Abstract
In addition to causing cirrhosis and hepatocellular carcinoma, hepatitis C virus (HCV) is thought to cause hypolipidemia, hepatic steatosis, insulin resistance, metabolic syndrome, and diabetes. The viral life cycle of HCV depends on cholesterol metabolism in host cells. HCV core protein and nonstructural protein 5A perturb crucial lipid and glucose pathways, such as the sterol regulatory element-binding protein pathway and the protein kinase B/mammalian target of rapamycin/S6 kinase 1 pathway. Although several lines of transgenic mice expressing core or full HCV proteins exhibit hepatic steatosis and/or dyslipidemia, whether they completely reflect the metabolic alterations in humans with HCV infection remains unknown. Many cross-sectional studies have demonstrated increased prevalences of metabolic alterations and cardiovascular events in patients with chronic hepatitis C (CHC); however, conflicting results exist, primarily due to unavoidable individual variations. Utilizing anti-HCV therapy, most longitudinal cohort studies of CHC patients have demonstrated the favorable effects of viral clearance in attenuating metabolic alterations and cardiovascular risks. To determine the risks of HCV-associated metabolic alterations and associated complications in patients with CHC, it is necessary to adjust for crucial confounders, such as HCV genotype and host baseline glucose metabolism, for a long follow-up period after anti-HCV treatment. Adipose tissue is an important endocrine organ due to its release of adipocytokines, which regulate lipid and glucose metabolism. However, most data on HCV infection and adipocytokine alteration are inconclusive. A comprehensive overview of HCV-associated metabolic and adipocytokine alterations, from bench to bedside, is presented in this topic highlight.
Keywords: Lipid, Glucose, Cardiovascular, Hepatitis C virus, Core, Nonstructural protein 5 A, Transgenic mice
Core tip: Hepatitis C virus (HCV) is thought to cause hypolipidemia, hepatic steatosis, insulin resistance, and diabetes. Its life cycle depends on host cholesterol metabolism. HCV core protein and nonstructural protein 5A perturb crucial metabolic pathways. Many cross-sectional studies have demonstrated increased cardiometabolic risks in HCV patients. Utilizing anti-HCV therapy, most cohort studies have demonstrated the favorable effects of HCV clearance in attenuating cardiometabolic risks. Adipose tissue is an important endocrine organ due to its release of adipocytokines, which strongly regulate metabolism. A comprehensive overview of HCV-associated metabolic and adipocytokine alterations, from bench to bedside, is presented in this topic highlight.
INTRODUCTION
Hepatitis C virus (HCV) infection is a substantial global health burden. HCV infects an estimated 170 million people globally, with millions newly infected each year, and chronic infections are established in the majority of infected individuals[1]. Its variants can be classified into 6 major genotypes, which differ in 30%-35% of the nucleotides in the complete genome[1]. HCV causes cirrhosis and hepatocellular carcinoma and is thought to cause metabolic alterations resulting in hypolipidemia, hepatic steatosis, insulin resistance (IR), metabolic syndrome (MS), and diabetes[2,3]. Much of the HCV life cycle is closely associated with lipid metabolism, and this association includes entry into naïve cells, infectivity, RNA replication, viral assembly and viral secretion[2]. Furthermore, HCV core protein[4-10] and nonstructural (NS) protein 5A participate in crucial lipid and glucose metabolic pathways in host cells[11-14]. Additionally, some organelles harbor virions and/or viral proteins during the HCV life cycle, leading to increased oxidative stress[15], which in turn modifies cellular metabolism. These factors contribute to a cascade of systemic metabolic alterations in the host. Although several lines of transgenic mice expressing either HCV core[16-21] or full HCV proteins[22,23] have demonstrated phenotypes revealing metabolic alterations and have revealed potentially altered pathways, whether these mice completely reflect the effects of human HCV infection remains unknown. Furthermore, conflicting data exist among various cross-sectional human studies on HCV-associated metabolic alterations. The inconsistencies primarily result from individual variations, including the different viral and host factors studied. The eradication of HCV by either interferon-based therapy[24] or direct-acting antiviral (DAA) drugs[25] provides the opportunity to study the causal relationship between HCV infection and metabolic alterations in the same individuals without individual variation. In contrast, hepatitis B virus (HBV), which infects 350 million individuals worldwide, is another main pathogen leading to liver cirrhosis and hepatocellular carcinoma[26], and conflicting data regarding its association with hypolipidemia have been reported[27,28]. Chronic HBV infection is not associated with hepatic steatosis, IR, or diabetes[29]. Hepatic steatosis may even promote spontaneous hepatitis B surface antigen seroconversion[30]. Furthermore, data on HBV-associated metabolic alterations are mainly based on case-control studies rather than cohort studies to view the influence of viral clearance[31]. Together, host metabolic alterations are much less associated with HBV infection than with HCV infection.
Adipose tissue has emerged as an important endocrine organ due to its release of adipocytokines[32], which regulate lipid and glucose metabolism via the adipoinsular axis[33]. Because both HCV infection and alterations in adipocytokines are critical in lipid and glucose metabolism, their potential relationship has attracted attention[34,35]. However, most data regarding HCV infection and adipocytokine alterations are inconclusive.
Thus, the current review aims to provide a comprehensive overview of HCV-associated metabolic and adipocytokine alterations, from bench to bedside, to serve as a cornerstone for future research and clinical practice.
HCV GENOME, PROTEINS AND LIFE CYCLE
HCV, a member of the Hepacivirus genus within the Flaviviridae family, has a viral genome consisting of single-stranded RNA with positive polarity that is approximately 9.5 kb long[36]. Untranslated regions (UTRs) located at the 5’ and 3’ ends of the genome flank a single open reading frame (ORF), which encodes a polyprotein of approximately 3000 amino acids[36]. The polyprotein is processed by viral and cellular proteases that produce mature viral structural and NS proteins. Structural proteins, including the core protein and envelope glycoprotein 1 (E1) and E2, are encoded in the N-terminal region of the ORF, whereas NS proteins, including NS1, NS2, NS3, NS4B, NS5A and NS5B, reside in the C-terminal region (Figure 1). HCV core protein is not only a component of the viral nucleocapsid but also a multifunctional protein that modulates viral and cellular gene expression[37]. The assembly of HCV requires a platform of cellular lipid droplets and interactions between NS5A and the core protein[38] (Figure 2). Thus, most HCV-associated metabolic alterations in hosts involve HCV core[4-10] and NS5A[11-14] proteins. Assembled particles bud into the endoplasmic reticulum (ER) and traffic through the secretory pathway, from which they are exported from the cell in conjunction with lipoprotein secretory pathways[39,40]. In the blood, HCV particles are heterogeneous in size and density as a result of their association with serum lipoproteins, namely, lipoviral particles (LVPs)[41] (Figure 2).
Figure 1.
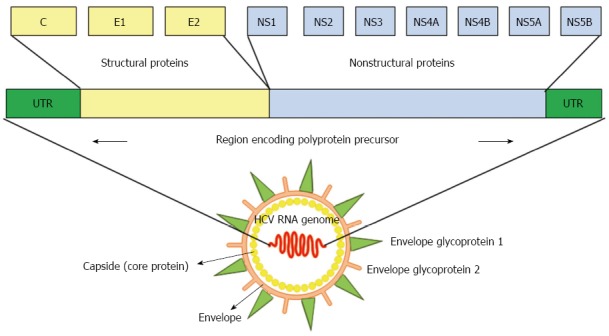
Diagram of the hepatitis C viral genome. Hepatitis C virus is a single-stranded RNA virus, and its genomic organization shows highly conserved 5’ and 3’ nonstructural proteins. UTR: Untranslated region; C: Core protein; E1 and E2: Envelope glycoprotein 1 and 2; NS: Nonstructural protein.
Figure 2.
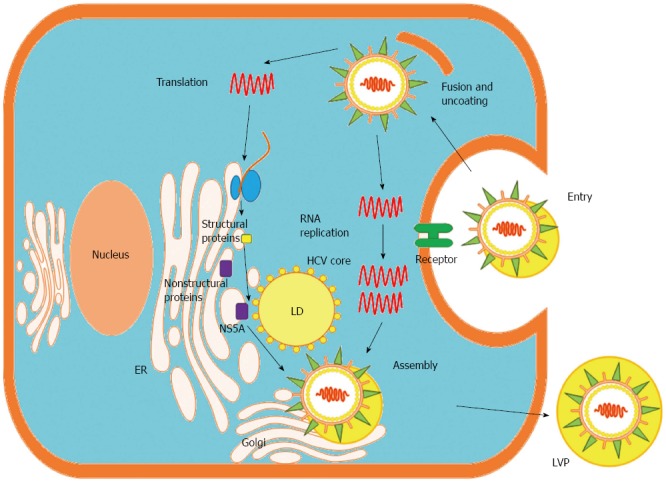
Life cycle of hepatitis C virus in the hepatocyte. Hepatitis C virus (HCV) LVPs enter hepatocytes via receptor-mediated endocytosis. Released viral RNA is translated at the endoplasmic reticulum (ER), producing a single polyprotein precursor that is cleaved by host and viral proteases. The viral NS proteins (e.g., NS5A protein) form RNA replication complexes in lipid rafts, where positive-strand RNA is replicated via a negative-strand intermediate. Newly synthesized positive-strand RNA is encapsidated by the HCV core protein in close proximity to LDs, and envelope glycoproteins are acquired through budding into the ER lumen. LVPs mature in the ER through interactions with lipoproteins and exit the cell via the cellular Golgi apparatus. LD: Lipid droplet; LVP: Lipoviral particle; Golgi: Golgi apparatus.
METABOLIC ALTERATIONS AND HCV: IN VITRO STUDIES
Most clinically isolated HCV is difficult to replicate in cultured cells[42]. Therefore, cells harboring HCV subgenomic replicons are widely used to study HCV replication[43]. Although very effective at replication, the replicon system is unable to produce infectious HCV particles. In contrast, a strain of genotype 2 (G2) HCV is capable of replicating in Huh7 cells and producing HCV particles that are infectious in cultured cells[44,45]. HCV particles produced through cell culture (referred to as HCVcc) are able to establish long-term infections in chimpanzees and in mice containing human liver grafts[46]. Pseudoparticles of HCV (HCVpps) are retroviral nucleocapsids surrounded by a lipid envelope containing authentic HCV glycoprotein complexes[47]. HCVpp is an ideal system for studying receptor binding and entry and has been used to characterize neutralizing antibodies[48]. Most data from in vitro HCV studies use the aforementioned systems.
Lipid metabolism
The HCV life cycle is closely associated with the cholesterol and lipogenesis pathways in hepatocytes. HCV influences host lipid metabolism in three ways, causing enhanced lipogenesis, impaired degradation and impaired export[2] (Figure 3). Hepatic steatosis arises from these conditions.
Figure 3.
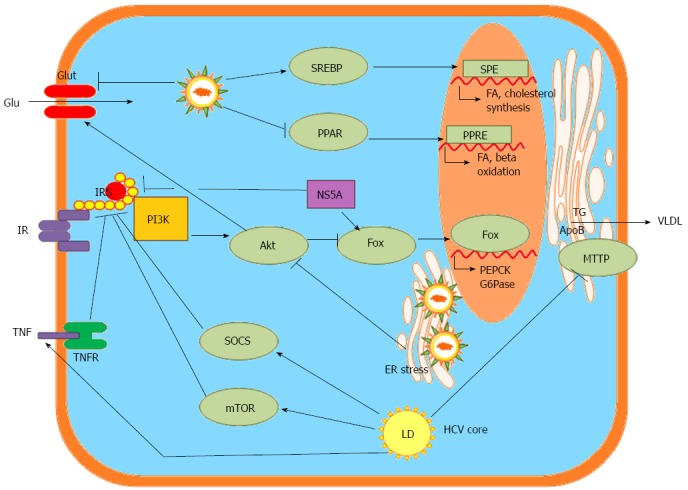
Hepatitis C virus-associated metabolic alterations in the hepatocyte, data from bench studies. SREBP: Sterol regulatory element-binding proteins; PPAR: Peroxisome proliferator-activated receptors; Glu: Glucose; Glut: Glucose transporter; ER: Endoplasmic reticulum; SRE: SREBP response element; FA: Fatty acid; PPRE: PPAR response element; PI3K: Phosphatidylinositide 3-kinase; Akt: Protein kinase B: Fox: Transcription factor forkhead box; PEPCK: Phosphoenolpyruvate carboxykinase; G6Pase: Glucose 6-phosphatase; IR: Insulin receptor; IRS: Insulin receptor sustrate; NS5A: HCV nonstructural protein 5 A; TG: Triglyceride; ApoB: Apoliporptoein B; MTTP: Microsomal triglyceride transfer protein; VLDL: Very low-density lipoprotein-cholesterol; SOCS: Suppressor of cytokine signaling proteins; mTOR: Mammalian target of rapamycin; TNF: Tumor necrosis factor; TNFR: Tumor necrosis factor receptor; LD: Lipid droplet.
Enhanced lipogenesis: Inhibiting sterol regulatory element-binding protein (SREBP) activation by treatment with 25-hydroxycholesterol[49], cholesterol 25-hydroxylase[50], nordihydroguaiaretic acid[51] and subtilisin/kexin-isozyme-1 or site-1 protease[52] blocks HCV replication. Fatty acid synthase (FAS), an enzyme primarily involved in the de novo synthesis of fatty acids, is up-regulated during HCV infection, and the inhibition of FAS activity inhibits HCV replication and release[53,54]. One product of the mevalonate pathway produced during the synthesis of cholesterol, geranylgeranyl lipid, that is required for HCV RNA replication, as shown by experiments in which the inhibition of HCV RNA replication by lovastatin was overcome by the addition of geranylgeraniol[55,56]. This finding is further supported by the observation that HCV replication can be blocked by an inhibitor of geranylgeranyl transferase I[55]. Inhibitors of the synthesis of triacylglycerides and cholesterol esters, triacsin C and YIC-C8-434, which inhibit long-chain acyl-CoA synthetase and acyl-CoA:cholesterol acyltransferase, respectively, reduce HCV RNA synthesis[57]. These observations highlight the importance of up-regulating the de novo synthesis of fatty acids and cholesterol to enhance the availability of important lipid constituents and to establish efficient HCV replication[2]. Altered pathways have been documented in HCV replicon-expressing cells using the Kyoto Encyclopedia of Genes and Genomes Pathway database; these pathways include mitogen-activated protein kinase, steroid biosynthesis, steroid biosynthesis and sphingolipid metabolism pathways, which are required for efficient HCV replication[58].
Impaired degradation: HCV impairs mitochondrial lipid β-oxidation, which results in low lipid combustion and the inhibition of mitochondrial trifunctional protein by HCV, as noted in HCV-infected hepatocytes[59]. Additionally, a systems biology approach identified the mitochondrial fatty acid oxidation enzyme dodecenoyl coenzyme A delta isomerase as a bottleneck protein controlling host metabolic reprogramming during HCV infection[60].
Impaired export: HCV has been shown to impede lipid export from the liver by reducing microsomal triglyceride transfer protein (MTTP) activity in animal studies[16].
Viral proteins and associated alterations to lipid metabolism: HCV infection, mainly through the activity of the HCV core protein, decreases the expression and activity of peroxisome proliferator-activating receptor (PPAR)-α/γ in hepatocytes[61]. HCV core protein localizes in the membrane of lipid vesicles and induces hepatic fat accumulation by activating SREBP-1c[6,7]. An in vitro interaction between HCV core protein and apolipoprotein AII has also been reported[8]. It has been suggested that HCV core protein directly interacts with retinoid X receptor α, a transcriptional regulator that controls many cellular functions, including lipid metabolism[9]. HCV core protein acts as a pathogenic factor involved in lipid droplet accumulation, changes in lipogenic gene expression, and/or the activity of lipogenic proteins in a genotype-specific manner[62]. Amino acid substitutions at positions 182 and 186 of genotype 3a (G3a) HCV and at amino acid 70/Q of genotype 1b (G1b) HCV affect lipid metabolism and contribute to the development of steatosis[63]. Hepatic steatosis is most common in patients infected with genotype 3 (G3) HCV, possibly due to the direct effects of G3 HCV core proteins[64]. However, HCV core protein may not be the only viral protein involved in HCV-induced steatosis. An interaction between HCV NS5A and apolipoprotein AI was observed in vitro, and core protein/NS5A colocalization was observed in cytoplasmic lipid droplets after transfection[12,13]. The expression of HCV NS5A in human hepatoma cells increased lipid droplet formation through enhanced lipogenesis and the transcriptional expression of PPARγ coactivator (PGC)-1α and diacylglycerol acyltransferase-1 but reduced the transcriptional expression of MTTP and PPARγ[11,12].
MicroRNA 122: Although microRNA 122 (miR-122) promotes the accumulation of HCV RNA through a direct interaction with viral RNA and stimulates the mevalonate pathway in the liver, the inhibition of miR-122 has negligible effects on the rate of 3-hydroxy-3-methyl-glutaryl-CoA reductase RNA synthesis. These findings suggest that miR-122 does not directly affect HCV RNA abundance through the mevalonate pathway[65].
Glucose metabolism
Altered pathways: HCV down-regulates glucose transporter 2 (GLUT2), which transports glucose to hepatocytes, and up-regulates the genes for phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6Pase), which are rate-limiting enzymes for hepatic gluconeogenesis. PEPCK and G6Pase are regulated by the transcription factor forkhead box O1 (FoxO1)[66]. The phosphorylation of FoxO1 was diminished in HCV-infected cells, resulting in an increased nuclear accumulation of FoxO1[67]. Additionally, increased hepatic expression of PGC-1α has been implicated in the elevation of G6Pase secondary to HCV infection[68]. HCV modulates the protein kinase B/mammalian target of rapamycin/S6 kinase 1 (Akt/mTOR/S6K1) signaling cascades by increasing tumor necrosis factor production while enhancing the activity of suppressor of cytokine signaling 3 (SOCS-3)[66,69] in inhibiting insulin receptor substrate (IRS) function to perturb glucose metabolism via the down-regulation of GLUT4 and the up-regulation of PCK2 for IR[10,70] (Figure 3).
Viral proteins and associated alterations to glucose metabolism: HCV proteins associate with mitochondria and the ER to promote oxidative stress, which involves p38 mitogen-activated protein kinase and activates nuclear factor kappa B[71]. HCV core protein induces the proteasomal degradation of IRS-1 and IRS-2, blocking intracellular insulin signaling[10]. Genotype-specific impairment of insulin signaling was observed during HCV infection; the expression of the G3 HCV core protein led to the down-regulation of PPARγ and the up-regulation of SOCS-7, whereas the G1 core protein activated mTOR[72]. HCV NS5A was directly linked to FoxO1-dependent increased gluconeogenesis[67] and to cellular hexokinase 2, the first rate-limiting enzyme of glycolysis[14]. HCV NS5A also increased the serine phosphorylation of IRS-1, thereby hampering metabolic activity and contributing to IR[11] (Figure 3).
HCV and cellular organelles: Evidence suggests that the impairment of mitochondrial functions, including the modification of metabolic fluxes, fatty acid oxidation, the generation and elimination of oxidative stress, Ca2+ signaling and apoptosis, plays a central role in HCV-associated metabolic alterations, particularly as several HCV proteins localize to mitochondria[73]. Attention has been focused on the PPARs due to their role in controlling liver lipid metabolism[74]. HCV infection also induces ER stress that results in the up-regulation of PGC-1α[75]. Upon envelopment at the ER, HCV exits the cell via the secretory pathway, as shown by the localization of HCV core protein to the Golgi and its co-trafficking with components of the recycling endosome[76].
Generalized metabolic alterations: Proteomic and lipidomic profiling performed in acute HCV-infected Huh-7.5 cells has shown that HCV induces early perturbations in glycolysis, the pentose phosphate pathway, and the citric acid cycle; these changes favor host biosynthetic activities supporting viral replication and propagation. These effects are followed by a compensatory shift in metabolism aimed at maintaining energy homeostasis and cell viability during elevated viral replication and increasing cellular stress. Thus, HCV infection may be associated with a delay in cell cycle progression that is accompanied by an adaptive metabolic response aimed at channeling substrates from synthetic to energetic purposes[77]. In a persistently HCV-infected cell line displaying prominent steatosis and supporting HCV infection for more than 2 years, the citric acid cycle was preferentially facilitated over the glycolysis pathway with marked increases in most amino acids[78]. Another study involving transcriptome sequencing, microarray analysis, and proteomic analyses of HCV infection in Huh 7.5 cells showed that HCV caused X receptor/retinoic acid receptor activation as a potential host antiviral response, and integrin-linked kinase signaling served as an entry factor. These responses also led to increases in cellular cholesterol and free fatty acid levels, which were associated with a profound and specific decrease in cellular glucose levels[79].
METABOLIC ALTERATIONS AND HCV: ANIMAL STUDIES
Studies using constitutional HCV core transgenic mice have demonstrated the augmented production of oxidative stress and the activation of the scavenging system, including catalase and glutathione[17,79]. Together with the observed activation of PPARα[80], these findings may account for the hepatic steatosis induced by HCV infection[17]. In another line of constitutional HCV core transgenic mice, core expression led to reductions in MTTP activity and in the particle size of nascent hepatic very low-density lipoprotein cholesterol, hampering lipid export from the liver[16]. Using a line of conditional HCV core transgenic mice, we have shown the topological relationship between HCV core protein and hepatic lipid vesicles[19]; we also demonstrated that HCV core-induced nonobese hepatic steatosis is associated with the down-regulation of the leptin gene in visceral fat and concurrent hypoadiponectinemia[20], and gene expression analyses in HCV core transgenic mice revealed SREBP pathway activation and the dysregulation of genes involved in lipid metabolism, including 3-hydroxy-3-methylglutaryl-coenzyme A synthase 1, apolipoprotein AII, apolipoprotein CI, acyl-CoA thioesterase I, and fatty acid binding protein 1[21]. In transgenic mice expressing the full-length HCV ORF, hepatic steatosis was associated with reduced plasma triglyceride levels. Triglyceride secretion was impaired, whereas activated lipogenesis was evidenced by increased lipogenic enzyme transcription resulting from the maturational activation and nuclear translocation of SREBP1c[22]. Another transgenic mouse line expressing all HCV proteins showed that fatty acid synthase was redistributed from its normal periportal expression into the midzone of the lobule. The alteration of zonation was not limited to lipogenic enzymes and appeared to be driven by systemic signaling via the Wnt/β-catenin pathway. These results help to explain the systemic effects of HCV on liver metabolism, which are triggered by a minority of infected cells[23]. HCV-infected Tupaia belangeri chinensis demonstrated a perturbation of the taurine, hypotaurine, ether lipid, glycerophospholipid, arachidonic acid, tryptophan, and primary bile acid metabolism pathways[81].
METABOLIC ALTERATIONS AND HCV: HUMAN STUDIES
Cross-sectional studies
In cross-sectional human studies, HCV genotype, baseline glucose profile and ethnicity were crucial confounders for metabolic alterations[82-90]. The metabolic alterations and complications associated with HCV infection are discussed below (Figure 4):
Figure 4.
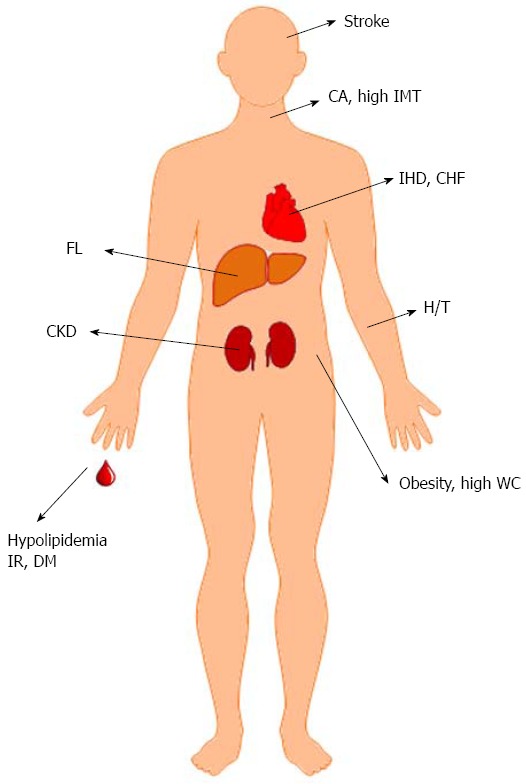
Hepatitis C virus-associated metabolic alterations and cardiovascular events, data from human studies. CA: Carotid atherosclerosis; IMT: Intima-media thickness; IHD: Ischemic heart disease; CHF: Congestive heart failure; FL: Fatty liver; H/T: Hypertension; WC: Waist circumference; IR: Insulin resistance; DM: Diabetes; CKD: Chronic kidney disease.
Hepatic steatosis and hypolipidemia: The overall prevalence of hepatic steatosis in patients with HCV infection is 55.54%, which is higher than in uninfected individuals[91]. In contrast to non-alcoholic fatty liver disease (NAFLD), which is usually associated with hyperlipidemia[92], chronic hepatitis C (CHC) is strongly linked to hypolipidemia, including hypo-cholesterolemia, hypo-triglyceridemia and low low-density lipoprotein (LDL) cholesterol levels[93,94]. The presence of NAFLD in patients with HCV is strongly associated with features of MS and is a risk factor for advanced fibrosis[95]. In G3-HCV infections, hepatic steatosis is related to viral load and hypolipidemia but not to metabolic factors and is termed “viral steatosis.” In G1, G2, or G4 HCV infections, steatosis appears to be secondary to IR/MS and is regarded as “metabolic steatosis”[85,96-98]. MTTP may play a central role in HCV-related steatosis because it is modulated by different genotype-specific mechanisms, mainly hyperinsulinemia in non-G3 CHC patients, and by more profound and direct virus-related effects in G3 CHC individuals[99]. Viral steatosis as observed in G3 CHC does not contribute significantly to liver fibrosis. IR, rather than steatosis, was independently associated with fibrosis for both G1 and G3 HCV[100]. However, other studies with 160 to 3026 CHC patients confirmed the positive role of steatosis in G3 CHC in accelerating fibrosis[101,102]. HCV-associated hypolipidemia is most prominent in G3 CHC[103]. A proportional relationship between serum lipid profiles and G2 HCV viral load has been reported based on a study of more than 500 Asian CHC patients[87]. Concordantly, triglyceride levels have been shown to be associated with HCV levels in Western G1 CHC patients[104]. In a study of the impacts of genetics on metabolic alterations, Caucasian G1 CHC patients with the CC polymorphism in interleukin-28B (IL28B) had higher levels of total cholesterol and LDL-cholesterol, lower levels of triglycerides, and a lower prevalence of IR and moderate-severe steatosis than patients without this genotype[105]. Moreover, an inverse correlation between microvesicular steatosis and level of autophagy was reported[106]. The activation of hepatic cannabinoid receptor 1 [CB(1)] is associated with steatosis and fibrosis, and CB(1) is up-regulated and is associated with increased steatosis in G3 CHC patients[107].
IR, diabetes, MS and obesity: High prevalences of IR, diabetes, MS and obesity (increased levels of mesenteric fat) in CHC patients compared with controls have been demonstrated in several studies[82,107-110]. IR was shown to be associated with high serum HCV RNA levels in G1, G2, G3 and G4 patients[82,83], but IR was more common in patients with G1 and G4 than in those with G2 and G3[84], or more common in those with G1, 2, and 4 than in those with G3 HCV infection[85]. Whether a dose-response relationship between the HCV RNA level and the presence of IR exists in Asian G1 and G2 CHC patients remains unclear[86,88,105]. MS was more frequent in G1 patients than in G2 patients, and MS and G1 were significantly related to SOCS-3 overexpression[89]. Among nondiabetic CHC patients, IR does not seem to be associated with viremia[90]. Ethnicity and BMI might be individually associated with the progression of fibrosis and the presence of cirrhosis[111,112] because Hispanics had the highest fibrosis indices and prevalences of cirrhosis, whereas African Americans had the lowest[112]. Visceral obesity was associated with high viral loads and histological damage in elderly (≥ 60 year) patients with reduced adiponectin levels[113]. In a study of the effects of genetics on IR, G2 CHC patients carrying the patatin-like phospholipase domain containing 3 protein (PNPLA3) I148M allele had increased IR and lower viral loads at baseline[114]. However, among non-diabetic G1 and G4 CHC patients, the rs738409 (PNPLA3) GG genotype was associated with advanced fibrosis and steatosis but not with IR. In contrast, the IL28B non-CC genotype was an independent risk factor for IR[115].
Cardiovascular events: Despite the favorable lipid profile caused by HCV infection noted above, many studies have shown an unfavorable role of HCV infection in cardiovascular events. Higher waist circumferences, hypertension rates[116,117], prevalences of late chronic kidney disease[118], stroke and past ischemic heart disease[119], intima-media thicknesses (IMT, an index of early atherosclerosis)[120], rates of congestive heart failure[117], serum homocysteine levels[121] and rates of carotid atherosclerosis (CA)[122] were noted in CHC patients compared with normal controls. However, a Japanese study of 88 CHC patients showed that carotid IMT was reduced in CHC patients compared with controls[123]. Notably, IMT, carotid plaques and coronary heart disease were found to be significantly associated with HCV infection only after adjustment for “classical” cardiovascular risk factors, particularly LDL cholesterol and systolic blood pressure[110]. These results may suggest that HCV affects cardiovascular risk mainly via non-conventional pathways rather than by virus-induced metabolic modifications, such as IR and good lipoprotein profiles, which may balance one another[124]. Furthermore, HCV RNA levels were found to be independently associated with CA in both the early phases of IMT lesions and the advanced phases of plaques[122]. Direct viral invasion of cardiovascular tissues and systemic inflammation caused by HCV infection are potentially responsible for the high rates of cardiovascular events in CHC patients[125].
Longitudinal studies
The combination of pegylated interferon (Peg-IFN)-α and ribavirin has provided a “cure” for a considerable proportion of patients with CHC, particularly in patients with the favorable IL28B genotype[24]. These cure rates were further improved by replacing interferon-based therapy with potent DAA drugs[25]. Thus, the many longitudinal studies of CHC patients receiving Peg-IFN-based or DAA therapy provide a landscape in which to study metabolic alterations and the associated manifestations caused by HCV clearance by comparing pre- and post-anti-HCV treatment metabolic profiles.
Predictors for the therapeutic failure of anti-HCV therapy: High waist circumference[126], high homeostatic model assessment (HOMA)-IR (for G1b, G2, G3 and G4 HCV infection)[105,127-129], high pre-anti-HCV treatment HCV RNA level, old age (for G1b)[128], low serum total and LDL-cholesterol and oxidative stress (for G1, 2 and 3)[130], steatosis (for non-G3)[131,132], DM[133], IL28B non-CC genotype (for G1 and 4)[129] and high serum uric acid level[134] predict anti-HCV treatment failure. Although hepatic steatosis is associated with lower sustained virological response (SVR), this effect is attenuated by IL28B in G1 Caucasian CHC patients[135]. Furthermore, studies of 96 to 932 CHC patients have shown that steatosis independently predicts relapse in G3 CHC patients with SVR[136,137]. However, IR does not predict rapid virologic responses or SVRs in CHC patients without MS[138,139].
Hepatic steatosis and hypolipidemia: Although the reversal of both hepatic steatosis and hypolipidemia has been reported only in G3 CHC patients and is not shared by other genotypes[140], accumulating evidence demonstrates that the reversal of hypolipidemia is not genotype specific[3,141]. In a study of genotype-specific HCV-associated lipid alteration, G2 CHC patients were shown to benefit more than G1 CHC patients from viral clearance resulting from lipid alterations, particularly in those without baseline IR[3]. Using a targeted paired cholesterol metabolomics study of CHC patients who received anti-HCV therapy, G3 but not G2 HCV was shown to selectively interfere with the late cholesterol synthesis pathway, as shown by lower distal sterol metabolites and preserved lanosterol levels. This distal interference resolves with SVR[142].
IR, diabetes, MS and obesity: Among CHC patients without baseline glucose abnormalities, HCV clearance did not reduce the risk of glucose intolerance[143]. However, another study showed that among non-diabetic CHC patients, HCV ameliorates β-cell function[144]. The data on genotype-specific effects are even more diverse. For example, the eradication of HCV was thought to reduce the incidences of type 2 diabetes in both G1 and G2 patients[145]. However, reduced IR at 12 wk after treatment was observed in G1 but not G2 or G3 patients with SVR[146]. Concordantly, a study based on Virahep-C showed that among G1 CHC patients with IR before treatment, viral clearance results in improvements in the HOMA-IR index 24 wk after treatment completion[147]. Although a prospective study that enrolled non-diabetic G1, 2, 3 and 4 CHC patients failed to demonstrate any differences between the mean pre- and 24 wk post-anti-HCV treatment HOMA-IR values in patients with SVR, there was an increased rate of de novo IR in non-SVR patients compared with SVR patients 24 mo after treatment completion, regardless of viral genotype[148]. All of the above results indicate that follow-up > 24 wk after treatment completion is essential for studying favorable glucose metabolism alterations after HCV viral clearance, especially in G1 CHC patients, after adjusting for baseline metabolism. In a study of patients who underwent orthotopic liver transplantation (OLT) in the setting of recurrent HCV after OLT, MS was strongly associated with long-term fibrosis progression[149]. CHC subjects were more likely to be overweight and obese at the time of transplant, and these conditions are associated with a higher risk of post-transplant diabetes that persists for up to 5 years post-transplant compared with that for CHB patients[150]. Interestingly, a study of G1 CHC patients showed that a high visceral adiposity index score is independently associated with steatosis and has a direct correlation with viral load[151].
Cardiovascular events: A retrospective United Kingdom cohort study of 4809 HCV-infected patients and 71668 controls failed to demonstrate different incidences of myocardial infarction between HCV-infected and HCV-uninfected patients during a median follow-up of 3.2 years[152]. In contrast, a community-based prospective Taiwanese cohort study of 1095 anti-HCV seropositive and 18541 anti-HCV seronegative patients showed higher circulatory and renal disease mortality in anti-HCV seropositive than in anti-HCV seronegative patients during an average follow-up period of 16.2 years[153]. Several large population-based cohort studies using the Taiwan National Health Insurance Research Database have recently shown that anti-HCV therapy is associated with decreased 8-year cumulative cardiovascular incidences in CHC patients[154,155]. These conflicting reports suggest the importance of follow-up duration (likely more than 8 years) when evaluating the effects of HCV infection on cardiovascular complications.
The HCV-associated cardiometabolic diseases and their recoverability after viral clearance, which were mainly obtained from longitudinal studies, are listed in Table 1.
Table 1.
The reversibility of hepatitis C virus-associated cardiometabolic diseases after viral clearance
| HCV-associated cardiometabolic diseases | Reversible after viral clearance | Ref. |
| Hypolipidemia | Yes | [3,110,140-142] |
| Hepatic steatosis | Yes | [140] |
| Obesity | No | [110] |
| Glucose intolerance, insulin resistance and diabetes | No | [110,143] |
| Yes | [145-148] | |
| Cardiovascular events | No | [152] |
| Yes | [153-155] |
HCV: Hepatitis C virus.
HCV infection and adipocytokine alterations
Adipocytokines, including leptin[156], adiponectin[157-162,164-167], plasminogen activator inhibitor-1 (PAI-1)[168], visfatin[169], retinol-binding protein 4 (RBP4)[170,171] and resistin[172-176], are discussed below:
Leptin: In a study of 42 patients, the serum leptin levels in CHC patients were higher than in controls[156].
Adiponectin: The data regarding adiponectin alteration and its correlation with HCV viral load are quite diverse among studies involving various HCV genotypes. For example, G3 CHC patients showed lower adiponectin levels than those of patients with other genotypes[157]. High HCV load and G2 were significantly associated with lower serum adiponectin levels[158]. Adiponectin level increases with the progression of hepatic fibrosis but is not related to viral load in G4 CHC patients[159]. In G1 or G3 CHC patients, adiponectin was associated with steatosis only in males and paradoxically increased with hepatic inflammation[136]. IR was associated with a decrease in adiponectin in G3 but not G1 CHC patients[160]. Adiponectin levels were significantly decreased in G1 and G3 CHC patients[161]. Whether HCV viral clearance leads to hyper- or hypo-adiponectinemia remains unclear and may differ between G3 and G4 HCV[159,163]. The lack of clarity regarding HCV infection and adiponectin alterations may stem from heterogeneous hepatic pathologies, metabolic conditions and immune reactions of the patients involved in various studies. In patients with CHC, fibrosis and steatosis are associated with hyperadiponectinemia and hypoadiponectinemia, respectively[164,165]. Furthermore, adiponectin is negatively correlated with IR, hepatic steatosis and MS[166]. The positive role of serum adiponectin in anti-HCV specific immune responses has been demonstrated[161]. Thus, after SVR, the decrease in adiponectin in G4[159] patients may reflect the reversal of hepatic fibrosis and hypo-triglyceridemia, whereas the increase in adiponectin in G3[163] patients may indicate an improvement in hepatic steatosis, which is most evident in G3 CHC[167].
PAI-1: Serum PAI-1 levels were identified as positive predictors of interferon-based therapeutic response[168].
Visfatin: No correlation between visfatin and HCV genotypes, viral load, or treatment response to Peg-IFN/ribavirin therapy has been shown[169].
RBP4: CHC patients had lower RBP4 levels than did control subjects. Higher RBP4 levels were linked to lower alanine aminotransferase, hyperlipidemia and high HOMA-IR in CHC patients[170]. Only patients with SVR had higher post-anti-HCV treatment RBP4 levels than pre-anti-HCV treatment levels[171].
Resistin: Hyper-resistinemia in CHC patients has been consistently reported[172-175]. This condition is reversed after viral clearance[176] and determines moderate to severe fibrosis[174] but is not associated with therapeutic response[176].
CONCLUSIONS AND PROSPECTIVE STUDIES
Using in vitro systems and animal models, the basis for HCV-associated metabolic alterations has been elucidated in detail in the literature. However, in human studies, various viral factors, especially HCV genotype, and host factors, including IL28B genotype, ethnicity and baseline metabolic conditions, may obscure metabolic alterations and complications attributed to HCV. By using anti-HCV therapy, prospective studies of CHC patients with viral clearance after anti-viral therapy followed by long periods of off-therapy observation provide the opportunity to study genuine metabolic homeostasis and establish personalized care for CHC patients. The future challenge for hepatologists, in an era in which almost all HCV is eradicable by potent DAAs, will be to determine whether hepatitis C virus-associated metabolic alterations and cardiovascular events are completely reversible or whether some are aggravated after viral clearance by anti-hepatitis C therapy. These discoveries will help to provide personalized care for patients with chronic or past HCV infection.
ACKNOWLEDGMENTS
The author thanks Mr. Chun-Ming Fan and Ms. Shiang-Chi Chen for their excellent assistance with delicate illustrations, and Mr. Chun-Kai Liang for his assistance with word processing.
Footnotes
Supported by Grants from the Chang Gung Medical Research Program, No. CMRPG380353, No. CMRPG3B1251, No. CMRPG3B0401, No. CMRPG3B1741, No. CMRPG3B1742 and No. XMRPG3A0521; and the National Science Council, Taiwan, No. 100-2314-B-182-069-, No. 101-2314-B-182-083- and No. 102-2628-B-182-021-MY3.
Conflict-of-interest statement: The author has no conflict of interest to report.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 4, 2015
First decision: July 14, 2015
Article in press: October 26, 2015
P- Reviewer: Montalto G, Takagi H S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Syed GH, Amako Y, Siddiqui A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21:33–40. doi: 10.1016/j.tem.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang ML, Tsou YK, Hu TH, Lin CH, Lin WR, Sung CM, Chen TH, Cheng ML, Chang KC, Chiu CT, et al. Distinct patterns of the lipid alterations between genotype 1 and 2 chronic hepatitis C patients after viral clearance. PLoS One. 2014;9:e104783. doi: 10.1371/journal.pone.0104783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koike K, Tsutsumi T, Yotsuyanagi H, Moriya K. Lipid metabolism and liver disease in hepatitis C viral infection. Oncology. 2010;78 Suppl 1:24–30. doi: 10.1159/000315226. [DOI] [PubMed] [Google Scholar]
- 5.Goldwasser J, Cohen PY, Lin W, Kitsberg D, Balaguer P, Polyak SJ, Chung RT, Yarmush ML, Nahmias Y. Naringenin inhibits the assembly and long-term production of infectious hepatitis C virus particles through a PPAR-mediated mechanism. J Hepatol. 2011;55:963–971. doi: 10.1016/j.jhep.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPherson S, Jonsson JR, Barrie HD, O’Rourke P, Clouston AD, Powell EE. Investigation of the role of SREBP-1c in the pathogenesis of HCV-related steatosis. J Hepatol. 2008;49:1046–1054. doi: 10.1016/j.jhep.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Enjoji M, Kohjima M, Kotoh K, Nakamuta M. Metabolic disorders and steatosis in patients with chronic hepatitis C: metabolic strategies for antiviral treatments. Int J Hepatol. 2012;2012:264017. doi: 10.1155/2012/264017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T, et al. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200–1205. doi: 10.1073/pnas.94.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsutsumi T, Suzuki T, Shimoike T, Suzuki R, Moriya K, Shintani Y, Fujie H, Matsuura Y, Koike K, Miyamura T. Interaction of hepatitis C virus core protein with retinoid X receptor alpha modulates its transcriptional activity. Hepatology. 2002;35:937–946. doi: 10.1053/jhep.2002.32470. [DOI] [PubMed] [Google Scholar]
- 10.Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127–2133. doi: 10.1002/hep.22269. [DOI] [PubMed] [Google Scholar]
- 11.Parvaiz F, Manzoor S, Iqbal J, McRae S, Javed F, Ahmed QL, Waris G. Hepatitis C virus nonstructural protein 5A favors upregulation of gluconeogenic and lipogenic gene expression leading towards insulin resistance: a metabolic syndrome. Arch Virol. 2014;159:1017–1025. doi: 10.1007/s00705-013-1892-3. [DOI] [PubMed] [Google Scholar]
- 12.Shi ST, Polyak SJ, Tu H, Taylor DR, Gretch DR, Lai MM. Hepatitis C virus NS5A colocalizes with the core protein on lipid droplets and interacts with apolipoproteins. Virology. 2002;292:198–210. doi: 10.1006/viro.2001.1225. [DOI] [PubMed] [Google Scholar]
- 13.Castera L, Chouteau P, Hezode C, Zafrani ES, Dhumeaux D, Pawlotsky JM. Hepatitis C virus-induced hepatocellular steatosis. Am J Gastroenterol. 2005;100:711–715. doi: 10.1111/j.1572-0241.2005.40898.x. [DOI] [PubMed] [Google Scholar]
- 14.Ramière C, Rodriguez J, Enache LS, Lotteau V, André P, Diaz O. Activity of hexokinase is increased by its interaction with hepatitis C virus protein NS5A. J Virol. 2014;88:3246–3254. doi: 10.1128/JVI.02862-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 16.Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185–194. doi: 10.1096/fj.01-0396com. [DOI] [PubMed] [Google Scholar]
- 17.Koike K. Hepatitis C virus contributes to hepatocarcinogenesis by modulating metabolic and intracellular signaling pathways. J Gastroenterol Hepatol. 2007;22 Suppl 1:S108–S111. doi: 10.1111/j.1440-1746.2006.04669.x. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka N, Moriya K, Kiyosawa K, Koike K, Gonzalez FJ, Aoyama T. PPARalpha activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in mice. J Clin Invest. 2008;118:683–694. doi: 10.1172/JCI33594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang ML, Chen JC, Yeh CT, Sheen IS, Tai DI, Chang MY, Chiu CT, Lin DY, Bissell DM. Topological and evolutional relationships between HCV core protein and hepatic lipid vesicles: studies in vitro and in conditionally transgenic mice. World J Gastroenterol. 2007;13:3472–3477. doi: 10.3748/wjg.v13.i25.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang ML, Yeh HC, Tsou YK, Wang CJ, Cheng HY, Sung CM, Ho YP, Chen TH, Yeh CT. HCV core-induced nonobese hepatic steatosis is associated with hypoadiponectinemia and is ameliorated by adiponectin administration. Obesity (Silver Spring) 2012;20:1474–1480. doi: 10.1038/oby.2012.45. [DOI] [PubMed] [Google Scholar]
- 21.Chang ML, Yeh CT, Chen JC, Huang CC, Lin SM, Sheen IS, Tai DI, Chu CM, Lin WP, Chang MY, et al. Altered expression patterns of lipid metabolism genes in an animal model of HCV core-related, nonobese, modest hepatic steatosis. BMC Genomics. 2008;9:109. doi: 10.1186/1471-2164-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerat H, Kammoun HL, Hainault I, Mérour E, Higgs MR, Callens C, Lemon SM, Foufelle F, Pawlotsky JM. Hepatitis C virus proteins induce lipogenesis and defective triglyceride secretion in transgenic mice. J Biol Chem. 2009;284:33466–33474. doi: 10.1074/jbc.M109.019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreau M, Rivière B, Vegna S, Aoun M, Gard C, Ramos J, Assenat E, Hibner U. Hepatitis C viral proteins perturb metabolic liver zonation. J Hepatol. 2015;62:278–285. doi: 10.1016/j.jhep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, Urban T, Afdhal NH, Jacobson IM, Esteban R, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120–9.e18. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Pawlotsky JM, Feld JJ, Zeuzem S, Hoofnagle JH. From non-A, non-B hepatitis to hepatitis C virus cure. J Hepatol. 2015;62:S87–S99. doi: 10.1016/j.jhep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Chan HL, Sung JJ. Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis. 2006;26:153–161. doi: 10.1055/s-2006-939753. [DOI] [PubMed] [Google Scholar]
- 27.Hsu CS, Liu CH, Wang CC, Tseng TC, Liu CJ, Chen CL, Chen PJ, Chen DS, Kao JH. Impact of hepatitis B virus infection on metabolic profiles and modifying factors. J Viral Hepat. 2012;19:e48–e57. doi: 10.1111/j.1365-2893.2011.01535.x. [DOI] [PubMed] [Google Scholar]
- 28.Chiang CH, Lai JS, Hung SH, Lee LT, Sheu JC, Huang KC. Serum adiponectin levels are associated with hepatitis B viral load in overweight to obese hepatitis B virus carriers. Obesity (Silver Spring) 2013;21:291–296. doi: 10.1002/oby.20000. [DOI] [PubMed] [Google Scholar]
- 29.Wong VW, Wong GL, Chu WC, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Chan HY, Woo J, et al. Hepatitis B virus infection and fatty liver in the general population. J Hepatol. 2012;56:533–540. doi: 10.1016/j.jhep.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Chu CM, Lin DY, Liaw YF. Does increased body mass index with hepatic steatosis contribute to seroclearance of hepatitis B virus (HBV) surface antigen in chronic HBV infection? Int J Obes (Lond) 2007;31:871–875. doi: 10.1038/sj.ijo.0803479. [DOI] [PubMed] [Google Scholar]
- 31.Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: pathogenesis, natural course, and management. J Hepatol. 2014;61:1407–1417. doi: 10.1016/j.jhep.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 32.Siasos G, Tousoulis D, Kollia C, Oikonomou E, Siasou Z, Stefanadis C, Papavassiliou AG. Adiponectin and cardiovascular disease: mechanisms and new therapeutic approaches. Curr Med Chem. 2012;19:1193–1209. doi: 10.2174/092986712799320583. [DOI] [PubMed] [Google Scholar]
- 33.Ballantyne GH, Gumbs A, Modlin IM. Changes in insulin resistance following bariatric surgery and the adipoinsular axis: role of the adipocytokines, leptin, adiponectin and resistin. Obes Surg. 2005;15:692–699. doi: 10.1381/0960892053923789. [DOI] [PubMed] [Google Scholar]
- 34.Cua IH, Hui JM, Bandara P, Kench JG, Farrell GC, McCaughan GW, George J. Insulin resistance and liver injury in hepatitis C is not associated with virus-specific changes in adipocytokines. Hepatology. 2007;46:66–73. doi: 10.1002/hep.21703. [DOI] [PubMed] [Google Scholar]
- 35.Jonsson JR, Moschen AR, Hickman IJ, Richardson MM, Kaser S, Clouston AD, Powell EE, Tilg H. Adiponectin and its receptors in patients with chronic hepatitis C. J Hepatol. 2005;43:929–936. doi: 10.1016/j.jhep.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 36.Choo QL, Richman KH, Han JH, Berger K, Lee C, Dong C, Gallegos C, Coit D, Medina-Selby R, Barr PJ. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci USA. 1991;88:2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piñeiro D, Martinez-Salas E. RNA structural elements of hepatitis C virus controlling viral RNA translation and the implications for viral pathogenesis. Viruses. 2012;4:2233–2250. doi: 10.3390/v4102233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masaki T, Suzuki R, Murakami K, Aizaki H, Ishii K, Murayama A, Date T, Matsuura Y, Miyamura T, Wakita T, et al. Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles. J Virol. 2008;82:7964–7976. doi: 10.1128/JVI.00826-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gastaminza P, Cheng G, Wieland S, Zhong J, Liao W, Chisari FV. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J Virol. 2008;82:2120–2129. doi: 10.1128/JVI.02053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colpitts CC, Lupberger J, Doerig C, Baumert TF. Host cell kinases and the hepatitis C virus life cycle. Biochim Biophys Acta. 2015;1854:1657–1662. doi: 10.1016/j.bbapap.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Boyer A, Dumans A, Beaumont E, Etienne L, Roingeard P, Meunier JC. The association of hepatitis C virus glycoproteins with apolipoproteins E and B early in assembly is conserved in lipoviral particles. J Biol Chem. 2014;289:18904–18913. doi: 10.1074/jbc.M113.538256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- 43.Lohmann V, Körner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 44.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Kräusslich HG, Mizokami M, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 46.Lindenbach BD, Meuleman P, Ploss A, Vanwolleghem T, Syder AJ, McKeating JA, Lanford RE, Feinstone SM, Major ME, Leroux-Roels G, et al. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci USA. 2006;103:3805–3809. doi: 10.1073/pnas.0511218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM, McKeating JA. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci USA. 2003;100:7271–7276. doi: 10.1073/pnas.0832180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye J. Reliance of host cholesterol metabolic pathways for the life cycle of hepatitis C virus. PLoS Pathog. 2007;3:e108. doi: 10.1371/journal.ppat.0030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J Biol Chem. 2004;279:52772–52780. doi: 10.1074/jbc.M410302200. [DOI] [PubMed] [Google Scholar]
- 50.Xiang Y, Tang JJ, Tao W, Cao X, Song BL, Zhong J. Identification of Cholesterol 25-Hydroxylase as a Novel Host Restriction Factor and a Part of the Primary Innate Immune Responses against Hepatitis C Virus Infection. J Virol. 2015;89:6805–6816. doi: 10.1128/JVI.00587-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Syed GH, Siddiqui A. Effects of hypolipidemic agent nordihydroguaiaretic acid on lipid droplets and hepatitis C virus. Hepatology. 2011;54:1936–1946. doi: 10.1002/hep.24619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olmstead AD, Knecht W, Lazarov I, Dixit SB, Jean F. Human subtilase SKI-1/S1P is a master regulator of the HCV Lifecycle and a potential host cell target for developing indirect-acting antiviral agents. PLoS Pathog. 2012;8:e1002468. doi: 10.1371/journal.ppat.1002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su AI, Pezacki JP, Wodicka L, Brideau AD, Supekova L, Thimme R, Wieland S, Bukh J, Purcell RH, Schultz PG, et al. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci USA. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang W, Hood BL, Chadwick SL, Liu S, Watkins SC, Luo G, Conrads TP, Wang T. Fatty acid synthase is up-regulated during hepatitis C virus infection and regulates hepatitis C virus entry and production. Hepatology. 2008;48:1396–1403. doi: 10.1002/hep.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye J, Wang C, Sumpter R, Brown MS, Goldstein JL, Gale M. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc Natl Acad Sci USA. 2003;100:15865–15870. doi: 10.1073/pnas.2237238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci USA. 2005;102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liefhebber JM, Hague CV, Zhang Q, Wakelam MJ, McLauchlan J. Modulation of triglyceride and cholesterol ester synthesis impairs assembly of infectious hepatitis C virus. J Biol Chem. 2014;289:21276–21288. doi: 10.1074/jbc.M114.582999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishimura-Sakurai Y, Sakamoto N, Mogushi K, Nagaie S, Nakagawa M, Itsui Y, Tasaka-Fujita M, Onuki-Karakama Y, Suda G, Mishima K, et al. Comparison of HCV-associated gene expression and cell signaling pathways in cells with or without HCV replicon and in replicon-cured cells. J Gastroenterol. 2010;45:523–536. doi: 10.1007/s00535-009-0162-3. [DOI] [PubMed] [Google Scholar]
- 59.Amako Y, Munakata T, Kohara M, Siddiqui A, Peers C, Harris M. Hepatitis C virus attenuates mitochondrial lipid β-oxidation by downregulating mitochondrial trifunctional-protein expression. J Virol. 2015;89:4092–4101. doi: 10.1128/JVI.01653-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rasmussen AL, Diamond DL, McDermott JE, Gao X, Metz TO, Matzke MM, Carter VS, Belisle SE, Korth MJ, Waters KM, et al. Systems virology identifies a mitochondrial fatty acid oxidation enzyme, dodecenoyl coenzyme A delta isomerase, required for hepatitis C virus replication and likely pathogenesis. J Virol. 2011;85:11646–11654. doi: 10.1128/JVI.05605-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dharancy S, Lemoine M, Mathurin P, Serfaty L, Dubuquoy L. Peroxisome proliferator-activated receptors in HCV-related infection. PPAR Res. 2009;2009:357204. doi: 10.1155/2009/357204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan M, Jahan S, Khaliq S, Ijaz B, Ahmad W, Samreen B, Hassan S. Interaction of the hepatitis C virus (HCV) core with cellular genes in the development of HCV-induced steatosis. Arch Virol. 2010;155:1735–1753. doi: 10.1007/s00705-010-0797-7. [DOI] [PubMed] [Google Scholar]
- 63.Jhaveri R, McHutchison J, Patel K, Qiang G, Diehl AM. Specific polymorphisms in hepatitis C virus genotype 3 core protein associated with intracellular lipid accumulation. J Infect Dis. 2008;197:283–291. doi: 10.1086/524846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roingeard P. Hepatitis C virus diversity and hepatic steatosis. J Viral Hepat. 2013;20:77–84. doi: 10.1111/jvh.12035. [DOI] [PubMed] [Google Scholar]
- 65.Norman KL, Sarnow P. Modulation of hepatitis C virus RNA abundance and the isoprenoid biosynthesis pathway by microRNA miR-122 involves distinct mechanisms. J Virol. 2010;84:666–670. doi: 10.1128/JVI.01156-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banerjee A, Meyer K, Mazumdar B, Ray RB, Ray R. Hepatitis C virus differentially modulates activation of forkhead transcription factors and insulin-induced metabolic gene expression. J Virol. 2010;84:5936–5946. doi: 10.1128/JVI.02344-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shoji I, Deng L, Hotta H. Molecular mechanism of hepatitis C virus-induced glucose metabolic disorders. Front Microbiol. 2011;2:278. doi: 10.3389/fmicb.2011.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shlomai A, Rechtman MM, Burdelova EO, Zilberberg A, Hoffman S, Solar I, Fishman S, Halpern Z, Sklan EH. The metabolic regulator PGC-1α links hepatitis C virus infection to hepatic insulin resistance. J Hepatol. 2012;57:867–873. doi: 10.1016/j.jhep.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 69.Romero-Gómez M. Insulin resistance and hepatitis C. World J Gastroenterol. 2006;12:7075–7080. doi: 10.3748/wjg.v12.i44.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bose SK, Shrivastava S, Meyer K, Ray RB, Ray R. Hepatitis C virus activates the mTOR/S6K1 signaling pathway in inhibiting IRS-1 function for insulin resistance. J Virol. 2012;86:6315–6322. doi: 10.1128/JVI.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qadri I, Iwahashi M, Capasso JM, Hopken MW, Flores S, Schaack J, Simon FR. Induced oxidative stress and activated expression of manganese superoxide dismutase during hepatitis C virus replication: role of JNK, p38 MAPK and AP-1. Biochem J. 2004;378:919–928. doi: 10.1042/BJ20031587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bugianesi E, Salamone F, Negro F. The interaction of metabolic factors with HCV infection: does it matter? J Hepatol. 2012;56 Suppl 1:S56–S65. doi: 10.1016/S0168-8278(12)60007-5. [DOI] [PubMed] [Google Scholar]
- 73.Brault C, Levy PL, Bartosch B. Hepatitis C virus-induced mitochondrial dysfunctions. Viruses. 2013;5:954–980. doi: 10.3390/v5030954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Agriesti F, Tataranni T, Ruggieri V, Capitanio N, Piccoli C. PPARs and HCV-Related Hepatocarcinoma: A Mitochondrial Point of View. PPAR Res. 2012;2012:605302. doi: 10.1155/2012/605302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yao W, Cai H, Li X, Li T, Hu L, Peng T. Endoplasmic reticulum stress links hepatitis C virus RNA replication to wild-type PGC-1α/liver-specific PGC-1α upregulation. J Virol. 2014;88:8361–8374. doi: 10.1128/JVI.01202-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coller KE, Heaton NS, Berger KL, Cooper JD, Saunders JL, Randall G. Molecular determinants and dynamics of hepatitis C virus secretion. PLoS Pathog. 2012;8:e1002466. doi: 10.1371/journal.ppat.1002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diamond DL, Syder AJ, Jacobs JM, Sorensen CM, Walters KA, Proll SC, McDermott JE, Gritsenko MA, Zhang Q, Zhao R, et al. Temporal proteome and lipidome profiles reveal hepatitis C virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog. 2010;6:e1000719. doi: 10.1371/journal.ppat.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sugiyama K, Ebinuma H, Nakamoto N, Sakasegawa N, Murakami Y, Chu PS, Usui S, Ishibashi Y, Wakayama Y, Taniki N, et al. Prominent steatosis with hypermetabolism of the cell line permissive for years of infection with hepatitis C virus. PLoS One. 2014;9:e94460. doi: 10.1371/journal.pone.0094460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woodhouse SD, Narayan R, Latham S, Lee S, Antrobus R, Gangadharan B, Luo S, Schroth GP, Klenerman P, Zitzmann N. Transcriptome sequencing, microarray, and proteomic analyses reveal cellular and metabolic impact of hepatitis C virus infection in vitro. Hepatology. 2010;52:443–453. doi: 10.1002/hep.23733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanaka N, Moriya K, Kiyosawa K, Koike K, Aoyama T. Hepatitis C virus core protein induces spontaneous and persistent activation of peroxisome proliferator-activated receptor alpha in transgenic mice: implications for HCV-associated hepatocarcinogenesis. Int J Cancer. 2008;122:124–131. doi: 10.1002/ijc.23056. [DOI] [PubMed] [Google Scholar]
- 81.Sun H, Zhang A, Yan G, Piao C, Li W, Sun C, Wu X, Li X, Chen Y, Wang X. Metabolomic analysis of key regulatory metabolites in hepatitis C virus-infected tree shrews. Mol Cell Proteomics. 2013;12:710–719. doi: 10.1074/mcp.M112.019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416–423. doi: 10.1053/j.gastro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 83.Patel K, Thompson AJ, Chuang WL, Lee CM, Peng CY, Shanmuganathan G, Thongsawat S, Tanwandee T, Mahachai V, Pramoolsinsap C, et al. Insulin resistance is independently associated with significant hepatic fibrosis in Asian chronic hepatitis C genotype 2 or 3 patients. J Gastroenterol Hepatol. 2011;26:1182–1188. doi: 10.1111/j.1440-1746.2011.06722.x. [DOI] [PubMed] [Google Scholar]
- 84.Sersté T, Nkuize M, Moucari R, Van Gossum M, Reynders M, Scheen R, Vertongen F, Buset M, Mulkay JP, Marcellin P. Metabolic disorders associated with chronic hepatitis C: impact of genotype and ethnicity. Liver Int. 2010;30:1131–1136. doi: 10.1111/j.1478-3231.2010.02291.x. [DOI] [PubMed] [Google Scholar]
- 85.Mihm S. Hepatitis C virus, diabetes and steatosis: clinical evidence in favor of a linkage and role of genotypes. Dig Dis. 2010;28:280–284. doi: 10.1159/000282103. [DOI] [PubMed] [Google Scholar]
- 86.Hsu CS, Liu CJ, Liu CH, Wang CC, Chen CL, Lai MY, Chen PJ, Kao JH, Chen DS. High hepatitis C viral load is associated with insulin resistance in patients with chronic hepatitis C. Liver Int. 2008;28:271–277. doi: 10.1111/j.1478-3231.2007.01626.x. [DOI] [PubMed] [Google Scholar]
- 87.Hsu CS, Liu CH, Liu CJ, Wang CC, Chen CL, Lai MY, Chen PJ, Chen DS, Kao JH. Association of lipid profiles with hepatitis C viral load in chronic hepatitis C patients with genotype 1 or 2 infection. Am J Gastroenterol. 2009;104:598–604. doi: 10.1038/ajg.2008.125. [DOI] [PubMed] [Google Scholar]
- 88.Huang HC, Chuang CS, Hsieh YY, Chang TS, Wei KL, Shen CH, Wu CS, Tung SY. Serum HCV RNA level is not associated with insulin resistance and metabolic syndrome in chronic hepatitis C patients with genotype 1 or 2 infection. Chang Gung Med J. 2011;34:487–495. [PubMed] [Google Scholar]
- 89.Persico M, Capasso M, Persico E, Svelto M, Russo R, Spano D, Crocè L, La Mura V, Moschella F, Masutti F, et al. Suppressor of cytokine signaling 3 (SOCS3) expression and hepatitis C virus-related chronic hepatitis: Insulin resistance and response to antiviral therapy. Hepatology. 2007;46:1009–1015. doi: 10.1002/hep.21782. [DOI] [PubMed] [Google Scholar]
- 90.Tsochatzis E, Manolakopoulos S, Papatheodoridis GV, Hadziyannis E, Triantos C, Zisimopoulos K, Goulis I, Tzourmakliotis D, Akriviadis E, Manesis EK, et al. Serum HCV RNA levels and HCV genotype do not affect insulin resistance in nondiabetic patients with chronic hepatitis C: a multicentre study. Aliment Pharmacol Ther. 2009;30:947–954. doi: 10.1111/j.1365-2036.2009.04094.x. [DOI] [PubMed] [Google Scholar]
- 91.Lonardo A, Loria P, Adinolfi LE, Carulli N, Ruggiero G. Hepatitis C and steatosis: a reappraisal. J Viral Hepat. 2006;13:73–80. doi: 10.1111/j.1365-2893.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 92.Chang ML, Hsu CM, Tseng JH, Tsou YK, Chen SC, Shiau SS, Yeh CT, Chiu CT. Plasminogen activator inhibitor-1 is independently associated with non-alcoholic fatty liver disease whereas leptin and adiponectin vary between genders. J Gastroenterol Hepatol. 2015;30:329–336. doi: 10.1111/jgh.12705. [DOI] [PubMed] [Google Scholar]
- 93.Marzouk D, Sass J, Bakr I, El Hosseiny M, Abdel-Hamid M, Rekacewicz C, Chaturvedi N, Mohamed MK, Fontanet A. Metabolic and cardiovascular risk profiles and hepatitis C virus infection in rural Egypt. Gut. 2007;56:1105–1110. doi: 10.1136/gut.2006.091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dai CY, Yeh ML, Huang CF, Hou CH, Hsieh MY, Huang JF, Lin IL, Lin ZY, Chen SC, Wang LY, et al. Chronic hepatitis C infection is associated with insulin resistance and lipid profiles. J Gastroenterol Hepatol. 2015;30:879–884. doi: 10.1111/jgh.12313. [DOI] [PubMed] [Google Scholar]
- 95.Sanyal AJ, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Stravitz RT, Mills AS. Nonalcoholic fatty liver disease in patients with hepatitis C is associated with features of the metabolic syndrome. Am J Gastroenterol. 2003;98:2064–2071. doi: 10.1111/j.1572-0241.2003.07640.x. [DOI] [PubMed] [Google Scholar]
- 96.Hézode C, Roudot-Thoraval F, Zafrani ES, Dhumeaux D, Pawlotsky JM. Different mechanisms of steatosis in hepatitis C virus genotypes 1 and 3 infections. J Viral Hepat. 2004;11:455–458. doi: 10.1111/j.1365-2893.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- 97.Hanouneh IA, Feldstein AE, Lopez R, Yerian L, Pillai A, Zein CO, Zein NN. Clinical significance of metabolic syndrome in the setting of chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2008;6:584–589. doi: 10.1016/j.cgh.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 98.Reddy KR, Govindarajan S, Marcellin P, Bernstein D, Dienstag JL, Bodenheimer H, Rakela J, Messinger D, Schmidt G, Ackrill A, et al. Hepatic steatosis in chronic hepatitis C: baseline host and viral characteristics and influence on response to therapy with peginterferon alpha-2a plus ribavirin. J Viral Hepat. 2008;15:129–136. doi: 10.1111/j.1365-2893.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 99.Mirandola S, Realdon S, Iqbal J, Gerotto M, Dal Pero F, Bortoletto G, Marcolongo M, Vario A, Datz C, Hussain MM, et al. Liver microsomal triglyceride transfer protein is involved in hepatitis C liver steatosis. Gastroenterology. 2006;130:1661–1669. doi: 10.1053/j.gastro.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 100.Bugianesi E, Marchesini G, Gentilcore E, Cua IH, Vanni E, Rizzetto M, George J. Fibrosis in genotype 3 chronic hepatitis C and nonalcoholic fatty liver disease: Role of insulin resistance and hepatic steatosis. Hepatology. 2006;44:1648–1655. doi: 10.1002/hep.21429. [DOI] [PubMed] [Google Scholar]
- 101.Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A, et al. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636–1642. doi: 10.1053/j.gastro.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 102.Hickman IJ, Powell EE, Prins JB, Clouston AD, Ash S, Purdie DM, Jonsson JR. In overweight patients with chronic hepatitis C, circulating insulin is associated with hepatic fibrosis: implications for therapy. J Hepatol. 2003;39:1042–1048. doi: 10.1016/s0168-8278(03)00463-x. [DOI] [PubMed] [Google Scholar]
- 103.Hofer H, Bankl HC, Wrba F, Steindl-Munda P, Peck-Radosavljevic M, Osterreicher C, Mueller C, Gangl A, Ferenci P. Hepatocellular fat accumulation and low serum cholesterol in patients infected with HCV-3a. Am J Gastroenterol. 2002;97:2880–2885. doi: 10.1111/j.1572-0241.2002.07056.x. [DOI] [PubMed] [Google Scholar]
- 104.Ramcharran D, Wahed AS, Conjeevaram HS, Evans RW, Wang T, Belle SH, Yee LJ. Serum lipids and their associations with viral levels and liver disease severity in a treatment-naïve chronic hepatitis C type 1-infected cohort. J Viral Hepat. 2011;18:e144–e152. doi: 10.1111/j.1365-2893.2010.01394.x. [DOI] [PubMed] [Google Scholar]
- 105.Petta S, Rosso C, Leung R, Abate ML, Booth D, Salomone F, Gambino R, Rizzetto M, Caviglia P, Smedile A, et al. Effects of IL28B rs12979860 CC genotype on metabolic profile and sustained virologic response in patients with genotype 1 chronic hepatitis C. Clin Gastroenterol Hepatol. 2013;11:311–7.e1. doi: 10.1016/j.cgh.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 106.Vescovo T, Romagnoli A, Perdomo AB, Corazzari M, Ciccosanti F, Alonzi T, Nardacci R, Ippolito G, Tripodi M, Garcia-Monzon C, et al. Autophagy protects cells from HCV-induced defects in lipid metabolism. Gastroenterology. 2012;142:644–653.e3. doi: 10.1053/j.gastro.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 107.van der Poorten D, Shahidi M, Tay E, Sesha J, Tran K, McLeod D, Milliken JS, Ho V, Hebbard LW, Douglas MW, et al. Hepatitis C virus induces the cannabinoid receptor 1. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rouabhia S, Malek R, Bounecer H, Dekaken A, Bendali Amor F, Sadelaoud M, Benouar A. Prevalence of type 2 diabetes in Algerian patients with hepatitis C virus infection. World J Gastroenterol. 2010;16:3427–3431. doi: 10.3748/wjg.v16.i27.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shaheen M, Echeverry D, Oblad MG, Montoya MI, Teklehaimanot S, Akhtar AJ. Hepatitis C, metabolic syndrome, and inflammatory markers: results from the Third National Health and Nutrition Examination Survey [NHANES III] Diabetes Res Clin Pract. 2007;75:320–326. doi: 10.1016/j.diabres.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 110.Mostafa A, Mohamed MK, Saeed M, Hasan A, Fontanet A, Godsland I, Coady E, Esmat G, El-Hoseiny M, Abdul-Hamid M, et al. Hepatitis C infection and clearance: impact on atherosclerosis and cardiometabolic risk factors. Gut. 2010;59:1135–1140. doi: 10.1136/gut.2009.202317. [DOI] [PubMed] [Google Scholar]
- 111.Petta S, Cammà C, Di Marco V, Alessi N, Cabibi D, Caldarella R, Licata A, Massenti F, Tarantino G, Marchesini G, et al. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol. 2008;103:1136–1144. doi: 10.1111/j.1572-0241.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 112.Kallwitz ER, Layden-Almer J, Dhamija M, Berkes J, Guzman G, Lepe R, Cotler SJ, Layden TJ. Ethnicity and body mass index are associated with hepatitis C presentation and progression. Clin Gastroenterol Hepatol. 2010;8:72–78. doi: 10.1016/j.cgh.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 113.Tsuzura H, Genda T, Sato S, Hirano K, Kanemitsu Y, Narita Y, Kikuchi T, Iijima K, Wada R, Ichida T. Association of visceral obesity with high viral load and histological findings in elderly patients with genotype 1 chronic hepatitis C. Intern Med. 2013;52:1665–1673. doi: 10.2169/internalmedicine.52.9430. [DOI] [PubMed] [Google Scholar]
- 114.Rembeck K, Maglio C, Lagging M, Christensen PB, Färkkilä M, Langeland N, Buhl MR, Pedersen C, Mørch K, Norkrans G, et al. PNPLA 3 I148M genetic variant associates with insulin resistance and baseline viral load in HCV genotype 2 but not in genotype 3 infection. BMC Med Genet. 2012;13:82. doi: 10.1186/1471-2350-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stättermayer AF, Rutter K, Beinhardt S, Scherzer TM, Stadlmayr A, Hofer H, Wrba F, Steindl-Munda P, Krebs M, Datz C, et al. Association of the IL28B genotype with insulin resistance in patients with chronic hepatitis C. J Hepatol. 2012;57:492–498. doi: 10.1016/j.jhep.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 116.Huang JF, Chuang WL, Yu ML, Yu SH, Huang CF, Huang CI, Yeh ML, Hsieh MH, Yang JF, Lin ZY, et al. Hepatitis C virus infection and metabolic syndrome---a community-based study in an endemic area of Taiwan. Kaohsiung J Med Sci. 2009;25:299–305. doi: 10.1016/S1607-551X(09)70520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37:647–652. doi: 10.1111/apt.12234. [DOI] [PubMed] [Google Scholar]
- 118.Li WC, Lee YY, Chen IC, Wang SH, Hsiao CT, Loke SS. Age and gender differences in the relationship between hepatitis C infection and all stages of Chronic kidney disease. J Viral Hepat. 2014;21:706–715. doi: 10.1111/jvh.12199. [DOI] [PubMed] [Google Scholar]
- 119.Adinolfi LE, Restivo L, Guerrera B, Sellitto A, Ciervo A, Iuliano N, Rinaldi L, Santoro A, Li Vigni G, Marrone A. Chronic HCV infection is a risk factor of ischemic stroke. Atherosclerosis. 2013;231:22–26. doi: 10.1016/j.atherosclerosis.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 120.Targher G, Bertolini L, Padovani R, Rodella S, Arcaro G, Day C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J Hepatol. 2007;46:1126–1132. doi: 10.1016/j.jhep.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 121.Petta S, Bellia C, Mazzola A, Cabibi D, Cammà C, Caruso A, Di Marco V, Craxì A, Ciaccio M. Methylenetetrahydrofolate reductase homozygosis and low-density lipoproteins in patients with genotype 1 chronic hepatitis C. J Viral Hepat. 2012;19:465–472. doi: 10.1111/j.1365-2893.2011.01557.x. [DOI] [PubMed] [Google Scholar]
- 122.Adinolfi LE, Restivo L, Zampino R, Guerrera B, Lonardo A, Ruggiero L, Riello F, Loria P, Florio A. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496–502. doi: 10.1016/j.atherosclerosis.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 123.Miyajima I, Kawaguchi T, Fukami A, Nagao Y, Adachi H, Sasaki S, Imaizumi T, Sata M. Chronic HCV infection was associated with severe insulin resistance and mild atherosclerosis: a population-based study in an HCV hyperendemic area. J Gastroenterol. 2013;48:93–100. doi: 10.1007/s00535-012-0610-3. [DOI] [PubMed] [Google Scholar]
- 124.Vespasiani-Gentilucci U, Gallo P, De Vincentis A, Galati G, Picardi A. Hepatitis C virus and metabolic disorder interactions towards liver damage and atherosclerosis. World J Gastroenterol. 2014;20:2825–2838. doi: 10.3748/wjg.v20.i11.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boddi M, Abbate R, Chellini B, Giusti B, Solazzo V, Soft F, Pratesi G, Pratesi C, Gensini G, Zignego AL. HCV infection facilitates asymptomatic carotid atherosclerosis: preliminary report of HCV RNA localization in human carotid plaques. Dig Liver Dis. 2007;39 Suppl 1:S55–S60. doi: 10.1016/s1590-8658(07)80012-0. [DOI] [PubMed] [Google Scholar]
- 126.Tarantino G, Conca P, Sorrentino P, Ariello M. Metabolic factors involved in the therapeutic response of patients with hepatitis C virus-related chronic hepatitis. J Gastroenterol Hepatol. 2006;21:1266–1268. doi: 10.1111/j.1440-1746.2006.04394.x. [DOI] [PubMed] [Google Scholar]
- 127.Poustchi H, Negro F, Hui J, Cua IH, Brandt LR, Kench JG, George J. Insulin resistance and response to therapy in patients infected with chronic hepatitis C virus genotypes 2 and 3. J Hepatol. 2008;48:28–34. doi: 10.1016/j.jhep.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 128.Dai CY, Huang JF, Hsieh MY, Hou NJ, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY, Chuang WL, et al. Insulin resistance predicts response to peginterferon-alpha/ribavirin combination therapy in chronic hepatitis C patients. J Hepatol. 2009;50:712–718. doi: 10.1016/j.jhep.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 129.Del Campo JA, Ampuero J, Rojas L, Conde M, Rojas A, Maraver M, Millán R, García-Valdecasas M, García-Lozano JR, González-Escribano MF, et al. Insulin resistance predicts sustained virological response to treatment of chronic hepatitis C independently of the IL28b rs12979860 polymorphism. Aliment Pharmacol Ther. 2013;37:74–80. doi: 10.1111/apt.12113. [DOI] [PubMed] [Google Scholar]
- 130.Angelico F, Francioso S, Del Ben M, Feole K, Carbone M, Pignatelli P, Violi F, Angelico M. Clinical trial: low plasma cholesterol and oxidative stress predict rapid virological response to standard therapy with peginterferon and ribavirin in HCV patients. Aliment Pharmacol Ther. 2009;30:444–451. doi: 10.1111/j.1365-2036.2009.04055.x. [DOI] [PubMed] [Google Scholar]
- 131.Westin J, Lagging M, Dhillon AP, Norkrans G, Romero AI, Pawlotsky JM, Zeuzem S, Schalm SW, Verheij-Hart E, Negro F, et al. Impact of hepatic steatosis on viral kinetics and treatment outcome during antiviral treatment of chronic HCV infection. J Viral Hepat. 2007;14:29–35. doi: 10.1111/j.1365-2893.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 132.Chu CJ, Lee SD, Hung TH, Lin HC, Hwang SJ, Lee FY, Lu RH, Yu MI, Chang CY, Yang PL, et al. Insulin resistance is a major determinant of sustained virological response in genotype 1 chronic hepatitis C patients receiving peginterferon alpha-2b plus ribavirin. Aliment Pharmacol Ther. 2009;29:46–54. doi: 10.1111/j.1365-2036.2008.03823.x. [DOI] [PubMed] [Google Scholar]
- 133.Elgouhari HM, Zein CO, Hanouneh I, Feldstein AE, Zein NN. Diabetes mellitus is associated with impaired response to antiviral therapy in chronic hepatitis C infection. Dig Dis Sci. 2009;54:2699–2705. doi: 10.1007/s10620-008-0683-2. [DOI] [PubMed] [Google Scholar]
- 134.Kosmacheva ED, Sergakova LM, At’kov OIu, Akchurin RS, Arabidze GG, Ataullakhanova DM. [Effects of surgical treatment on hypertrophy of the myocardium of the left ventricle in patients with symptomatic arterial hypertension] Kardiologiia. 1991;31:25–28. [PubMed] [Google Scholar]
- 135.Clark PJ, Thompson AJ, Zhu Q, Vock DM, Zhu M, Patel K, Harrison SA, Naggie S, Ge D, Tillmann HL, et al. The association of genetic variants with hepatic steatosis in patients with genotype 1 chronic hepatitis C infection. Dig Dis Sci. 2012;57:2213–2221. doi: 10.1007/s10620-012-2171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Restivo L, Zampino R, Guerrera B, Ruggiero L, Adinolfi LE. Steatosis is the predictor of relapse in HCV genotype 3- but not 2-infected patients treated with 12 weeks of pegylated interferon-α-2a plus ribavirin and RVR. J Viral Hepat. 2012;19:346–352. doi: 10.1111/j.1365-2893.2011.01555.x. [DOI] [PubMed] [Google Scholar]
- 137.Shah SR, Patel K, Marcellin P, Foster GR, Manns M, Kottilil S, Healey L, Pulkstenis E, Subramanian GM, McHutchison JG, et al. Steatosis is an independent predictor of relapse following rapid virologic response in patients with HCV genotype 3. Clin Gastroenterol Hepatol. 2011;9:688–693. doi: 10.1016/j.cgh.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fattovich G, Baroni GS, Pasino M, Pierantonelli I, Covolo L, Ieluzzi D, Passigato N, Tonon A, Faraci MG, Guido M, et al. Post-load insulin resistance does not predict virological response to treatment of chronic hepatitis C patients without the metabolic syndrome. Dig Liver Dis. 2012;44:419–425. doi: 10.1016/j.dld.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 139.Fattovich G, Covolo L, Pasino M, Perini E, Rossi L, Brocco G, Guido M, Cristofori C, Belotti C, Puoti M, et al. The homeostasis model assessment of the insulin resistance score is not predictive of a sustained virological response in chronic hepatitis C patients. Liver Int. 2011;31:66–74. doi: 10.1111/j.1478-3231.2010.02343.x. [DOI] [PubMed] [Google Scholar]
- 140.Fernández-Rodríguez CM, López-Serrano P, Alonso S, Gutiérrez ML, Lledó JL, Pérez-Calle JL, Temiño R, Cacho G, Nevado M, Casas ML, et al. Long-term reversal of hypocholesterolaemia in patients with chronic hepatitis C is related to sustained viral response and viral genotype. Aliment Pharmacol Ther. 2006;24:507–512. doi: 10.1111/j.1365-2036.2006.03000.x. [DOI] [PubMed] [Google Scholar]
- 141.Ramcharran D, Wahed AS, Conjeevaram HS, Evans RW, Wang T, Belle SH, Yee LJ. Associations between serum lipids and hepatitis C antiviral treatment efficacy. Hepatology. 2010;52:854–863. doi: 10.1002/hep.23796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Clark PJ, Thompson AJ, Vock DM, Kratz LE, Tolun AA, Muir AJ, McHutchison JG, Subramanian M, Millington DM, Kelley RI, et al. Hepatitis C virus selectively perturbs the distal cholesterol synthesis pathway in a genotype-specific manner. Hepatology. 2012;56:49–56. doi: 10.1002/hep.25631. [DOI] [PubMed] [Google Scholar]
- 143.Giordanino C, Bugianesi E, Smedile A, Ciancio A, Abate ML, Olivero A, Pellicano R, Cassader M, Gambino R, Bo S, et al. Incidence of type 2 diabetes mellitus and glucose abnormalities in patients with chronic hepatitis C infection by response to treatment: results of a cohort study. Am J Gastroenterol. 2008;103:2481–2487. doi: 10.1111/j.1572-0241.2008.02002.x. [DOI] [PubMed] [Google Scholar]
- 144.Huang JF, Dai CY, Yu ML, Huang CF, Huang CI, Yeh ML, Yang JF, Hou NJ, Hsiao PJ, Lin ZY, et al. Pegylated interferon plus ribavirin therapy improves pancreatic β-cell function in chronic hepatitis C patients. Liver Int. 2011;31:1155–1162. doi: 10.1111/j.1478-3231.2011.02545.x. [DOI] [PubMed] [Google Scholar]
- 145.Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Hirakawa M, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739–744. doi: 10.1002/hep.22703. [DOI] [PubMed] [Google Scholar]
- 146.Thompson AJ, Patel K, Chuang WL, Lawitz EJ, Rodriguez-Torres M, Rustgi VK, Flisiak R, Pianko S, Diago M, Arora S, et al. Viral clearance is associated with improved insulin resistance in genotype 1 chronic hepatitis C but not genotype 2/3. Gut. 2012;61:128–134. doi: 10.1136/gut.2010.236158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Conjeevaram HS, Wahed AS, Afdhal N, Howell CD, Everhart JE, Hoofnagle JH. Changes in insulin sensitivity and body weight during and after peginterferon and ribavirin therapy for hepatitis C. Gastroenterology. 2011;140:469–477. doi: 10.1053/j.gastro.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 148.Aghemo A, Prati GM, Rumi MG, Soffredini R, D’Ambrosio R, Orsi E, De Nicola S, Degasperi E, Grancini V, Colombo M. Sustained virological response prevents the development of insulin resistance in patients with chronic hepatitis C. Hepatology. 2012;56:1681–1687. doi: 10.1002/hep.25867. [DOI] [PubMed] [Google Scholar]
- 149.Hanouneh IA, Feldstein AE, McCullough AJ, Miller C, Aucejo F, Yerian L, Lopez R, Zein NN. The significance of metabolic syndrome in the setting of recurrent hepatitis C after liver transplantation. Liver Transpl. 2008;14:1287–1293. doi: 10.1002/lt.21524. [DOI] [PubMed] [Google Scholar]
- 150.Younossi Z, Stepanova M, Saab S, Trimble G, Mishra A, Henry L. The association of hepatitis C virus infection and post-liver transplant diabetes: data from 17 000 HCV-infected transplant recipients. Aliment Pharmacol Ther. 2015;41:209–217. doi: 10.1111/apt.13027. [DOI] [PubMed] [Google Scholar]
- 151.Petta S, Amato M, Cabibi D, Cammà C, Di Marco V, Giordano C, Galluzzo A, Craxì A. Visceral adiposity index is associated with histological findings and high viral load in patients with chronic hepatitis C due to genotype 1. Hepatology. 2010;52:1543–1552. doi: 10.1002/hep.23859. [DOI] [PubMed] [Google Scholar]
- 152.Forde KA, Haynes K, Troxel AB, Trooskin S, Osterman MT, Kimmel SE, Lewis JD, Lo Re V. Risk of myocardial infarction associated with chronic hepatitis C virus infection: a population-based cohort study. J Viral Hepat. 2012;19:271–277. doi: 10.1111/j.1365-2893.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, Wang CH, Chen WJ, Chen CJ. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 154.Hsu YC, Lin JT, Ho HJ, Kao YH, Huang YT, Hsiao NW, Wu MS, Liu YY, Wu CY. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 155.Hsu YC, Ho HJ, Huang YT, Wang HH, Wu MS, Lin JT, Wu CY. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64:495–503. doi: 10.1136/gutjnl-2014-308163. [DOI] [PubMed] [Google Scholar]
- 156.Liu ZW, Zhang N, Han QY, Zeng JT, Chu YL, Qiu JM, Wang YW, Ma LT, Wang XQ. Correlation of serum leptin levels with anthropometric and metabolic parameters and biochemical liver function in Chinese patients with chronic hepatitis C virus infection. World J Gastroenterol. 2005;11:3357–3362. doi: 10.3748/wjg.v11.i22.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Durante-Mangoni E, Zampino R, Marrone A, Tripodi MF, Rinaldi L, Restivo L, Cioffi M, Ruggiero G, Adinolfi LE. Hepatic steatosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-alpha in chronic hepatitis C patients. Aliment Pharmacol Ther. 2006;24:1349–1357. doi: 10.1111/j.1365-2036.2006.03114.x. [DOI] [PubMed] [Google Scholar]
- 158.Liu CJ, Chen PJ, Jeng YM, Huang WL, Yang WS, Lai MY, Kao JH, Chen DS. Serum adiponectin correlates with viral characteristics but not histologic features in patients with chronic hepatitis C. J Hepatol. 2005;43:235–242. doi: 10.1016/j.jhep.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 159.Derbala M, Rizk N, Al-Kaabi S, Amer A, Shebl F, Al Marri A, Aigha I, Alyaesi D, Mohamed H, Aman H, et al. Adiponectin changes in HCV-Genotype 4: relation to liver histology and response to treatment. J Viral Hepat. 2009;16:689–696. doi: 10.1111/j.1365-2893.2009.01096.x. [DOI] [PubMed] [Google Scholar]
- 160.Wang AY, Hickman IJ, Richards AA, Whitehead JP, Prins JB, Macdonald GA. High molecular weight adiponectin correlates with insulin sensitivity in patients with hepatitis C genotype 3, but not genotype 1 infection. Am J Gastroenterol. 2005;100:2717–2723. doi: 10.1111/j.1572-0241.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 161.Palmer C, Hampartzoumian T, Lloyd A, Zekry A. A novel role for adiponectin in regulating the immune responses in chronic hepatitis C virus infection. Hepatology. 2008;48:374–384. doi: 10.1002/hep.22387. [DOI] [PubMed] [Google Scholar]
- 162.Khattab MA, Eslam M, Shatat M, Abd-Aalhalim H, Mousa YI, Samir F, Aly H, Shaker O, Shaker Y. Changes in adipocytokines and insulin sensitivity during and after antiviral therapy for hepatitis C genotype 4. J Gastrointestin Liver Dis. 2012;21:59–65. [PubMed] [Google Scholar]
- 163.Zografos TA, Liaskos C, Rigopoulou EI, Togousidis E, Makaritsis K, Germenis A, Dalekos GN. Adiponectin: a new independent predictor of liver steatosis and response to IFN-alpha treatment in chronic hepatitis C. Am J Gastroenterol. 2008;103:605–614. doi: 10.1111/j.1572-0241.2007.01729.x. [DOI] [PubMed] [Google Scholar]
- 164.Corbetta S, Redaelli A, Pozzi M, Bovo G, Ratti L, Redaelli E, Pellegrini C, Beck-Peccoz P, Spada A. Fibrosis is associated with adiponectin resistance in chronic hepatitis C virus infection. Eur J Clin Invest. 2011;41:898–905. doi: 10.1111/j.1365-2362.2011.02498.x. [DOI] [PubMed] [Google Scholar]
- 165.Korah TE, El-Sayed S, Elshafie MK, Hammoda GE, Safan MA. Significance of serum leptin and adiponectin levels in Egyptian patients with chronic hepatitis C virus associated hepatic steatosis and fibrosis. World J Hepatol. 2013;5:74–81. doi: 10.4254/wjh.v5.i2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lago F, Dieguez C, Gómez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 167.Fartoux L, Poujol-Robert A, Guéchot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003–1008. doi: 10.1136/gut.2004.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Miki D, Ohishi W, Ochi H, Hayes CN, Abe H, Tsuge M, Imamura M, Kamatani N, Nakamura Y, Chayama K. Serum PAI-1 is a novel predictor for response to pegylated interferon-α-2b plus ribavirin therapy in chronic hepatitis C virus infection. J Viral Hepat. 2012;19:e126–e133. doi: 10.1111/j.1365-2893.2011.01516.x. [DOI] [PubMed] [Google Scholar]
- 169.Huang JF, Huang CF, Yu ML, Dai CY, Huang CI, Yeh ML, Hsieh MH, Yang JF, Hsieh MY, Lin ZY, et al. Serum visfatin is correlated with disease severity and metabolic syndrome in chronic hepatitis C infection. J Gastroenterol Hepatol. 2011;26:530–535. doi: 10.1111/j.1440-1746.2010.06438.x. [DOI] [PubMed] [Google Scholar]
- 170.Huang JF, Dai CY, Yu ML, Shin SJ, Hsieh MY, Huang CF, Lee LP, Lin KD, Lin ZY, Chen SC, et al. Serum retinol-binding protein 4 is inversely correlated with disease severity of chronic hepatitis C. J Hepatol. 2009;50:471–478. doi: 10.1016/j.jhep.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 171.Iwasa M, Hara N, Miyachi H, Tanaka H, Takeo M, Fujita N, Kobayashi Y, Kojima Y, Kaito M, Takei Y. Patients achieving clearance of HCV with interferon therapy recover from decreased retinol-binding protein 4 levels. J Viral Hepat. 2009;16:716–723. doi: 10.1111/j.1365-2893.2009.01119.x. [DOI] [PubMed] [Google Scholar]
- 172.Tiftikci A, Atug O, Yilmaz Y, Eren F, Ozdemir FT, Yapali S, Ozdogan O, Celikel CA, Imeryuz N, Tozun N. Serum levels of adipokines in patients with chronic HCV infection: relationship with steatosis and fibrosis. Arch Med Res. 2009;40:294–298. doi: 10.1016/j.arcmed.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 173.Baranova A, Jarrar MH, Stepanova M, Johnson A, Rafiq N, Gramlich T, Chandhoke V, Younossi ZM. Association of serum adipocytokines with hepatic steatosis and fibrosis in patients with chronic hepatitis C. Digestion. 2011;83:32–40. doi: 10.1159/000314592. [DOI] [PubMed] [Google Scholar]
- 174.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–969. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 175.Sjöwall C, Cardell K, Boström EA, Bokarewa MI, Enocsson H, Ekstedt M, Lindvall L, Frydén A, Almer S. High prevalence of autoantibodies to C-reactive protein in patients with chronic hepatitis C infection: association with liver fibrosis and portal inflammation. Hum Immunol. 2012;73:382–388. doi: 10.1016/j.humimm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 176.Durazzo M, Belci P, Niro G, Collo A, Grisoglio E, Ambrogio V, Spandre M, Fontana R, Gambino R, Cassader M, et al. Variations of serum levels of adiponectin and resistin in chronic viral hepatitis. J Endocrinol Invest. 2013;36:600–605. doi: 10.3275/8883. [DOI] [PubMed] [Google Scholar]


