Abstract
Background. Chlamydia trachomatis genital tract infection is a major cause of female reproductive morbidity. Risk factors for ascending infection are unknown, and the role for antibody in protection is not well established.
Methods. We recruited 225 women from urban outpatient clinics and followed them for a median of 12 months. We performed a cross-sectional analysis of serum anti-chlamydial immunoglobulin G (IgG), behavioral factors, and microbiological factors associated with endometrial infection at enrollment, and a longitudinal analysis of factors associated with incident infection.
Results. Oral contraceptives (adjusted relative risk [RR], 2.02 [95% confidence interval {CI}, 1.38–2.97]) and gonorrhea (adjusted RR, 1.66 [95% CI, 1.07–2.60]) were associated with endometrial infection. Gonorrhea (adjusted hazard ratio [HR], 3.09 [95% CI, 1.41–6.78]), cervical infection at enrollment (adjusted HR, 2.33 [95% CI, 1.07–5.11]), and exposure to uncircumcised partners (adjusted HR, 2.65 [95% CI, 1.21–5.82]) or infected partners (adjusted HR, 4.99 [95% CI, 2.66–9.39]) significantly increased the risk of incident infection. Seropositivity was associated with a reduced cervical burden (P < .05) but no differences in rates of ascending infection (adjusted RR, 1.24 [95% CI, .71–2.19]) or incident infection (adjusted HR, 0.94 [95% CI, .52–1.69]).
Conclusions. Serum anti-chlamydial IgG is not associated with a lowered rate of ascending or repeat infection. Identification of factors associated with ascending infection and increased risk of incident infection provide guidance for targeted screening of women at increased risk for sequelae.
Keywords: Chlamydia trachomatis, pelvic inflammatory disease, serum antibody, risk factors, reproductive hormones
Chlamydia trachomatis causes >100 million genital tract infections annually and remains a significant global health problem [1]. In some infected women, C. trachomatis ascends to the upper genital tract and leads to pelvic inflammatory disease (PID), chronic pelvic pain, ectopic pregnancy, and tubal factor infertility [2]. Multiple studies have revealed risks for incident infection in women, including young age, minority race, gonorrhea, and new or multiple sex partners [3–5]. To our knowledge, no studies have comprehensively examined risk factors for ascending infection. Defining these could aid clinicians in providing targeted screening for women at greatest risk.
The role of antibody in response to C. trachomatis genital tract infection remains unclear. Antibody is dispensable for normal resolution of primary infection in the murine model of chlamydial genital infection but contributes to resistance from reinfection [6]. Data from mouse and guinea pig models link serum anti-chlamydial immunoglobulin G (IgG) to reduced bacterial load, duration of infection, and pathology [6, 7]. In contrast, data from human studies indicate minimal to no role for serum antibody in resistance. Several studies correlated high titers of serum anti-chlamydial IgG with increased morbidity [8–11], while other studies described protective effects for IgG and immunoglobulin A (IgA) [12, 13]. In contrast, Cohen et al reported that levels of IgG or IgA specific for chlamydial elementary bodies (EBs) or heat shock protein 60 (HSP60) were not associated with a decreased risk of infection [14]. IgG is the major antibody subclass in genital secretions, where it predominates over IgA [15]. Serum IgG transudates into cervical secretions, so levels of IgG in serum and endocervical mucus are highly correlated [16]. Since IgG is the dominant antibody to C. trachomatis [17], the role of IgG in protection from chlamydia requires clarification.
Our objectives were to define risk factors for ascending infection, confirm risk factors for incident infection, and determine the relationship of serum anti-chlamydial IgG to bacterial burden and to ascending and incident infection. We hypothesized that although antibody would not associate with resistance to reinfection, high titers would correlate with a reduced bacterial burden.
METHODS
Patient Population
The institutional review boards for human subject research at the University of Pittsburgh and the University of North Carolina approved the study. Women aged 15–35 years who had lower genital tract infections or were at risk for chlamydia infection were approached for enrollment from February 2011 through May 2014. Women were recruited from the Allegheny County Health Department's Sexually Transmitted Diseases Clinic, Magee-Womens Hospital (MWH) Ambulatory Care Clinic, and the Reproductive Infectious Disease Research Unit at MWH. Eligibility criteria were clinical evidence of mucopurulent cervicitis, diagnosis of gonorrhea or chlamydia prior to treatment, or reported sexual contact with a male who received a diagnosis of gonorrhea, chlamydia, or nongonococcal urethritis. Exclusion criteria included pregnancy, uterine procedure or miscarriage in the preceding 60 days, menopause, hysterectomy, antibiotic therapy in the preceding 14 days, and allergy to study medications. Women with acute PID were excluded.
Subjects provided informed consent at the time of enrollment and agreed to attend follow-up visits 1, 4, 8, and 12 months after enrollment. At enrollment, study personnel obtained demographic data and a standardized medical history. Subjects completed a questionnaire regarding behavioral practices, sex exposure, contraceptive methods, and symptoms. General physical and pelvic examinations were performed; vaginal fluid was collected for pH measurement, whiff testing for the presence of amines, and microscopy to detect clue cells, as well as for subsequent diagnosis of bacterial vaginosis by use of Amsel criteria [18]. Vaginal swab specimens were collected for culture and molecular testing for Trichomonas vaginalis (Aptima TV; Gen-Probe, San Diego, California). Nucleic acid amplification tests (NAATs) were performed on cervical swabs for detection of C. trachomatis and Neisseria gonorrhoeae (Aptima Combo 2, Gen-Probe, San Diego, California) and Mycoplasma genitalium (Aptima MG: Gen-Probe, San Diego, California). Serum was collected for analysis of anti-chlamydial antibody titers, human immunodeficiency virus (HIV) antibody, and syphilis testing.
Women underwent endometrial sampling at enrollment. The cervix was cleaned with Betadine, a sterile endometrial sampler (Unimar Pipelle de Cornier, CooperSurgical, Shelton, Connecticut) was placed into the endometrial cavity, and a tissue sample was aspirated into the cannula. The tissue specimen was discharged into a sterile Petri dish. Tissue proximal to the sampling portal of the cannula was placed in 10% formalin fixative, distal tissue was used for microbiologic culture, and a swab absorbed 5 mm of distal tissue for qualitative NAAT (Aptima). All participants received single-dose agents for gonorrhea (ceftriaxone, 125 mg intramuscularly), chlamydia (azithromycin, 1 g orally), and bacterial vaginosis (metronidazole, 2 g orally).
At follow-up visits, subjects completed a questionnaire addressing symptomatology and interim medical and sexual history. Vaginal fluid and vaginal and cervical swab specimens were collected and analyzed as described above.
Serum Antibody Titers
Enrollment antibody titers were measured by a microimmunofluorescence assay, performed at the University of Washington. The assay identifies IgG and immunoglobulin M (IgM) antibodies to chlamydial EBs from the 14 major serovars of C. trachomatis, Chlamydia pneumoniae, and Chlamydia psittaci and detects chlamydia anti-lipopolysaccharide (genus-specific) and anti-major outer membrane protein (MOMP; serovar-specific) antibodies [19]. IgG titers of ≥1:16 were considered positive.
Cervical and Endometrial Bacterial Load
DNA was extracted from 180 µL of the reserved cervical or endometrial swab eluate to generate a template for the quantitation of chlamydial burden. Genomic DNA was extracted using a Quick-DNA Universal Kit (Zymo Research, Irvine, California) according to the manufacturer's protocol. The abundance of chlamydial DNA present in the extracted material was determined by quantitative PCR with SsoAdvanced SYBR mix (Bio-Rad) and primers directed against chlamydial 16S ribosomal DNA, using a CFX iCycler (Bio-Rad) [20]. Each specimen was assayed in triplicate.
Statistical Analysis
Risk factors for ascending infection were determined by log-binomial regression of cross-sectional enrollment data [21]. Risk factors for incident chlamydia infection were determined using the Wei–Lin–Weissfeld method, a marginal Cox model accounting for multiple chlamydial infections per person that adjusts for within-subject correlations [22]. Incident C. trachomatis infection was defined as any C. trachomatis–positive test result during follow-up, and the incidence rate was defined as the number of incident infections per person-years at risk. Sociodemographic factors analyzed as potential risk factors for incident infection were determined at enrollment, whereas microbiologic, sexual history and exposure, and contraception data used for these analyses were gathered at each follow-up visit. For ascending and incident infection, we calculated the unadjusted relative risk (RR) and hazard ratio (HR), using a univariable model that measured 1 independent variable at a time. We then developed final multivariable parsimonious regression models to determine the best subset of risk factors for ascending and incident infection by stepwise regression, with the α to enter set at P ≤ .2 and the α to be maintained set at P ≤ .1. We reported the adjusted RR or HR for variables maintained in the final model. In a separate analysis, independent variables that were tested but not retained in the final models were adjusted for the variables that were retained.
To assess the relationship between IgG titer and chlamydial burden, we log transformed the cervical and endometrial burden data, performed Shapiro–Wilk normality tests to ensure normal distribution, and compared chlamydial burdens of seronegative and seropositive women, using unpaired t tests. Linear regression assessed the dependence of the endometrial chlamydial burden on the cervical chlamydial burden.
We compared the frequency of clinical symptoms among women with chlamydia infection to that among women with sexually transmitted coinfections, using the Fisher exact test. The frequency of incident infection among women infected at enrollment was compared to that among uninfected women with reported chlamydial exposure within 3 weeks of enrollment, using the Fisher exact test. We used χ2 tests to compare the frequency of seropositivity among women with cervix-only and cervix plus endometrium infection and to compare the frequency of cervical ectopy (encompassing ≥25% of the cervical face) among oral contraceptive pill (OCP) users and non-OCP users.
Risk factor analyses were conducted using R (version 3.1.1). Other tests were performed using GraphPad Prism, version 6.05 for Windows (GraphPad Software, La Jolla, California). P values of <.05 were considered statistically significant.
RESULTS
Baseline Patient Characteristics
The data set included 225 women who attended 933 visits, with a median follow-up of 12 months (interquartile range, 8–12 months). Within the cohort, 172 women (76%) completed at least 3 follow-up visits, and 128 (57%) completed 4. There were no sociodemographic differences between women who attended all visits and those who did not (data not shown). Table 1 shows demographic characteristics of the cohort. The cohort comprised mainly young (median age 21 years; range, 18–35 years), single (89%), African American (66%) women who were enrolled from ambulatory clinics. The majority (55%) reported having previously received a diagnosis of chlamydia, and 24% reported having had ≥2 prior infections. Subjects commonly reported past infections with N. gonorrhoeae (21%) and/or T. vaginalis (28%). Two-thirds of women (67%) reported a past history of at least 1 bacterial STI. There were no reports of HIV infection, and reports of genital herpes (8%) and genital warts (6%) were uncommon.
Table 1.
Baseline Sociodemographic Characteristics for Cohort of Highly Exposed Women in Pittsburgh, Pennsylvania
| Characteristic | Value (n = 225) |
|---|---|
| Age, y, median (range) | 21 (18–35) |
| Race/ethnicity | |
| African American | 148 (66) |
| White | 48 (21) |
| Hispanic or Latino | 13 (6) |
| Multiracial | 23 (10) |
| Other | 6 (3) |
| Marital status | |
| Married | 1 (0.4) |
| Living with partner for ≥4 mo | 21 (9) |
| Divorced or separated | 3 (1) |
| Single | 200 (89) |
| Education level | |
| Less than high school graduate | 36 (16) |
| High school graduate or GED degree | 82 (36) |
| Vocational training | 15 (7) |
| Some college work | 73 (32) |
| College graduate | 19 (8) |
| Insurance | |
| Private | 57 (25) |
| Medicaid | 105 (47) |
| Other | 8 (4) |
| None | 55 (24) |
| Substance use | |
| Smoking | 114 (51) |
| Marijuana use | 85 (38) |
| Alcohol use | 114 (51) |
Data are no. (%) of subjects, unless otherwise indicated.
Abbreviation: GED, general education development.
The cohort was sexually active, reporting prior pregnancy (55%), a miscarriage or abortion (33%), an ectopic pregnancy (5%), and a prior PID (10%). Eighty-seven percent reported sex with a male within 30 days preceding enrollment, and 20% reported multiple male partners during this period. Many women (42%) reported having had sex in the preceding 3 months with a male who had a sexually transmitted infection. Thirty (13%) reported an untreated chlamydia diagnosis within 3 weeks of enrollment; none reported a gonorrhea diagnosis during this period. Although 58% reported condom use prior to enrollment, only 12% reported use 100% of the time. Twelve percent reported using combined OCPs, and none reported using progestin-only pills, but women receiving OCPs did not have a higher frequency of cervical ectopy on examination, compared with non-OCP users (P = .15).
Many women in the cohort were asymptomatic. Of 149 women with chlamydia at enrollment, vaginal discharge and urinary symptoms were reported in 38% and 21%, respectively. Pelvic examination revealed moderate-to-profuse vaginal or cervical discharge in 57% and 44%, respectively. Twenty-three women (15%) were coinfected with M. genitalium, and 15 (10%) were coinfected with N. gonorrhoeae; 3 subjects were infected with all 3 pathogens at enrollment. Women with chlamydia and M. genitalium coinfection were more likely to report dysuria than those with chlamydia infection alone (17.4% vs 3.7%; P = .03); otherwise, symptoms and signs were not higher in women with coinfections (data not shown). At the 1-month visit, C. trachomatis was redetected in 6% of women, which could represent treatment failure or repeat infection.
Factors Associated With the Presence of Endometrial Infection
Of 149 women with cervical chlamydial infection, 63 (42%) also had chlamydiae detected in the swab of their endometrial biopsy specimen. Factors associated with ascending infection are reported in Table 2. Sex with a new partner in the preceding 30 days (RR, 1.62 [95% CI, 1.13–2.33]), sex during previous menstrual period (RR, 1.69 [95% CI, 1.12–2.57]), and OCP use (RR, 2.02 [95% CI, 1.43–2.84]) were significantly associated with an increased risk of ascension in this unadjusted analysis. Stepwise regression identified 3 variables (exposure to a new partner in the preceding 30 days, OCP use, and gonorrhea) that met criteria for retention in the final multivariable model. After adjusting for these variables, OCP use (adjusted RR, 2.02 [95% CI, 1.38–2.97) and gonorrhea (adjusted RR, 1.66 [95% CI, 1.07–2.60]) remained significantly associated with ascending infection, but sex with a new partner was no longer significant (adjusted RR, 1.36 [95% CI, .96–1.92]). Four additional variables (exposure to multiple partners, sex with an uncircumcised male, sex during a previous menstrual period, and historical chlamydia infection) were tested but not maintained, and none were significant after adjustment for retained variables. Age, race, and marital status were not associated with an altered rate of endometrial infection (not shown).
Table 2.
Factors Associated With Endometrial Chlamydia trachomatis Infection at Enrollment
| Factor | Site of Infection, No. (%) |
Univariable Analysis |
Multivariable Model |
|||
|---|---|---|---|---|---|---|
| Cervical Infection (n = 86a) | Endometrial Infection (n = 63a) | Unadjusted RR (95% CI)b |
P Value | Adjusted RR (95% CI)c |
P Value | |
| At enrollment | ||||||
| Neisseria gonorrhoeae infection | 9 (10) | 11 (16) | 1.34 (.86–2.09) | .19 | 1.66 (1.07–2.60)d | .03 |
| Mycoplasma genitalium infection | 17 (22) | 9 (15) | 0.76 (.43–1.34) | .34 | NA | NA |
| Trichomonas vaginalis infection | 14 (21) | 9 (18) | 0.90 (.51–1.57) | .70 | NA | NA |
| Bacterial vaginosis | 21 (26) | 16 (26) | 1.02 (.66–1.57) | .93 | NA | NA |
| Before enrollment | ||||||
| C. trachomatis infection | 53 (62) | 30 (48) | 0.72 (.50–1.05) | .09 | 0.76 (.51–1.13)e | .17 |
| Sexual activity | ||||||
| ≥2 partners in prior 30 d | 13 (15) | 15 (24) | 1.35 (.90–2.03) | .15 | 0.91 (.57–1.48)e | .72 |
| New partner in prior 30 d | 15 (17) | 22 (35) | 1.62 (1.13–2.33) | .01 | 1.36 (.96–1.92)d | .08 |
| New partner in prior 12 mo | 55 (64) | 45 (71) | 1.23 (.80–1.88) | .35 | ||
| Uncircumcised partner in prior 3 mo | 3 (4) | 5 (8) | 1.53 (.86–2.71) | .15 | 0.80 (.40–1.61)e | .53 |
| Sex during last menstrual period | 4 (5) | 9 (14) | 1.69 (1.12–2.57) | .01 | 1.17 (.78–1.76)e | .45 |
| Contraception (At enrollment) | ||||||
| OCPs | 4 (5) | 13 (21) | 2.02 (1.43–2.84) | <.01 | 2.02 (1.38–2.97)d | .0003 |
| DMPA | 13 (15) | 9 (14) | 0.96 (.56–1.65) | .89 | NA | NA |
| Intrauterine device | 11 (13) | 9 (14) | 1.08 (.64–1.82) | .79 | NA | NA |
| Condoms | 49 (57) | 35 (56) | 0.97 (.66–1.41) | .86 | NA | NA |
| Coitus interruptus | 33 (38) | 22 (35) | 0.92 (.62–1.36) | .67 | NA | NA |
| Abstinence | 9 (10) | 7 (11) | 1.04 (.58–1.88) | .90 | NA | NA |
| None | 13 (15) | 8 (13) | 0.89 (.50–1.58) | .68 | NA | NA |
Abbreviations: CI, confidence interval; DMPA, depot medroxyprogesterone; NA, not applicable; OCP, oral contraceptive pills; RR, relative risk.
a Data are for women who reported or had the factor.
b Data were determined by log binomial regression. Unless otherwise indicated, reference groups are composed of all women who did not report or have the specified factor.
c Any independent variable with an unadjusted P value of ≤.2 was tested in a final multivariable model, using stepwise regression, and variables with a P value of ≤.1 were maintained. No adjusted RR is provided for variables that were not tested in the model, as indicated by “NA.” Unless otherwise indicated, reference groups are composed of all women who did not report or have the specified factor.
d Variable was maintained in the final multivariable model.
e Variable was tested but not maintained in the final multivariable model.
Risk Factors for Incident Infection During Follow-up
During 2058 person-months of risk, 48 women (21.3%) were positive for C. trachomatis, and the incidence rate was 28 cases per 100 person-years (95% CI, 22–36 cases per 100 person-years). Our univariable analysis indicated that older age (HR, 0.87 [95% CI, .78–.97]) was associated with a reduced rate of incident infection (Table 3). Race, ethnicity, and marital status were not associated with an altered rate of incident infection (data not shown), but there was a trend toward a reduced risk with increasing education level. Detection of gonorrhea at any follow-up visit was associated with increased incident chlamydia rates (HR, 3.99 [95% CI, 2.22–7.15]), but neither M. genitalium infection, T. vaginalis infection, nor bacterial vaginosis significantly increased the risk of incident chlamydial infection. Exposure to a partner with a sexually transmitted infection (HR, 3.76 [95% CI, 2.21–6.41]) or C. trachomatis infection (HR, 5.07 [95% CI, 3.01–8.53]), ≥3 partners (HR, 5.38 [95% CI, 1.22–23.76]), new partners (HR, 1.74 [95% CI, 1.10–2.76]), and uncircumcised partners (HR, 3.17 [95% CI, 1.68–5.98]) were associated with increased rates of incident chlamydia. Women reporting depot medroxyprogesterone (DMPA) injection receipt (HR, 1.80 [95% CI, 1.08–3.01]), coitus interruptus (HR, 1.89 [95% CI, 1.14–3.12]), or condom use (HR, 1.67 [95% CI, 1.04–2.70]) were at significantly increased risk of infection.
Table 3.
Risk Factors for Incident Chlamydia trachomatis Infection
| Risk Factor | Univariable Analysis |
Multivariable Model |
||
|---|---|---|---|---|
| Unadjusted HR (95% CI)a | P Value | Adjusted HR (95% CI)b | P Value | |
| Age, y | 0.87 (.78–.97) | .01 | 0.92 (.84–1.00)c | .06 |
| Education level | ||||
| Less than high school graduate | Reference | … | Reference | … |
| High school graduate or GED degree | 0.66 (.39–1.14) | .14 | 0.85 (.46–1.56)d | .60 |
| Vocational training | 1.52 (.69–3.33) | .30 | 1.73 (.89–3.38)d | .11 |
| Some college education | 0.56 (.30–1.05) | .07 | 0.93 (.47–1.83)d | .84 |
| College graduate | 0.24 (.06–1.01) | .05 | 0.50 (.11–2.16)d | .35 |
| Substance use | ||||
| Smoking | 1.13 (.71–1.78) | .61 | NA | NA |
| Marijuana use | 1.32 (.84–2.06) | .23 | NA | NA |
| Alcohol use | 0.83 (.52–1.30) | .41 | NA | NA |
| Infection type during follow-up | ||||
| N. gonorrhoeae infection | 3.99 (2.22–7.15) | <.0001 | 3.09 (1.41–6.78)c | .005 |
| M. genitalium infection | 1.48 (.80–2.72) | .21 | NA | NA |
| T. vaginalis infection | 1.16 (.51–2.66) | .72 | NA | NA |
| Bacterial vaginosis | 1.36 (.57–3.23) | .49 | NA | NA |
| Site of C. trachomatis infection at enrollment | ||||
| Uninfected | Reference | … | Reference | … |
| Cervix only | 3.14 (1.56–6.33) | .001 | 2.33 (1.07–5.11)c | .03 |
| Cervix/endometrium | 2.10 (.98–4.53) | .06 | 1.54 (.66–3.59)c | .32 |
| STI diagnosis received by partner during follow-up | ||||
| C. trachomatis infection, N. gonorrhoeae infection, or nongonococcal urethritis |
3.76 (2.21–6.41) | <.0001 | 0.94 (.30–2.98)d | .91 |
| C. trachomatis infection | 5.07 (3.01–8.53) | <.0001 | 4.99 (2.66–9.39)c | <.0001 |
| N. gonorrhoeae infection | 2.16 (.50–9.34) | .30 | NA | NA |
| Sex exposure | ||||
| Male partner(s) since last visit, no. | ||||
| 0 | Reference | … | Reference | … |
| 1 | 2.24 (.51–9.74) | .28 | 1.69 (.38–7.50)d | .49 |
| 2 | 4.10 (.94–17.86) | .06 | 1.99 (.41–9.65)d | .39 |
| ≥3 | 5.38 (1.22–23.76) | .03 | 2.86 (.61–13.42)d | .18 |
| New male partner(s) since last visit, no. | ||||
| 0 | Reference | … | Reference | … |
| ≥1 | 1.74 (1.10–2.76) | .02 | 1.56 (.95–2.57)c | .08 |
| Sex with uncircumcised male in last 3 mo | 3.17 (1.68–5.98) | .0004 | 2.65 (1.21–5.82)c | .02 |
| Sex during last menstrual period | 0.99 (.47–2.07) | .98 | NA | NA |
| Contraception (Reported at any visit) | ||||
| OCPs | 0.75 (.40–1.38) | .35 | NA | NA |
| DMPA | 1.80 (1.08–3.01) | .02 | 1.03 (.59–1.78)d | .92 |
| Condoms | 1.67 (1.04–2.70) | .04 | 1.39 (.79–2.45)d | .26 |
| Coitus interruptus | 1.89 (1.14–3.12) | .01 | 1.52 (.89–2.59)d | .13 |
| Abstinence | 0.36 (.09–1.53) | .17 | NA | NA |
| None | 1.05 (.53–2.04) | .90 | NA | NA |
| Cervical ectopy | 0.78 (.49–1.23) | .29 | NA | NA |
Abbreviations: CI, confidence interval; DMPA, depot medroxyprogesterone; GED, general education development; HR, hazard ratio; NA, not applicable; M. genitalium, Mycoplasma genitalium; N. gonorrhoeae, Neisseria gonorrhoeae; OCP, oral contraceptive pills; STI, sexually transmitted infection; T. vaginalis, Trichomonas vaginalis.
a Analysis was performed using the Wei–Lin–Weissfeld method, which is a marginal Cox model for repeated data. Unless otherwise indicated, reference groups are composed of all women who did not report or have the specified factor.
b Any independent variable with an unadjusted P value of ≤.2 was tested in a final multivariable model, using the stepwise regression, and variables with a P value of ≤.1 were maintained. No adjusted HR is provided for variables that were not tested in the model, as indicated by “NA.” Unless otherwise indicated, reference groups are composed of all women who did not report or have the specified factor.
c Variable was maintained in the final multivariable model.
d Variable was tested but not maintained in the final multivariable model.
Six factors were retained in our final multivariable model: age, gonorrhea, chlamydia infection at enrollment, and sex with new, uncircumcised, or infected partners. After adjustment for these variables, gonorrhea (adjusted HR, 3.09 [95% CI, 1.41–6.78]), sex with an infected partner (adjusted HR, 4.99 [95% CI, 2.66–9.39]), sex with an uncircumcised male (adjusted HR, 2.65 [95% CI, 1.21–5.82]), and cervical chlamydial infection at enrollment (adjusted HR, 2.33 [95% CI, 1.07–5.11]) remained significant risk factors for incident infection. Table 3 shows the adjusted HR for tested variables not retained in the multivariable model; none remained a significant risk factor after adjustment for maintained variables.
Unadjusted analyses revealed that women infected at enrollment had an increased risk of incident infection, compared with uninfected women, regardless of infection site (HR, 3.14 [95% CI, 1.56–6.33] for the cervix and 2.10 [95% CI, .98–4.53] for the cervix/endometrium). The risk of incident infection was lower for women with endometrium infection, compared with those with cervix-only infection, although nonsignificantly so (HR, 0.67 [95% CI, .41–1.10]; P = .10). After adjustment for the variables in the final model, only cervical infection increased the risk of incident infection (adjusted HR, 2.33 [95% CI, 1.07–5.11]).
Of 49 (65%) women reporting exposure to an infected partner within 3 months of enrollment, 46 (94%) denied receiving treatment, suggesting that they either resisted infection or became infected and experienced spontaneous clearance of infection. Only 11% of these women became infected during follow-up, compared with 36% of those infected at enrollment (P = .0008). Thus, women who resisted infection or resolved infection spontaneously exhibited a reduced risk of incident infection, as described by Geisler et al [23].
Relationship of Anti-chlamydial Antibody Titers at Enrollment to Ascending and Incident Infection
Sixteen percent of subjects were seronegative and 83% were seropositive at enrollment. Nineteen (46%) of 35 women who denied prior infection and were uninfected at enrollment had titers of 1:16–1:8192, indicating that they had resolved an undetected infection. The frequency of seropositivity was similar for women with cervical and endometrial infection (P = .48). Seropositivity was not associated with a reduced risk of ascending (adjusted RR, 1.24 [95% CI, .71–2.19]) or incident infection (adjusted HR, 0.94 [95% CI, .52–1.69]) after adjustment for risk factors included in the final multivariable models (Table 4).
Table 4.
Relationship of Serum Anti-Chlamydia trachomatis Immunoglobulin G (IgG) Titer to Ascending Infection at Enrollment and Incident Infection Over 1 Year of Follow-up
| IgG Titer | Ascending Infection at Enrollment |
Incident Infection During Follow-up |
||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted RR (95% CI)a |
P Value | Adjusted RR (95% CI)b |
P Value | Unadjusted HR (95% CI)c |
P Value | Adjusted HR (95% CI)d |
P Value | |
| ≤1:8 | Reference | … | Reference | … | Reference | … | Reference | … |
| 1:16–1:8192 | 0.29 (.47–1.26) | .77 | 1.24 (.71–2.19) | .45 | 1.19 (.58–2.46) | .63 | 0.94 (.52–1.69) | .82 |
Abbreviations: CI, confidence interval; HR, hazard ratio; RR, relative risk.
a Data were determined by log binomial logistic regression.
b Analysis adjusted for the 3 variables included in our final multivariate model for ascending infection: Neisseria gonorrhoeae coinfection, sex with new male partners, and oral contraceptive pill use.
c Analysis was performed using the Wei–Lin–Weissfeld method, which is a marginal Cox model for repeated data.
d Analysis adjusted for the 6 variables included in final multivariate model for incident infection over time: age, Neisseria gonorrhoeae infection, C. trachomatis infection at enrollment, exposure to a partner with C. trachomatis, sex with a new partner, and sex with an uncircumcised male.
Relationship of Anti-chlamydial Antibody Titers at Enrollment to Upper and Lower Tract Chlamydial Burden
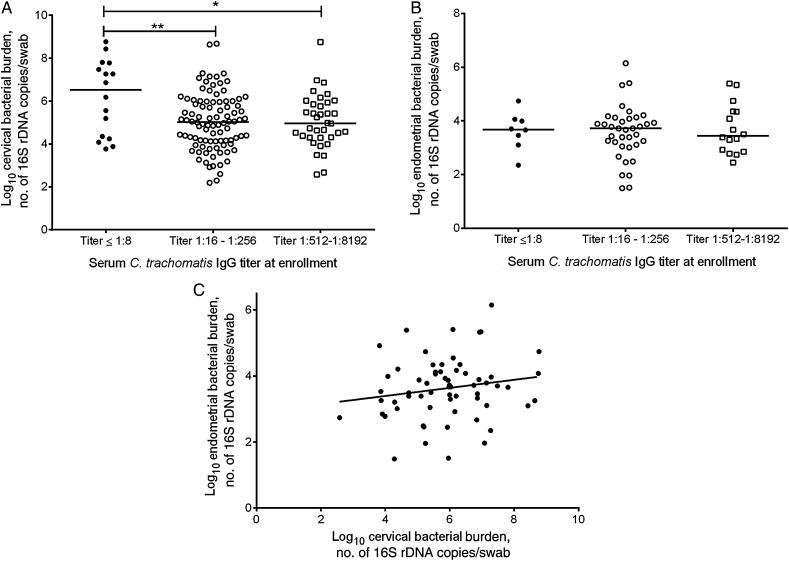
Seropositive women with midrange (1:16–1:256) and high (1:512–1:8192) titers of anti-chlamydial serum IgG had lower bacterial burdens in cervical swab specimens, compared with seronegative women (Figure 1A; seronegative vs titers of 1:16–1:256, P < .005; seronegative vs titers of 1:512–1:8192, P = .01). There was no difference in endometrial chlamydial burden between seronegative and seropositive women (Figure 1B; seronegative vs titers of 1:16–1:256, P = .85; seronegative vs titers of 1:512–1:8192; P = .86). Linear regression did not reveal a statistically significant interaction between the endometrial and cervical burdens (P = .17; Figure 1C).
Figure 1.
Comparison of serum anti-Chlamydia trachomatis immunoglobulin G (IgG) titer at enrollment to cervical (A) and endometrial (B) bacterial burden and relationship between endometrial and cervical bacterial burden (C). A, Cervical bacterial burden measured by quantitative polymerase chain reaction (qPCR) performed on eluates of cervical swab specimens obtained from women with infection at enrollment. Subjects were categorized as seronegative (titer ≤1:8; black circles) or seropositive with midrange titers (1:16 to 1:256; white circles) or high titers (1:512 to 1:8192; squares) of anti-C. trachomatis IgG, based on enrollment titers measured by a microimmunofluorescence (MIF) assay. Compared with seronegative women, seropositive women with midrange titers (P = .003, by the t test) and high titers (P = .01, by the t test) had a significantly lower bacterial burden in the cervix. B, Endometrial bacterial burden measured by qPCR on eluates of swabs of biopsied tissue specimens obtained from all women with endometrial infection at enrollment. Subjects were categorized as seronegative (titer ≤1:8; black circles) or seropositive with midrange titers (1:16 to 1:256; white circles) or high titers (1:512 to 1:8192; squares) of anti-C. trachomatis IgG, based on enrollment titers measured by a MIF assay. There was no difference in upper genital tract burden among seronegative and seropositive women with midrange titers (P = .85, by the t test) or high titers (P = .86, by the t test) of serum IgG. C, Linear regression of endometrial bacterial burden and cervical bacterial burden of all women with endometrial infection at enrollment. The linear regression equation was as follows: y = 0.1224x + 2.906 (P = .17). Abbreviation: rDNA, ribosomal DNA.
DISCUSSION
Long-term sequelae caused by C. trachomatis infection in women occur because it ascends from the cervix to the upper genital tract. Our recruitment of women with likely or known exposure to C. trachomatis led to the inclusion of a high frequency of women (66%) with active infection. We determined that OCP use and coinfection with N. gonorrhoeae were associated with an increased rate of endometrial infection. Risk factors for incident infection included the presence of infection limited to the cervix at the time of enrollment, gonorrhea at any follow-up visit, sex with a partner who received a diagnosis of chlamydia at any time during follow-up, and sex with an uncircumcised male in the previous 3 months.
We found a highly significantly increased risk of ascending infection in women using OCPs, but DMPA use was not associated with endometrial infection. In guinea pigs, treatment with estrogen [24] or estrogen and progesterone [25] leads to increased ascension and upper genital tract pathology. Additionally, in vitro studies have shown that estrogen increases chlamydial attachment to human endometrial epithelial cells [26]; this is one potential mechanism for increased ascension in OCP users. In a secondary analysis of a cohort of patients with PID treated in the United States during the 1980s, OCPs were not associated with upper genital tract chlamydial infection [27]. Our study excluded women with symptomatic PID. Although OCP use may increase ascension, other factors may be more important in determining the development of PID symptoms.
N. gonorrhoeae coinfection also significantly increased the risk of endometrial infection in our final multivariable model, suggesting that N. gonorrhoeae may impair the host immune response to C. trachomatis. Neutrophils elicited in response to gonorrhea may lead to enhanced sloughing of chlamydia-infected epithelial cells and promote ascension [28]. As another possible mechanism for this effect, N. gonorrhoeae can inhibit proliferation and expansion of antigen-specific CD4+ T cells through effects on host antigen-presenting cells [29].
Recent cohort studies by Hwang et al and Aghaizue et al examined risk factors for incident chlamydial infection in young women and reported infection rates of 4% and 4.9%, respectively, at enrollment and incident infection rates of 15% and 5.7%, respectively [4, 5]. Our recruitment strategy resulted in higher rates of infection at enrollment (66%) and incident infection during follow-up (28%), reflecting ongoing exposure among our cohort. This allowed us to confirm reported risk factors for incident infection, including young age, gonorrhea, exposure to a partner with urethritis, and sex with new or multiple partners [3–5]. Consistent with prior studies, we found a statistical trend toward a reduction of risk with increasing education level [30, 31]. Most of our cohort (71%) was African American, and we were unable to reaffirm the reported increased risk among minority races [3, 4]. Hwang et al and Aghaizu et al observed that smoking significantly increased the risk of incident infection; we found a nonsignificantly increased risk in smokers among our smaller cohort [4, 5].
DMPA use has recently been reported as a risk factor for HIV infection [32–34]. Animal models of simian immunodeficiency virus reveal that progesterone treatment depresses cellular immunity and causes thinning of lower genital tract epithelium, increasing pathogen uptake [35, 36]. In our univariable analysis, we found that DMPA increased the risk of incident chlamydial infection, which is consistent with prior studies [37–39]. However, after adjustment for age and other variables maintained in the model, DMPA was no longer significantly associated with incident infection.
Women who were uninfected at enrollment (n = 76) had the lowest risk of incident infection. Most (74%) were seropositive, indicating past exposure, and 65% reported exposure to an infected partner within 3 months of enrollment. Women with cervical infection at enrollment had an increased risk of incident infection, compared with these uninfected women. However, women with cervical and endometrial infection did not. All women enrolled in the study received antibiotics. Presumably, women with endometrium infection at enrollment were treated later in their infection course than women presenting with infection in the cervix only, allowing the host immune system adequate time to develop an adaptive response that provided protection over the subsequent year. These data support the “arrested immunity hypothesis” proposed by Brunham et al in response to increasing rates of reinfection in areas where screening and treatment programs have been instituted [40].
Our second objective was to investigate the role of serum antibody in the host immune response to chlamydia. We found that anti-chlamydial serum IgG titers were similar for women with infection limited to the cervix and those with endometrium infection. This is consistent with the report by Miettinen et al, who found no correlation between serum IgG or IgA to MOMP and tissue site of chlamydial isolation in a small cohort of patients with acute PID [41]. Furthermore, we did not find an association between antibody and protection from ascending or incident infection. This confirms prior work by Cohen et al that showed no association between anti-chlamydial EB or anti-chlamydial HSP60 serum IgG and the risk of incident infection among female sex workers [14]. We found that seropositivity was associated with reduced bacterial burden at the cervix but not at the endometrium. Whether serum IgG plays a direct role in reducing cervical bacterial burden or serves as a surrogate marker for an alternate adaptive immune response remains unclear.
A weakness of our study includes the potential overestimation of the rate of incident infection, since we were unable to distinguish treatment failure from reinfection in 11 women infected at sequential visits. Analysis of the role of antibody in protection would be improved by neutralization studies using relevant human cells and sera from women with documented resistance to reinfection. Last, use of NAAT to estimate the chlamydial burden may be less meaningful than quantitative cell culture that enumerates live bacteria. Strengths of our study include recruitment of high-risk but otherwise normal healthy young women, a high rate of follow-up, and extensive data on sexual exposure, enabling adjustment for this important confounder. Infection of the upper genital tract is likely required for induction of infertility. Repeated chlamydial infection has been associated with an increased risk of long-term sequelae. Thus, a determination of factors associated with endometrial infection and repeat infection may aid in identification of factors that increase a woman's risk for tubal disease.
Notes
Acknowledgments. We thank the women who agreed to participate in this study; Ingrid Macio, Melinda Petrina, Carol Priest, Abi Jett, and Lorna Rabe, for their efforts in the clinic and the microbiology laboratory; and the staff at the Allegheny County Health Department STD Clinic, for their efforts.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant U19 AI084024).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infections – 2008, 2012.
- 2.Haggerty CL, Ness RB. Epidemiology, pathogenesis and treatment of pelvic inflammatory disease. Exp Rev Anti-infect Therap 2006; 4:235–47. [DOI] [PubMed] [Google Scholar]
- 3.Navarro C, Jolly A, Nair R, Chen Y. Risk factors for genital chlamydial infection. Can J Infect Dis 2002; 13:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang LY, Ma Y, Moscicki AB. Biological and behavioral risks for incident Chlamydia trachomatis infection in a prospective cohort. Obstet Gynecol 2014; 124:954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aghaizu A, Reid F, Kerry S et al. Frequency and risk factors for incident and redetected Chlamydia trachomatis infection in sexually active, young, multi-ethnic women: a community based cohort study. Sex Transm Infect 2014; 90:524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol 2005; 175:7536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rank RG, Batteiger BE. Protective role of serum antibody in immunity to chlamydial genital infection. Infect Immun 1989; 57:299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ness RB, Soper DE, Richter HE et al. Chlamydia antibodies, chlamydia heat shock protein, and adverse sequelae after pelvic inflammatory disease: the PID evaluation and clinical health (PEACH) study. Sex Transm Dis 2008; 35:129–35. [DOI] [PubMed] [Google Scholar]
- 9.Tiitinen A, Surcel HM, Halttunen M et al. Chlamydia trachomatis and chlamydial heat shock protein 60-specific antibody and cell-mediated responses predict tubal factor infertility. Hum Reprod 2006; 21:1533–8. [DOI] [PubMed] [Google Scholar]
- 10.El Hakim EA, Gordon UD, Akande VA. The relationship between serum Chlamydia antibody levels and severity of disease in infertile women with tubal damage. Arch Gynecol Obstet 2010; 281:727–33. [DOI] [PubMed] [Google Scholar]
- 11.Eckert LO, Hawes SE, Wolner-Hanssen P et al. Prevalence and correlates of antibody to chlamydial heat shock protein in women attending sexually transmitted disease clinics and women with confirmed pelvic inflammatory disease. J Infect Dis 1997; 175:1453–8. [DOI] [PubMed] [Google Scholar]
- 12.Brunham RC, Kuo CC, Cles L, Holmes KK. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect Immun 1983; 39:1491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osser S, Persson K. Postabortal pelvic infection associated with Chlamydia trachomatis and the influence of humoral immunity. Am J Obstet Gynecol 1984; 150:699–703. [DOI] [PubMed] [Google Scholar]
- 14.Cohen CR, Koochesfahani KM, Meier AS et al. Immunoepidemiologic profile of Chlamydia trachomatis infection: importance of heat-shock protein 60 and interferon- gamma. J Infect Dis 2005; 192:591–9. [DOI] [PubMed] [Google Scholar]
- 15.Schumacher GFB, Yang SL, Insler V, Bettendorf G. Cyclic changes of immunoglobulins and specific antibodies in human and rhesus monkey cervical mucus. The uterine cervix and reproduction. Stuttgart: Georg Thieme Verlag, 1977:187–203. [Google Scholar]
- 16.Masson PL, Heremans JF, Ferin J. Clinical importance of the biochemical changes in the female genital tract I. Studies on the proteins of cervical mucus. Int J Fertil 1969; 14:1–7. [PubMed] [Google Scholar]
- 17.Richmond SJ, Milne JD, Hilton AL, Caul EO. Antibodies to Chlamydia trachomatis in cervicovaginal secretions: relation to serum antibodies and current chlamydial infection. Sex Transm Dis 1980; 7:11–5. [DOI] [PubMed] [Google Scholar]
- 18.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983; 74:14–22. [DOI] [PubMed] [Google Scholar]
- 19.Wang SP, Grayston JT. Human serology in Chlamydia trachomatis infection with microimmunofluorescence. J Infect Dis 1974; 130:388–97. [DOI] [PubMed] [Google Scholar]
- 20.He X, Nair A, Mekasha S, Alroy J, O'Connell CM, Ingalls RR. Enhanced virulence of Chlamydia muridarum respiratory infections in the absence of TLR2 activation. PloS One 2011; 6:e20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNutt L-A, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 2003; 157:940–3. [DOI] [PubMed] [Google Scholar]
- 22.Wei LJ, Lin DY, Weissfeld L. Regression-analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc 1989; 84:1065–73. [Google Scholar]
- 23.Geisler WM, Lensing SY, Press CG, Hook EW III. Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection. J Infect Dis 2013; 207:1850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rank RG, White HJ, Hough AJ Jr, Pasley JN, Barron AL. Effect of estradiol on chlamydial genital infection of female guinea pigs. Infect Immun 1982; 38:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barron AL, Pasley JN, Rank RG, White HJ, Mrak RE. Chlamydial salpingitis in female guinea pigs receiving oral contraceptives. Sex Transm Dis 1988; 15:169–73. [DOI] [PubMed] [Google Scholar]
- 26.Maslow AS, Davis CH, Choong J, Wyrick PB. Estrogen enhances attachment of Chlamydia trachomatis to human endometrial epithelial cells in vitro. Am J Obstet Gynecol 1988; 159:1006–14. [DOI] [PubMed] [Google Scholar]
- 27.Ness RB, Soper DE, Holley RL et al. Hormonal and barrier contraception and risk of upper genital tract disease in the PID Evaluation and Clinical Health (PEACH) study. Am J Obstet Gynecol 2001; 185:121–7. [DOI] [PubMed] [Google Scholar]
- 28.Rank RG, Whittimore J, Bowlin AK, Wyrick PB. The intimate relationship between polymorphonuclear leukocytes and the chlamydial developmental cycle: an in vivo ultrastructural analysis. Infect Immun 2011; 79:3291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu W, Ventevogel MS, Knilans KJ et al. Neisseria gonorrhoeae suppresses dendritic cell-induced, antigen-dependent CD4 T cell proliferation. PloS One 2012; 7:e41260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Annang L, Walsemann KM, Maitra D, Kerr JC. Does education matter? Examining racial differences in the association between education and STI diagnosis among black and white young adult females in the U.S. Pub Health Rep 2010; 125(suppl 4):110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller HG, Cain VS, Rogers SM, Gribble JN, Turner CF. Correlates of sexually transmitted bacterial infections among U.S. women in 1995. Fam Plan Perspect 1999; 31:4–9, 23. [PubMed] [Google Scholar]
- 32.Ralph LJ, McCoy SI, Shiu K, Padian NS. Hormonal contraceptive use and women's risk of HIV acquisition: a meta-analysis of observational studies. Lancet 2015; 15:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison CS, Chen PL, Kwok C et al. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med 2015; 12:e1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noguchi LM, Richardson BA, Baeten JM et al. Risk of HIV-1 acquisition among women who use different types of injectable progestin contraception in South Africa: a prospective cohort study. Lancet 2015; 2:e279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marx PA, Spira AI, Gettie A et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med 1996; 2:1084–9. [DOI] [PubMed] [Google Scholar]
- 36.Trunova N, Tsai L, Tung S et al. Progestin-based contraceptive suppresses cellular immune responses in SHIV-infected rhesus macaques. Virol 2006; 352:169–77. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson DL, Peralta L, Farmer M, Graham NM, Gaydos C, Zenilman J. Relationship of hormonal contraception and cervical ectopy as measured by computerized planimetry to chlamydial infection in adolescents. Sex Transm Dis 2000; 27:313–9. [DOI] [PubMed] [Google Scholar]
- 38.Baeten JM, Nyange PM, Richardson BA et al. Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol 2001; 185:380–5. [DOI] [PubMed] [Google Scholar]
- 39.Morrison CS, Bright P, Wong EL et al. Hormonal contraceptive use, cervical ectopy, and the acquisition of cervical infections. Sex Transm Dis 2004; 31:561–7. [DOI] [PubMed] [Google Scholar]
- 40.Brunham RC, Rekart ML. The arrested immunity hypothesis and the epidemiology of chlamydia control. Sex Transm Dis 2008; 35:53–4. [DOI] [PubMed] [Google Scholar]
- 41.Miettinen A, Heinonen PK, Teisala K, Punnonen R, Paavonen J. Antigen specific serum antibody response to Chlamydia trachomatis in patients with acute pelvic inflammatory disease. J Clin Pathol 1990; 43:758–61. [DOI] [PMC free article] [PubMed] [Google Scholar]