Abstract
Objective
Functional imaging studies have shown that control of planned movement involves a distributed network that involves the premotor (PMv) and posterior parietal cortices (PPC). Similarly, anatomical studies show that these regions are densely interconnected via white matter tracts. We therefore hypothesized that the PPC influence over the motor cortex is partly via a connection with the PMv.
Methods
Using a novel three-pulse ipsilateral transcranial magnetic stimulation technique, we preconditioned the PPC (80%RMT) at ISIs from 4–15ms prior to stimulating the PMv and M1 at ISIs of 4 and 6ms.
Results
As previously shown, PMv-M1 paired-pulse stimulation resulted in inhibition of the MEP (90% RMT, 4–6ms) and PPC-M1 paired-pulse stimulation resulted in facilitation of the MEP (90% RMT, 4–8ms). PPC-M1 paired-pulse stimulation at 80%RMT preconditioning had no effect on M1. PPC-PMv-M1 stimulation resulted in reversal of inhibition observed with PMv-M1 stimulation at ISIs ranging from 6–15ms.
Conclusions
The reversal of inhibition observed with PPC-PMv-M1 stimulation suggests that the parietal connection to the PMv plays a role in the modulation of M1.
Significance
This is the first study to stimulate three intrahemispheric regions in order to test a disynaptic connection with M1. The described network may be important in a variety of movement disorders.
Keywords: Parietal cortex, connectivity, motor cortex, transcranial magnetic stimulation
Introduction
Control of planned movement involves a distributed network that involves the frontal and parietal cortices (Cohen and Andersen, 2002, Croxson et al., 2005). Looking at the cortical connections in the non-human primate, the majority of the cortical connections from the posterior parietal cortex (PPC) project primarily to the premotor cortex (Matelli et al., 1998). The parietal and premotor cortices are densely interconnected via the superior longitudinal fasciculus (Makris et al., 2005). Other studies have found an association between diffusion tensor imaging and motor functional connectivity observed with transcranial magnetic stimulation (TMS) (Boorman et al., 2007, Wahl et al., 2007). Specifically, individual differences in inhibition measured using TMS have been positively correlated with fractional anisotropy in the premotor and parietal cortices (Buch et al., 2010). Thus it is reasonable to hypothesize that the parietal influence on the primary motor cortex (M1) is principally via a connection with the premotor cortex.
Paired-pulse TMS paradigms are useful because they can test the role of an interconnected region on the motor cortex. As numerous paired-pulse paradigms have shown, the premotor cortex has an inhibitory role over the motor cortex (Civardi et al., 2001, Davare et al., 2006, Davare et al., 2008, Houdayer et al., 2012). Similarly, the PPC has a facilitatory or inhibitory role depending on exact location [anterior-inhibition, central/posterior-facilitation](Koch et al., 2007, Koch et al., 2010, Karabanov et al., 2013). In a study designed to test the PPC influence on grasping movements, repetitive theta-burst stimulation of the ventral premotor cortex (PMv) disrupted the parietal facilitation of motor evoked potential (MEP) amplitudes, suggesting that the PPC likely influences M1 via a connection with the premotor cortex (Koch et al., 2010). These results highlight the importance of the fronto-parieto-motor network and further investigating its connections.
Paired-pulse paradigms have allowed study of parietal-motor and premotor-motor connections; however, they do not allow the study of the important parietal-premotor connection because such paradigms require stimulation of the motor cortex for readout. We designed a three pulse paradigm composed of sequential single pulses over PPC and PMv followed by a third pulse over M1. We refer to these pulses as the preconditioning stimulus (over PPC), the conditioning stimulus (over PMv) and the test stimulus (over M1). The use of three pulses allows for probing the roles and latencies of the interaction of the proposed network between PPC-PMv-M1. We hypothesized that subthreshold PPC preconditioning of the PMv will reverse the inhibition observed with PMv stimulation alone. Similarly, we expect that the latency of this connection will give us insight into the nature of these connections. To the best of our knowledge, this is the first study to stimulate three intrahemispheric regions in order to evaluate a disynaptic relationship.
Methods
Subjects
Thirteen right-handed healthy volunteers took part in the main experiment. Two subjects had to be excluded since space limitation on the scalp did not allow proper coil placement with the three-coil configuration, therefore only 11 participants (mean age 29.5 years, 3 female) were included in the main analysis. A smaller group (n=8, mean age 38.6, 4 female) participated in an additional experiment. During the experiment, subjects were instructed to remain at rest with their eyes open. The study was approved by the Institutional Review Board of the National Institutes of Health. All participants gave their informed oral and written consent before the experiments in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and National Institutes of Health guidelines.
Electromyography
Electromyogram (EMG) activity of the right first dorsal interosseus muscle (FDI) was recorded throughout the experiment in a belly-tendon montage using Ag-AgCl surface electrodes. Impedances were kept below 30 kOhms. EMG signals were collected using a Viking IV EMG machine (Nicolet Biomedical, Madison, Wisconsin), bandpass-filtered at 20–2000 Hz. The amplified analogue outputs from the Viking were digitized at 5 kHz using Signal software (Cambridge Electronic Devices, Cambridge, UK) and stored for offline analysis. Measurements were made on each individual trial and the mean peak-to-peak amplitude of the conditioned MEP was expressed as a percentage of the mean amplitude size of the unconditioned test stimulus (TS).
Transcranial Magnetic Stimulation
We used a three-pulse stimulation technique, stimulating sequentially the left central intraparietal lobule (IPL), left PMv, and left M1. Magnetic stimulation was delivered using three custom-made figure-of-eight coils with handles perpendicular to the coil windings (‘branding iron style’, 40 mm external diameter) connected to three high power Magstim 2002 stimulators (Magstim Company Ltd, Whitland, Dyfed, UK). Stimulation of M1 was applied at the point that evoked the largest MEP in the contralateral FDI (“motor hotspot”); this position was marked on a tight fitting cap to ensure proper coil placement throughout the experiment. The M1 coil was held tangential to the scalp at a 45° angle to the mid-sagittal plane such that it induced a posterior-anterior directed current in the brain. The IPL coil was held tangentially to the scalp at a 10° angle to induce a posterior-anterior directed current in the underlying tissue (Koch et al., 2007). The PMv coil was positioned to induce a current directed antero-posteriorly (Houdayer et al., 2012).
Neuronavigation (Brainsight, Rogue Research, Inc., Rogue Resolutions Ltd, Cardiff, UK) was used for precise positioning of the coil over the PMv and IPL. Anatomical magnetic resonance imaging data specific to each participant were used to ensure correct placement of the coil, which was placed over the caudal portion of the pars opercularis of the inferior frontal gyrus (Davare et al., 2006) and in the central portion of the IPL (Karabanov et al., 2013). Each individual magnetic resonance image was normalized, a posteriori, onto the Montreal Neurological Institute brain template using the same software. PMv stimulation coordinates were then expressed with respect to the Montreal Neurological Institute standard space. The mean normalized Montreal Neurological Institute coordinates of the PMv stimulation sites were (x, y, z; mean ± SEM in mm): −47.1 ± 1.3, 16.6 ± 2.5, 3.3 ± 1.2, similarly the co-ordinates for the IPL stimulation sites were 8.6 ± 1.4, −66.3 ± 2.2, 38.8 ± 4.0. These two mean coordinates belong to inferior frontal gyrus and the IPL according to the Talairach atlas and fit within the coordinates previously published (Mayka et al., 2006). The positions of the two coils were marked on a tight-fitting cap to ensure proper coil placement throughout the experiment.
Resting motor threshold (RMT) of the FDI was measured for each subject by a recruitment curve method. For this method, TMS pulses evenly distributed in 5% increments between 5% and 100% stimulator output were randomly administered. Curve fitting of the Boltzmann equation was done using the Levenberg-Marquardt algorithm (Seber and Wild, 2003) in Matlab (nlinfit function, The MathWorks, Inc.). This method has been described previously (Kukke et al., 2014). The test stimulus (TS) was S50, or the stimulation intensity that produces an MEP equal to 50% of MEPmax. The MEPmax represents a value in which all the motor neurons from magnetic stimulation are excited. Therefore, S50 is an appropriate value to stimulate because it represents each subject’s optimal intensity where the corticospinal system may be modulated (i.e., inhibited or facilitated). RMT was defined as the stimulus intensity where the line tangential to the recruitment curve at the S50 point intersects the baseline (baseline-intercept method; Carroll et al., 2001; Devanne et al., 1997). The conditioning stimulus (CS) at the PMv, the stimulation intensity was set to 90% RMT. The preconditioning stimulus (PCS) at the IPL, the stimulation intensity was set to either 80 or 90% RMT depending on the stimulation parameters (see below).
Experiment 1: PPC-M1 and PMv-M1 interactions in the left hemisphere
We wanted to reproduce that the conditioning stimuli alone applied over the IPL (90% RMT) and PMv (90% RMT) produced excitation and inhibition, respectively. Interstimulus intervals (ISIs) between PCS and TS were 4, 6, and 8 ms for the IPL and between CS and TS were 4 and 6 ms for the PMv based on previously published results showing facilitation and inhibition, respectively. There were 16 trials for each condition (TS, PCS+TS, CS+TS) for a total of 96 pulses (summarized in Figure 1A). For this and the following experiment, the order of presentation of the pulses varied pseudorandomly across subjects. There was 4 seconds between each trial. Following this portion of the experiment, some subjects did not show facilitation for the parietal site (a result which has been previously shown in our lab) and the coil was moved slightly and the position was re-tested. Once the proper site for facilitation was obtained, this site was resampled using Brainsight.
Figure 1.
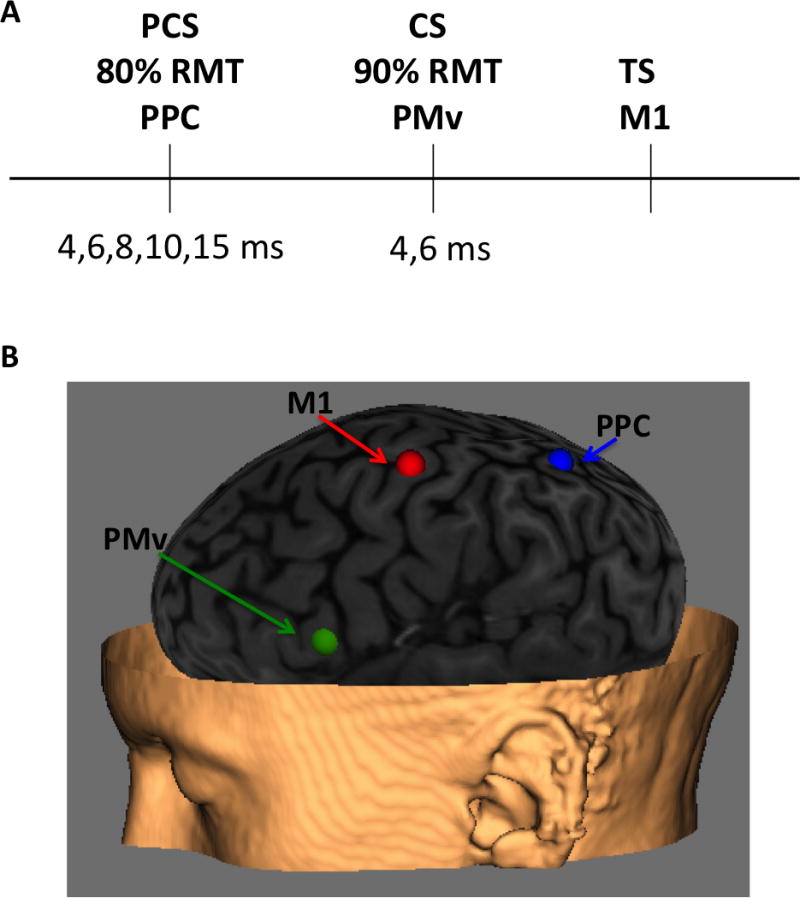
Experimental set-up. (A) Summarized three pulse design. (B) Localization of PMv and PPC using three-dimensional structural MRI. PMv was defined using a neuronavigation system (Brainsight, Rogue Research, Inc., Rogue Resolutions Ltd, Cardiff, UK) and placed over the caudal portion of the pars opercularis of the inferior frontal gyrus. Similarly, PPC was defined as the central portion of the inferior parietal lobule.
Experiment 2: PPC-PMv-M1 interaction in the left hemisphere
For the next experiment, we wanted to test the interaction between the three regions. We therefore lowered the stimulation intensity of the parietal site to 80% RMT in order to control for effects of summation of stimulating both regions at 90% RMT. This was done since the parietal site has previously been shown to cause facilitation at 90% RMT (but not 80% RMT) and we did not want the effects on inhibition at the PMv to be solely due to facilitation of the MEP. For these sets of experiments, the PCS over PPC was applied at ISIs of 4, 6, 8, 10 and 15 ms prior to CS over PMv. The CS was applied over the PMv at 4 and 6 ms prior to the TS over M1. There were 16 trials for each condition (TS, PCS+CS+TS) for a total of 176 pulses.
Experiment 3: PPC-M1 interaction at increased ISIs
A separate set of subjects (n=8) underwent further testing of the PPC site to include longer ISIs (PCS+TS, 12 and 14 ms, 80% RMT) to ensure there was no facilitation at these longer time periods using the same parameters described above. These data were compared with pairwise comparisons to the data in Experiment 2.
Statistical Analysis
Statistical analyses of normalized MEP amplitudes obtained in each experiment were performed using a repeated-measures analysis of variance (RM-ANOVA) with ISI as a factor, RM-ANOVAs were checked for violation of sphericity. The significance level was p<.05.
For the parietal and premotor conditioning experiments, RM-ANOVA was performed to examine the effect of a conditioning pulse on the MEP. Therefore baseline (TS amplitude) and the different ISIs used for either PMv (4 and 6 ms) or PPC stimulation (4, 6, 8, 12, and14 ms) were analyzed.
For the three-pulse experiment, RM-ANOVA was performed to examine the difference between the baseline (PMv stimulation alone) and the different ISIs of PPC stimulation (4, 6, 8, 10, 15 ms). The Dunnett method was used to correct for multiple comparisons with baseline as a control. Where applicable, the unequal variance model was used. Dunnett-Hsu was used to further test any post-hoc interactions. For experiment 3, the data of the triple pulse experiment was compared with the larger subset (experiment 2).
Results
The mean RMT and mean S50 were 56.9%±12.3 and 68.2%±12.7, respectively. The mean MEP amplitude induced by stimulating at S50 was 1.58 mV± 0.54.
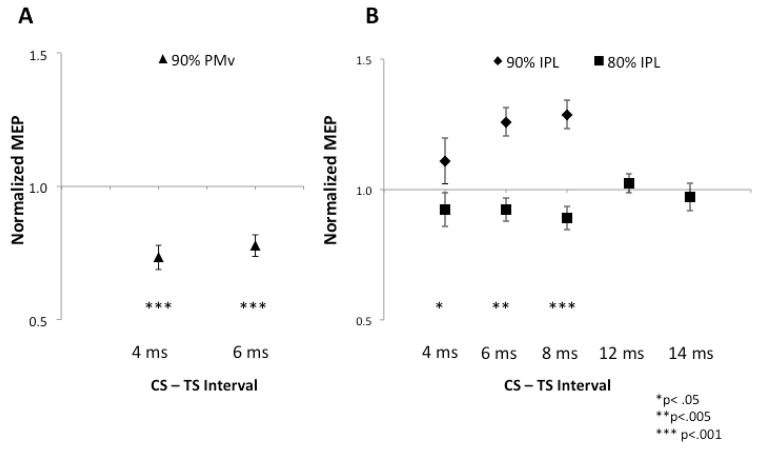
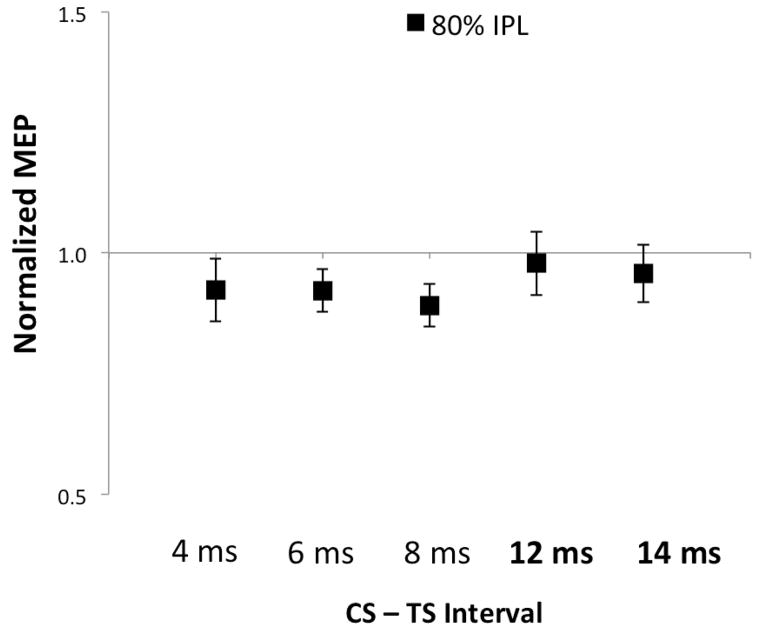
First, we wanted to replicate previous studies and show that we could elicit inhibition and facilitation with premotor and parietal stimulation, respectively. Conditioning the PMv resulted in inhibition (Figure 2A, ISI, F1,10=22.6, p<.001) at both 4 (p<.001) and 6 ms (p<.001). Conditioning of the IPL resulted in facilitation at 4, 6, and 8 ms (Figure 2B, ISI, F1,10=15.6, p=.005). There was a difference between the stimulation intensities used to elicit facilitation (90% RMT) and the intensity used for the three-pulse experiment (80% RMT) (Figure 2B, Intensity, F1,51=48.7, p<.001). There was a trend showing peak facilitation in the 8 ms condition (90%RMT, t=−2.18, p=.057). There was no facilitation observed in the subset of longer ISIs 12 and 14ms (80%RMT, p=0.46, Figure 3) described in Experiment 3.
Figure 2.
Premotor-motor and parietal-motor interactions are expressed as percentage of unconditioned MEP amplitude (normalized MEP) during paired-pulse TMS. Error bars signify SEM. (A) Conditioning stimuli of the PMv at both 4 and 6 ms caused inhibition of the motor cortex. (B) Conditioning of the caudal IPL caused facilitation when the stimulation intensity was 90% RMT that peaked at 8 ms.
Figure 3.
Parietal stimulation (80% RMT) at increased ISIs in Experiment 3 (12–14ms) did not show facilitation or inhibition (p>.05).
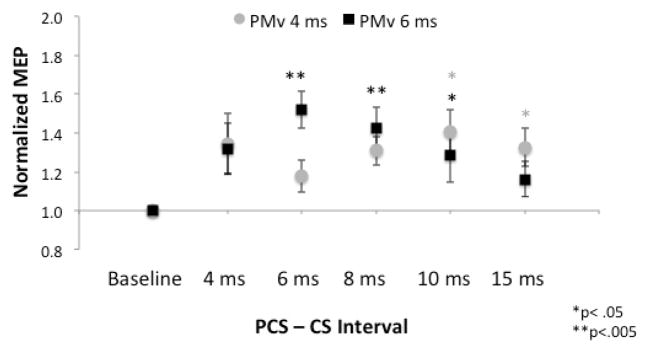
For the three-pulse experiment, there was no significant difference between the two PMv time points (4 and 6 ms) (p>0.05). When examining these time points separately for effects of reversal of inhibition, at 4ms, we observed an overall effect of reversal of inhibition (Figure 4, ISI, F5,24=3.5, p=.017), that was significant at 10 (t=3.4, p=.01) and 15 ms(t=3.3, p=.013). Similarly, when conditioning the PMv at 6 ms, we observed an overall effect of reversal of inhibition (ISI, F5,24=6.2, p<.001), that was significant at 6 (t=4.9, p<.001), 8 (t=4.1, p=.002), and 10ms (t=3.3, p=.01). Additionally, at the 6ms interval, there was a maximal reversal of inhibition when compared to the 15 ms interval (t=2.71, p=.022).
Figure 4.
Interaction of the parietal (PCS, 80% RMT) and premotor (CS, 90% RMT) cortices on the motor cortex. Error bars signify SEM. When conditioning the PMv at 4 ms, preconditioning of the parietal cortex reversed the premotor inhibition at 10 and 15 ms. When conditioning the PMv at 6 ms, preconditioning of the parietal cortex reversed the premotor inhibition at 6, 8, and 10 ms. The peak of the PPC-PMv interaction was observed at PMv 6ms-PPC 6 ms, which is earlier than the peak facilitation ISI (8 ms) for PPC stimulation alone.
Discussion
The main finding of the current study is that the inhibition observed with PMv paired-pulse stimuli can be reversed with pre-conditioning of the PPC. This finding is consistent with the major neural pathway between the PPC and M1 being a disynaptic connection with the PMv (Matelli et al., 1998). The interaction between the parietal and premotor cortex peaked at an ISI of 6 ms, which is earlier than the peak facilitation ISI (8 ms) for parietal stimulation alone. The latencies of these interactions give insight into the connections of this network, which will be discussed below.
As numerous paired-pulse paradigms have shown, the premotor cortex has an inhibitory role over the motor cortex (Civardi et al., 2001, Davare et al., 2006, Davare et al., 2008, Houdayer et al., 2012). The latency of this inhibition has previously been shown to have maximal effects at 6 and 8 ms (Davare et al., 2008). The current study shows equivalent inhibition at 4 and 6 ms. Similarly, the PPC has a facilitatory or inhibitory role depending on exact location [anterior-inhibition, central/posterior-facilitation] (Koch et al., 2007, Koch et al., 2010, Karabanov et al., 2013). The peak of the facilitatory interaction has been described at 8 ms (Karabanov et al., 2013), which was also observed in the current study. When using cTBS to create a transient lesion in the PMv, Koch et al. (2010) observed that parietal facilitation was disrupted providing further evidence for the PPC-PMv-M1 network. When testing the connection between the premotor and parietal cortices, we expanded the ISIs to 15 ms since other studies have found later effects of parietal stimulation up to this time point (Koch et al., 2007).
The combined latencies of the maximum reversal of inhibition (PPC to PMv to M1) connections that we observed were 12 (PMv 6ms, PPC 6ms) and 14 ms (PMv 4ms, PPC 10ms). Since this latency is greater than that of the tested and previously reported IPL to M1 facilitation alone (4–8 ms), we can postulate that this may be a result of the PPC-PMv-M1 network. Evidence that it is not due to a direct effect of PPC on M1 is that we did not observe any inhibition or facilitation when stimulating the PPC at these time periods (12 and 14ms). Koch et al. (2007) had reported PPC-M1 facilitation up to 15ms in the right hemisphere, but no long latency effect in the left hemisphere. Thus, the longer latency of this connection suggests that the longer IPL-M1 effect is via a serial connection with PMv, and may provide a pathway for parietal modulation of PMv-M1 input. Since Koch et al. (2007) showed right hemisphere PPC-M1 facilitation up to 15msec, these results may differ in the non-dominant hemisphere. Given the summation of electrophysiologic effects that must occur between cortico-cortical connections, the balance between these regions helps to explain the broad latencies over which the parietal cortex affects M1.
Much of what we know about these networks comes from the non-human primate literature. Stimulation of the ventral premotor (F5 in the non-human primate) can facilitate M1 corticospinal volleys within 3 ms (Shimazu et al., 2004, Schmidlin et al., 2008). Additionally, stimulation of F5 alone yields I waves in the corticospinal tract in the absence of D waves. The I waves produced by F5 stimulation are longer in latency and smaller in amplitude compared to those from M1 stimulation alone, which is proposed to be via a cortico-cortical connection with M1 (Maier et al., 2013). These studies provide physiologic evidence for the influence of the premotor cortex on the motor cortex. The present study expands upon the current knowledge by providing physiologic evidence in humans that the parietal cortex is also connected to this network and may further influence I waves in the corticospinal tract via a disynaptic connection with the premotor cortex.
A limitation of the current study is that it is just the beginning of our understanding of the physiology of this network. The effects of the IPL on M1 stimulation could change in parallel to the excitability of M1 and lead to a different interaction of the PMv with the motor cortex. In order to test the effect of the proposed network on a different population of M1 neurons, future studies could change the direction of M1 stimulation (PA vs AP) as well as test this network during an active motor task. Similarly, as we only stimulated ISIs later than 4ms, we cannot ignore that other studies have found facilitation in primates and humans (Groppa et al., 2012) as early as 0.8ms. Thus, there may be short-latency interconnections influencing this network that must be probed in further experiments.
To the best of our knowledge, this is the first study to stimulate three intrahemispheric regions in order to test the relationship of regions connected via a known neural pathway. Our findings show that stimulation of the parietal cortex prior to stimulation of the premotor cortex can reverse the effects of stimulation of the premotor cortex alone. Furthermore, the ability to stimulate three intrahemispheric regions is an important technique that will allow for the intricate study of neural networks in the human brain.
Highlights.
A three-pulse, three site, transcranial magnetic stimulation technique can be used for examining a cortical network in human subjects.
Preconditioning the posterior parietal cortex reverses the motor cortex inhibition produced by stimulation of premotor cortex.
These experiments reveal a disynaptic connection from parietal to motor cortex via the premotor cortex.
Acknowledgments
The study was supported by the NINDS Intramural Program. The authors would like to thank Nguyet Dang for her technical assistance and Tianxia Wu for her statistical assistance. This research was made possible through the National Institutes of Health Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, The Leona M. and Harry B. Helmsley Charitable Trust, and the HHMI, as well as other private donors.
Footnotes
Conflict of Interest Statement
Dr. Hallett serves as Chair of the Medical Advisory Board for and receives honoraria and funding for travel from the Neurotoxin Institute. He may accrue revenue on US Patent #6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent #7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil); in relation to the latter, he has received license fee payments from the NIH (from Brainsway) for licensing of this patent. He is on the Editorial Board of 20 journals, and received royalties and/or honoraria from publishing from Cambridge University Press, Oxford University Press, John Wiley & Sons, Wolters Kluwer, Springer, and Elsevier. He has received honoraria for lecturing from Columbia University. Dr. Hallett’s research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds have been granted by the Kinetics Foundation for studies of instrumental methods to monitor Parkinson’s disease, BCN Peptides, S.A. for treatment studies of blepharospasm, Medtronics, Inc., for studies of deep brain stimulation, Parkinson Alliance for studies of eye movements in Parkinson’s disease, UniQure for a clinical trial of AAV2-GDNF for Parkinson Disease, Merz for treatment studies of focal hand dystonia, and Allergan for studies of methods to inject botulinum toxins.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boorman ED, O’Shea J, Sebastian C, Rushworth MF, Johansen-Berg H. Individual differences in white-matter microstructure reflect variation in functional connectivity during choice. Curr Biol. 2007;17:1426–1431. doi: 10.1016/j.cub.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Buch ER, Mars RB, Boorman ED, Rushworth MF. A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming. J Neurosci. 2010;30:1395–1401. doi: 10.1523/JNEUROSCI.4882-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Riek S, Carson RG. Reliability of the input-output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. J Neurosci Methods. 2001;112:193–202. doi: 10.1016/s0165-0270(01)00468-x. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuro Image. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nature Rev Neurosci. 2002;3:553–562. doi: 10.1038/nrn873. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MF. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25:8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci. 2006;26:2260–2268. doi: 10.1523/JNEUROSCI.3386-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, shi R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol. 2008;586:2735–2742. doi: 10.1113/jphysiol.2008.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- Groppa S, Werner-Petroll N, Munchau A, Deuschl G, Ruschworth MF, Siebner HR. A novel dual-site transcranial magnetic stimulation paradigm to probe fast facilitatory inputs from ipsilateral dorsal premotor cortex to primary motor cortex. Neuroimage. 2012;62:500–9. doi: 10.1016/j.neuroimage.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Houdayer E, Beck S, Karabanov A, Poston B, Hallett M. The differential modulation of the ventral premotor-motor interaction during movement initiation is deficient in patients with focal hand dystonia. Eur J Neurosci. 2012;35:478–485. doi: 10.1111/j.1460-9568.2011.07960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabanov AN, Chao CC, Paine R, Hallett M. Mapping Different Intra-Hemispheric Parietal-Motor Networks Using Twin Coil TMS. Brain Stimul. 2013;6:384–389. doi: 10.1016/j.brs.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Cercignani M, Pecchioli C, Versace V, Oliveri M, Caltagirone C, Rothwell J, Bozzali M. In vivo definition of parieto-motor connections involved in planning of grasping movements. Neuro Image. 2010;51:300–312. doi: 10.1016/j.neuroimage.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Ruge D, Schippling S, Caltagirone C, Rothwell JC. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci. 2007;27:6815–6822. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukke SN, Paine RW, Chao CC, de Campos AC, Hallett M. Efficient and reliable characterization of the corticospinal system using transcranial magnetic stimulation. J Clin Neurophysiol. 2014 Jun;31(3):246–52. doi: 10.1097/WNP.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier MA, Kirkwood PA, Brochier TG, Lemon RN. Responses of single corticospinal neurons to intracortical stimulation of primary motor and premotor cortex in the anesthetized macaque monkey. J Neurophys. 2013;109:2982–98. doi: 10.1152/jn.01080.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31:1453–74. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matelli M, Govoni P, Galletti C, Kutz DF, Luppino G. Superior area 6 afferents from the superior parietal lobule in the macaque monkey. J Comp Neuro. 1998;402:327–352. [PubMed] [Google Scholar]
- Schmidlin E, Brochier T, Maier MA, Kirkwood PA, Lemon RN. Pronounced reduction of digit motor responses evoked from macaque ventral premotor cortex after reversible inactivation of the primary motor cortex hand area. J Neurosci. 2008;28:5772–5783. doi: 10.1523/JNEUROSCI.0944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seber GAF, Wild CJ. Nonlinear Regression. Wiley-Interscience; Hoboken, NJ: 2003. [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J Neurosci. 2004;24:1200–1211. doi: 10.1523/JNEUROSCI.4731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]