Abstract
The retropharyngeal internal carotid artery (ICA) is a well-described arterial anomaly with important implications for patients undergoing pharyngeal approach surgical procedures. Existing clinical and imaging classification schemes for a retropharyngeal ICA take into account arterial distance to the pharyngeal mucosal wall. We describe a case of mobility of a retropharyngeal ICA between short-interval imaging studies. The possibility of respiratory variability or other etiologies causing such changes in retropharyngeal carotid position have not been described previously. Our findings suggest that imaging findings from a single study alone may not be sufficient to confidently exclude this clinically significant arterial anomaly.
Keywords: Carotid artery, retropharyngeal, pharynx, mobility
INTRODUCTION
Variations in the course and position of the cervical internal carotid arteries (ICA) are well known and described in both the radiology and otorhinolaryngology literature.1,2 Given the frequency with which common surgical procedures, such as tonsillectomy and adenoidectomy, involve a pharyngeal approach, one such anatomic variation that is of clinical significance is the retropharyngeal ICA. The retropharyngeal space located immediately posterior to the mucosa of the nasopharynx, oropharynx, and hypopharynx can be the site of a number of pathologies such as infection, lymphadenopathy, or neoplasm. An aberrant course of the ICA can present as a submucosal pseudomass on direct inspection of the pharynx and thereby pose a significant potential hazard in pharyngeal surgery or biopsy.2 Although interpatient variability in the course of the cervical ICA has been described, to our knowledge intrapatient variation in the location of the cervical ICA over time has not been described.
CASE REPORT
An otherwise healthy 28-year-old African American female presented with sore throat and cervical lymphadenopathy. Despite a short course of antibiotics, the patient developed increasing lymphadenopathy, fever, and a 13-pound weight loss. Imaging showed diffuse enlarged bilateral nodes in the neck (up to 3.8 × 2 cm), chest, and abdomen. Excisional biopsy of a left axillary node was initially reported as nodular sclerosing Hodgkin lymphoma, but rereview at a tertiary care cancer center revealed only reactive nodes with marked follicular hyperplasia. Reactive lymphadenopathy was confirmed after excision of a left intraparotid node. Retropharyngeal ICAs at the level of the oropharynx seen on an initial scan (Fig. 1) returned to normal nonretropharyngeal positions on a subsequent scan obtained 7 months later (Fig. 2). The patient had no history of neck surgery or radiation therapy. The patient’s body weight at the initial neck computed tomography (CT) was 102 kg, and 103 kg at the 7 month follow-up. She has no diagnosis of malignancy and is now asymptomatic. She continues to be followed clinically.
Fig. 1.
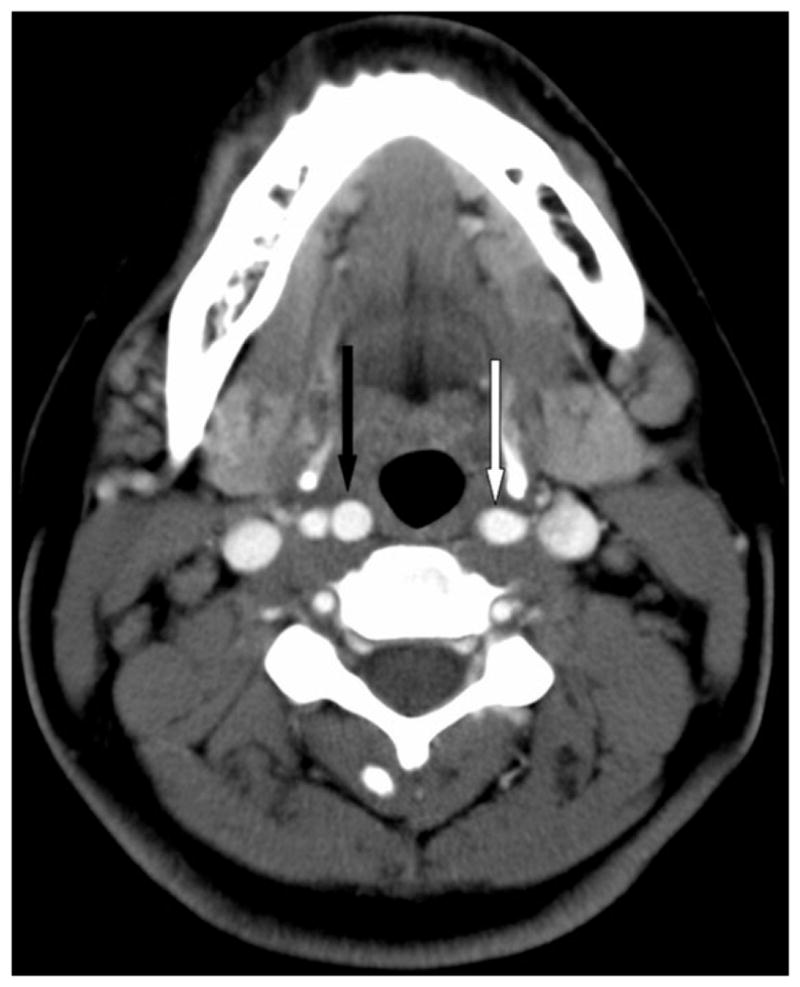
Baseline axial contrast-enhanced computed tomography at the level of the oropharynx. The left bifurcation is slightly higher than the right, with the left common carotid artery (white arrow) present at the same level that the proximal-most axial image of the right internal carotid artery (ICA) (black arrow). Note the position of the carotid arteries relative to the oropharyngeal mucosa, with the carotids encroaching on the retropharyngeal space. At this level, the distances of the ICA from the oropharyngeal mucosa are 3.8 and 4.8 mm on the right and left, respectively.
Fig. 2.
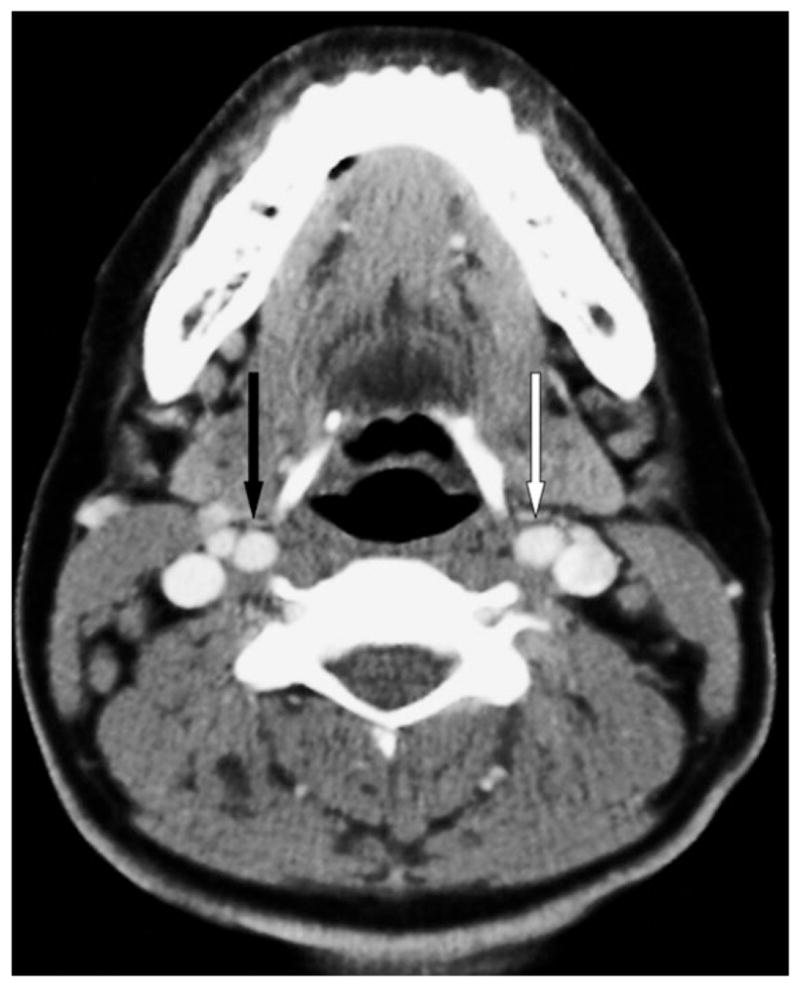
Axial contrast-enhanced computed tomography at the level of the oropharynx 7 months after the image shown in Figure 1. This image is at an equivalent craniocaudal position as the baseline image, confirmed by the presence of the distal left common carotid artery (white arrow) seen at the same level of the proximal-most axial image of the right internal carotid artery (black arrow). Note the change in apparent position of the carotid arteries compared to Figure 1, with a normal course of the bilateral carotid arteries at this level outside of the retropharyngeal space. At this level, the distances of the carotid arteries from the oropharyngeal mucosa are 10.4 and 10.0 mm on the right and left, respectively.
DISCUSSION
In this patient, CT imaging demonstrates shift in the position of the cervical carotid arteries bilaterally relative to the mucosal surface of the pharynx. On the baseline CT scan, the carotid arteries are in a retropharyngeal position, and could, on direct visual inspection, appear as a submucosal pseudomass. This patient received no local or regional therapy (surgery or radiation), which would be expected to alter the fascial planes of the deep neck or the spatial relationships of the pharyngeal mucosal, parapharyngeal, and carotid spaces. Also, the patient’s weight remained relatively stable over this time period, and although she had evidence of lymphadenopathy, none was present at the level of the apparently mobile cervical carotid artery.
The potentially lethal complications arising from tonsillectomy or adenoidectomy in patients with a retropharyngeal course of the cervical ICA have been known for nearly a century. Skillern warned in 1913,3 that ‘‘since this anomaly may be present in any given case, thorough ocular and digital exploration of the pharynx for arterial pulsations must never be omitted’’ in any pharyngeal surgery. Aberrant or variant courses of the cervical internal carotid artery have been described with incidences ranging from 10% to 40% of the population.1,4,5 In their cadaveric study of anatomic variations of the cervical ICA relative to the pharynx in 292 head and neck specimens, Paulsen et al.1 found a 32.3% incidence of anatomic variability in the carotid arteries with curving (27.2%) more common than kinking or coiling (6%), and approximately 10% of all specimens with medial curvature of the cervical ICA causing a decreased distance to the pharyngeal wall.
Imaging techniques such as magnetic resonance imaging (MRI) or CT have been used to define the distance of the cervical ICA to the pharyngeal wall. Deutsch et al.,6 in their evaluation of 100 patients with MRI, demonstrated that with increasing age and weight in childhood, the average distance between the cervical ICA and the pharyngeal wall increases to approach approximately 2.5 cm by adulthood. Based on such normative values, Pfeiffer and Ridder7 recently proposed a clinicoradiologic classification of parapharyngeal ICA aberrations, taking into account risk for potential for ICA injury, location within the pharynx (naso-, oro-, or hypo-), and minimum distance to the pharyngeal wall. For example, grade 1 patients in this classification have a relatively low potential for ICA injury, with a minimum distance to the pharyngeal wall of 10 mm or more at the nasopharynx or oropharynx, in contrast to grade 4 patients who have a very high potential for ICA injury and have an ICA that contacts or is within 2 mm of the pharyngeal wall in the nasopharynx or oropharynx. The role of radiologic assessment of aberrant cervical ICA position has also been supported by other investigators, such as Galletti et al.,8 who contended that three-dimensional time-of-flight magnetic resonance angiography along with Doppler ultrasonography are highly accurate means of characterizing these anomalies.
Our case highlights the potential limitations of relying solely on imaging criteria to determine the course of the cervical ICA relative to pharyngeal wall. In our case, despite stable patient weight and absence of treatment changes to account for ICA position variability, within 7 months this patient went from a retropharyngeal ICA, classified as a Pfeiffer grade 3, which implies a high risk for ICA injury in pharyngeal wall surgery, to a normal position relative to the pharyngeal wall at 7-month follow-up. Although the change over time in position of the cervical ICA within a given individual has been described as a normal developmental phenomenon in pediatric subjects6 or as a function of the development of senescent arteriosclerotic changes of the ICA,1 the extent of change over the relatively short time period has to our knowledge never been reported in the literature.
The physiology underlying the intrapatient variability of ICA position in our case remains unclear. Whether this case represents a new fixed alteration in the spatial relationship of the carotid to the pharyngeal wall, or a transient apparent variation in ICA position captured at the time of imaging is uncertain. In a study of the variability of pharyngeal diameters in 148 pediatric subjects requiring sedation for MRI, Donnelly et al.9 noted that variations in pharyngeal wall diameter were frequently present using cine magnetic resonance techniques. In particular, dynamic changes and intermittent collapse was frequently observed, with variability of near 3 mm in diameter noted in the oropharynx. It is conceivable that in our patient, in part the variation of pharyngeal surface to ICA distance could be attributed to such variability in phase of respiration. Of note, the diameter of the oropharynx is significantly smaller in the baseline imaging (Fig. 1) compared to 7-month follow-up (Fig. 2), suggesting that some degree of respiratory variability is present between these two scans. Furthermore, although the images are taken at identical craniocaudal levels with respect to the carotid vasculature, there is slight variation in the appearance of the configuration of the hyoid bone suggesting relative motion of the carotid sheath to the adjacent anatomic imaging landmarks. However, even allowing for the differences in respiration, there does appear to be significantly less intervening tissue between the ICA and pharyngeal wall when the two imaging studies are compared. Potential causes of a new fixed alteration in the spatial relationship of the carotid to the pharyngeal wall include accelerated idiopathic arteriosclerotic changes or idiopathic atrophy of parapharyngeal tissue thickness. There are no specific findings in the patient’s physical or history to support these potential etiologies, however, which are also unlikely given the short time interval between scans. Clearly, additional work is warranted in a larger patient cohort to assess the frequency and incidence of variability or mobility of aberrant cervical ICAs.
CONCLUSION
Regardless of the underlying mechanism of this intrapatient change in position of the cervical ICA (transient respiration-dependent change vs. a new fixed altered position), our finding is of significant clinical interest. Imaging findings from a single remote cross-sectional exam alone may not be sufficient to confidently exclude the possibility that a submucosal pharyngeal mass may represent an aberrant ICA. Additional investigation is needed to assess whether the classification schema of Pfeiffer et al. may need to take into account the respiratory phase of the imaging study used to assess aberrant ICA position. Continued recognition of the potential for this anatomic variation with both careful direct visual and tactile inspection during pharyngeal surgery remains of significant clinical value.
Footnotes
The authors have no funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Paulsen F, Tillmann B, Christofides C, Richter W, Koebke J. Curving and looping of the internal carotid artery in relation to the pharynx: frequency, embryology and clinical implications. J Anat. 2000;197(pt 3):373–381. doi: 10.1046/j.1469-7580.2000.19730373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz A, De Vergas J, Crespo J. Imaging and clinical findings in patients with aberrant course of the cervical internal carotid arteries. Open Neuroimag J. 2010;4:174–181. doi: 10.2174/1874440001004010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skillern P. Anomalous internal carotid artery and its clinical significance in operations on tonsils. JAMA. 1913;60:172–173. [Google Scholar]
- 4.Wasserman JM, Sclafani SJ, Goldstein NA. Intraoperative evaluation of a pulsatile oropharyngeal mass during adenotonsillectomy. Int J Pediatr Otorhinolaryngol. 2006;70:371–375. doi: 10.1016/j.ijporl.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Tillmann B, Christofides C. The ‘‘dangerous loop’’ of the internal carotid artery. An anatomic study [in German] HNO. 1995;43:601–604. [PubMed] [Google Scholar]
- 6.Deutsch MD, Kriss VM, Willging JP. Distance between the tonsillar fossa and internal carotid artery in children. Arch Otolaryngol Head Neck Surg. 1995;121:1410–1412. doi: 10.1001/archotol.1995.01890120066013. [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer J, Ridder GJ. A clinical classification system for aberrant internal carotid arteries. Laryngoscope. 2008;118:1931–1936. doi: 10.1097/MLG.0b013e318180213b. [DOI] [PubMed] [Google Scholar]
- 8.Galletti B, Bucolo S, Abbate G, et al. Internal carotid artery transposition as risk factor in pharyngeal surgery. Laryngoscope. 2002;112:1845–1848. doi: 10.1097/00005537-200210000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly LF, Casper KA, Chen B, Koch BL. Defining normal upper airway motion in asymptomatic children during sleep by means of cine MR techniques. Radiology. 2002;223:176–180. doi: 10.1148/radiol.2231011023. [DOI] [PubMed] [Google Scholar]


