Abstract
Hemagglutination inhibition (HAI) antibody responses to anti–influenza virus hyperimmune intravenous immunoglobulin (hIVIG) were characterized. Thirty-one patients with influenza during the 2013–2014 season were randomly assigned to receive 0.25 g/kg of hIVIG (n = 16) or placebo (n = 15). For hIVIG recipients, the ratio of geometric mean titers (1 hour after infusion/before infusion) was 4.00 (95% confidence interval [CI], 2.61–6.13) for 2009 pandemic influenza A(H1N1) and 1.76 (95% CI, 1.33–2.32) for influenza A(H3N2) and influenza B. Among patients with 2009 pandemic influenza A(H1N1), ratios for hIVIG (n = 9) versus placebo (n = 8) were higher 1 hour after infusion (3.9 [95% CI, 2.3–6.7]) and sustained through day 3 (2.0 [95% CI, 1.0–4.0]). hIVIG administration significantly increases HAI titer levels among patients with influenza, supporting the need to perform a clinical outcomes study. Clinical trials registration: NCT02008578.
Keywords: anti–influenza virus hIVIG, influenza, antibody titers, randomized trial
Despite widespread access to antivirals such as oseltamivir and to seasonal vaccines, influenza has been associated with an estimated average of 226 000 excess hospitalizations and 34 000–49 000 deaths each year in the United States [1, 2]. There is a need for more effective treatments for severe, hospitalized cases of influenza [3–10]. One therapeutic strategy is infusion of convalescent plasma obtained from patients who have recovered from documented influenza. In studies dating back to the 1918 influenza A(H1N1) pandemic, investigators have attempted to determine whether treatment with convalescent blood products can improve the outcome of severe cases of influenza [6]. While continued interest in this approach has often been based on successes with the use of convalescent plasma in other viral diseases [7], the underlying rationale is predicated upon the as yet unproven assumption that rapidly raising the level of influenza virus–specific antibody in patients with influenza to levels achievable with recent vaccination might favorably modulate the disease course.
Recent investigations have explored the safety and efficacy of anti–influenza virus hyperimmune intravenous immunoglobulin (hIVIG) prepared from pooled plasma obtained from convalescent patients or from healthy volunteers recently vaccinated against either seasonal or pandemic strains of influenza viruses. Some reports suggest that the administration of anti–influenza virus hIVIG may be beneficial in patients with severe influenza [8–10]. To determine the pharmacokinetic contribution of infused IVIG to the evolution of the hemagglutination inhibition (HAI) antibody titer during natural infection, as well as the feasibility of conducting such a trial, a pilot study was performed during the 2013–2014 Northern Hemisphere influenza season.
METHODS
As an extension of global observational studies of influenza conducted since 2009 [5], the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) initiated a pilot study of anti–influenza virus hIVIG (FLU-IVIG Pilot) at clinical sites in the United States. This study was approved by the institutional review boards of participating sites.
Design and Study Population
Eight sites participated in a randomized, double-blind clinical trial of inpatients or outpatients with a diagnosis of seasonal influenza during the 2013–2014 Northern Hemisphere influenza season. Eligible patients were adults ≥18 years of age who were positive for influenza A or B by reverse-transcription polymerase chain reaction (RT-PCR) analysis or rapid antigen testing of upper respiratory tract specimens within 48 hours prior to randomization, had onset of clinical illness no more than 6 days prior to randomization (with onset defined as when the patient first experienced at least one respiratory symptom, constitutional symptom, or fever), and were able to provide written informed consent. Local diagnoses of positive influenza virus results were later confirmed by RT-PCR to identify virus strain at a central laboratory (Leidos, Frederick, Maryland).
The purpose of this study was to determine the pharmacokinetic profile of anti–influenza virus hIVIG and assess whether those levels, as measured by HAI antibody titers, were similar to predicted titers based on product HAI antibody titers and volume of distribution. For this objective, the maximum concentration of anti–influenza virus hIVIG was evaluated using the HAI antibody titer 1 hour after infusion for the IVIG group.
A major secondary objective was to compare HAI antibody titers in the anti–influenza virus hIVIG and placebo groups at 1 hour after infusion and on study days 1, 3, 7, and 28 according to the infecting virus strain. The difference in HAI titers between treatment groups was projected to represent the effect of the natural immune response plus anti–influenza virus hIVIG (IVIG group) as compared to the natural immune response alone (placebo group).
Study Product and Treatment
The anti–influenza virus hIVIG used in this pilot was manufactured by Emergent Biosolutions (previously known as Cangene) according to good manufacturing practices under contract to the National Institute of Allergy and Infectious Diseases, National Institutes of Health. All units were screened for the following adventitious agents: human immunodeficiency virus types 1 and 2; human T-lymphotropic virus types I and II; hepatitis A, B, and C viruses; Treponema palladium; West Nile virus; and parvovirus B19.
The lot of IVIG used had the following reciprocal geometric mean HAI antibody titers (rGMT): A/California/7/2009/A(H1N1)pdm09, 1:640; A/Victoria/361/2011/A(H3N2), 1:320; and B/Massachusetts/2/2012, 1:160. The dose of 0.25 g/kg was based on a volume of distribution of 0.05 L/kg and targeted to achieve a rGMT of ≥ 1:40 against 2009 pandemic influenza A(H1N1) (hereafter, “A[H1N1]pdm09”), the strain predicted to have the highest prevalence during the study period (Supplementary Data).
Randomization and Blinding
Patients were randomly assigned equally to receive standard of care therapy plus either a single dose of anti–influenza virus hIVIG or placebo. Standard of care therapy included provision of licensed antiviral therapy (eg, oseltamivir) at standard treatment doses.
Only the site pharmacist was aware of an individual's treatment assignment. IVIG or saline placebo was administered in 500-mL plastic bags shielded within an amber-tinted bag to maintain double-blind status. Infusions were administered over a minimum time of 2 hours, per institutional IVIG infusion guidelines.
Baseline and Follow-up Measurements
Prior to randomization and infusion, demographic characteristics, medical history, vital signs, targeted symptoms, and use of antiviral and antibacterial treatments were recorded. Blood specimens were collected for measurement of HAI antibody titers of each major strain of influenza virus (ie, influenza A[H1N1], influenza A[H3N2], and influenza B), before infusion and on days 1, 3, 7, and 28 after infusion; for safety laboratory tests, before infusion and on days 3, 7, and 28 after infusion; and for storage for future research. A nasopharyngeal swab specimen was collected at baseline and day 3 for central quantitative RT-PCR analysis of influenza viral RNA.
Event severity was assessed using the Division of AIDS Toxicity Table (available at: http://rsc.tech-res.com/safetyandpharmacovigilance/gradingtables.aspx), and grade 3 and 4 events were reported.
Statistical Considerations
Descriptive statistics were used to compare the treatment groups at study entry. Paired t tests were used to summarize the difference in log-transformed levels of HAI antibody titers for the IVIG group. HAI antibody titers of <10 were imputed as 10. Average differences (calculated as the log value after infusion minus the log value before infusion) were back transformed and summarized as the ratio of geometric means. Analysis of covariance with the log-transformed preinfusion titer as a covariate was used to compare treatment groups for log-transformed HAI antibody titer levels at each follow-up time point. Treatment differences for log-transformed titer levels were back transformed to obtain geometric means and were only carried out for those infected with A(H1N1)pdm09 because the numbers of persons infected with influenza A(H3N2) or influenza B were too small. For viral load analyses see Supplementary data. Statistical analyses were performed using SAS (version 9.3).
Clinical information including adverse events was kept blinded to study investigators because data from participants in the FLU-IVIG Pilot will be used together with data from the outcome study to evaluate the efficacy of anti–influenza virus hIVIG.
RESULTS
Thirty-one patients with laboratory-confirmed influenza were enrolled across 8 U.S. sites (Table 1). Anti–influenza virus hIVIG was administered to 16 participants and placebo to 15. The median age of study participants was 53 years of age, and 77% were enrolled while inpatient in a general hospital ward. 9/16 IVIG and 5/15 placebo recipients had previously received the 2013–2014 trivalent influenza vaccine; and all but 2 persons in each group had started oseltamivir on or before the day of randomization. Local influenza diagnosis was confirmed centrally for all enrollees, with A(H1N1)pdm09 being the predominant influenza virus strain (55%). Two patients randomly assigned to receive placebo did not receive the full dose: one person refused the infusion following randomization, and the infusion for the second patient was interrupted owing to anxiety and subjective dyspnea.
Table 1.
Baseline Characteristics of the Study Participants
| Characteristic | IVIG Group (n = 16) | Placebo Group (n = 15) | Total (n = 31) |
|---|---|---|---|
| Age, y | |||
| <40 | 7 (43.8) | 4 (26.7) | 11 (35.5) |
| 40–59 | 6 (37.5) | 5 (33.3) | 11 (35.5) |
| ≥60 | 3 (18.8) | 6 (40.0) | 9 (29.0) |
| Overall | 48 (37–57) | 55 (39–62) | 53 (37–62) |
| Sex | |||
| Male | 8 (50.0) | 4 (26.7) | 12 (38.7) |
| Female | 8 (50.0) | 11 (73.3) | 19 (61.3) |
| Race | |||
| Asian | 0 (0.0) | 1 (6.7) | 1 (3.2) |
| Black | 5 (31.3) | 7 (46.7) | 12 (38.7) |
| White | 11 (68.8) | 7 (46.7) | 18 (58.1) |
| Patient statusa | |||
| Outpatient | 2 (12.5) | 2 (13.3) | 4 (12.9) |
| General ward | 14 (87.5) | 10 (66.7) | 24 (77.4) |
| Intensive care unit | 0 (0.0) | 3 (20.0) | 3 (9.7) |
| Local influenza virus test type | |||
| RT-PCR | 12 (75.0) | 10 (66.7) | 22 (71.0) |
| Rapid antigen test | 4 (25.0) | 5 (33.3) | 9 (29.0) |
| Influenza virus strain | |||
| A(H1N1)pdm09 | 9 (56.3) | 8 (53.3) | 17 (54.8) |
| A(H3N2) | 1 (6.3) | 2 (13.3) | 3 (9.7) |
| B | 5 (31.3) | 4 (26.7) | 9 (29.0) |
| A, subtype unknown | 1 (6.3) | 1 (6.7) | 2 (6.5) |
| Time since onset, d | 3 (3–4) | 4 (3–4) | 4 (3–4) |
| Weight, kg | 82.8 (71.9–97.9) | 83.0 (74.8–104.4) | 83.0 (74.8–100.7) |
| Dose, gb | 20.8 (18.0–24.4) | 20.8 (18.7–25.0) | 20.8 (18.7–25.0) |
Data are no. (%) of subjects or median value (interquartile range).
Abbreviations: A(H1N1)pdm09, 2009 pandemic influenza A(H1N1); IVIG, intravenous immunoglobulin; RT-PCR, reverse-transcription polymerase chain reaction.
a Location of the patient at the time of randomization.
b Calculated dose to be used if randomly assigned to active arm.
HAI Titers for Patients in the IVIG Group
For IVIG recipients, targeted HAI titer levels were achieved in 100% of patients infected with A/California/07/2009/A(H1N1)pdm09 (target, 1:64), 100% of those infected with A/Texas/50/2012/A(H3N2) (target, 1:32), and 93.8% of patients infected with B/Massachusetts/02/2012 (target, 1:16; Supplementary Data).
Treatment Differences in HAI Titers for Patients Infected With A(H1N1)pdm09
For the patients infected with A(H1N1)pdm09, HAI titer levels differed significantly between the IVIG and placebo groups through day 3 of follow-up (Figure 1). Owing to the evolution in natural immunity during the first week after enrollment, initial treatment differences in HAI titer levels diminished with longer follow-up. At 1 hour after infusion and on days 1, 3, 7, and 28, the ratios of rGMTs for IVIG versus placebo were 3.9 (95% confidence interval [CI], 2.3–6.7), 3.1 (95% CI, 1.8–5.4), 2.0 (95% CI, 1.0–4.0), 1.4 (95% CI, .5–4.2), and 1.1 (95% CI, .3–4.6), respectively.
Figure 1.
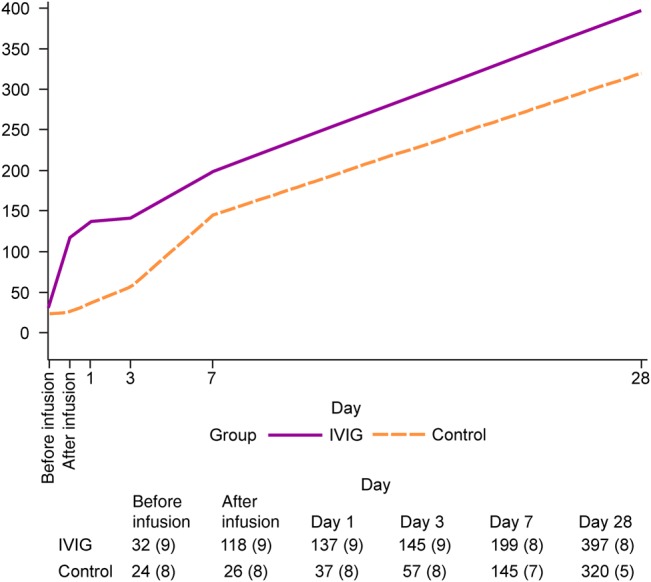
The kinetics of serum hemagglutination inhibition (HAI) antibody titer changes in the intravenous immunoglobulin (IVIG) and saline placebo treatment arms are shown over the 28-day course of study participation among patients infected with 2009 pandemic influenza A(H1N1). The reciprocal geometric mean titers of HAI antibody levels are summarized in the table 1 at 6 study-defined time points; numbers of observations are specified in the parentheses.
Owing to small numbers of individuals infected with influenza A(H3N2) or influenza B, influenza viral RNA levels from nasopharyngeal swabs were only compared in persons infected with A(H1N1)pdm09. There were no significant differences in median values of viral titers before infusion and after infusion between hIVIG and placebo recipients (Supplementary Data).
Safety Summary
Grade 4 adverse events were reported for 3 patients during the study. One of these patients had 3 grade 4 events (elevated bilirubin level, elevated platelet count, and renal failure) and ultimately died. These events were attributed to advanced cancer and not to study treatment. Two others each experienced 1 grade 4 event (hyperkalemia and worsened dysthymic disorder) of uncertain etiology.
DISCUSSION
This pilot study of adults with seasonal influenza demonstrated that anti–influenza virus hIVIG administration resulted in an initial spike in a strain-specific HAI antibody response that occurred at least 3 days earlier than that of natural infection alone, that after infusion titers were within the protective range (based on inactivated vaccine studies) [11–14], and that a clinical outcomes trial of anti–influenza virus hIVIG was feasible.
While this study showed that it is possible to rapidly raise antibody titers to postvaccination levels, protective preexposure prophylactic titers and postexposure disease modification titers are distinct concepts [11]. Past experience suggests that postexposure administration of immune globulin may confer clinical benefit, but neither its timing nor it relationship to HAI antibody titer has been defined.
In a meta-analysis of 8 studies of individuals with influenza virus–associated pneumonia treated during the 1918 influenza A(H1N1) pandemic, 336 recipients of convalescent sera, plasma, or blood were compared to 1219 contemporaneous untreated controls or to individuals treated late in the course of infection [6]. The pooled risk difference was 21% and favored the treatment group. Compared with later therapy, the pooled risk difference was 41%, favoring those treated within 4 days of pneumonia diagnosis. However, these studies were not randomized or placebo controlled, and the blood products studied and their preparations differed.
Investigators in Hong Kong enrolled 93 patients with influenza A(H1N1)pdm09 infection requiring intensive care support within 7 days of illness onset [8]. All were offered treatment with 500 mL of convalescent plasma with a neutralizing A(H1N1)pdm09 antibody titer of >1:160. Twenty persons (21.5%) agreed to receive the plasma treatment, whereas 73 declined and served as the control group. Mortality among those who received convalescent plasma was significantly lower than that for controls (20.0% vs 54.8%; adjusted odds ratio, 0.20 [95% CI, .06–.69]). The nonrandomized design precludes definitive conclusions about efficacy.
The potential therapeutic value of anti–influenza virus hIVIG was suggested by a small randomized, double-blind, placebo-controlled trial. Thirty-five patients with A(H1N1)pdm09 infection who required intensive care and ventilation support were randomly assigned to receive anti–influenza virus hIVIG (prepared from individuals who had recovered from A[H1N1]pdm09 infection) or control IVIG lacking activity against A(H1N1)pdm09 [9]. Although overall results indicated no survival difference, a post hoc subgroup analysis demonstrated reduced mortality among anti–influenza virus hIVIG recipients treated within 5 days of symptom onset (0 of 12 deaths among anti–influenza virus hIVIG recipients versus 4 of 10 among controls). However, for those treated later than 5 days after onset, this relationship was reversed.
Our pilot study was limited by low enrollment numbers and did not attain the planned sample size of 40 participants (Supplementary Data). This was in part due to slow start-up and an early peak in the 2013–2014 influenza season. However, data for the 31 participants provides sufficient supportive information to justify conducting a clinical end point study currently underway. This trial is seeking to evaluate the efficacy and safety of anti–influenza virus hIVIG in patients hospitalized with severe influenza, beginning with the 2014–2015 Northern Hemisphere influenza season (available at: https://clinicaltrials.gov/ct2/show/NCT02287467?term=INSIGHT+AND+influenza&rank=3). Owing to the ongoing threat of antigenic drift, one major challenge of such a trial will be the need to keep the antibody specificity of the manufactured IVIG product contemporaneous with circulating virus subtypes throughout the projected course of the study (ie, ≥2 influenza seasons).
STUDY GROUP MEMBERS
Members of the INSIGHT FLU005 IVIG Pilot Study Group are as follows: Richard T. Davey, Jr, Norman Markowitz, John Beigel, Deborah Wentworth, Abdel Babiker, Tauseef Rehman, Robin Dewar, Julia Metcalf, Timothy M. Uyeki, Elizabeth B. Finley, Barbara Standridge, Paul Riska, H. Clifford Lane, Fred Gordin, and James D. Neaton (writing group); E. Denning, A. DuChene, N. Engen, M. Harrison, K. Quan, and G. Thompson (statistical and data management center); Adriana Sanchez (international coordinating center); Marie Hoover and Venn Natarajan (laboratory support); H. Preston Holley, John Tierney, and Jocelyn Voell (National Institute of Allergy and Infectious Diseases); and J. Baxter, D. Bigley, P. Coburn, L. Faber, E. Gardner, L. Harlow, M. Jain, L. Makohon, R. McConnell, J. Moghe, R. Nahra, B. Omotosho, T. Petersen, H. Polenakovik, S. Rizza, J. Scott, A. Shoen, C. Solorzano, Z. Temesgen, and J. Whitaker (clinical sites).
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgment. We thank the patients who participated in the study.
Disclaimer. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. The views expressed are those of the authors and do not reflect the policy of the National Institute of Allergy and Infectious Diseases (NIAID) or the Centers for Disease Control and Prevention. The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported by the NIAID Intramural Research Program and the NIAID Division of Clinical Research, through a contract with the University of Minnesota (Leidos prime contract HHSN261200800001E).
Potential conflicts of interest. Dr Babiker received grant support from the University of Minnesota and the United Kingdom Medical Research Council during the conduct of the study. Dr Riska received grant support (to the National Institutes of Health) and work-related travel support from and served as a consultant for Gilead; salary support from Montefiore Medical Center; and grant support (to his institution) from Sanofi-Pasteur, Cubist, Actelion, Rempex, Summit, and Scynexis outside the conduct of this study. Dr Neaton received grant support from the University of Minnesota during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: INSIGHT FLU005 IVIG Pilot Study Group, Richard T. Davey, Jr, Norman Markowitz, John Beigel, Deborah Wentworth, Abdel Babiker, Tauseef Rehman, Robin Dewar, Julia Metcalf, Timothy M. Uyeki, Elizabeth B. Finley, Barbara Standridge, Paul Riska, H. Clifford Lane, Fred Gordin, James D. Neaton, E. Denning, A. DuChene, N. Engen, M. Harrison, K. Quan, G. Thompson, Adriana Sanchez, Marie Hoover, Venn Natarajan, H. Preston Holley, John Tierney, Jocelyn Voell, J. Baxter, D. Bigley, P. Coburn, L. Faber, E. Gardner, L. Harlow, M. Jain, L. Makohon, R. McConnell, J. Moghe, R. Nahra, B. Omotosho, T. Petersen, H. Polenakovik, S. Rizza, J. Scott, A. Shoen, C. Solorzano, Z. Temesgen, and J. Whittaker
References
- 1.Thompson WW, Shay DK, Weintraub E et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–40. [DOI] [PubMed] [Google Scholar]
- 2.Thompson MG, Shay DK, Zhou H et al. Estimates of deaths associated with seasonal influenza – United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057–62. [PubMed] [Google Scholar]
- 3.Kumar A, Zarychanski R, Pinto R et al. Critically ill patients with 2009 influenza A (H1N1) infection in Canada. JAMA 2009; 302:1872–9. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S et al. Pneumonia and respiratory failure from swine origin influenza A (H1N1) in Mexico. N Engl J Med 2009; 361:680–9. [DOI] [PubMed] [Google Scholar]
- 5.Dwyer DE; The INSIGHT Influenza Study Group. Surveillance of illness associated with pandemic (H1N1) 2009 virus infection among adults using a global clinical site network approach: The INSIGHT FLU 002 and FLU 003 studies. Vaccine 2011; 29S:B56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luke TR, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: future H5N1 treatment? Ann Intern Med 2006; 145:599–609. [DOI] [PubMed] [Google Scholar]
- 7.Mair-Jenkins J, Saavedra-Campos M, Baillie JK et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis 2015; 211:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung IF, To KK, Lee CK et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis 2011; 52:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hung IF, To KK, Lee CK et al. Hyperimmune intravenous immunoglobulin treatment: a multicentre double-blind randomized controlled trial for patients with severe A(H1N1)pdm09 infection. Chest 2013; 144:464–73. [DOI] [PubMed] [Google Scholar]
- 10.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med 2007; 357:1450–1. [DOI] [PubMed] [Google Scholar]
- 11.Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis 2011; 204:1879–85. [DOI] [PubMed] [Google Scholar]
- 12.Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines. US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, 2007. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/. Accessed 10 August 2015. [Google Scholar]
- 13.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hygiene (Camb) 1972; 70:767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Jong JC, Palache AM, Beyer WEP et al. Haemagglutination-inhibiting antibody to influenza virus. In: Brown F, Haaheim LR, Schild, eds. Laboratory correlates of immunity to influenza—a reassessment. Basel, Switzerland: Karger, 2003:63–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


