Abstract
Many lines of evidence indicate that postsynaptic dendritic spines are plastic during development and largely stable in adulthood. It remains unclear to what degree presynaptic axonal terminals undergo changes in the developing and mature cortex. In this study, we examined the formation and elimination of fluorescently-labeled axonal boutons in the living mouse barrel cortex with transcranial two-photon microscopy. We found that the turnover of axonal boutons was significantly higher in 3-week-old young mice than in adult mice (older than 3 months). There was a slight but significant net loss of axonal boutons in mice from 1 to 2 months of age. In both young and adult barrel cortex, axonal boutons existed for at least one week were less likely to be eliminated than those recently-formed boutons. In adulthood, ~80% of axonal boutons persisted over 12 months and enriched sensory experience caused a slight but not significant increase in the turnover of axonal boutons over 2–4 weeks. Thus, similar to postsynaptic dendritic spines, presynaptic axonal boutons show remarkable stability after development ends. This long-term stability of synaptic connections is likely important for reliable sensory processing in the mature somatosensory cortex.
Keywords: axonal bouton, two-photon imaging, synaptic plasticity, sensory enrichment
INTRODUCTION
Changes of synaptic connections are essential for the development and function of the nervous system (Bailey and Kandel, 1993; Bhatt et al., 2009; Buonomano and Merzenich, 1998; Cline and Haas, 2008; De Roo et al., 2008; Fox, 2002; Hua and Smith, 2004; Katz and Shatz, 1996; Malenka and Nicoll, 1999). In vivo imaging studies in the mouse cortex have revealed that dendritic spines, the postsynaptic elements of most excitatory synapses, are highly plastic during development and largely maintained in adulthood (Grutzendler et al., 2002; Holtmaat et al., 2005; Yang et al., 2009; Zuo et al., 2005a). Multiple lines of evidence indicate that sensory and learning experience play an important role in regulating dendritic spine remodeling throughout life (Holtmaat et al., 2006; Kleim et al., 1998; Knott et al., 2002; Lai et al., 2012; Yang et al., 2009; Zuo et al., 2005b). For example, whisker stimulation over 24 hours causes a transient increase in the number of dendritic spines in Layer 4 of the mouse barrel cortex (Knott et al., 2002). Sensory enrichment over days increases dendritic spine formation and elimination of Layer 5 pyramidal neurons in the barrel cortex (Yang et al., 2009). Recent studies have shown that motor learning induces the formation of new dendritic spines over hours in the motor cortex. The degree of persistent new spines strongly correlates with performance improvement over days (Liston et al., 2013; Yang et al., 2014; Yang et al., 2009). Together, these studies suggest important functions of dendritic spine formation and maintenance in information encoding and storage.
Compared to postsynaptic dendritic spines, much less is known about the development and plasticity of presynaptic axonal terminals in vivo. Early imaging studies have revealed different degrees of presynaptic terminal dynamics over days to months at neuromuscular junctions and in autonomic ganglia (Gan et al., 2003; Lichtman et al., 1987; Purves et al., 1987). Rapid dynamics of axonal terminals has been observed during the development of the mouse neuromuscular junctions (Gan and Lichtman, 1998; Walsh and Lichtman, 2003), the optical tectum of Xenopus and zebrafish (Hu et al., 2005; Hua et al., 2005; Ruthazer et al., 2003), and the mouse cortex (Portera-Cailliau et al., 2005). In adult sensory cortices, in vivo two-photon imaging studies have shown high turnover rates of presynaptic axonal boutons over weeks in both monkeys and mice. In the primary visual cortex of adult Macaques, ~7% of axonal boutons were found to be eliminated and formed within one week (Stettler et al., 2006). High turnover rates of axonal boutons were also observed in the adult mouse barrel cortex. Depending on cell types, 15–60% of axonal boutons were eliminated and formed within one month (De Paola et al., 2006). While these findings suggest that axonal boutons are highly plastic even in the adult cortex, one study shows that axonal terminals in the somatosensory and auditory cortices of 40 day-old mice are largely stable, with a turnover rate of <10% over 3 weeks (Majewska et al., 2006). The reason for different turnover rates of axonal boutons in different mouse cortices is unclear. Moreover, it remains unclear whether the majority of axonal boutons can persist throughout adulthood, similar to postsynaptic dendritic spines (Grutzendler et al., 2002; Yang et al., 2009; Zuo et al., 2005a).
To better understand the plasticity of presynaptic axonal boutons, we repeatedly imaged axonal boutons over days to months in the developing and mature barrel cortex using transcranial two-photon microscopy and transgenic mice expressing yellow fluorescent proteins (YFP) in a subset of Layer 5 pyramidal neurons (YFP-H line). Our results indicate that turnover rates of axonal boutons are lower than those of dendritic spines in young and adult barrel cortex. After development ends, the majority of axonal boutons are stably maintained in adulthood. Furthermore, enriched sensory experience has no significant effects on the turnover rates of axonal boutons over weeks. These findings underscore the remarkable stability of presynaptic terminals in the mature somatosensory cortex.
MATERIALS AND METHODS
Experimental Animals
Transgenic mice expressing YFP in Layer 5 pyramidal neurons (Thy1-YFP H line) were purchased from the Jackson Laboratories. Wild type ICR mice were purchased from Taconic, New York. All experiments were performed in accordance with the institutional guidelines.
Sensory Enrichment
Sensory enrichment was conducted by placing mice in standard mouse cages containing strings of beads whose positions were changed daily. Mice could move freely in these cages and had to navigate through the strings of beads to obtain food and water. The enriched environment used in this study was the same as described previously (Yang et al., 2009). Such enriched environment has been shown to increase the remodeling of dendritic spines of Layer 5 pyramidal neuron over days in the barrel cortex (Yang et al., 2009).
In Utero Electroporation to Label Layer 2/3 Pyramidal Neurons
Plasmids containing EYFP under the CAG promoter were transfected into Layer 2/3 pyramidal neurons by intraventricular injection of mouse embryos in utero followed by electroporation as previously described (Tsai et al., 2005). In brief, pregnant ICR mice (Taconic, NY) were used, and 1–2 μl of cDNAs (1–5 μg/μl) were injected into the ventricle of embryonic brains at E14-15. A pair of copper alloy oval plates attached to the electroporation generator ECM830 (Harvard Apparatus, MA) transmitted 5 square electric pulses at 50 V for 50 ms at 1-s intervals through the uterine wall. All experiments were done in accordance with protocols approved by the institutional animal committee.
Transcranial Two-Photon Imaging of Axonal Boutons And Dendritic Spines
The procedures of transcranial two-photon imaging were performed in anesthetized mice as previously described (Grutzendler et al., 2002; Yang et al., 2010). Briefly, for repeated imaging of axonal boutons in Layer 1, YFP-H transgenic mice were anesthetized with an intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (15 mg/kg). The mouse head was shaved and the skull surface was exposed with a midline scalp incision. The region (0.2 mm in diameter) to be imaged was identified over the barrel cortex based on stereotaxic coordinates (1.1 mm posterior to the bregma and 3.4 mm lateral from the midline). After removing the periosteum tissue, a custom-made steel plate was glued onto the skull to immobilize the head. A high-speed micro-drill was used to remove the external layer of the compact bone and most spongy bone to create a cranial window for imaging. A microsurgical blade was used to continue the thinning process until the skull was 15–20 μm in thickness. A high quality picture of the brain vasculature was taken with a CCD camera mounted on the dissection microscope and used as a landmark for future relocation. For two-photon imaging, the steel plate on the animal’s head was screwed to two metal bars that were located on both sides of the animal’s head and fixed to a solid metal base and the entire animal was placed under a custom-made two-photon microscope. Image stacks of neuronal processes within a depth of 100 μm from the pial surface were obtained with two-photon laser tuned to 920 nm and a 1.1 NA 60 X water-immersion objective, yielding a full three-dimensional data set of axons in the area of interest. After imaging, the plate was gently detached from the skull and the scalp was sutured with 6-0 silk. The animals were returned to their home cages until the next viewing.
Dendritic spines of Layer 2/3 pyramidal neurons in the barrel cortex were imaged through a thinned skull window with two-photon microscopy using the procedure described above. Image stacks of dendritic branches within a depth of 100 μm from the pial surface were obtained. Different from repeated imaging of axonal boutons, dendritic spines of Layer 2/3 pyramidal neurons were imaged once in either 1 month- or 6 month-old mice.
Quantification of Axonal Bouton Turnover
We identified en passant boutons (EPBs) on axonal branches in Layer 1 of barrel cortex according to criteria described in previous studies (De Paola et al., 2006; Grillo et al., 2013; Majewska et al., 2006). Only regions exhibiting high signal-to-noise ratio in all imaging sessions were considered for data analysis. EPBs appeared as swellings along the axonal shaft and were at least two times brighter than nearby shaft in at least one imaging session. To score bouton elimination, the brightness of boutons had to drop below 1.3 times of the neighbor axon shaft brightness. Previous studies have suggested that up to 10% of axonal varicosities may not form synaptic contact with postsynaptic dendritic spines in hippocampal pyramidal neurons (Becker et al., 2008; Shepherd and Harris, 1998). Using serial section electron microscopy, a recent study has reconstructed nine boutons that were imaged previously in Layer 1 of the barrel cortex (Grillo et al., 2013). All reconstructed boutons made synaptic contacts with postsynaptic structure; the smallest of these boutons had a relative intensity 1.92 times the intensity of the axonal backbone. Because axonal boutons identified in our study were > 2 times brighter than the axonal backbone, the vast majority of these axonal boutons likely formed synapses. It is possible that few small EPBs may be non-synaptic swellings in the axon and may not bear synapses. Nevertheless, the inclusion of such EPBs would likely underestimate the stability of presynaptic axonal boutons.
The formation and elimination rates of axonal boutons are defined as the number of boutons formed and eliminated divided by the total number of axonal boutons at the first image session.
Quantification of Dendritic Spine Density of Layer 2/3 Pyramidal Neurons
Dendritic branches of Layer 2/3 pyramidal neurons in Layer 1 were identified from 3D image stacks with high image quality (signal-to-background-noise ratio > 4-fold). Dendritic filopodia were identified as long thin structures (head diameter/neck diameter <1.2-fold and length/neck diameter >3-fold) with fluorescence intensity comparable to those of thin spine necks. The remaining protrusions were classified as spines. For quantifying spine or filopodial density on individual dendritic branches in the barrel cortex, branches longer than 20 μm were included in order to minimize the variation in spine density. Spine or filopodial density was calculated as the total number of spines or filopodia divided by the total length of dendritic segments measured in each animal.
Statistics
All data except in Fig. 2B were presented as mean ± S.E.M.. A Student t-test was used to determine the difference between groups. P < 0.05 was considered statistically significant.
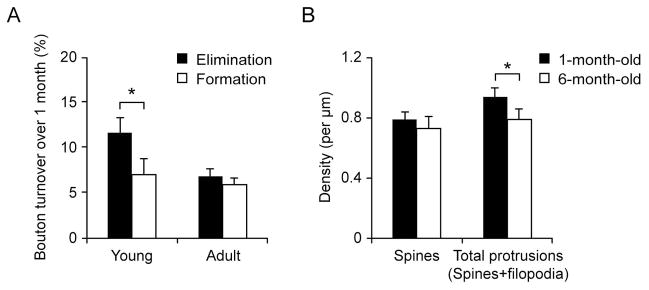
Figure 2. A slight net loss of axonal boutons in young adolescent mice.
(A) Elimination and formation rates of axonal boutons in young and adult mice over one month. Young mice showed a significantly higher rate of bouton elimination than bouton formation, resulting in a net loss (~4.7%) in young mice over a one-month interval. No significance was found between the rate of bouton elimination and formation over a one-month interval in adult mice. Data are presented as (mean ± S.E.M.). (B) Spine density on apical dendrites of Layer 2/3 pyramidal neurons was not significantly different between 1-month-old and 6-month-old mice (P > 0.2). The density of total dendritic protrusions (spines and filopodia) of Layer 2/3 pyramidal neurons was significantly higher in 1-month-old mice than in 6-month-old mice. Data are presented as mean ± S.D. * P < 0.05.
RESULTS
Higher turnover of axonal boutons during development than in adulthood
To determine axonal bouton dynamics in Layer 1 of the barrel cortex, we used Thy1-YFP H line transgenic mice in which YFP is predominantly expressed in a small subset of Layer 5 pyramidal neurons in the cortex, as well as in the hippocampus and amygdala (Feng et al., 2000; Porrero et al., 2010). Previous studies have shown that axonal inputs to Layer 1 of the barrel cortex come from various sources including the thalamus, motor cortex, as well as local projection neurons within the barrel cortex (De Paola et al., 2006; Lu and Lin, 1993; Lubke et al., 1996; Petersen et al., 2003; Petreanu et al., 2009; Shepherd et al., 2005; Veinante and Deschenes, 2003). Because YFP is not expressed in the thalamocortical neurons in YFP H line mice (Porrero et al., 2010), many of axonal terminals imaged in this study were likely from YFP-expressing Layer 5 pyramidal neurons in the motor and barrel cortices.
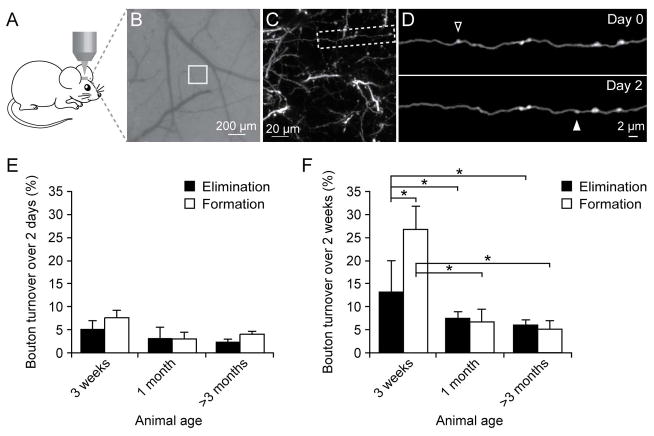
Using transcranial two-photon microscopy, we imaged the same axonal branches over time in Layer 1 of the barrel cortex at various ages (Fig. 1A–D). We focused our studies on en passant boutons (EPBs) because they were the most abundant presynaptic structures in the barrel cortex of YFP H line mice. EPBs were identified according to the criteria described in previous studies (De Paola et al., 2006; Grillo et al., 2013; Majewska et al., 2006). They appeared as swellings along the axonal shaft and were at least two times brighter than the shaft in at least one imaging session (Fig. 1D). We found that over 2 days (Fig. 1E), the elimination and formation rates of axonal boutons were 5.3 ± 1.7% and 7.5 ± 2.1% in 3-week-old mice (5 mice, n = 238 boutons), 3.3 ± 2.4% and 3.1 ± 1.4% in 1 month-old mice (4 mice, n = 273), 2.9 ± 0.6% and 3.9 ± 0.6% in adult mice (7 mice, n = 580). No significant difference in the formation or elimination rate of axonal boutons was found over 2 days among mice of various ages (P > 0.1).
Figure 1. Axonal bouton turnover in young and adult barrel cortex.
(A) Transcranial two-photon imaging of the mouse barrel cortex. (B) CCD camera view of the vasculature of the barrel cortex below the thinned skull. The white box indicates the region where the two-photon image in (C) was obtained. (C) A low magnification image of dendrites and axons in Layer 1. A higher-magnification view of the axon segment in (C) is shown in (D). (D) Repeated imaging of an axonal branch over 2 days. The open and filled arrowheads indicate eliminated and newly formed axonal boutons over two days. (E) Elimination and formation rates of axonal boutons in three-week-old, one-month-old (young) and >3-month-old (adult) mice over a two-day interval. No significant differences were found in the elimination or formation rate among mice of various ages. (F) Elimination and formation rates of axonal boutons over two weeks in mice of various ages. Both the formation and elimination rates in 3-week-old mice were significantly higher than those in 1-month-old or adult mice. The formation rate of axonal boutons was significantly higher than the elimination rate at the age of 3 weeks. No significant difference in bouton formation or elimination rates was found over 2 weeks between 1-month-old and > 3-month-old mice. Data are presented as mean ± S.E.M. * P < 0.05.
Over a period of 2 weeks (Fig. 1F), the elimination and formation rates of axonal boutons were 13.3 ± 6.7% and 26.8 ± 5.0% in 3-week-old mice (3 mice, n = 121), 7.5 ± 1.5% and 6.8 ± 2.7% in 1-month-old mice (7 mice, n = 448), 6.2 ± 1.0% and 5.3 ± 1.6% in adult mice (4 mice, n = 439). Both the elimination and formation rates of axonal boutons over 2 weeks were significantly higher in 3-week-old mice than those in 1-month-old and adult mice (unpaired t test, P < 0.05), indicating a progressive stabilization of axonal boutons as development proceeds.
Regardless of the ages of animals, we found that > 90% of axonal boutons persisted over 2 weeks in mice older than 1 month of age. The overall stability of axonal boutons was slightly higher than that of postsynaptic dendritic spines of Layer 5 pyramidal neurons in mice of the same age (Zuo et al., 2005a; Zuo et al., 2005b).
A slight net loss of axonal boutons in young adolescent mice
Previous studies have shown that there is a ~20% net loss of spines on apical tuft dendrites of Layer 5 pyramidal neurons in the barrel and visual cortices in mice from 1 month to 2 months of age (Grutzendler et al., 2002; Zuo et al., 2005a). To examine whether a similar net loss of axonal boutons might occur in the barrel cortex, we imaged axonal boutons over a 1-month interval in 1-month-old mice (Fig. 2A). We found that the elimination rate of boutons was slightly but significantly higher than the formation rate over one month (11.7 ± 1.6% versus 7.9 ± 1.7%, 5 mice, n = 320; P < 0.05, paired t test). As a result, there is a ~4.8% reduction in the number of axonal boutons in mice from 1 month to 2 months of age. In adult mice, no significant difference in the rates of botuon elimination and formation were observed over a one-month interval (Fig. 2A; 6.9 ± 0.8% versus 5.9 ± 0.8%, 6 mice, n = 491; P > 0.2).
The above results indicate that the net loss of axonal boutons is much smaller than that of dendritic spines on apical tufts of Layer 5 pyramidal neurons in mice from 1 month of age to adulthood. Because axonal boutons in Layer 1 form synapses with not only Layer 5 pyramidal neurons but also other cell types including inhibitory neurons and Layer 2/3 pyramidal neurons, we examined whether dendritic spines of Layer 2/3 neurons in Layer 1 of the barrel cortex undergo a net loss similar to axonal boutons from 1 month of age to adulthood. In this experiment, we transfected YFP into Layer 2/3 pyramidal neurons in the barrel cortex with an in utero electroporation technique and subsequently quantified the density of dendritic spines on apical tuft dendrites of Layer 2/3 pyramidal neurons in mice at 1 month and 6 months of age (Fig. 2B).
We found that dendritic spine density was slightly but not significantly higher at 1 month of age than at 6 months of age (0.79 ± 0.05/μm (n = 7) versus 0.73 ± 0.08/μm (n = 4); P > 0.2). In addition to dendritic spines, there was a higher percentage of dendritic filopodia, long and thin protrusions without bulbous spine heads, in 1-month-old mice (11.7 ± 5.7% of dendritic protrusions; n = 7) than in 6-month-old mice (6.3 ± 2.2%; n = 4). The density of total dendritic protrusions (spines and filopodia) was 0.94 ± 0.06/μm (n = 7) at 1 month of age, significantly higher than that at 6 month of age (0.79 ± 0.07/μm, n = 4) (P < 0.05). Similar to axonal boutons, the reduction in the density of dendritic spines or total dendritic protrusions on apical tufts of Layer 2/3 pyramidal neurons is less than that of Layer 5 pyramidal neurons in mice from 1 month of age to adulthood. This finding suggests that a small reduction in the number of axonal boutons in young adolescent barrel cortex could be in part due to the fact that axonal boutons form synaptic contact with spines on Layer 2/3 pyramidal cells.
Boutons existed for more than 1 week show higher stability than those formed recently
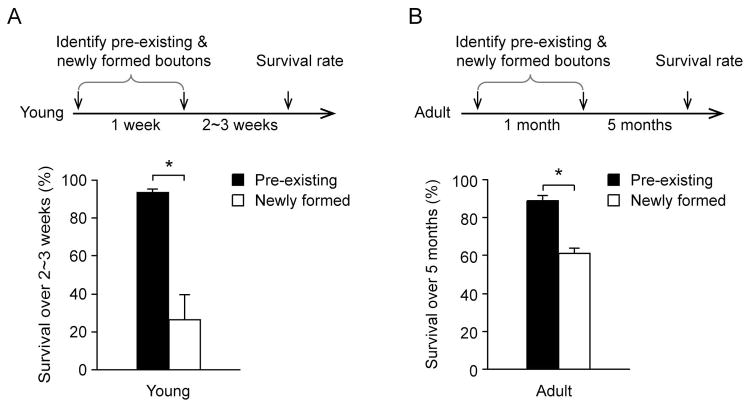
Previous studies have shown that newly-formed spines are mostly eliminated while spines existed for weeks are largely maintained over time (Yang et al., 2009). We found that the survival rates of newly-formed and pre-existing axonal boutons were also substantially different. In 1-month-old mice (Fig. 3A), only 24.3 ± 12.7% of axonal boutons formed during the previous one week persisted over the next 2 to 3 weeks (3 mice, n = 18), whereas 93.4 ± 2.0% of boutons existed for at least one week persisted over the same 2–3 week period (3 mice, n = 169). In adult mice (Fig. 3B), 61.3% ± 2.8% of boutons formed over the past one month persisted over the next 5 months (3 mice, n = 15), whereas 88.7% ± 2.8% of axonal boutons existed for at least one month were maintained over the same 5 month period (3 mice, n = 229). Thus, similar to dendritic spines, recently-formed axonal boutons are largely eliminated over subsequent days to weeks. Axonal boutons existed for weeks in the barrel cortex are largely maintained over time.
Figure 3. Boutons existed for weeks showed higher stability than those newly formed.
(A) Axonal boutons formed within the previous week and pre-existing boutons were identified. The survival rates of pre-existing and newly formed spines were measured over the next 2–3 weeks. Boutons pre-existed for more than one-week showed a significantly higher survival rate than those formed during the previous week (P < 0.05). (B) Boutons existed for at least one month showed a significantly higher survival rate during the next five months than those formed over the previous month. Data are presented as mean ± S.E.M. * P < 0.05.
Long-term stability of axonal boutons in adulthood
The above findings show that >85% of axonal boutons in the adult barrel cortex persist over more than 5 months. To better understand the persistence of axonal boutons, we examined the formation and elimination of axonal boutons in the barrel cortex over various time intervals, up to 12 months (Fig. 4). We found that the survival rates of axonal boutons were 89.2 ± 1.2% (4 mice, n = 201), 85.8 ± 1.5% (3 mice, n = 292), 79.2 ± 2.1% (2 mice, n = 265) over 2 months, 5.5 months, and 12 months respectively. The survival of boutons over time could be fitted with a single-exponential equation (Fig. 4; 0.9583e−0.015x, R2 = 0.9235). Assuming a stochastic turnover of axonal boutons, we estimated that axonal boutons existed for two months would be eliminated at an average rate of ~1% per month. Consistent with previous findings of long-term stability of adult dendritic spines, we estimated that ~60% of axonal boutons in the mature barrel cortex could last over 3 years.
Figure 4. Long-term stability of axonal boutons in adult barrel cortex.
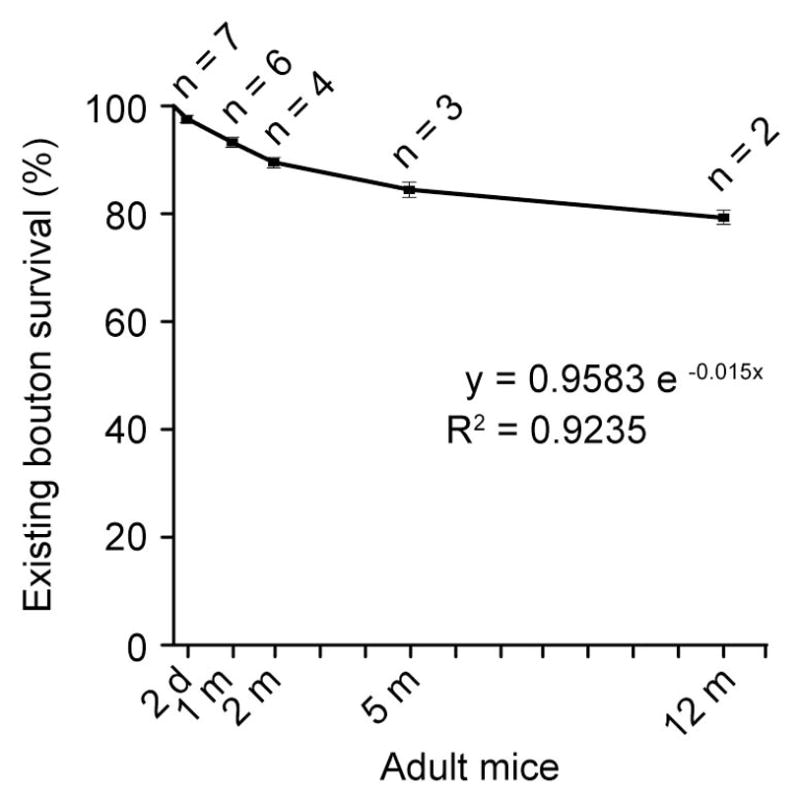
The survival rate of axonal boutons over various time intervals in adult barrel cortex. The number of animals examined over each time interval is indicated in the figure. The total number of axonal boutons quantified are 580, 491, 201, 292, 265 over 2 days, 1 month, 2 months, 5.5 months, and 12 months respectively. The line represents a single-exponential regression fitting (y=0.9583e−0.015x, R2 =0.9235). Data are presented as mean ± S.E.M.
Sensory enrichment over 2–4 weeks has no significant effects on axonal bouton turnover
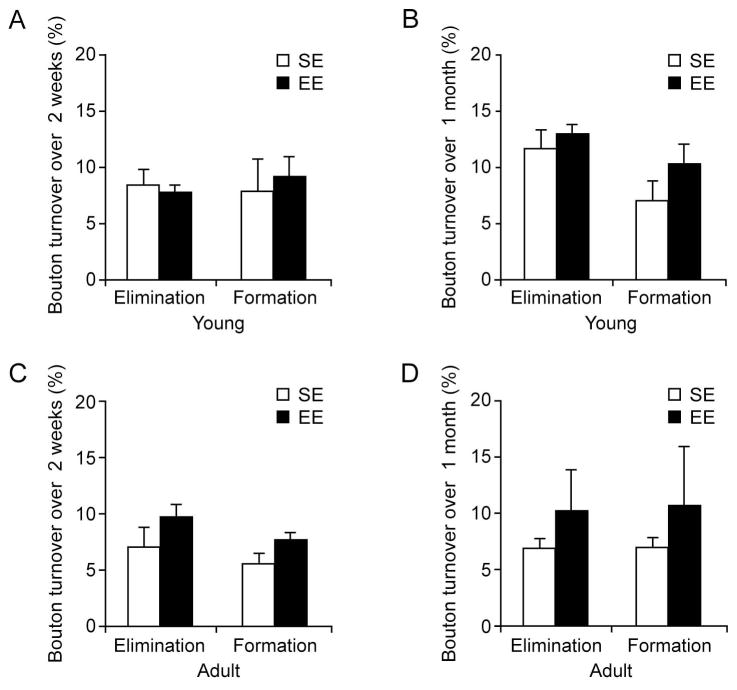
Many lines of evidence indicate that sensory experience plays an important role in regulating synaptic strength and number (Buonomano and Merzenich, 1998; Gogolla et al., 2007; Knott et al., 2002; Majewska et al., 2006; Yang et al., 2009; Zuo et al., 2005b). It has been shown previously that whisker stimulation over 24 hours or sensory enrichment over days causes a transient increase in the formation of new dendritic spines in the barrel cortex (Knott et al., 2002; Yang et al., 2009). To investigate the effects of enriched sensory experience on the remodeling of axonal boutons, we compared the rates of axonal bouton formation and elimination in the barrel cortex in mice under enriched environment (EE) and standard housing environment (SE, control group) (Fig. 5). Sensory enrichment was provided by placing mice in standard housing cages containing strings of beads whose positions were changed daily. The same sensory environment has been shown to increase the remodeling of dendritic spines of Layer 5 pyramidal neuron over days in the barrel cortex (Yang et al., 2009). Notably, we found no significant differences in the turnover rates of axonal boutons in 1-month-old mice housed under SE and EE over either 2 weeks or 1 month (Fig. 5A, B, P > 0.05). Similarly, in adult mice, the elimination and formation of axonal boutons were slightly but not significantly higher under EE than those under SE over 2 weeks or 1 month (Fig. 5C, D; P > 0.05). The observations indicate that sensory enrichment over weeks has no significant effects on the turnover rates of axonal boutons.
Figure 5. Sensory enriched environment has no significant effects on bouton turnover rates over weeks in young and adult barrel cortex.
(A–B) In young mice, there was no significant difference in the elimination or formation rate of axonal boutons between ‘EE’ and ‘SE’ groups over either two weeks (A) or one month (B). (C–D) In adult mice, there was a slight, but not significant, difference in the elimination or formation rate of axonal boutons between ‘EE’ and ‘SE’ groups over two weeks (C) or one month (D). Data are presented as mean ± S.E.M. P > 0.05.
DISCUSSION
In this study, we examined the remodeling of presynaptic axonal boutons in Layer 1 of the mouse barrel cortex using transcranial two-photon microscopy. Similar to dendritic spines on apical tuft dendrites of Layer 5 pyramidal neurons, axonal boutons located in Layer 1 are more plastic during development than in adulthood. In adult mice (> 3 months), the majority of axonal boutons persist over many months. Furthermore, enriched sensory environment cause a slight but not significant increase in the turnover rates of axonal boutons over 2–4 weeks. Together with previous findings of dendritic spine stability (Grutzendler et al., 2002; Yang et al., 2009; Zuo et al., 2005a), our findings underscore the overall stability of synaptic connections in the mature barrel cortex.
Our studies show that presynaptic axonal boutons are more persistent than dendritic spines on apical dendrites of Layer 5 pyramidal neurons in Layer 1 of the developing and mature barrel cortex (Yang et al., 2009; Zuo et al., 2005a; Zuo et al., 2005b). In the adult barrel cortex, it has been shown that axonal boutons with a relative intensity ~2 times the intensity of the axonal backbones make synaptic contacts with postsynaptic structures (Grillo et al., 2013). Because axonal boutons included in our analysis were > 2 times brighter than the axonal backbone, it is likely that the vast majority of these boutons bear synapses. Several lines of evidence indicate that a fraction of axonal boutons make synaptic contacts with multiple spines (Knott et al., 2006; Shepherd and Harris, 1998; Toni et al., 1999; Toni et al., 2007). It is possible that one spine in contact with such a multi-spine bouton could be formed or eliminated whereas the bouton would persist. This would explain a lower turnover rate of axonal boutons as compared with that of dendritic spines. Furthermore, because axonal boutons likely make synaptic contacts with both excitatory and inhibitory neurons, it is also possible that axonal boutons in contact with inhibitory neurons were more stable than those with dendritic spines on pyramidal neurons. Further studies with trans-synaptic labeling approaches would be required to resolve this issue.
Previous studies from fixed tissue show that there is a substantial reduction in synaptic density in the mammalian cortex from infancy until puberty (Huttenlocher, 1990; Huttenlocher and Dabholkar, 1997; Lubke and Albus, 1989; Markus and Petit, 1987; Rakic et al., 1986). Our results show that there is a slight net loss of axonal boutons or spines of Layer 2/3 pyramidal neurons in the mouse barrel cortex from 1 month of age to adulthood. Nevertheless, the reduction in the number of axonal boutons or spines of Layer 2/3 pyramidal cells is substantially less than that of dendritic spines on apical tuft of Layer 5 pyramidal neurons (Grutzendler et al., 2002; Majewska et al., 2006; Yang et al., 2009; Zuo et al., 2005a). Therefore, dendritic spine loss on apical tuft of Layer 5 pyramidal neurons appears to be a major contributor to the total loss of synaptic connectivity in the superficial layer of the developing barrel cortex. In the optic tectum of Xenopus tadpoles, the number of axonal boutons in contact with multiple dendrites decrease as animals mature (Li et al., 2011). It would be interesting to determine whether there is a transition from multiple-spine boutons to single-spine boutons in the barrel cortex as development proceeds. Such a transition would be consistent with a substantial loss of dendritic spines of Layer 5 pyramidal neurons, but not axonal boutons, in young adolescent barrel cortex.
The turnover rates of axonal boutons (~5% over one month) in the adult barrel cortex are comparable to those reported in somatosensory, and auditory cortices (Majewska et al., 2006), but are lower than those in the barrel cortex reported in a previous study (De Paola et al., 2006). In our study, we focused on the dynamics of fluorescently-labeled en passant boutons as they are the most abundant ones in the barrel cortex of Thy1-H line mice. These en passant boutons likely come from YFP-expressing Layer 5 pyramidal projection neurons within the barrel cortex as well as other cortical regions because YFP expression is absent in the thalamocortical neurons in YFP H line mice. In the previous study, different transgenic mouse lines were used to identify two types of axons with en-passant boutons in the barrel cortex, one coming from the thalamus and another from local projection neurons in the barrel cortex (De Paola et al., 2006). ~25% of en-passant boutons, likely from projection neurons within the barrel cortex and other cortical regions, were found to undergo turnover within one month. Furthermore, ~60% of terminaux boutons, likely from Layer 6 pyramidal neurons, undergo turnover over one month. In addition to the use of different transgenic lines, it is important to note the differences in imaging approaches between these two studies. In our study, axonal boutons were imaged through a thinned-intact skull (~15 μm in thickness). In the previous study, axonal boutons were imaged through a cranial window that involves removing a piece of the skull and implanting a coverslip, as well as adding dexamethasone to minimize swelling at the surgical sites. It has been shown that dendritic spine dynamics is high under open-skull window than under a thinned skull window, presumably due to various factors related to open-skull surgery and window implantation (Holtmaat et al., 2005; Trachtenberg et al., 2002; Xu et al., 2007; Yang et al., 2010). Therefore, the difference in the measurement of axonal bouton dynamics could be at least in part due to the use of different cranial windows for in vivo imaging.
Previous studies have shown sensory enrichment over days leads to a significant increase in new spine formation on apical dendrites of Layer 5 pyramidal neurons in the barrel cortex (Yang et al., 2009). Furthermore, a small fraction of newly-formed spines persist over extended periods of time. We found that the same sensory enrichment causes a slight, but not significant, increase in the formation and elimination of axonal boutons over 2–4 weeks. Several lines of evidence indicate that some newly-formed spines are in contact with multi-spine boutons (Knott et al., 2006; Toni et al., 1999; Toni et al., 2007). It is thus possible that some of dendritic spines formed in response to enriched sensory experiences may form synaptic contact with multiple-spine boutons. This may explain in part why the de novo formation of axonal boutons in the barrel cortex occurs to a lesser degree than that of new dendritic spines in response to enriched sensory experiences. Furthermore, it is important to note that while the majority of axonal boutons are stably maintained over time under different sensory environment, the morphology of these boutons could undergo activity-dependent changes during development and adulthood (Becker et al., 2008; Galimberti et al., 2006). Because the size of synapses correlates with synaptic strength (Harris and Stevens, 1989; Knott et al., 2006; Matsuzaki et al., 2001), changes in axonal bouton morphology indicate that synaptic strength may be modified without axonal bouton turnover. Future studies are needed to investigate this issue to better understand how changes in the morphology of pre-synaptic terminals contribute to adult plasticity (Becker et al., 2008; Buonomano and Merzenich, 1998; Grossman et al., 2002).
Acknowledgments
This study was supported by the funding from Peking University Shenzhen Graduate School and National Institutes of Health P01 NS074972 to W.-B.G., by grants from Whitehall Foundation and National Institutes of Health R01 GM107469 and R21 AG048410 to G.Y., and by the National Natural Science Foundation of China (No. 81100839), Shenzhen Science and Technology Innovation Funds (GJHS20120628101219327) to W.L.
References
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Becker N, Wierenga CJ, Fonseca R, Bonhoeffer T, Nagerl UV. LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron. 2008;60:590–597. doi: 10.1016/j.neuron.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Cline H, Haas K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: a review of the synaptotrophic hypothesis. J Physiol. 2008;586:1509–1517. doi: 10.1113/jphysiol.2007.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res. 2008;169:199–207. doi: 10.1016/S0079-6123(07)00011-8. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Fox K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience. 2002;111:799–814. doi: 10.1016/s0306-4522(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Galimberti I, Gogolla N, Alberi S, Santos AF, Muller D, Caroni P. Long-term rearrangements of hippocampal mossy fiber terminal connectivity in the adult regulated by experience. Neuron. 2006;50:749–763. doi: 10.1016/j.neuron.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Gan WB, Kwon E, Feng G, Sanes JR, Lichtman JW. Synaptic dynamism measured over minutes to months: age-dependent decline in an autonomic ganglion. Nat Neurosci. 2003;6:956–960. doi: 10.1038/nn1115. [DOI] [PubMed] [Google Scholar]
- Gan WB, Lichtman JW. Synaptic segregation at the developing neuromuscular junction. Science. 1998;282:1508–1511. doi: 10.1126/science.282.5393.1508. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, Caroni P. Structural plasticity of axon terminals in the adult. Curr Opin Neurobiol. 2007;17:516–524. doi: 10.1016/j.conb.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Grillo FW, Song S, Teles-Grilo Ruivo LM, Huang L, Gao G, Knott GW, Maco B, Ferretti V, Thompson D, Little GE, De Paola V. Increased axonal bouton dynamics in the aging mouse cortex. Proc Natl Acad Sci U S A. 2013;110:E1514–1523. doi: 10.1073/pnas.1218731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AW, Churchill JD, Bates KE, Kleim JA, Greenough WT. A brain adaptation view of plasticity: is synaptic plasticity an overly limited concept? Prog Brain Res. 2002;138:91–108. doi: 10.1016/S0079-6123(02)38073-7. [DOI] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Hu B, Nikolakopoulou AM, Cohen-Cory S. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development. 2005;132:4285–4298. doi: 10.1242/dev.02017. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–1026. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Swain RA, Armstrong KA, Napper RM, Jones TA, Greenough WT. Selective synaptic plasticity within the cerebellar cortex following complex motor skill learning. Neurobiol Learn Mem. 1998;69:274–289. doi: 10.1006/nlme.1998.3827. [DOI] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–273. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- Li J, Erisir A, Cline H. In vivo time-lapse imaging and serial section electron microscopy reveal developmental synaptic rearrangements. Neuron. 2011;69:273–286. doi: 10.1016/j.neuron.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman JW, Magrassi L, Purves D. Visualization of neuromuscular junctions over periods of several months in living mice. J Neurosci. 1987;7:1215–1222. doi: 10.1523/JNEUROSCI.07-04-01215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SM, Lin RC. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosensory & motor research. 1993;10:1–16. doi: 10.3109/08990229309028819. [DOI] [PubMed] [Google Scholar]
- Lubke J, Albus K. The postnatal development of layer VI pyramidal neurons in the cat's striate cortex, as visualized by intracellular Lucifer yellow injections in aldehyde-fixed tissue. Brain Res Dev Brain Res. 1989;45:29–38. doi: 10.1016/0165-3806(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Lubke J, Markram H, Frotscher M, Sakmann B. Frequency and dendritic distribution of autapses established by layer 5 pyramidal neurons in the developing rat neocortex: comparison with synaptic innervation of adjacent neurons of the same class. J Neurosci. 1996;16:3209–3218. doi: 10.1523/JNEUROSCI.16-10-03209.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska AK, Newton JR, Sur M. Remodeling of synaptic structure in sensory cortical areas in vivo. J Neurosci. 2006;26:3021–3029. doi: 10.1523/JNEUROSCI.4454-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Petit TL. Neocortical synaptogenesis, aging, and behavior: lifespan development in the motor-sensory system of the rat. Exp Neurol. 1987;96:262–278. doi: 10.1016/0014-4886(87)90045-8. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC, Grinvald A, Sakmann B. Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J Neurosci. 2003;23:1298–1309. doi: 10.1523/JNEUROSCI.23-04-01298.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrero C, Rubio-Garrido P, Avendano C, Clasca F. Mapping of fluorescent protein-expressing neurons and axon pathways in adult and developing Thy1-eYFP-H transgenic mice. Brain Res. 2010;1345:59–72. doi: 10.1016/j.brainres.2010.05.061. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C, Weimer RM, De Paola V, Caroni P, Svoboda K. Diverse modes of axon elaboration in the developing neocortex. PLoS Biol. 2005;3:e272. doi: 10.1371/journal.pbio.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Voyvodic JT, Magrassi L, Yawo H. Nerve terminal remodeling visualized in living mice by repeated examination of the same neuron. Science. 1987;238:1122–1126. doi: 10.1126/science.3685967. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Akerman CJ, Cline HT. Control of axon branch dynamics by correlated activity in vivo. Science. 2003;301:66–70. doi: 10.1126/science.1082545. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3-->CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Stepanyants A, Bureau I, Chklovskii D, Svoboda K. Geometric and functional organization of cortical circuits. Nat Neurosci. 2005;8:782–790. doi: 10.1038/nn1447. [DOI] [PubMed] [Google Scholar]
- Stettler DD, Yamahachi H, Li W, Denk W, Gilbert CD. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron. 2006;49:877–887. doi: 10.1016/j.neuron.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J Cell Biol. 2005;170:935–945. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Deschenes M. Single-cell study of motor cortex projections to the barrel field in rats. J Comp Neurol. 2003;464:98–103. doi: 10.1002/cne.10769. [DOI] [PubMed] [Google Scholar]
- Walsh MK, Lichtman JW. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 2003;37:67–73. doi: 10.1016/s0896-6273(02)01142-x. [DOI] [PubMed] [Google Scholar]
- Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci. 2007;10:549–551. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- Yang G, Lai CS, Cichon J, Ma L, Li W, Gan WB. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344:1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc. 2010;5:201–208. doi: 10.1038/nprot.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005a;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005b;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]