualized with Sytox green, and multiple images were obtained per well. Images were thresholded using the default thresholding algorithm in ImageJ (NIH, Bethesda, Maryland) and gated to include extruded NETs and exclude stained nuclei. NET formation was quantified as the percentage area of the totaled imaged field. Well averages were then ensemble averaged. Data represent 4–20 wells per condition.
Statistical Analysis
Data were pooled from a minimum of 4 independent experiments representing at least 4 different donors, as indicated. Analysis of variance with Newman–Keuls post hoc analysis or a paired-sample Student t test were used as appropriate, using MATLAB (Mathworks, Natick, Massachusetts) or Excel (Microsoft, Redmond, Washington) running the statistiXL data package (statistiXL, Nedlands, Australia). The null hypothesis was rejected if P < .01.
RESULTS
Neonatal Neutrophils Form Rapid NETs in Response to Fn + β-glucan
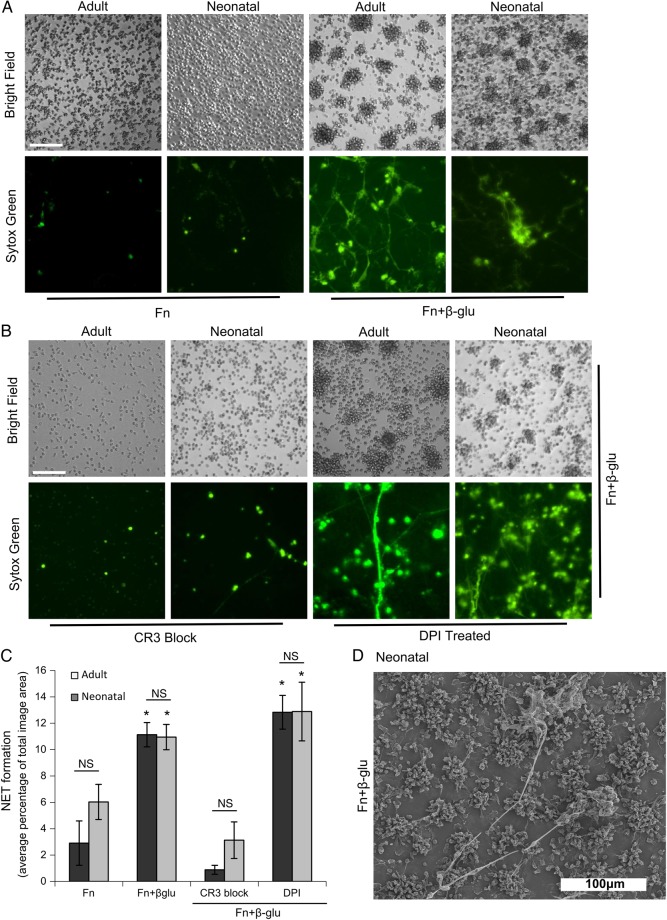
In a prior report, our laboratory showed that neutrophils from healthy adult donors undergo aggregation and NET formation in response to immobilized fungal β-glucan and the ECM protein Fn but not to either alone [8]. Here, we exposed neonatal neutrophils to Fn, with or without β-glucan, and found they also cluster and form NETs in response to Fn plus β-glucan, but not Fn alone after 30 minutes (Figure 1A). Yost et al [11] reported that neonatal cells are not capable of forming significant extruded NETs in response to PMA, bacterial LPS, and PAF, as seen in adult neutrophils. Instead, neonatal neutrophils showed a minimal NETotic response and some swollen nuclei, revealed by the cell impermanent DNA-intercalating dye, Sytox green. Under our assay conditions, we consistently observed minimal response to these agonists in neonatal cells after a 60-minute incubation period sufficient to induce significant extruded NETs from adult neutrophils (Supplementary Figure 1). As we reported for adult cells, neonatal neutrophils depend on CR3 for homotypic aggregation and NETosis and do not require ROS because DPI addition was not limiting (Figure 1B and 1C). The neonatal extruded NET response to Fn plus β-glucan was quantified using multiple Sytox green images per well that were gated to extruded NETs and excluded stained nuclei. NET formation was quantified as an average percentage of the total imaged field and was quantitatively comparable to the NET formation by adult neutrophils (Figure 1C). In support of NETs identified by Sytox green staining in Figure 1A, Figure 1D provides physical evidence by scanning electron microscopy for NET structures released by neonatal neutrophils on exposure to Fn plus β-glucan.
Figure 1.
Neonatal neutrophils form CR3-mediated clusters and rapid neutrophil extracellular traps (NETs) in response to fibronectin (Fn) plus β-glucan, which is independent of respiratory burst. Neonatal neutrophils on fibronectin plus β-glucan form aggregates and NETs. A, Micrographs show adult and neonatal polymorphonuclear leukocytes (PMNs) that were adhered to wells precoated with Fn alone (left columns) or Fn supplemented with β-glucan (right columns). PMNs were pretreated with 10−9 M fMLF and 1 mM Mn+2 immediately before adding cells to the coated wells and incubating for 30 minutes at 37°C. Data represent at least 5 independent experiments done using neutrophils from different individual donors. All images were obtained at 20× original magnification (bar, 100 µm). B, Adult and neonatal PMNs prepared as described above were pretreated with CR3-blocking monoclonal antibody (clone 44abc; left columns) or the respiratory burst inhibitor DPI (right columns) before they were adhered to wells coated with Fn plus β-glucan. Images were taken at 20× original magnification (bar, 100 µm). These results represent at least 4 independent experiments, using neutrophils from different individual donors. C, Quantification of NET formation as the percentage of the total imaged field. Data represent 4–20 wells per condition. Error bars represent standard errors of the mean (SEM). *P < .01 vs cells treated with Fn alone and those treated with CR3-blocking monoclonal antibody. D, Scanning electron microscopy images of neonatal PMNs obtained at 40× original magnification, demonstrating NET elaboration. Neonatal neutrophils were prepared as described above and were then fixed and prepared for scanning electron microscopy. Abbreviation: NS, nonsignificant.
Neonatal Neutrophils Form Rapid NETs in Response to Fn + C. albicans Hyphae
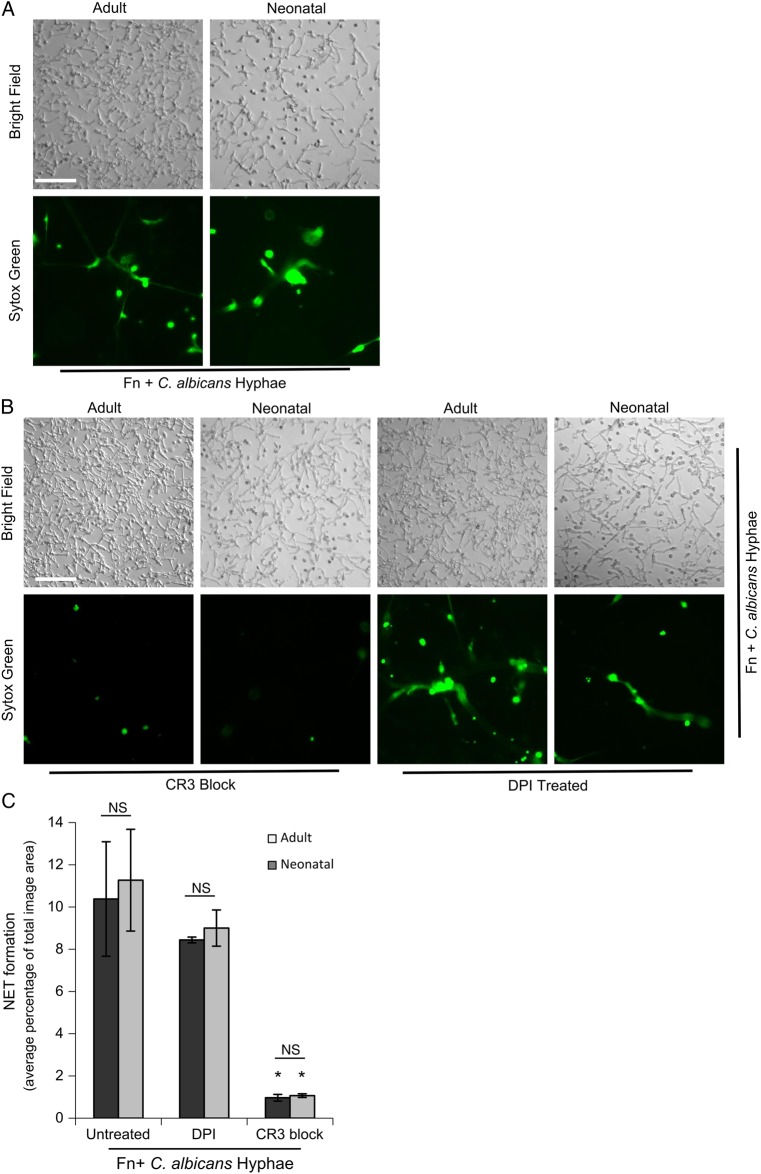
Figure 2 demonstrates rapid NETosis of adult and neonatal neutrophils to C. albicans hyphae elaborated on Fn. Differences in hyphal density among images reflects both different seeding densities across the wells and hyphal adhesion to the wells after washing. Neutrophils were added in 3.5-fold excess to hyphae elaborated by seeding 2 × 106 C. albicans yeast per Fn- coated well. Similar densities of NETs (as a percentage of the image area) released by neutrophils on immobilized β-glucan and Fn were observed with hyphae on Fn-coated wells initially seeded with 5 ×x 105–5 × 106 C. albicans. NET production by adult and neonatal cells required CR3 but not ROS (Figure 2B). Quantification of NETosis is shown in Figure 2C, demonstrating the similarity in magnitude, CR3 dependence, and ROS independence characterizing NET release by adult and neonatal neutrophils in response to C. albicans and Fn.
Figure 2.
Neonatal neutrophils form CR3-mediated clusters and rapid NETs in response to Candida albicans hyphae in the context of fibronectin (Fn), which is independent of respiratory burst. Neonatal neutrophils form aggregates and NETs in response to C. albicans hyphae elaborated on Fn. A, Micrographs show adult and neonatal polymorphonuclear leukocytes (PMNs) that were adhered to wells precoated with 10 µg/mL Fn, upon which C. albicans hyphae were grown. PMNs were pretreated with 10−9 M fMLF and 1 mM Mn+2 before adding cells to the coated wells and incubating for 30 minutes at 37°C. Data represent at least 5 independent experiments done using neutrophils from different individual donors. All images were obtained at 20× original magnification (bar, 100 µm). B, Adult and neonatal PMNs prepared as described above were pretreated with CR3-blocking monoclonal antibody (clone 44abc; left columns) or the respiratory burst inhibitor DPI (right columns) before they were adhered to wells coated with Fn and hyphae, as described above, and incubated at 37°C for 30 minutes. Images were obtained at 20× original magnification (bar, 100 µm). These results represent at least 4 independent experiments performed using neutrophils from different individual donors. C, Quantification of NET formation as the percentage of the total imaged field. Data represent 4–20 wells per condition. Error bars represent standard errors of the mean. *P < .01 vs both untreated PMNs and PMNs treated with DPI. Abbreviation: NS, nonsignificant.
DISCUSSION
Neutrophil impairment and other characteristics of a compromised immune system underlie the morbidity and mortality of the neonatal patient population to sepsis and other infectious complications [9]. Candida organisms are an emerging cause of sepsis in neonates [10]. Neutrophils play an instrumental role in host defense against fungal infections, which is why they were the focus of this study.
Our findings demonstrate that, like adult cells, neonatal neutrophils release NETs in response to fungal β-glucan in the context of ECM in an ROS-independent manner. NET release was demonstrable in response to both purified β-glucan and C. albicans hyphae in the context of Fn. The ability of immobilized, purified β-glucan in isolation from the fungal cell wall to mimic the response of neutrophils to intact hyphae supports the importance of this cell wall component in innate immune responsiveness. Unlike small unicellular blastoconidia, intact C. albicans hyphae are too large to be ingested by phagocytosis, so antifungal neutrophil activity takes place under conditions in which internalization cannot occur. In this regard, immobilization of β-glucan thereby works as a reductionist model of neutrophil responsiveness to this key fungal PAMP in the absence of phagocytosis. Moreover, as in the adult, the β2 integrin CR3 on the neutrophil cell surface was essential for NET release and aggregation. In a report by Yost et al [11], neutrophils from premature and healthy term infants were shown to be incapable of forming significant extruded NETs when challenged with inflammatory agonists, including PMA, PAF, and LPS. Here, we conducted experiments that supplement and extend these data, to further understand the role of NETs in neonatal immune responses. We found that neonatal PMNs formed minimal NETs to inflammatory agonists (Supplementary Figure 1), confirming results by Yost et al [11]. Yost et al [11] did not challenge with fungal stimuli as examined here, so the ability of neonatal neutrophils to self-aggregate and generate NETs may prove to be stimulus and/or ECM dependent. Technically, this result is not dependent on the use of density gradient versus magnetic bead isolation of neutrophils from whole blood, as we found robust aggregation and NETosis of neonatal PMNs on Fn plus β-glucan, using either isolation method (data not shown).
Previously, we showed that neutrophils from healthy adult donors undergo a robust respiratory burst in response to immobilized fungal β-glucan, which is actively suppressed by ECM, and we hypothesized that the purpose of this activity is to limit consequent tissue damage of migrating neutrophils until multifocal contact with fungal hyphae [12]. Previous work from our laboratory [8] assayed NET killing of C. albicans by adult neutrophils and confirmed that NETs released by neutrophils exposed to immobilized Fn with β-glucan, when ROS production was inhibited, maintained their fungicidal activity. In the current study, we showed that neonatal neutrophil NET formation is unimpeded by the addition of DPI, demonstrating that oxidative burst and rapid NET formation are uncoupled during the response to the fungal PAMP β-glucan or intact C. albicans hyphae.
Our neonatal neutrophils were from umbilical cord blood specimens obtained from term infants delivered by elective cesarean section. It is reported that labor deliveries increase the surface expression of Toll-like receptors on monocytes and that interleukin 8–induced neutrophil chemotaxis is enhanced due to the stress of birth [13]. Future studies comparing the effect of cesarean section versus labor delivery on the neonatal response to fungal pathogens will provide a better understanding of the immune response in this particular population.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Financial support. This work was supported by the National Institutes of Health (NIH) (grant GM066194 to J. S. R., grant P20GM103537 to J. M. B., and grant F31DE023726 to M. P. H.) and a United Negro College Fund/Merck Graduate Science Research Dissertation Fellowship (to A. S. B.).
Potential conflicts of interest. Dr Byrd, Dr O'Brien, Ms Laforce-Nesbitt, Ms Parisi, Mr Hirakawa, Dr Bliss, and Dr Reichner received grants from the NIH during the conduct of the study. Dr Byrd, Dr O'Brien, Mr Hirakawa, and Dr Reichner received nonfinancial support from Biothera (Eagan, Minnesota) in the form of Imprime-PGG glucan for use in the current and related studies. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol 2014; 9:181–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardoel BW, Kenny EF, Sollberger G, Zychlinsky A. The balancing act of neutrophils. Cell Host Microbe 2014; 15:526–36. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532–5. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol 2012; 198:773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan C, Ding A. Nonresolving inflammation. Cell 2010; 140:871–82. [DOI] [PubMed] [Google Scholar]
- 6.Harler MB, Wakshull E, Filardo EJ, Albina JE, Reichner JS. Promotion of neutrophil chemotaxis through differential regulation of beta 1 and beta 2 integrins. J Immunol 1999; 162:6792–9. [PubMed] [Google Scholar]
- 7.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007; 7:678–89. [DOI] [PubMed] [Google Scholar]
- 8.Byrd AS, O'Brien XM, Johnson CM, Lavigne L, Reichner JS. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to C. albicans. J Immunol 2013; 190:4136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melvan JN, Bagby GJ, Welsh DA, Nelson S, Zhang P. Neonatal sepsis and neutrophil insufficiencies. Int Rev Immunol 2010; 29:315–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. Am J Perinatol 2013; 30:131–41. [DOI] [PubMed] [Google Scholar]
- 11.Yost CC, Cody MJ, Harris ES et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood 2009; 113:6419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavigne LM, O'Brien XM, Kim M, Janowski JW, Albina JE, Reichner JS. Integrin engagement mediates the human polymorphonuclear leukocyte response to a fungal pathogen-associated molecular pattern. J Immunol 2007; 178:7276–82. [DOI] [PubMed] [Google Scholar]
- 13.Yektaei-Karin E, Moshfegh A, Lundahl J, Berggren V, Hansson LO, Marchini G. The stress of birth enhances in vitro spontaneous and IL-8-induced neutrophil chemotaxis in the human newborn. Pediatr Allergy Immunol 2007; 18:643–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.