Abstract
Liver biopsy evaluation plays a critical role in management of patients with viral hepatitis C. In patients with acute viral hepatitis, a liver biopsy, though uncommonly performed, helps to rule out other non-viral causes of deranged liver function. In chronic viral hepatitis C, it is considered the gold standard in assessment of the degree of necroinflammation and the stage of fibrosis, to help guide treatment and determine prognosis. It also helps rule out any concomitant diseases such as steatohepatitis, hemochromatosis or others. In patients with chronic progressive liver disease with cirrhosis and dominant nodules, a targeted liver biopsy is helpful in differentiating a regenerative nodule from dysplastic nodule or hepatocellular carcinoma. In the setting of transplantation, the liver biopsy helps distinguish recurrent hepatitis C from acute rejection and also is invaluable in the diagnosis of fibrosing cholestatic hepatitis, a rare variant of recurrent hepatitis C. This comprehensive review discusses the entire spectrum of pathologic findings in the course of hepatitis C infection.
Keywords: Hepatitis C, Liver pathology, Biopsy, Grading, Staging, Neoplasia
Core tip: The manuscript is a comprehensive review of liver pathology of hepatitis C infection. It delves into the historical literature and terminology of chronic viral hepatitis. It further focuses on the entire spectrum of histopathological findings related to different stages of hepatitis C infection. The diagnostic dilemmas in a post-transplantation setting such as recurrent hepatitis C, are also addressed. Relevant illustrations and tables support the histological descriptions. This article would be of educational benefit for pathologists as well as hepatologists.
INTRODUCTION
Hepatitis C virus (HCV) infection is the most common cause of necroinflammation in liver biopsies in the United States. HCV is a single stranded RNA virus of flaviviridae family that is transmitted parenterally and is hepatotropic[1]. The overall prevalence of HCV infection in the United States is 1.6%[2]. HCV infection is a slow and progressive disease. More commonly, it causes a subclinical acute infection that can progress to persistent chronic infection in about 75%-85% individuals, 10%-20% of which develop cirrhosis over a period of 20-30 years[2]. There is a 1%-5% annual risk of development of hepatocellular carcinoma (HCC) in cirrhotic patients[3]. Cirrhosis secondary to HCV is the leading cause of liver transplantation in the United States. Recurrence of hepatitis C within the first 6 mo post-transplantation is almost universal and occurs more commonly as recurrent hepatitis C and uncommonly as fibrosing cholestatic hepatitis or the cholestatic variant of hepatitis C[3]. In keeping with the natural history of HCV infection, the liver pathology of hepatitis C infection encompasses a wide spectrum of histomorphologic features, depending on what phase of liver injury a biopsy is performed (Table 1). In general, it is a necroinflammatory process of the hepatic parenchyma. This review focuses on the entire histopathologic spectrum of liver damage in HCV infection, dwells on the historical aspects and the evolution of pathological changes with advent of newer drugs.
Table 1.
Liver histology in hepatitis C infection
| Histological features of acute hepatitis C | |
| Cholestatic injury | |
| Bile duct injury (Poulsen Christofferson lesion) | |
| Bile duct injury (Poulsen Christofferson lesion) | |
| Cholangiolar proliferation (mild) | |
| Hepatocytic injury | |
| Lobular necroinflammation with disarray | |
| Steatosis | |
| Sinusoidal inflammation | |
| Histological features of chronic hepatitis C | |
| Portal changes | |
| Portal lymphoid aggregates/reactive lymphoid follicles | |
| Interface hepatitis | |
| Bile duct damage | |
| Isolated clusters of hepatocytes in portal tracts | |
| Phlebitis | |
| Lobular injury | |
| Necroinflammation | |
| Steatosis | |
| Multinucleation and nuclear pleomorphism | |
| Sinusoidal cell hyperplasia | |
| Microgranulomas | |
| Fibrosis | |
| Preneoplastic changes | |
| Large cell change | |
| Small cell change | |
| Iron free foci | |
Historical perspective
Historically, the terminology used for chronic viral hepatitis dates back to early 1980s and includes “chronic persistent hepatitis”, “chronic active hepatitis” and “chronic lobular hepatitis”[4]. Chronic persistent hepatitis referred to portal inflammation without piecemeal necrosis (now called as interface hepatitis), chronic active hepatitis referred to portal inflammation with piecemeal necrosis and chronic lobular hepatitis was used when there was lobular necroinflammation with no significant piecemeal necrosis[5]. These terms dates back to era when serologic tests for hepatitis C had not been developed, not much was known about this virus and it was referred to as non-A-non-B hepatitis. No antiviral treatments were available. These terms had prognostic value, where “chronic persistent hepatitis” was considered self-limited, and “chronic active hepatitis” as well as “chronic lobular hepatitis” progressed to chronic liver disease, if left untreated. Subsequently hepatitis C was discovered and with better understanding of the biologic progression of the infection, the limitations of the classification were recognized and the terms became obsolete. It became apparent that disease progression to chronic liver disease, fibrosis and ultimately cirrhosis occurred with all three pathologic descriptors[6,7].
Liver biopsy sample size
With the advent of effective anti-viral treatments, the liver biopsy became an integral part of patient care to assess the histological severity of inflammation and for prognostication in chronic hepatitis C. Liver biopsy size is critical for staging and grading of disease[8-10]. Colloredo et al[9] reported that liver biopsies shorter than 3 cm in length and less than 1.4 mm in width show milder/lower grades and stage of disease. The recommended sample size is at least 2 cm or 2.5 cm in length with at least 10 portal tracts using a 16 or 18 gauge biopsy needle for appropriate grading and staging of disease[10,11] Heterogeneity in distribution of fibrosis and sampling variation can affect assessment of fibrosis. For example, assessment of fibrosis in a subcapsular liver biopsy can be misleading.
LIVER PATHOLOGY OF ACUTE HEPATITIS C
Acute HCV hepatitis in immunocompetent individuals is largely asymptomatic and, hence, it is rarely biopsied. However, in symptomatic patients with deranged liver function tests, a liver biopsy plays an integral role in clinical and laboratory work-up to determine the etiology[12]. The clinical picture, in this setting, may be purely hepatitic, or a mixed hepatitic and cholestatic type. The clinical differential diagnosis is broad and includes autoimmune hepatitis, steatohepatitis, Wilson disease, biliary disorders including primary biliary cirrhosis, primary sclerosing cholangitis, or a drug effect. Liver biopsy helps in arriving at a definitive diagnosis in a majority of cases.
Since this is an uncommon scenario for liver biopsy, there is limited literature on liver histology in acute phase of HCV infection[13-16]. The histological spectrum is broad and includes both bile duct and lobular hepatocytic injury. Early phase of disease may show a cholestatic picture characterized by mixed portal inflammation composed of lymphocytes and neutrophils, cholangiolar proliferation, cholestasis, canalicular or hepatocellular, and mild to moderate lobular inflammation (Figure 1). The biopsies done in the later part of the acute phase show mild nonspecific portal and lobular inflammation[16]. Other histologic features include a distinctive bile duct lesion “Poulsen-Christoffersen lesion” that shows duct-centric lymphoid aggregates associated with lymphocytic inflammation and injury to the bile duct proper (Figure 2), mixed portal inflammation, lobular necroinflammation with disarray, steatosis and prominent sinusoidal inflammatory infiltrate[12].
Figure 1.
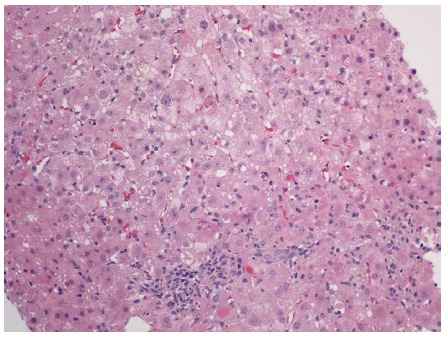
Liver biopsy with cholestasis, mild portal and lobular inflammation, and apoptotic bodies. Hematoxylin and eosin stain, magnification × 100.
Figure 2.
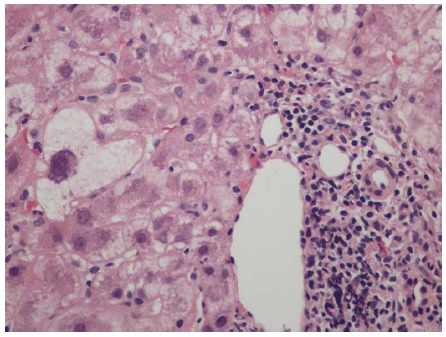
Lymphocyte predominant portal inflammation and a damaged bile duct (Poulsen Christofferson lesion). Periportal hepatocytes show ballooning/feathery degeneration. Hematoxylin and eosin stain, magnification × 200.
Acute hepatitis C in immunocompromised host, specifically in the setting of concurrent human immunodeficiency virus (HIV) infection, shows lymphoplasmacytic portal inflammation, interface hepatitis, and necroinflammatory lobular changes, features of chronic viral hepatitis. In addition, liver biopsies from these patients show moderately advanced fibrosis at presentation[17] (Figure 3) or rapid progression to fibrosis over a period of time[18,19].
Figure 3.
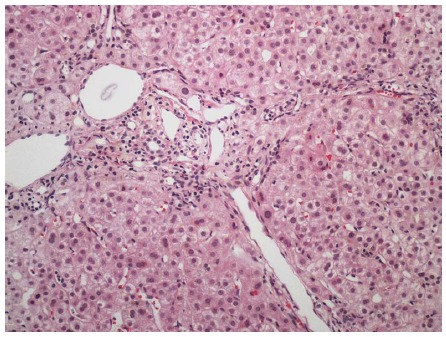
Portal and periportal fibrosis in a patient with concurrent human immunodeficiency virus and acute hepatitis C infection. Hematoxylin and eosin stain, magnification × 100.
LIVER PATHOLOGY OF CHRONIC HEPATITIS C
Chronic hepatitis is defined as persistence of infection for at least 6 mo after the onset of infection[2]. Histologically, it is characterized by necroinflammation accompanied by variable degree of fibrosis. The pathologic features of chronic viral hepatitis, in general, have been well characterized[20-24]. These features are not specific to chronic hepatitis C but can also be seen in chronic hepatitis due to hepatitis B virus with or without hepatitis D virus, and drug-induced hepatitis.
Portal inflammation
The distinctive feature of chronic hepatitis is portal based inflammation with or without lobular inflammation. The patchy portal expansion by chronic lymphoplasmacytic inflammation is readily appreciable under low magnification as “blue portal tracts”. The portal inflammation is composed predominantly of lymphocytes admixed with few plasma cells and rare eosinophil, the so called lymphoid follicles or lymphoid aggregates (Figure 4). The inflammation is of variable intensity, and varies among different portal tracts in one biopsy, among serial biopsies from one patient, and varies from patient to patient. In some cases, plasma cells may be prominent and these can be described as having autoimmune features. If clinical and serological features of autoimmune hepatitis are present, this can represent an overlap syndrome of autoimmune hepatitis and hepatitis C (Figure 5). Portal and lobular macrophages can be present and may contain engulfed debris in the form of pigment or PAS positive-diastase resistant material and is considered to be an evidence of recent activity (Figure 6). Portal and/or uncommonly central endothelialitis can be seen.
Figure 4.
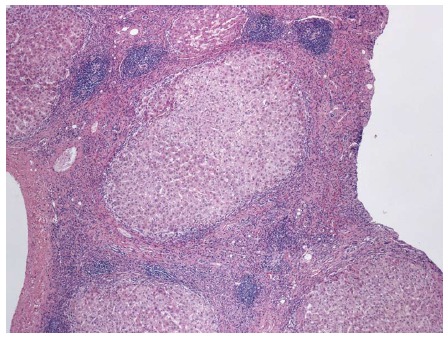
Fibrous septa with lymphocyte predominant inflammatory infiltrates and multiple lymphoid aggregates, in a patient with cirrhosis secondary to hepatitis C. Hematoxylin and eosin stain, magnification × 40.
Figure 5.
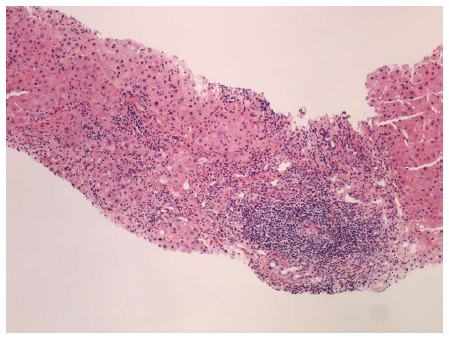
Portal lymphoid aggregate and lymphoplasmacytic inflammation with severe interface hepatitis, indicative of an overlap syndrome of autoimmune hepatitis and hepatitis C. Hematoxylin and eosin stain, magnification × 100.
Figure 6.
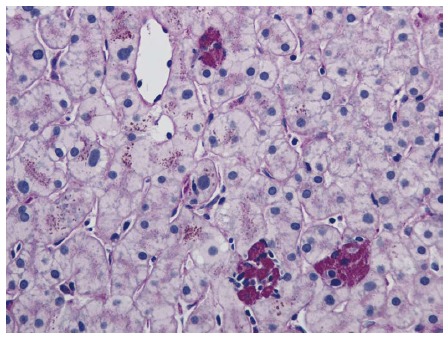
Small clusters of lobular macrophages containing periodic acid Schiff positive-diastase resistant cytoplasmic debris. Periodic acid Schiff with diastase stain, magnification × 200.
Interface hepatitis
The portal inflammation may remain confined to portal tracts. More commonly, it involves the adjacent hepatic parenchyma, thus obscuring the sharp demarcation of portal-parenchymal interface (limiting plate), and this is called interface hepatitis. Interface hepatitis (formerly called as piecemeal necrosis) is another distinctive lesion of chronic hepatitis and is characterized by spillage of lymphoplasmacytic inflammation across the portal-parenchymal interface, into the periportal hepatocytes (Figure 7)[25,26]. Interface hepatitis is focal and often associated with hepatocytic damage, loss and apoptosis. Emperipolesis characterized by penetration of lymphocytes into hepatocytes has also been described in chronic hepatitis[27]. Isolated clusters of hepatocytes present in portal tracts, separated from lobules, sometimes seen in liver biopsies from patients with chronic hepatitis, are indicative of past episode of interface hepatitis (Figure 8). Ductular reaction is another manifestation of regenerative response to hepatocytic injury at the limiting plate.
Figure 7.
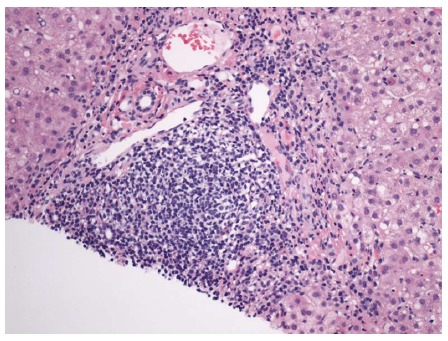
Portal lymphoid aggregate and lymphoplasmacytic inflammation with interface hepatitis and occasional apoptotic hepatocyte at the portal-periportal interface. Hematoxylin and eosin stain, magnification × 200.
Figure 8.
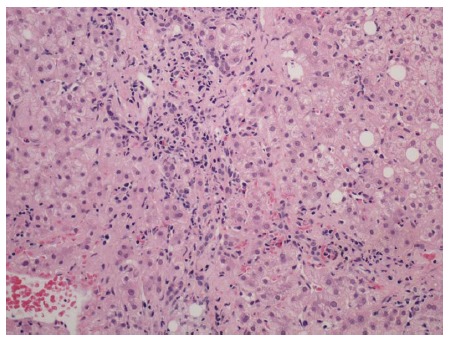
Portal tract with lymphoplasmacytic inflammation, and enclosing small clusters of hepatocytes indicative of recent episode of active hepatitis. Hematoxylin and eosin stain, magnification × 200.
Lobular inflammation
Lobular hepatitis is an active necroinflammatory component of chronic hepatitis. It manifests as isolated hepatocytic apoptosis, spotty necrosis, and bridging or confluent necrosis. Isolated apoptotic hepatocyte, also known as Councilman body, is identified as a small-angulated hepatocyte without a nucleus or with a small nuclear fragment and bright eosinophilic cytoplasm. Spotty necrosis is composed of small clusters of lymphocytes and/or macrophages enclosing damaged/apoptotic hepatocytes (Figure 9). Small clusters of macrophages containing PAS-positive diastase-resistant material indicate prior foci of spotty necrosis. Bridging necrosis, a severe form of necrosis, extending between two vascular structures, and confluent necrosis that spans several lobules[4], are uncommon in chronic hepatitis C. Other features of lobular injury include multinucleation of hepatocytes[28], Mallory-Denk hyaline[29], and sinusoidal Kupffer cell hyperplasia[29].
Figure 9.
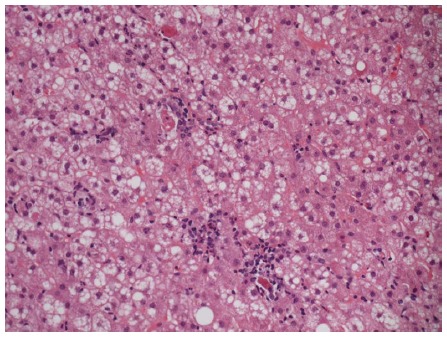
Spotty necrosis characterized by small foci of lobular necroinflammation and associated apoptotic hepatocytes. Hematoxylin and eosin stain, magnification × 200.
Portal lymphoid aggregates, bile duct damage, steatosis
Features characteristically seen in chronic hepatitis C are portal lymphoid aggregates and/or lymphoid follicles, bile duct damage, macrovesicular or mixed microvesicular and macrovesicular steatosis and Mallory-Denk-like material[29]. These are not specific and can be seen in other subtypes of viral hepatitis or in non-viral hepatitis too. Portal lymphoid infiltrates have been reported to be present in 33% to 78% of cases and are composed of small lymphocytes, some with germinal centers[28,30,31]. These occur more frequently in HCV genotype 1B infection[31].
The lymphoid aggregates occur in proximity to bile ducts and are sometimes associated with damage to bile duct epithelium that was originally described by Poulsen and Christoffersen[32]. The bile duct damage is mild and non-destructive, and is characterized by cellular vacuolation, cytoplasmic eosinophilia, nuclear overlapping and nuclear dropout. The bile duct lesions are frequent and have been reported in as high as 91% of cases[28], and are more prevalent in hepatitis C genotype 3a[33].
Steatosis, macrovesicular or mixed macrovesicular and microvesicular type, is a viral cytopathic effect[29], that when present is a characteristic feature of chronic hepatitis C and is commonly seen in association with hepatitis C genotype 3a[33]. The steatosis in this setting is usually mild and is periportal or azonal in distribution. The steatosis is postulated to be secondary to free radical mediated peroxidation that is elicited by increased virus-induced iron storage[34]. Presence of moderate to marked steatosis in a liver biopsy, especially in a centrilobular/perivenular distribution, from a patient with hepatitis C should raise a clinical consideration for concomitant fatty liver disease secondary to obesity, alcohol, hyperlipidemia or diabetes. Such cases may, in addition, show neutrophilic infiltration, ballooning degeneration, and Mallory-Denk-hyaline and pericellular/perisinusoidal fibrosis.
Iron deposition
Other features of chronic hepatitis C include iron deposition. Mild iron deposition occurs in both hepatocytes and sinusoidal lining Kupffer cells in chronic viral hepatitis. Assessment of iron in liver biopsy is pertinent, as hepatocellular iron deposition has been shown to be an inverse predictor of response to interferon treatment[35-38]. Perls stain is the most commonly used stain for assessing hemosiderosis in liver because of high sensitivity. The iron deposition in hepatocytes is assessed and commonly graded using the Searle’ modification[39] of Scheuer scoring system[40] for iron deposition. Hereditary hemochromatosis is always a clinical consideration in presence of hepatocellular iron in liver biopsies, even in small amounts, because of variable penetrance of the disease.
Fibrosis
Fibrosis is a dynamic reparative process and is a consequence of chronic inflammation, hepatocyte loss and regeneration in viral hepatitis. Fibrosis usually starts in the portal tracts resulting in expansion, and then extends to periportal tissue as thin fibrous septa that account for the irregular contours of the portal tracts. As the disease progresses over time (usually 10-20 years), the fibrous septa link to adjacent portal tracts or central veins and evolves into bridging fibrosis. This combined with hepatocytic regeneration leads to architectural distortion, formation of nodules and eventual cirrhosis (Figure 10). Cholestasis is not a feature of hepatitis C, but can be seen in cirrhotic livers with decompensation. The stage of fibrosis is best assessed on connective tissue stains such as Masson’s trichrome stain.
Figure 10.
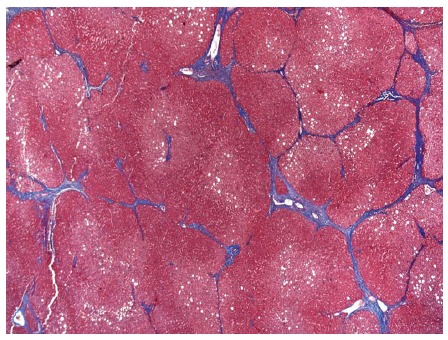
Cirrhosis characterized by bridging fibrosis with nodule formation. Masson’s trichrome stain, magnification × 40.
Fibrosis has generally been regarded as an irreversible process. Several studies have reported decrease in fibrosis scores following treatment of the etiological process in a variety of diseases such as steatohepatitis[41], hemochromatosis[42], Wilson disease[43], Indian childhood cirrhosis[44], biliary obstruction[45], autoimmune hepatitis[46], chronic viral hepatitis[47]. In liver tissue of patients with chronic viral hepatitis, the fibrosis has been seen to regress with antiviral treatment and sustained viral response and eradication[48]. The regression is limited to decrease or disappearance of fibrous septa on histological evaluation, however, the sequelae of cirrhosis such as arteriovenous shunt may persist[49].
GRADING AND STAGING OF CHRONIC VIRAL HEPATITIS
Assessment of liver biopsy in chronic hepatitis C is important to determine the grade (severity) of necroinflammation and evidence of disease progression, which is measured by stage (fibrosis) of disease. This has therapeutic implications, as it is helpful in determining the response to antiviral treatment. It also has prognostic implications as it predicts the progression of disease. Several numerical grading and staging systems were developed to objectively assess the histological findings.
The earliest scoring system was proposed in 1981 by Knodell et al[50] and formed the basis of subsequent scoring systems that include Batts and Ludwig[51], Scheuer[52], Ishak (modified Knodell)[53] and the METAVIR[54] (Table 2). Each system has its strengths and weaknesses, but all are reproducible, easy to use and convey the information required for patient care[22].
Table 2.
Various staging systems for assessment of fibrosis in chronic hepatitis
| Score | System |
| Ishak modification of Knodell staging system (1995)[53] | |
| 0 | No fibrosis |
| 1 | Fibrous expansion of some portal areas with or without short fibrous septa |
| 2 | Fibrous expansion of most portal areas with or without short fibrous septa |
| 3 | Fibrous expansion of most portal areas with occasional portal to portal (P-P) |
| 4 | Fibrous expansion of portal areas with marked P-P and portal-central bridging |
| 5 | Marked bridging with occasional nodules (incomplete cirrhosis) |
| 6 | Cirrhosis, probable or definite (Bridging fibrosis with nodule formation) |
| METAVIR system (1996)[54] | |
| F0 | No fibrosis |
| F1 | Portal fibrosis without septa |
| F2 | Portal fibrosis with rare fibrous septa |
| F3 | Numerous septae without cirrhosis |
| F4 | Cirrhosis |
| Scheuer system (1991)[52] | |
| 0 | No fibrosis |
| 1 | Enlarged, fibrotic portal tracts |
| 2 | Periportal or portal-portal septa but intact architecture |
| 3 | Fibrosis with architectural distortion but no obvious cirrhosis |
| 4 | Probable or definite cirrhosis |
| Batts and Ludwig (1995)[51] | |
| 0 | No fibrosis (normal connective tissue) |
| 1 | Portal fibrosis (Fibrous portal expansion) |
| 2 | Periportal fibrosis (Periportal or rare portal-portal septa) |
| 3 | Septal fibrosis (Fibrous septa with architectural distortion; no obvious cirrhosis) |
| 4 | Cirrhosis |
The grading of activity is based on semi-quantitative assessment of the amount of interface hepatitis and lobular necroinflammation. Interface hepatitis is graded based on the involvement of number of portal tracts, and the amount of circumferential involvement of portal tracts in a biopsy. Lobular inflammation is graded based on number of foci and presence of bridging/confluent necrosis. The stage of disease is also assessed semi-quantitatively on a scale of 0-4 or 0-6 based on the portal fibrosis, extension of fibrotic septa beyond the portal tracts, bridging fibrosis with architectural distortion and cirrhosis.
Preneoplastic/neoplastic lesions
The preneoplastic lesions that can be seen in cirrhotic or non-cirrhotic liver in the setting of hepatitis C comprise of large cell change, small cell change, iron free foci, low-grade dysplastic nodule and high-grade dysplastic nodule. It is recommended that these features if present must be commented upon in the liver biopsies.
Large cell change is characterized by hepatocytes with abundant cytoplasm, large atypical nuclei with multinucleation and with preserved nuclear to cytoplasmic ratio. The nuclear changes reflect aneuploidy. The hepatocytes with large cell change are irregularly intermixed with regenerating hepatocytes and are reported to represent cellular senescence, which is a safeguard against malignant transformation[55], or replicative senescence, predictive of malignancy in the surrounding liver[56]. However, others have shown that this is a dysplastic change[57].
Small cell change is a preneoplastic/dysplastic lesion[58], characterized by expansile clusters of small hepatocytes with high nuclear to cytoplasmic ratio with hyperchromatic nuclei, irregular nuclear contours and cytoplasmic basophilia. Reticulin stain shows focal loss of reticulin with mildly thickened trabeculae (> 1-2 layers)[59,60].
Iron free foci
Some liver tissues with advanced fibrosis can have abundant iron deposition. In such situation, presence of iron free foci is indicative of dysplastic change[61].
Dysplastic nodules are expansile lesions, with size > 0.8 cm, visible on gross examination with a bulging cut surface and with a color and texture that is distinct from the surrounding liver[62]. Low-grade dysplastic nodules (LGDN) morphologically mimic the regenerative nodule in cirrhotic liver because of retained portal tracts. In addition, the LGDN may have unpaired arteries and show features suggestive of clonality that include fatty metamorphosis, iron/copper accumulation and/or cytologic features of large cell change[59]. In contrast, high-grade dysplastic nodules have unpaired arteries and rare portal tracts, along with monotonous expansile populations of hepatocytes with cytologic features of small cell change and/or iron free foci. Atypical architectural changes such as thickened trabeculae, nodule within nodule, and rare acinar transformation can be present[59]. Early hepatocellular carcinoma can develop in high-grade dysplastic nodule or can develop independent of dysplastic nodule. Invasion of portal tract is a feature of malignancy in a HGDN[63].
POST TRANSPLANTATION PATHOLOGY OF HEPATITIS C
Recurrent hepatitis C
Status-post orthotopic liver transplantation (OLT), virologic recurrence of hepatitis C is almost universal, and is an important cause of graft loss and mortality. Histological evidence of recurrent hepatitis C is seen in about 70% of cases[64]. In liver biopsies, performed within the first 6 mo of OLT, early recurrent hepatitis C is an important diagnostic consideration/dilemma vs mild acute cellular rejection. Lobular inflammation, apoptotic bodies, spotty necrosis and lobular disarray, with portal lymphocyte predominance characterize early recurrent hepatitis C[65,66]. Saxena et al[67] reported that presence of an average of 55 apoptotic bodies per linear cm favor a diagnosis of recurrent hepatitis C. In contrast, acute cellular rejection is characterized by mixed portal/periportal inflammation composed of lymphocytes, plasma cells and eosinophils, lymphocytic cholangitis and endothelialitis[68]. Yeh et al[69] found that minimal to mild portal endothelialitis can be seen in viral hepatitis C, however, presence of severe endothelialitis favors a diagnosis of acute cellular rejection.
Fibrosing cholestatic hepatitis
Fibrosing cholestatic hepatitis or cholestatic variant of hepatitis C is an enigmatic phenomenon seen in patients with chronic viral hepatitis C and is characterized by an onset within 1 year of transplantation, either liver[70], kidney[71] or hematopoietic stem cell transplant[72]. It is associated with poor prognosis due to rapid progression of fibrosis, and resistance to conventional antiviral therapies. Histologically, it presents as hepatocytic injury characterized by ballooning degeneration, apoptotic bodies, spotty necrosis along with features of cholestasis including predominantly canalicular cholestasis, ductular reaction, biliary-type piecemeal necrosis, and periportal and perisinusoidal/pericellular fibrosis (Figures 11A and B)[73]. The differential diagnosis includes other causes of cholestasis such as biliary complications, drug/toxic effect among others.
Figure 11.
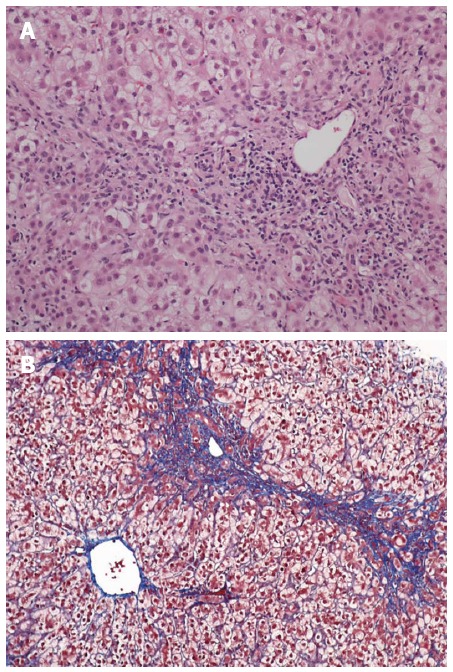
Fibrosing cholestatic hepatitis with mixed portal inflammation, bile duct damage, interface hepatitis, ductular reaction and fibrosis. Hematoxylin and eosin stain, magnification × 100 (A); and extensive pericellular and perisinusoidal fibrosis (B; Trichrome stain, magnification × 100).
FUTURE OF LIVER BIOPSY IN HEPATITIS C
As described in this review, the histopathology of chronic hepatitis C encompasses a wide spectrum of features that correspond to the evolution and progression of hepatitis C infection. Increasing use of newer direct acting antiviral drugs- serine protease inhibitors, with or without interferon, is expected to have sustained viral response (SVR) for 12 mo in about 90% of patients[74]. This will markedly slow down the progression to cirrhosis. In addition, increasing clinical use of noninvasive methods to assess fibrosis such as ultrasonic transient elastography (fibroscan)[75] will decrease the role of liver biopsies as a tool to monitor the disease activity and stage in chronic hepatitis C.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest to report.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 7, 2015
First decision: August 31, 2015
Article in press: December 1, 2015
P- Reviewer: A-Kader HH, Chetty R S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.Hytiroglou P. Hepatitis C. In: Saxena R, editor. Practical Hepatic Pathology. A Diagnostic Approach. 1st ed. Philadelphia: Elsevier Saunders; 2011. pp. 225–233. [Google Scholar]
- 2.Rustgi VK. The epidemiology of hepatitis C infection in the United States. J Gastroenterol. 2007;42:513–521. doi: 10.1007/s00535-007-2064-6. [DOI] [PubMed] [Google Scholar]
- 3.Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58–S68. doi: 10.1016/j.jhep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Popper H, Schaffner F. The vocabulary of chronic hepatitis. N Engl J Med. 1971;284:1154–1156. doi: 10.1056/NEJM197105202842011. [DOI] [PubMed] [Google Scholar]
- 5.Ishak KG. Chronic hepatitis: morphology and nomenclature. Mod Pathol. 1994;7:690–713. [PubMed] [Google Scholar]
- 6.Ludwig J. The nomenclature of chronic active hepatitis: an obituary. Gastroenterology. 1993;105:274–278. doi: 10.1016/0016-5085(93)90037-d. [DOI] [PubMed] [Google Scholar]
- 7.Scheuer PJ. The nomenclature of chronic hepatitis: time for a change. J Hepatol. 1995;22:112–114. doi: 10.1016/0168-8278(95)80269-x. [DOI] [PubMed] [Google Scholar]
- 8.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 9.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239–244. doi: 10.1016/s0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 10.Schiano TD, Azeem S, Bodian CA, Bodenheimer HC, Merati S, Thung SN, Hytiroglou P. Importance of specimen size in accurate needle liver biopsy evaluation of patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2005;3:930–935. doi: 10.1016/s1542-3565(05)00541-0. [DOI] [PubMed] [Google Scholar]
- 11.Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Tsuang W, Subramanian R, Liu Q, Hart J, Mohanty SR. The need for liver biopsy in a patient with acute HCV infection. Nat Clin Pract Gastroenterol Hepatol. 2008;5:54–57. doi: 10.1038/ncpgasthep1030. [DOI] [PubMed] [Google Scholar]
- 13.Omata M, Iwama S, Sumida M, Ito Y, Okuda K. Clinico-pathological study of acute non-A, non-B post-transfusion hepatitis: histological features of liver biopsies in acute phase. Liver. 1981;1:201–208. doi: 10.1111/j.1600-0676.1981.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 14.Schmid M, Pirovino M, Altorfer J, Gudat F, Bianchi L. Acute hepatitis non-A, non-B; are there any specific light microscopic features? Liver. 1982;2:61–67. doi: 10.1111/j.1600-0676.1982.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi K, Hashimoto E, Ludwig J, Hisamitsu T, Obata H. Liver biopsy features of acute hepatitis C compared with hepatitis A, B, and non-A, non-B, non-C. Liver. 1993;13:69–72. doi: 10.1111/j.1600-0676.1993.tb00609.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson K, Kotiesh A, Boitnott JK, Torbenson M. Histology of symptomatic acute hepatitis C infection in immunocompetent adults. Am J Surg Pathol. 2007;31:1754–1758. doi: 10.1097/PAS.0b013e318093f90e. [DOI] [PubMed] [Google Scholar]
- 17.Fierer DS, Uriel AJ, Carriero DC, Klepper A, Dieterich DT, Mullen MP, Thung SN, Fiel MI, Branch AD. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J Infect Dis. 2008;198:683–686. doi: 10.1086/590430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel M, Page E, Boesecke C, Reiberger T, Schwarze-Zander C, Mauss S, Baumgarten A, Wasmuth JC, Nelson M, Rockstroh JK. Liver fibrosis progression after acute hepatitis C virus infection in HIV-positive individuals. Clin Infect Dis. 2012;54:556–559. doi: 10.1093/cid/cir854. [DOI] [PubMed] [Google Scholar]
- 19.Konerman MA, Mehta SH, Sutcliffe CG, Vu T, Higgins Y, Torbenson MS, Moore RD, Thomas DL, Sulkowski MS. Fibrosis progression in human immunodeficiency virus/hepatitis C virus coinfected adults: prospective analysis of 435 liver biopsy pairs. Hepatology. 2014;59:767–775. doi: 10.1002/hep.26741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geller SA. Hepatitis B and hepatitis C. Clin Liver Dis. 2002;6:317–334. doi: 10.1016/s1089-3261(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 21.Lefkowitch JH. Liver biopsy assessment in chronic hepatitis. Arch Med Res. 2007;38:634–643. doi: 10.1016/j.arcmed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Theise ND. Liver biopsy assessment in chronic viral hepatitis: a personal, practical approach. Mod Pathol. 2007;20 Suppl 1:S3–S14. doi: 10.1038/modpathol.3800693. [DOI] [PubMed] [Google Scholar]
- 23.Fiel MI. Pathology of chronic hepatitis B and chronic hepatitis C. Clin Liver Dis. 2010;14:555–575. doi: 10.1016/j.cld.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Guido M, Mangia A, Faa G. Chronic viral hepatitis: the histology report. Dig Liver Dis. 2011;43 Suppl 4:S331–S343. doi: 10.1016/S1590-8658(11)60589-6. [DOI] [PubMed] [Google Scholar]
- 25.Popper H. Changing concepts of the evolution of chronic hepatitis and the role of piecemeal necrosis. Hepatology. 1983;3:758–762. doi: 10.1002/hep.1840030522. [DOI] [PubMed] [Google Scholar]
- 26.Baptista A, Bianchi L, De Groote J, Desmet VJ, Ishak KG, Korb G, MacSween RN, Popper H, Poulsen H, Scheuer PJ. The diagnostic significance of periportal hepatic necrosis and inflammation. Histopathology. 1988;12:569–579. doi: 10.1111/j.1365-2559.1988.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 27.Bechtelsheimer H, Gedgik P, Müller R, Klein H. [Aggressive emperipolesis in chronic hepatitis (author’s transl)] Klin Wochenschr. 1976;54:137–140. doi: 10.1007/BF01468791. [DOI] [PubMed] [Google Scholar]
- 28.Bach N, Thung SN, Schaffner F. The histological features of chronic hepatitis C and autoimmune chronic hepatitis: a comparative analysis. Hepatology. 1992;15:572–577. doi: 10.1002/hep.1840150403. [DOI] [PubMed] [Google Scholar]
- 29.Lefkowitch JH, Schiff ER, Davis GL, Perrillo RP, Lindsay K, Bodenheimer HC, Balart LA, Ortego TJ, Payne J, Dienstag JL. Pathological diagnosis of chronic hepatitis C: a multicenter comparative study with chronic hepatitis B. The Hepatitis Interventional Therapy Group. Gastroenterology. 1993;104:595–603. doi: 10.1016/0016-5085(93)90432-c. [DOI] [PubMed] [Google Scholar]
- 30.Scheuer PJ, Ashrafzadeh P, Sherlock S, Brown D, Dusheiko GM. The pathology of hepatitis C. Hepatology. 1992;15:567–571. doi: 10.1002/hep.1840150402. [DOI] [PubMed] [Google Scholar]
- 31.Luo JC, Hwang SJ, Lai CR, Lu CL, Li CP, Tsay SH, Wu JC, Chang FY, Lee SD. Clinical significance of portal lymphoid aggregates/follicles in Chinese patients with chronic hepatitis C. Am J Gastroenterol. 1999;94:1006–1011. doi: 10.1111/j.1572-0241.1999.01004.x. [DOI] [PubMed] [Google Scholar]
- 32.Poulsen H, Christoffersen P. Abnormal bile duct epithelium in liver biopsies with histological signs of viral hepatitis. Acta Pathol Microbiol Scand. 1969;76:383–390. doi: 10.1111/j.1699-0463.1969.tb03269.x. [DOI] [PubMed] [Google Scholar]
- 33.Mihm S, Fayyazi A, Hartmann H, Ramadori G. Analysis of histopathological manifestations of chronic hepatitis C virus infection with respect to virus genotype. Hepatology. 1997;25:735–739. doi: 10.1002/hep.510250340. [DOI] [PubMed] [Google Scholar]
- 34.Farinati F, Cardin R, De Maria N, Della Libera G, Marafin C, Lecis E, Burra P, Floreani A, Cecchetto A, Naccarato R. Iron storage, lipid peroxidation and glutathione turnover in chronic anti-HCV positive hepatitis. J Hepatol. 1995;22:449–456. doi: 10.1016/0168-8278(95)80108-1. [DOI] [PubMed] [Google Scholar]
- 35.Barton AL, Banner BF, Cable EE, Bonkovsky HL. Distribution of iron in the liver predicts the response of chronic hepatitis C infection to interferon therapy. Am J Clin Pathol. 1995;103:419–424. doi: 10.1093/ajcp/103.4.419. [DOI] [PubMed] [Google Scholar]
- 36.Ikura Y, Morimoto H, Johmura H, Fukui M, Sakurai M. Relationship between hepatic iron deposits and response to interferon in chronic hepatitis C. Am J Gastroenterol. 1996;91:1367–1373. [PubMed] [Google Scholar]
- 37.Izumi N, Enomoto N, Uchihara M, Murakami T, Ono K, Noguchi O, Miyake S, Nouchi T, Fujisawa K, Marumo F, et al. Hepatic iron contents and response to interferon-alpha in patients with chronic hepatitis C. Relationship to genotypes of hepatitis C virus. Dig Dis Sci. 1996;41:989–994. doi: 10.1007/BF02091542. [DOI] [PubMed] [Google Scholar]
- 38.Boucher E, Bourienne A, Adams P, Turlin B, Brissot P, Deugnier Y. Liver iron concentration and distribution in chronic hepatitis C before and after interferon treatment. Gut. 1997;41:115–120. doi: 10.1136/gut.41.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Searle J, Kerr JRF, Halliday JW. Iron storage disease. In: MacSween RNM, Anthony PP, Sheuer PJ, editors. Pathology of Liver. 3rd ed. London: Churchill Livingstone; 1994. pp. 219–241. [Google Scholar]
- 40.Scheuer PJ, Williams R, Muir AR. Hepatic pathology in relatives of patients with haemochromatosis. J Pathol Bacteriol. 1962;84:53–64. [PubMed] [Google Scholar]
- 41.Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–841. doi: 10.1136/gutjnl-2014-306842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falize L, Guillygomarc’h A, Perrin M, Lainé F, Guyader D, Brissot P, Turlin B, Deugnier Y. Reversibility of hepatic fibrosis in treated genetic hemochromatosis: a study of 36 cases. Hepatology. 2006;44:472–477. doi: 10.1002/hep.21260. [DOI] [PubMed] [Google Scholar]
- 43.Sini M, Sorbello O, Sanna F, Battolu F, Civolani A, Fanni D, Faa G, Demelia L. Histologic evolution and long-term outcome of Wilson’s disease: results of a single-center experience. Eur J Gastroenterol Hepatol. 2013;25:111–117. doi: 10.1097/MEG.0b013e328358f7da. [DOI] [PubMed] [Google Scholar]
- 44.Pradhan AM, Bhave SA, Joshi VV, Bavdekar AR, Pandit AN, Tanner MS. Reversal of Indian childhood cirrhosis by D-penicillamine therapy. J Pediatr Gastroenterol Nutr. 1995;20:28–35. doi: 10.1097/00005176-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Hammel P, Couvelard A, O’Toole D, Ratouis A, Sauvanet A, Fléjou JF, Degott C, Belghiti J, Bernades P, Valla D, et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001;344:418–423. doi: 10.1056/NEJM200102083440604. [DOI] [PubMed] [Google Scholar]
- 46.Valera JM, Smok G, Márquez S, Poniachik J, Brahm J. [Histological regression of liver fibrosis with immunosuppressive therapy in autoimmune hepatitis] Gastroenterol Hepatol. 2011;34:10–15. doi: 10.1016/j.gastrohep.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Bedossa P. Reversibility of hepatitis B virus cirrhosis after therapy: who and why? Liver Int. 2015;35 Suppl 1:78–81. doi: 10.1111/liv.12710. [DOI] [PubMed] [Google Scholar]
- 48.Haseltine EL, Penney MS, George S, Kieffer TL. Successful treatment with telaprevir-based regimens for chronic hepatitis C results in significant improvements to serum markers of liver fibrosis. J Viral Hepat. 2015;22:701–707. doi: 10.1111/jvh.12382. [DOI] [PubMed] [Google Scholar]
- 49.Wanless IR, Nakashima E, Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med. 2000;124:1599–1607. doi: 10.5858/2000-124-1599-ROHC. [DOI] [PubMed] [Google Scholar]
- 50.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 51.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 53.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 54.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 55.Ikeda H, Sasaki M, Sato Y, Harada K, Zen Y, Mitsui T, Nakanuma Y. Large cell change of hepatocytes in chronic viral hepatitis represents a senescent-related lesion. Hum Pathol. 2009;40:1774–1782. doi: 10.1016/j.humpath.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Paradis V, Youssef N, Dargère D, Bâ N, Bonvoust F, Deschatrette J, Bedossa P. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001;32:327–332. doi: 10.1053/hupa.2001.22747. [DOI] [PubMed] [Google Scholar]
- 57.Kim H, Oh BK, Roncalli M, Park C, Yoon SM, Yoo JE, Park YN. Large liver cell change in hepatitis B virus-related liver cirrhosis. Hepatology. 2009;50:752–762. doi: 10.1002/hep.23072. [DOI] [PubMed] [Google Scholar]
- 58.Hytiroglou P. Morphological changes of early human hepatocarcinogenesis. Semin Liver Dis. 2004;24:65–75. doi: 10.1055/s-2004-823097. [DOI] [PubMed] [Google Scholar]
- 59.Hytiroglou P, Park YN, Krinsky G, Theise ND. Hepatic precancerous lesions and small hepatocellular carcinoma. Gastroenterol Clin North Am. 2007;36:867–887, vii. doi: 10.1016/j.gtc.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 60.Chang O, Yano Y, Masuzawa A, Fukushima N, Teramura K, Hayashi Y. The cytological characteristics of small cell change of dysplasia in small hepatic nodules. Oncol Rep. 2010;23:1229–1232. doi: 10.3892/or_00000754. [DOI] [PubMed] [Google Scholar]
- 61.Deugnier YM, Charalambous P, Le Quilleuc D, Turlin B, Searle J, Brissot P, Powell LW, Halliday JW. Preneoplastic significance of hepatic iron-free foci in genetic hemochromatosis: a study of 185 patients. Hepatology. 1993;18:1363–1369. [PubMed] [Google Scholar]
- 62.International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983–993. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 63.Park YN, Kojiro M, Di Tommaso L, Dhillon AP, Kondo F, Nakano M, Sakamoto M, Theise ND, Roncalli M. Ductular reaction is helpful in defining early stromal invasion, small hepatocellular carcinomas, and dysplastic nodules. Cancer. 2007;109:915–923. doi: 10.1002/cncr.22460. [DOI] [PubMed] [Google Scholar]
- 64.Vasuri F, Malvi D, Gruppioni E, Grigioni WF, D’Errico-Grigioni A. Histopathological evaluation of recurrent hepatitis C after liver transplantation: a review. World J Gastroenterol. 2014;20:2810–2824. doi: 10.3748/wjg.v20.i11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park YN, Boros P, Zhang DY, Sheiner P, Kim-Schluger L, Thung SN. Serum hepatitis C virus RNA levels and histologic findings in liver allografts with early recurrent hepatitis C. Arch Pathol Lab Med. 2000;124:1623–1627. doi: 10.5858/2000-124-1623-SHCVRL. [DOI] [PubMed] [Google Scholar]
- 66.Demetris AJ, Eghtesad B, Marcos A, Ruppert K, Nalesnik MA, Randhawa P, Wu T, Krasinskas A, Fontes P, Cacciarelli T, et al. Recurrent hepatitis C in liver allografts: prospective assessment of diagnostic accuracy, identification of pitfalls, and observations about pathogenesis. Am J Surg Pathol. 2004;28:658–669. doi: 10.1097/00000478-200405000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saxena R, Crawford JM, Navarro VJ, Friedman AL, Robert ME. Utilization of acidophil bodies in the diagnosis of recurrent hepatitis C infection after orthotopic liver transplantation. Mod Pathol. 2002;15:897–903. doi: 10.1038/modpathol.3880626. [DOI] [PubMed] [Google Scholar]
- 68.Moreira RK. Recurrent hepatitis C and acute allograft rejection: clinicopathologic features with emphasis on the differential diagnosis between these entities. Adv Anat Pathol. 2011;18:393–405. doi: 10.1097/PAP.0b013e31822a5a10. [DOI] [PubMed] [Google Scholar]
- 69.Yeh MM, Larson AM, Tung BY, Swanson PE, Upton MP. Endotheliitis in chronic viral hepatitis: a comparison with acute cellular rejection and non-alcoholic steatohepatitis. Am J Surg Pathol. 2006;30:727–733. doi: 10.1097/00000478-200606000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Murakami K, Kawagishi N, Ishida K, Sekiguchi S, Fujishima F, Sasano H, Ohuchi N. Fibrosing cholestatic hepatitis developing within one month after living donor liver transplantation for chronic hepatitis C-related cirrhosis: a case report. Transplant Proc. 2014;46:995–998. doi: 10.1016/j.transproceed.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 71.Li DL, Fang J, Zheng Z, Wu W, Wu Z. Successful treatment of fibrosing cholestatic hepatitis following kidney transplantation with allogeneic hematopoietic stem cell transplantation: a case report. Medicine (Baltimore) 2015;94:e480. doi: 10.1097/MD.0000000000000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evans AT, Loeb KR, Shulman HM, Hassan S, Qiu WC, Hockenbery DM, Ioannou GN, Chauncey TR, Gretch DR, McDonald GB. Fibrosing cholestatic hepatitis C after hematopoietic cell transplantation: report of 3 fatal cases. Am J Surg Pathol. 2015;39:212–220. doi: 10.1097/PAS.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 73.Xiao SY, Lu L, Wang HL. Fibrosing cholestatic hepatitis: clinicopathologic spectrum, diagnosis and pathogenesis. Int J Clin Exp Pathol. 2008;1:396–402. [PMC free article] [PubMed] [Google Scholar]
- 74.Kattakuzhy S, Levy R, Kottilil S. Sofosbuvir for treatment of chronic hepatitis C. Hepatol Int. 2015;9:161–173. doi: 10.1007/s12072-014-9606-9. [DOI] [PubMed] [Google Scholar]
- 75.Nguyen-Khac E, Capron D. Noninvasive diagnosis of liver fibrosis by ultrasonic transient elastography (Fibroscan) Eur J Gastroenterol Hepatol. 2006;18:1321–1325. doi: 10.1097/01.meg.0000243884.55562.37. [DOI] [PubMed] [Google Scholar]


