Abstract
Implanted vestibular neurostimulators are effective in driving slow phase eye movements in monkeys and humans. Furthermore, increases in slow phase velocity and electrically evoked compound action potential (vECAP) amplitudes occur with increasing current amplitude of electrical stimulation. In intact monkeys, protracted intermittent stimulation continues to produce robust behavioral responses and preserved vECAPs. In lesioned monkeys, shorter duration studies show preserved but with somewhat lower or higher velocity behavioral responses. It has been proposed that such changes are due to central adaptive changes in the electrically elicited vestibulo-ocular reflex (VOR). It is equally possible that these differences are due to changes in the vestibular periphery in response to activation of the vestibular efferent system. In order to investigate the site of adaptive change in response to electrical stimulation, we performed transtympanic gentamicin perfusions to induce rapid changes in vestibular input in monkeys with long-standing stably functioning vestibular neurostimulators, disambiguating the effects of implantation from the effects of ototoxic lesion. Gentamicin injection was effective in producing a large reduction in natural VOR only when it was performed in the non-implanted ear, suggesting that the implanted ear contributed little to the natural rotational response before injection. Injection of the implanted ear produced a reduction in the vECAP responses in that ear, suggesting that the intact hair cells in the non-functional ipsilateral ear were successfully lesioned by gentamicin, reducing the efficacy of stimulation in that ear. Despite this, injection of both ears produced central plastic changes that resulted in a dramatically increased slow phase velocity nystagmus elicited by electrical stimulation. These results suggest that loss of vestibular afferent activity, and a concurrent loss of electrically elicited vestibular input, produces an increase in the efficacy of a vestibular neurostimulator by eliciting centrally adapted behavioral responses without concurrent adaptive increase of galvanic afferent activation in the periphery.
Keywords: gentamicin, labyrinthectomy, prosthesis, vestibular, vestibulo-ocular reflex
INTRODUCTION
The vestibular end organs located in the inner ear transduce rotational and translational acceleration of the head into neural signals. These signals in turn contribute to perception of motion, postural control, and control of eye and head movements. A loss of functionality in the vestibular end organs is debilitating. No restorative options currently exist for patients with vestibular loss. Encouraged by the success of cochlear implant technology for the treatment of sensorineural hearing loss, much work over the past decade has been devoted to the study and development of a vestibular neural prosthesis (see Fridman & Della Santina 2012 for a review).
Implantable, single- and multi-channel vestibular neurostimulators, which deliver trains of biphasic pulses to afferents of individual branches of the ampullar nerves innervating the three semicircular canals, have been extensively studied in animal models, particularly non-human primates, using devices developed by several groups (Davidovics et al. 2011; Della Santina et al. 2005, 2007; Gong et al. 2008; Dai et al. 2011a, b; Lewis et al. 2001, 2002, 2010; Nie et al. 2013; Phillips et al. 2011, 2012, 2015b; Rubinstein et al. 2012). This type of electrical stimulation has long been known to modulate the behavior of vestibular afferents (Goldberg et al. 1984) and to elicit eye movements (Cohen et al. 1964; Cohen and Suzuki 1963; Suzuki and Cohen 1964) comparable to those produced by the angular vestibulo-ocular reflex (aVOR). Indeed, vestibular neurostimulators have been shown to be capable of producing aVOR-like nystagmus, the slow phase velocity of which can be controlled by modulating the current amplitude or pulse rate of the stimulation train and the direction by varying which canal nerves receive stimulation. Further studies have demonstrated that such a stimulator, combined with a head-mounted rotational sensor, constitutes a functional vestibular prosthesis that is capable of partially restoring vestibulo-ocular reflex (VOR) (Davidovics et al. 2013; Perez Fornos et al. 2014; Golub et al. 2014; Guyot et al. 2011a, b, 2012; Pelizzone et al. 2014; Phillips et al. 2015a, b; Thompson et al. 2012; Wall et al. 2007), producing head and postural movements (Mitchell et al. 2013; Phillips et al. 2013), and eliciting perceptual responses (Lewis et al. 2013, Phillips et al. 2015a).
A number of challenges still remain prior to full implementation of this technology. Although there has been some study of bilateral implantation, most current approaches focus on unilateral implantation, since the potential risk of bilateral implantation to hearing is significant. This means that stimulation with the device is inherently asymmetric (e.g., Dai et al. 2011b). The device can drive contralaterally directed slow phase eye movements directly, but it must rely on adaptation to a base rate of stimulation to drive ipsilaterally directed slow phase eye movements. In addition, the relationships between stimulation parameters (such as pulse rate or pulse current amplitude) and the velocity of the elicited eye movements are highly variable from canal to canal and highly non-linear. This means that there may be poor initial correspondence between the commanded eye movement direction and velocity and the resulting eye movements. Adaptive changes in the response to electrical stimulation must occur to allow the devices to become more effective over time.
Such adaptive mechanisms have been demonstrated in animal models. Merfeld et al. studied bilaterally lesioned animals receiving chronic, pulse rate-modulated, unilateral stimulation of the lateral canal (Merfeld et al. 2007). Initial responses exhibited low VOR gain, misalignment, and asymmetry. Chronic stimulation was able to reduce asymmetry and produce bidirectional VOR. Furthermore, they showed that the gain of the VOR response to stimulation remains low (Lewis et al. 2010; Thompson et al. 2012). Dai et al. (2013) showed that the response to stimulation of individual canals in bilaterally lesioned macaque became somewhat better directionally aligned with the canal plane with chronic stimulation, although the gains decreased over time. In both cases, transtympanic gentamicin was used to produce loss of vestibular function prior to implantation of the device.
Mechanisms for VOR adaptation to changes in peripheral sensitivity, such as those arising from loss of hair cells performing sensory transduction or contexts where visual feedback mismatches afferent signals, are necessary during sickness or injury and relatively robust (Curthoys and Halmagyi 1995; Fetter and Zee 1988; Peng et al. 1994; Schultheis and Robinson 1981). The bulk of these adaptive processes act centrally, by modifying the sensitivity of secondary vestibular neurons to afferent input (Cullen et al. 2009, Lisberger et al. 1994, Lisberger 1994). However, Sadeghi et al. (2007) have also demonstrated that peripheral changes in afferent diversity occur following contralateral vestibular lesion, resulting in a greater proportion of irregular afferents post-lesion. Irregular afferents are more galvanically sensitive than regular afferents (Goldberg et al. 1984). Central mechanisms may have a significant effect on how secondary vestibular neurons respond to the synchronous, pulsed afferent input produced by a vestibular prosthesis. Conversely, the changes in the periphery that produce irregular afferent discharge after peripheral loss may also greatly alter the recruitment dynamics during stimulation. How and where such natural vestibular adaptive mechanisms modify behavioral responses to electrical stimulation delivered by a vestibular prosthesis is currently unknown and deserves further study.
To assess the mechanism of adaptive changes in the response to electrical stimulation with a vestibular prosthesis, we studied animals that had working vestibular prosthetic devices that had been implanted long before the adaptation experiments. We did this to disambiguate the effects of implantation of the prosthetic device from the effects of a post-lesion adaptive change. Our adaptation stimulus was a bilateral ototoxic lesion of the vestibular end organs. Reduction of vestibular input was chosen to drive adaptation because such changes produce reliable adaptive changes in both behavior and neural discharge (e.g., Angelaki et al. 2000; Cullen et al. 2009; Newlands et al. 2014; Yu et al. 2014). Transtympanic gentamicin was chosen because, unlike canal plugging, it could produce a change in natural vestibular input without altering the structure of the canal adjacent to the implanted arrays.
We assessed to what extent adaptation following bilateral chemical lesion by transtympanic gentamicin is localized in the periphery versus centrally by comparing the changes in vestibular evoked compound action potentials (vECAPs), a measure of local activation of neural fibers in the end organ, with changes in elicited nystagmus, which also involve adaptive changes occurring centrally. By comparing and contrasting changes in these two measures, using rotationally elicited aVOR as a measure of the efficacy of our ototoxic lesion, we evaluated the mechanism of the adaptive changes in prosthetically elicited behavioral responses.
MATERIALS AND METHODS
The experiments presented in this study were performed in accordance with the recommendations of the Society for Neuroscience and the National Research Council (1997, 2003). They exceeded the requirements recommended by the Institute of Laboratory Animal Resource and the Association for Assessment and Accreditation of Laboratory Animal Care International. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Washington.
Device and Surgery
In separate sterile surgeries, four rhesus macaque monkeys were implanted with a vestibular stimulator (UW/Cochlear), scleral eye coils, and head stabilization lugs and finally with a neural recording chamber. The UW/Cochlear prosthesis was implanted in the right ear in each animal. Detailed descriptions of the UW/Cochlear prosthesis employed in this study (Nie et al. 2013) and the surgical implantation approach (Rubinstein et al. 2012) have been published previously. To summarize, the UW/Cochlear prosthesis was based on a Nucleus Freedom cochlear implant (Cochlear, Ltd.). It consisted of a chronically implantable neurostimulator connected to an external processor via an RF link, such that the stimulating device required no percutaneous leads. The neurostimulator contained a trifurcated lead, the distal ends (closest to the tip of the inserted array) of which each contained an electrode array 2.5 mm in length with three 250 μm x 120 μm stimulation sites. During surgical implantation, a fenestration was made in the bony labyrinth adjacent to the ampullae of the three semicircular canals. The electrode array tip of each lead was inserted through this fenestration into the perilymphatic space of the canal. vECAPs were utilized during surgery to optimize the placement of the stimulating electrode within the canal (Nie et al. 2011). A remote ground ball electrode was placed under the temporalis muscle. If, following implantation, electrical stimulation failed to elicit behavioral responses, an additional surgery was undertaken in which the electrode leads were repositioned. In these experiments, a NIC-2 clinical research processor sent stimulation instructions to the neurostimulator. The UW/Cochlear prosthesis, though capable of continuous modulated stimulation, was only activated during testing sessions, typically two to three times per week, and was inactive at all other times.
Chemical Lesion
In order to elicit a robust adaptive change in the aVOR that could modify the response to electrical stimulation, we created bilateral ototoxic lesions of the vestibular end organ with intratympanic gentamicin. This was done sequentially with serial unilateral lesions, so that we could evaluate the base state of vestibular input to the CNS in our animals before provoking an adaptive change. We assumed that vestibular input would be symmetrical from both ears. We had previous data (Rubinstein et al. 2012) suggesting that this was true shortly following implantation, and we had longitudinal data suggesting that little changed in the response to stimulation over time (Phillips et al. 2015a). We subdivided our animals into two groups, those that received ipsilateral gentamicin initially and those that received contralateral gentamicin initially. If inputs were symmetric from both ears at the outset, then both initial injections would produce comparable behavioral changes. If there was asymmetric input, then injection of the better functioning ear would produce a larger change in behavior.
Under anesthesia, two myringotomies were performed. Approximately 0.2 mL gentamicin was injected through one opening until the solution was seen flowing from the other. For the first two injections in monkey M2, a 10-mg/mL gentamicin concentration was used. This produced limited changes in observed function, so for subsequent injections in all animals, a 40-mg/mL concentration was utilized to minimize the number of injections needed to effectively lesion the end organ. In monkeys M1 and M2, injections were performed first to the ear ipsilateral to the implantation (right) then to the contralateral non-implanted (left) ear. In monkeys M3 and M4, the injections were first to the non-implanted ear and then to the implanted ear. Thus, each animal was studied longitudinally through three sequential time periods: (1) post-implantation of the neurostimulator and pre-injection, (2) following gentamicin perfusion of one ear (ipsilateral in M1 and M2, contralateral in M3 and M4), and (3) following gentamicin perfusion of both ears. These periods are referred to as the pre-injection, unilaterally lesioned, and bilaterally lesioned periods, respectively, throughout the text. In all cases, perfusions ceased when observed VOR gains decreased to <0.2 in response to en bloc rotational chair testing.
Vestibular Testing and Stimulation
Four sets of data were collected longitudinally over the course of chemical lesions from each animal: (1) slow phase eye movements in response to passive rotations, (2) slow phase eye movements in response to biphasic pulse electrical stimulation trains from the UW/Cochlear prosthesis, (3) vECAPs, and (4) electrode impedance measurements. Rotational testing and electrical stimulation testing were conducted in separate sessions on separate days, while vECAPs and impedance measurements were taken prior to either rotational or stimulation testing sessions.
Passive whole-body rotation testing was conducted to assess the natural state of natural vestibular function over the course of the gentamicin perfusions. Such testing provided a behavioral measure that should be sensitive to bilateral or unilateral vestibular loss and should provide insights into the preexisting state of the vestibular inputs at the outset of the experiments. This testing consisted of both velocity steps and sinusoidal rotations. In both cases, testing was conducted in the dark while the animal was seated in a 3 df rotational chair with its head restrained by stabilization lugs. Horizontal and vertical eye movements were collected using a magnetic search coil with driver coils mounted on the rotary chair (CNC Engineering, Seattle, WA). Eye and chair position data were recorded online with a sampling rate of 1,000 Hz with a CED Power 1401 (Cambridge, UK). Velocity steps were conducted in the yaw plane by accelerating the chair at 1,000°/s/s to a velocity of 100°/s, which was held for 45 s, then decelerating at the same rate to a static position for the same amount of time. Steps were to the ipsilateral (implanted and right) ear first, then to the contralateral (non-implanted and left) ear twice, and then again to the ipsilateral ear. These stimulation parameters were chosen since they had previously been shown to be sensitive to unilateral canal plugging in prosthesis-implanted animals (Rubinstein et al. 2012). Two types of sinusoidal rotation testing were conducted over a range of frequencies: constant peak amplitude (CPA) sinusoidal rotations and constant peak velocity (CPV) sinusoidal rotations. Constant peak velocity rotations of ±80°/s were conducted in the yaw plane between 0.01 and 1 Hz. Constant peak amplitude rotations of ±10° were conducted both in the yaw and pitch planes between 0.05 and 2 Hz. These stimuli were chosen specifically to reproduce the frequencies and amplitude characteristics of rotational stimuli used in previously published reports of functional rotation modulated electrically elicited aVOR. The use of constant peak amplitude rotation was required during pitch rotation due to pitch amplitude limitations in our rotational apparatus and reproduced horizontally for comparison to the more traditional constant velocity vertical axis rotations that the device was capable of.
Electrical stimulation testing was conducted to assess the efficacy of the UW/Cochlear prosthesis at eliciting slow phase eye movements before and after adaptation to ototoxic lesioning. As with rotational testing, animals were seated in the dark with their heads restrained. Prior to the study, all animals were trained to direct their gaze toward an illuminated spot projected on a drum in front of them. During electrical stimulation testing sessions, the animal was tasked with making saccades to direct their gaze at a randomly stepping spot. At random intervals, the spot was moved to a straight ahead position and then turned off. A 2-s train of electrical stimulation was subsequently delivered from the UW/Cochlear device. In this experiment, stimulation consisted of 2 s trains of biphasic pulses, all with a pulse width of 100 μs/phase and a 8-μs interphase gap, delivered between one of the implanted canal sites and a combination of the remote and case grounds of the device. To determine the relationship between stimulation pulse rate and current amplitude and the elicited slow phase eye velocities, a set of stimulation trains of differing pulse rates and amplitudes were tested during each session: pulse rate frequencies of 75, 150, 300, and 600 pulses/s (PPS) and current amplitudes of 25–250 μA (in steps of 25 or 50 μA). This set of stimulation parameters provided a range of stimulation that extended from below the threshold for eliciting eye movements through the highest stimulation current that could be delivered without activating the facial nerve. Stimulation pulse markers from the NIC-2 processor were recorded simultaneously with eye position data for later offline data analysis.
vECAPs and impedance data were collected prior to rotation or electrical stimulation testing sessions with Nucleus Freedom Custom Sound EP software (Cochlear, Ltd.). Monopolar stimulation occurred between the most distal electrode on an array and a remote ground, and the ECAP was recorded from an adjacent electrode (intracanal recording) or electrode in an adjoining canal (transcanal recording). A forward masking paradigm was utilized to reduce the stimulation artifact (see Nie et al. 2011). For each current, the vECAP was characterized automatically by the Custom Sound software by measuring the N1-P1 amplitude of the response and determining a threshold current (Fig. 1B, D). Impedance measurements were also collected between each stimulating electrode and the common ground during these sessions.
FIG. 1.
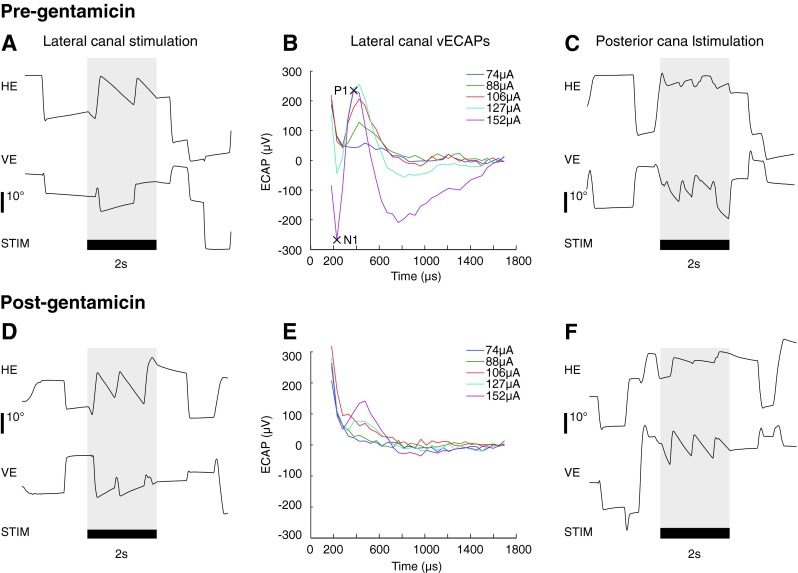
Electrically evoked eye movement position traces (A, C, D, F) and vestibular evoked compound action potentials (ECAP, B, E). All panels are taken from M1. A, B, and C are from pre-gentamicin injection test sessions (days −27 (A, C) and −10 (B)), while D, E, and F are taken from test sessions following bilateral gentamicin injections (days 53 (D, F) and 47 (E)). Eye movements were characterized by calculating the average slow phase velocity produced during the stimulation train (shaded areas). Eye position is plotted such that a negative value indicates a leftward or downward eye movement. Panels A and D plot eye movements from lateral canal stimulation at 75 μA, while panels C and F plot eye movements from posterior canal stimulation at 75 μA. vECAPs were characterized by calculating the N1-P1 amplitude for each stimulation current (e.g., B, 152 μA).
Data Analysis
Analysis of recorded eye movements was conducted offline using custom written Spike2 (CED, Cambridge, UK) and Matlab (Mathworks, Natick, MA) scripts. First, eye position records were marked, based on a chosen velocity criterion, to indicate the location of saccades or fast-phase eye movements in a period of recording in the absence of electrical stimulation taken during each session while the animal was in the dark. A linear regression using a least-squares method was applied to calculate a velocity for each resultant slow phase. A time-weighted average of the horizontal and vertical eye velocity of this period was used as a measure of the average slow phase velocity of the animal’s underlying spontaneous drift on that day.
For electrical stimulation trials, the eye position records during the stimulation train (e.g., Fig. 1A, shaded region) were desaccaded by the same method as that used for the spontaneous drift. A time-weighted average of the slow phase velocity was calculated from all slow phases at the same stimulation parameters (electrode, pulse rate, and current amplitude) on a given test date. The spontaneous drift value, described above, was then subtracted from the velocity of the slow phases from the same session to provide an indication of the change in slow phase velocity produced by electrical stimulation.
For rotational velocity step tests, nystagmus velocities were calculated for each slow phase by the method described above. A gain was calculated for each step by averaging the maximum slow phase velocity of the first five slow phases after step onset, subtracting the spontaneous drift, and dividing the resultant value by the velocity of the stimulus step (100°/s). A time constant was also calculated for each step using an exponential fit to the velocities of all slow phases against time within the period of constant velocity following the velocity step. Gains and time constants of like conditions were averaged (e.g., per-ipsilateral rotation step and post-contralateral rotation step) for each test session. An overall gain was also calculated by averaging the gains of all four conditions. For sinusoidal rotational testing, eye position records during rotational testing were desaccaded as described above. A least-squares fit was applied to the sinusoidal eye velocity to calculate a phase, amplitude, and offset of the sinusoidal eye velocity. From this sine fit, a gain was calculated as the ratio of the amplitudes of the sine fit of eye velocity to that of chair velocity.
Statistical analyses were performed using a post hoc ANOVA in Statview. For eye position and velocity measurements, negative values indicate a leftward or downward position or velocity. Values are reported as the mean ± 1 standard deviation.
RESULTS
Implantation
Four rhesus macaques were included in this study. All were previously implanted with a UW/Cochlear vestibular neurostimulator in their right ear using identical techniques. Two of the animals (M1 and M2) were implanted with electrodes in the lateral and posterior canals only. M3 and M4 were implanted with electrodes in those canals using an identical procedure, and then, the anterior canal was implanted with an identical array. One revision surgery, conducted to reposition electrode leads within the canal, was performed on M2. Before gentamicin injection, the animals were followed to make sure that they had stable rotational vestibular function, vECAPs, and electrically elicited VOR. The first gentamicin injection was conducted on an average of 426 days post-implantation or revision surgery (M1—652 days, M2—252, M3—679, M4—119). This was done to fully disambiguate the adaptive changes studied here from possible transient effects of device implantation. At this point, electrical stimulation delivered to six of ten total implanted canals elicited relatively robust, directionally appropriate nystagmus that scaled with stimulation current and pulse rate. Responses from these six canals (M1—lateral, posterior; M2—lateral; M3—lateral, posterior; M4—posterior) are examined in the present study.
Vestibular Testing
Velocity step testing was performed prior to any gentamicin injection to evaluate the state of vestibular input from both ears prior to bilateral ototoxic lesion. Such testing had been shown to be sensitive to unilateral loss of function due to canal plugging in implanted animals (Rubinstein et al. 2012). The step tests showed overall gains of 0.78 ± 0.02 for all four animals. However, across three of four animals, an asymmetry was present between velocity steps ipsilateral to the implanted ear (gain = 0.70 ± 0.067) and contralateral to the implanted ear (0.86 ± 0.061), which was significant (T6 = 3.557, p = 0.012). In one animal, M4, no asymmetry was observed between velocity step directions (ipsi gain = 0.794, contra = 0.795). These results suggested that some vestibular loss was present prior to chemical lesion in the implanted ear for three of the four animals at these late post-implantation times. However, the findings of small rotational asymmetries at any frequency must be interpreted with caution in animals that have undergone surgically induced structural changes to the end organ during prior implantation surgery. Pre-gentamicin sinusoidal rotary chair testing was partly consistent with velocity step results. During CPV testing, animals similarly showed gains of 0.74 ± 0.03 at 0.05 Hz and 0.78 ± 0.03 at 0.5 Hz. VOR phases averaged 170.71° ± 5.03° out of phase to chair velocity at 0.05 Hz and 176.24° ± 4.69° at 0.5 Hz during CPV testing. In two animals, M1 and M2, positive (rightward) eye velocity offsets were observed (M1—11.55°/s and 9.14°/s, M2—15.71°/s and 7.52°/s for the same conditions), which suggest a rightward eye velocity bias or a leftward head velocity bias. This finding is consistent with a pre-gentamicin vestibular loss in the right, implanted ear. In the other two animals, M3 and M4, no offsets were observed. The data for animal M4 is consistent with the step velocity testing, whereas the monkey M3 data was not. Gains from sinusoidal yaw and pitch CPA testing are plotted against time in Figure 2. Pre-injection gains from this test are comparable to those obtained by velocity step and CPV testing. While these results suggest a functional asymmetry in vestibular input between the two ears consequent to surgery and prior to our ototoxic lesion study, they are not conclusive. Any rotational test, or even a caloric test, applied to a surgically altered inner ear could be affected by the resulting structural changes to that ear, resulting in subtle asymmetries.
FIG. 2.
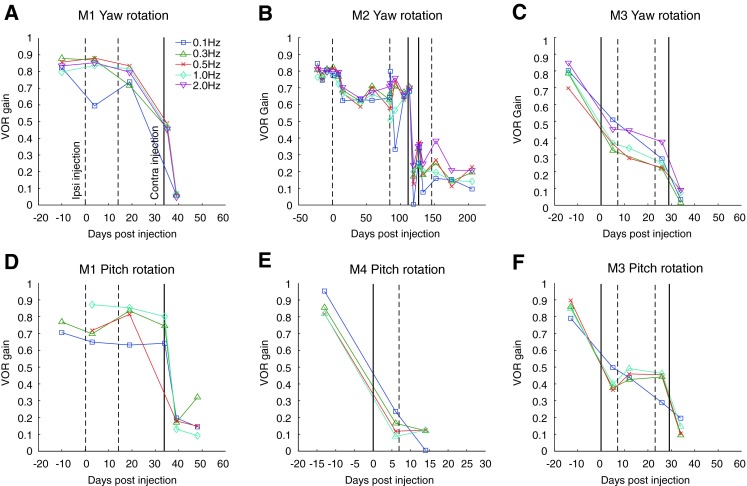
Longitudinal plot of VOR gain from yaw and pitch constant peak amplitude sinusoidal rotation testing (±10°) at different frequencies for all animals. Vertical solid and dashed lines denote dates for gentamicin perfusions in the ipsilateral (dashed line) or contralateral (solid line) ear.
To provide more conclusive demonstration of any preexisting asymmetry in functional vestibular input between the two ears in our animals before bilateral lesion, we performed unilateral transtympanic injections of gentamicin in the ipsilateral and contralateral ear. We reasoned that a large preexisting asymmetry in function would be dramatically revealed by such a test. Ototoxic lesion to a non-functioning ear would produce little or no change in function regardless of the state of the opposite ear. Ototoxic lesion to a functioning ear would produce a small but reliable change in aVOR if the opposite ear was providing comparable input. Ototoxic lesion to a functioning ear would produce a dramatic change in aVOR, if the opposite ear was non-functional. This paradigm could provide a clear indication of the state of vestibular input from both ears without relying on small asymmetries present following unilateral loss in rhesus monkeys (Newlands et al. 2014).
All animals were unilaterally chemically lesioned by transtympanic gentamicin injections to either the ear ipsilateral to the vestibular prosthesis (M1 and M2) or contralateral to the implanted ear (M3 and M4). Fisher’s protected least significant difference (PLSD) (post hoc ANOVA) was used to calculate the significance of changes to rotary chair testing results before and after chemical lesion. The p values for these tests for all animals are collected in Table 1.
TABLE 1.
Fisher’s PLSD (post hoc ANOVA) p values for rotational test VOR gains between pre-injection, unilaterally lesioned, and bilaterally lesioned longitudinal time periods
| M1 | M2 | M3 | M4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cond. | Pre-Uni | Uni-Bi | Pre-Bi | Pre-Uni | Uni-Bi | Pre-Bi | Pre-Uni | Uni-Bi | Pre-Bi | Pre-Uni | Uni-Bi | Pre-Bi |
| Step | NS | <0.0001 | <0.0001 | NS | 0.0015 | 0.0002 | 0.0023 | NS | 0.0003 | <0.0001 | NS | <0.0001 |
| CPV | NS | <0.0001 | <0.0001 | NS | <0.0001 | <0.0001 | <0.0001 | 0.0003 | <0.0001 | <0.0001 | NS | <0.0001 |
| CPA-yaw | NS | <0.0001 | <0.0001 | 0.0488 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | – | – | – |
| CPA-pitch | NS | <0.0001 | <0.0001 | – | – | – | <0.0001 | NS | <0.0001 | <0.0001 | NS | <0.0001 |
Data is grouped by animal and rotary chair test condition (velocity step test, constant peak velocity sinusoidal rotary chair test, constant peak amplitude sinusoidal rotary chair test)
Pre pre-injection, Uni unilaterally lesioned, Bi bilaterally lesioned, Step velocity step test, CPV constant peak velocity sinusoidal rotary chair test, CPA constant peak amplitude sinusoidal rotary chair test), NS non-significant changes (p > 0.05)
For animals M3 and M4, which received gentamicin injections in the ear contralateral to the implanted prosthesis, overall velocity step gains decreased from 0.75 to 0.42 in M3 and from 0.79 to 0.09 in M4. This suggested that both animals had a substantial loss of lateral semicircular canal function in the right (implanted) ear prior to injection of the unimplanted left ear. Likewise, CPA gains decreased considerably across all tested frequencies (Fig. 2C, E, and F). Fisher’s PLSD (post hoc ANOVA) found these changes and comparable changes in CPV gains to be significant. In animal M3, no significant changes to VOR phase were observed during sinusoidal rotary chair testing following injection of gentamicin in the ear contralateral to the implant. In animal M4, phase could not be reliably determined following such an injection due to the extremely low gains that resulted. For animals M1 and M2, which received gentamicin injections in the ear ipsilateral to the implanted prosthesis, overall velocity step gains decreased little or not at all. This suggested that the animals had a loss of lateral semicircular canal function in the right (implanted) ear, and good function in the left (unimplanted) ear, prior to the injection. In animal M1, there were no significant VOR gain reductions in step, CPV, or CPA testing and no significant changes to VOR phase during sinusoidal testing (Fig. 2A, D). In animal M2, ipsilateral gentamicin perfusions caused a small decrease in VOR gain (Fig. 2B). Step gains decreased from 0.74 to 0.67, which were not significant. A significant decrease in gains was observed only in CPV results. No significant changes to VOR phase were observed during sinusoidal rotational testing.
After unilateral lesion, all animals subsequently received unilateral injections of gentamicin to the ear opposite the previously injected ear (contralateral to the implant in M1 and M2 and ipsilateral to the implant in M3 and M4), producing a bilateral ototoxic lesion to drive adaptation of the aVOR.
Following gentamicin injections to the opposite ear, which was ipsilateral to the implant, animal M4 showed no subsequent significant decrease in step or sinusoidal aVOR gain, which was already very low from previous injection of the ear contralateral to the implant (Table 1). A similar injection in animal M3 produced only a small decrease in step, CPV, and yaw CPA aVOR gains. Fisher’s PLSD (post hoc ANOVA) found the changes in yaw CPA and CPV gains to be significant. No significant changes to VOR phase during sinusoidal testing were observed. In animal M3, a final injection of gentamicin to the ear contralateral to the implant resulted in dramatically decreased VOR gains, such that velocity step gain was 0.08 and the CPV and CPA gains were <0.2 (see Fig. 2C, F). The decrease was statistically significant (Fisher’s PLSD, p = 0.0019, <0.0001, <0.0001, and <0.0001 for step, CPV, and yaw CPA and pitch CPA gains, respectively). For both M1 and M2, a large drop in VOR gain occurred following gentamicin perfusion contralateral to the implanted ear (i.e., in the ear opposite to the initial injection). Overall velocity step gains decreased from 0.78 to 0.02 in M1 and from 0.67 to 0.27 in M2. Sinusoidal, CPV, and CPA rotation gains decreased across all frequencies (Fig. 2A, B, and D). These changes were found to be significant by a post hoc ANOVA.
Taken together, asymmetries in the VOR gains of three animals during rotary chair testing suggest some vestibular loss in the implanted ear during the period prior to gentamicin injections, although all four animals exhibited normal averaged VOR gains during the same period. Sequential unilateral injections of gentamicin in the ears ipsilateral and contralateral to the implanted ear confirmed conclusively that all animals had a similar preexisting loss of vestibular function in the implanted ear prior to any gentamicin injections. Bilateral transtympanic gentamicin injection produced a profound decrease in VOR gains across all rotational testing conditions, effectively producing a monkey with a loss function in both ears. Again, in all animals, the vast majority of this decrease occurred following gentamicin perfusions to the contralateral ear, irrespective of the order in which injections were administered. Smaller decreases in VOR gain were observed following ipsilateral injections. These results were consistent with some preexisting vestibular loss, over time, in the implanted ear and demonstrated that we had performed a successful bilateral ototoxic lesion to drive adaptive changes in the vestibular system.
Electrical Stimulation
To assess the changes in the efficacy of electrical stimulation in activating vestibular afferent fibers following a perturbation in vestibular function, we monitored vECAPs before, during, and after serial injections of aminoglycoside antibiotic to the ipsilateral and contralateral ears. vECAPS were elicited by electrical stimulation and recording in all of the canals studied. Pre-gentamicin vECAP thresholds ranged from 61 to 152 μA, and N1-P1 peak amplitude increased with increasing stimulation current (Fig. 3). Following gentamicin injections to the ipsilateral ear, electrical stimulation in the lateral and posterior canals of monkey M1 and the lateral canal of monkey M2 produced decreased vECAP amplitudes and increased thresholds, suggesting a loss of galvanic sensitivity of the ampullar nerves after injection. In monkey M1, N1-P1 peak amplitude at 152 μA decreased from 275 to 137 μV during electrical stimulation of the lateral canal and from 284 to 123 μV during stimulation of the posterior canal. Thresholds increased from 88 to 127 μA for stimulation in the lateral canal and from 74 to 127 μA in the posterior canal in the same monkey. These changes were statistically significant (Fisher’s PLSD, p = 0.0007 and p < 0.0001 for stimulation of the lateral and posterior canals, respectively).
FIG. 3.
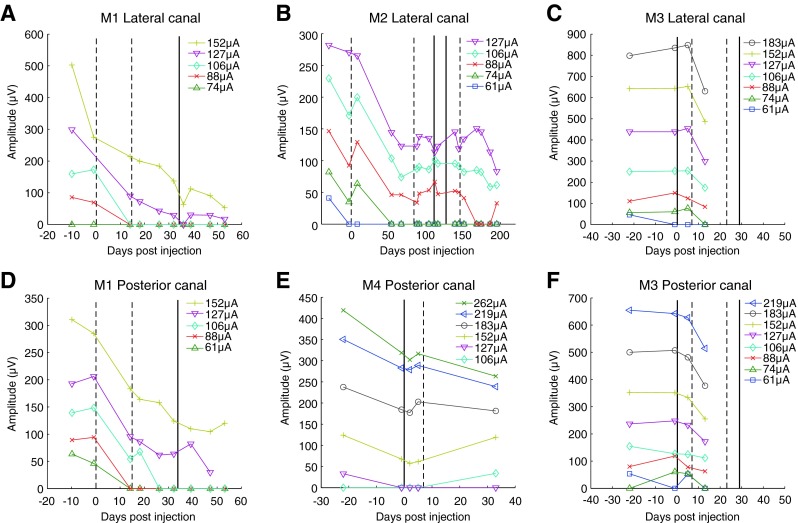
Longitudinal plot of vestibular evoked compound action potential (vECAP) N1-P1 peak amplitude at different stimulation currents for all animals. vECAPs were collected from a return electrode located in the same canal as the stimulating electrode (A, B) or from a return electrode located in an adjacent canal (C, D, E, F). Vertical solid and dashed lines denote dates for gentamicin perfusions in the ipsilateral (dashed line) or contralateral (solid line) ear.
To evaluate any change in galvanic sensitivity due to changes in efferent modulation of the activity of stimulated afferents, we lesioned the contralateral ear and looked for changes in the vECAP. Following contralateral gentamicin injections, vECAP amplitudes continued to decrease slightly in both canals, although these changes were not significant. A similar trend was observed in monkey M2. A significant decrease in vECAP amplitudes was observed following ipsilateral gentamicin injection (Fisher’s PLSD p < 0.0001), but the small decreases in vECAP amplitude following contralateral injection were not found to be significant.
In monkey M3, vECAP N1-P1 peak amplitudes exhibited no significant changes in either the lateral or posterior canal following contralateral transtympanic gentamicin injection. Following ipsilateral injection, however, a significant decrease in vECAP N1-P1 peak amplitudes was observed in both canals (Fisher’s PLSD, p = 0.0152 and p = 0.0186 for the lateral and posterior canals, respectively), while thresholds increased slightly from 74 μA in the lateral canal and 61 μA in the posterior canal to 88 μA in both canals. In the posterior canal of M4, no significant changes in vECAPs were observed following contralateral or subsequent ipsilateral gentamicin injections. Thus, in five of six canals, vECAP amplitudes exhibited a significant decrease following transtympanic injection of gentamicin into the ipsilateral ear, but no significant changes occurred following contralateral injections. These results suggest that injection of aminoglycoside into the implanted ear did reduce the efficacy of the electrical stimulation in driving compound action potentials, presumably via activation of the fibers of the ampullar nerves. vECAPS were unaffected by contralateral injections. This was true despite the fact that the contralateral injections produced significant changes in natural rotational VOR gain, indicating that the injections were effective. Again, no increase in afferent sensitivity, as evidenced by vECAP, was seen following transtympanic injection of gentamicin either before or after transtympanic injection into the ipsilateral ear.
The final step in evaluating the site of adaptive change during electrical stimulation was to compare the efficacy of electrical stimulation in producing overt slow phase eye movement behavior following a perturbation in vestibular function. To do this, we monitored responses to short trains of biphasic stimuli before, during, and after ipsilateral, contralateral or bilateral transtympanic injection of gentamicin. Two-second trains of biphasic pulses of electrical stimulation (100 μs pulse width with an 8-μs interphase gap) were delivered to the ampullae of individual canals, which elicited sustained nystagmus in all tested canals tested prior to gentamicin perfusions (see Fig. 1A, C). Current amplitude thresholds for eliciting nystagmus ranged from 50 to 100 μA with a pulse rate of 300 PPS. Eye movements were largely directionally appropriate: stimulation delivered to the lateral canal elicited a nystagmus with a predominantly leftward slow phase. Stimulation delivered to the posterior canal elicited a nystagmus with a primarily downward slow phase. Vertical components to the nystagmus were present during stimulation of the lateral canal (Fig. 1A, D), as were horizontal components to stimulation of the posterior canal (Fig. 1C, F). These typically increased with increasing current but were always lower velocity than the expected direction component. The recording paradigm utilized in this experiment was incapable of recording torsional eye movements, and therefore, the precise rotational vector could not be determined from these recordings. With these limitations in mind, the slow phase velocities reported here and presented in the figures are the dimensional primary components of the slow phase eye movements elicited by stimulation of a specific canal (i.e., horizontal for the lateral canal and vertical for the posterior canal).
Figures 4 and 5 show that the primary component slow phase eye velocity of the elicited nystagmus increased with increasing pulse rate (Fig. 4; larger negative numbers indicate great leftward and downward velocities) or current amplitude (Fig. 5) as has been reported previously in monkeys (Dai et al. 2013, Phillips et al. 2015a) and humans (Phillips et al. 2013; Golub et al. 2014; Phillips et al. 2015a). The slow phase velocities were quite high, despite the fact that these animals had been implanted up to 650 days before these recordings were obtained.
FIG. 4.
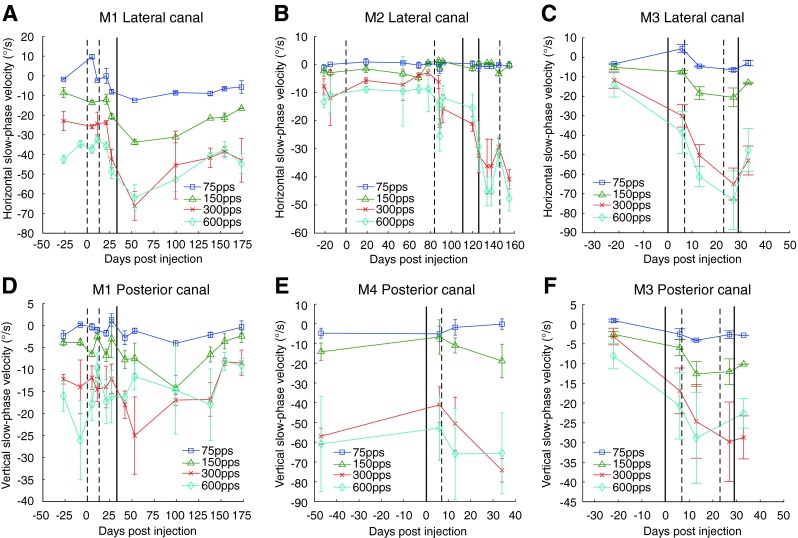
Longitudinal plot of mean slow phase velocity of electrically evoked nystagmus during 2-s trains of stimulation delivered to the lateral (A, B, C) or posterior canal (D, E, F) at different stimulation pulse rates in all animals. Velocities are from mid-range stimulation currents for each canal: either 100 μA (A, B, C, D, F) or 200 μA (E). Negative values indicate leftward or downward velocities for lateral canal or posterior canal stimulation, respectively. Vertical solid and dashed lines denote dates for gentamicin perfusions in the ipsilateral (dashed line) or contralateral (solid line) ear. Error bars are ±1 standard deviation for all slow phases recorded on that test date.
FIG. 5.
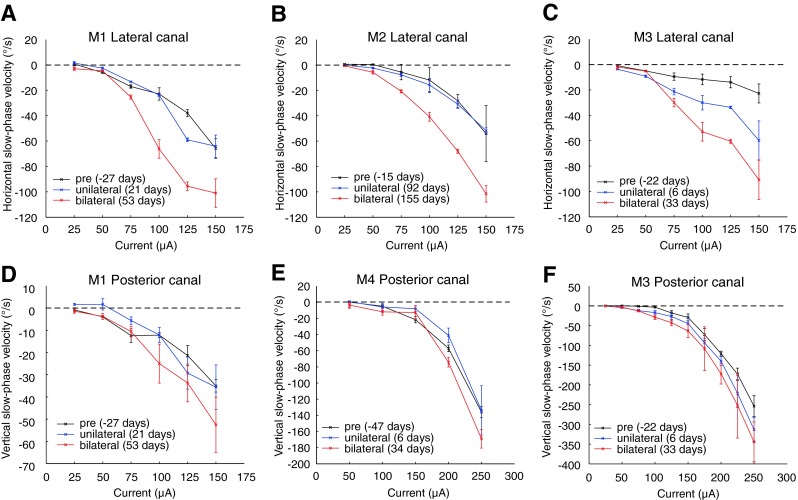
Mean slow phase velocity plotted against stimulation current electrically evoked nystagmus during 2-s trains of 300 PPS stimulation delivered to the lateral (A, B, C) or posterior canal (D, E, F) at three longitudinal time points in all animals. Time points are prior to gentamicin perfusions (black, “pre”), following unilateral perfusions and prior to bilateral gentamicin perfusions (blue, “unilateral”), and following bilateral perfusions (red, “bilateral”). Negative values indicate leftward or downward velocities for lateral canal or posterior canal stimulation, respectively. Error bars are ±standard deviation for all slow phases recorded on that test date.
Following unilateral gentamicin injection, significant increases in the slow phase velocities of elicited eye movements were observed in five of the six canals studied, and one monkey (M4) showed no significant increase or decrease. These increases occurred despite the fact that there was a significant decrease in the vECAP amplitude and increase in vECAP thresholds occurring as a consequence of the injections. Therefore, in the absence of any increase in peripheral galvanic sensitivity, and indeed in the face of a dramatic loss in peripheral galvanic sensitivity, the overall behavioral response to electrical stimulation actually increased. This was consistent with central but not peripheral adaptive change as the underlying mechanism.
Changes in slow phase velocity were generally significant at the higher pulse rates but not significant for some of the lower pulse rates due to the very low response at those pulse frequencies. Table 2 presents the results of post hoc ANOVA analyses for all animals, canals, and pulse rates. At 300 PPS, mean slow phase velocities of elicited eye movements were increased in all canals in all animals following bilateral transtympanic injection of gentamicin (Fig. 5). These changes were significant in four of six canals. This increase was substantial, particularly in the lateral canals where the mean slow phase velocity at current amplitudes of 125 μA increased from 37.9°/s to 95.6°/s in M1, 28.3°/s to 68.0°/s in M2, and 11.9°/s to 65.5°/s in M3. As seen in Figure 5, these increases in elicited slow phase velocity are a product of a substantial increase in response gain to current rather than a reduction in current threshold to elicit eye movements. This was again consistent with a central adaptive change as opposed to a peripheral adaptation due to efferent modulation. In three canals (M3—lateral, M2—lateral, and M1—posterior), velocities elicited following bilateral injections were significantly increased from those following unilateral injections. In one canal (M3—lateral), velocities elicited following a contralateral injection alone significantly increased from those recorded prior to gentamicin injections.
TABLE 2.
Fisher’s PLSD (post hoc ANOVA) p values for elicited eye movements between pre-injection, unilaterally lesioned, and bilaterally lesioned longitudinal time periods
| M1 | Lateral | M2 | Lateral | M3 | Lateral | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PPS | Pre-Uni | Uni-Bi | Pre-Bi | Pre-Uni | Uni-Bi | Pre-Bi | Pre-Uni | Uni-Bi | Pre-Bi | ||
| 75 | 0.0101 | NS | <0.0001 | 75 | <0.0001 | NS | 0.0049 | 75 | NS | 0.0002 | 0.0016 |
| 150 | 0.0046 | 0.0002 | <0.0001 | 150 | <0.0001 | NS | <0.0001 | 150 | NS | <0.0001 | <0.0001 |
| 300 | 0.0374 | NS | 0.0009 | 300 | NS | 0.0074 | 0.003 | 300 | 0.0002 | <0.0001 | <0.0001 |
| 600 | 0.0005 | NS | 0.0033 | 600 | NS | 0.0001 | 0.0111 | 600 | 0.001 | <0.0001 | <0.0001 |
| M1 | Posterior | M4 | Posterior | M3 | Posterior | ||||||
| PPS | Pre-Uni | Uni-Bi | Pre-Bi | Pre-Uni | Uni-Bi | Pre-Bi | Pre-Uni | Uni-Bi | Pre-Bi | ||
| 75 | NS | NS | 0.0009 | 75 | NS | NS | NS | 75 | 0.0005 | NS | 0.0002 |
| 150 | 0.0024 | 0.0001 | <0.0001 | 150 | NS | NS | NS | 150 | NS | 0.0053 | <0.0001 |
| 300 | NS | 0.0115 | 0.0228 | 300 | NS | NS | NS | 300 | NS | NS | NS |
| 600 | NS | NS | NS | 600 | NS | NS | NS | 600 | NS | NS | NS |
Data is grouped by animal, canal, and stimulation rate (pulses/s)
Pre pre-injection, Uni unilaterally lesioned, Bi bilaterally lesioned, PPS pulses/s, NS non-significant changes (p > 0.05)
Electrode Impedances
We assumed that the effect of gentamicin was to eliminate hair cell contributions to either the direct activation of the afferent fibers or to the galvanic sensitivity of those fibers. However, it was possible that the gentamicin produced changes to the electrical environment where our electrodes were placed, perhaps by creating scarring or accumulation of tissue around the electrodes. In order to evaluate this, we recorded electrode impedances between the stimulating electrode of each canal and the common ground. The impedances varied between animals and canals within animals. However, across all animals, impedance measurements changed no more than 12 % (mean 4.9 %, SD 4.3 %) from their pre-injection measurements over the course of all gentamicin injections (Table 3). This suggested that the electrical environment remained relatively stable throughout our experiments.
TABLE 3.
Electrode impedances measured between the stimulating electrode and common ground for canal studied on three longitudinal time periods: pre-injection, unilaterally lesioned, and bilaterally lesioned
| Canal | Pre | Uni | Bi |
|---|---|---|---|
| M1—lateral | 13.93 | 14.6 | 14.25 |
| M1—posterior | 33.38 | 36.04 | 32.66 |
| M2—lateral | 19.21 | 16.95 | 16.96 |
| M4—posterior | 29.06 | 32.3 | 30.21 |
| M3—lateral | 15.24 | 14.88 | 13.92 |
| M3—posterior | 19.31 | 19.31 | 19.39 |
Pre pre-injection, Uni unilaterally lesioned, Bi bilaterally lesioned
DISCUSSION
This study was designed to examine two aspects of the adaptive response to electrical stimulation delivered by a vestibular neurostimulator. First, we sought to examine relative contributions of peripheral and central adaptive mechanisms to generating VOR-like nystagmus in response to peripheral stimulation following vestibular loss. Second, we sought to study how vestibular adaptive mechanisms overall altered the relationship between stimulation parameters and elicited nystagmus in a context where any sensitization or habituation to the stimulus itself was limited. Based on earlier published results, we knew that it was plausible that changes might occur both peripherally, due to efferent modulation of the afferent fibers, and centrally, due to modulation of the central neurons receiving vestibular input (see Sadeghi et al. 2007). Our results suggested that adaptation was a strictly central phenomenon. Efferent modulation of peripheral afferents in response to vestibular loss seemed to play no role in the adaptive changes, which increased the behavioral response to prosthetic electrical stimulation. Furthermore, our results demonstrated the dramatic effect of such central adaptive changes. Even though bilateral ototoxic lesion significantly reduced the galvanic response of the peripheral afferent fibers, the overall behavioral response was strongly increased by the same lesions.
Preexisting Peripheral loss
There were several interesting results that came out of this work which were not central to the overall objectives. For example, we studied four animals with previously implanted vestibular neurostimulators, all of which retained effective electrically elicited nystagmus in response to short trains of biphasic stimuli delivered by the neurostimulator. In addition, all four animals also retained seemingly normal overall vestibular responses to natural rotation at the frequencies most commonly studied in vestibular prosthesis experiments, as assessed by rotary chair measures. However, rotational studies conducted at the onset of these experiments suggested that although they exhibited overall normal VOR gains, these animals had potentially lost some natural vestibular function in the implanted ear over the 119 to 679 days following implantation of the neurostimulator prior to experimental intervention in this study. We evaluated this further in the experiments reported here by first injecting gentamicin ipsilaterally in two animals and contralaterally in two animals and then adding later injection of the opposite ear to produce bilateral lesions. As is well documented, transtympanic gentamicin is highly effective at inducing peripheral vestibular loss (Halmagyi et al. 1994; Black et al. 2004). Uniformly, injection of the ipsilateral ear alone produced smaller changes in rotational VOR than injections of the contralateral ear alone. Furthermore, secondary injection of the opposite ear had greater effects on preserved rotational VOR, in the three animals that had preserved function following unilateral injection, when that ear was contralateral to the implant. This confirmed that at the outset of the experiments, the animals had some loss of peripheral vestibular function in the implanted ear.
From our experiments, we cannot tell for certain whether this loss of function was mechanical or due to hair cell loss in the implanted ear or both. It is possible that other techniques, such as caloric stimulation, a head thrust during fixation, or high frequency rotation, could have resolved this issue, but this is not certain due to the fact that the canals and ampullae were already compromised by fenestration of the canal and implantation of the electrode arrays into, or immediately adjacent to, the ampulla. Also, this was not a main objective of the study. Our rotational stimuli were used simply to demonstrate the efficacy of our gentamicin injections.
Some preexisting loss may have occurred, and injection of gentamicin was less effective in eliminating VOR in the ear with that presumed preexisting loss. Previous studies (e.g., Rubinstein et al. 2012) demonstrated that natural vestibular rotational sensitivity could be preserved in the short term following implantation of a vestibular neurostimulator in monkeys when the electrode placement and design maintained the patency of the lumen of the implanted semicircular canal. This finding was not replicated in human subjects with a diseased ear prior to implantation (Phillips et al. 2013; Golub et al. 2014). The data from this study suggest that over longer periods, there is a reduction in natural function associated with device implantation and/or repeated electrical stimulation in monkeys.
Second, we recorded electrically elicited compound action potentials from the implanted canals (vECAPs). If the preexisting loss of function resulted from a progressive loss of functioning hair cells, then we might expect to see little or no loss in the amplitude of the vECAP with injection of gentamicin in the implanted ear. This is because the gentamicin affects the function of hair cells directly and it is thought to have little direct effect on vestibular afferents, although it may directly affect cochlear afferents (Hirvonen et al. 2005; Nakagawa et al. 1997; Steyger et al. 2008; Zhang et al. 2012). Therefore, if the afferents were being directly electrically activated before injection and the hair cells were already dead, this activation should be unaffected by our injection just as the ipsilateral injection had largely failed to produce changes in the already asymmetric rotational VOR. However, if there was a reduction in vECAP following ipsilateral injection of the gentamicin, then the effect would presumably be due to changes in hair cell function. In this case, a mechanical lesion of the end organ, perhaps due to progressive plugging of the canal with connective tissue, could potentially be the cause of the observed vestibular deficit. We found that electrical activation of vECAP did indeed change dramatically following ipsilateral gentamicin injections, producing smaller amplitudes at a given current level and higher current thresholds for eliciting a response. Furthermore, contralateral injection had little effect on elicited vECAPs. Thus, these findings were consistent with the idea that long-term loss of residual peripheral function ipsilateral to the implantation was possibly mechanical and that hair cells contributed to the sustained vECAP response.
While it is thought that the electrical stimulation employed here activates afferent fibers directly (Goldberg et al. 1984), there are several potential explanations for why the activation of afferents, as indicated by vECAP, changed with loss of hair cells. One possibility is that there was a loss of direct electrical activation of hair cells, whose depolarization contributes directly to the observed response. A second is that the electrical stimulation is driving depolarization and hair cell neurotransmitter release and that this contributes to the afferent fiber compound action potentials observed in the vECAP. Studies recording ECAPs from stimulation of the auditory nerve have suggested that hair cells themselves can contribute directly to ECAP amplitudes (Miller et al. 2006; Nourski et al. 2007; Van Den Honert and Stypulkowski 1984). It is also possible that the afferent fibers were at a lower galvanic threshold due to tonic unstimulated neurotransmitter release prior to gentamicin lesion of the hair cells and were therefore more responsive to direct galvanic stimulation. A final possibility is that the gentamicin had a direct toxic affect on the afferents. We could not distinguish between these possibilities directly in our experiments. However, from the changes in vECAP amplitude and potential, we could predict that there would be a simultaneous change in the efficacy of the electrical stimulation in driving vestibular-mediated behavior. Indeed, in a comparable study, human patients with gentamicin vestibulotoxicity exhibited reduced VOR responses to transmastoid galvanic stimulation (Aw et al. 2008). Thus, we would expect that the response to electrical stimulation would decrease dramatically with a loss of the vECAP in response to comparable current levels of electrical stimulation. We observed the opposite.
Central and Peripheral Adaptation
Bilateral peripheral vestibular lesion with transtympanic gentamicin significantly increased the efficacy of electrical stimulation in driving eye movements. The vECAP results, discussed above, suggested that the combined galvanic sensitivity of the afferent fibers in the stimulated canal was typically reduced after bilateral transtympanic injection. Efferent modulation of peripheral afferents did not increase the galvanic sensitivity of the afferents, producing an increase in the behavioral response. Rather, the injections had reduced the peripheral vestibular input to the central nervous system in response to electrical stimulation. Therefore, the increase in slow phase velocity observed with electrical stimulation following bilateral injection of gentamicin appears to have been a central adaptive change alone and was not driven by any increase in galvanic sensitivity.
Previous work by Sadeghi et al. (2007) had suggested that efferent-mediated adaptive mechanisms might produce changes in afferent diversity. In particular, they found a greater proportion of irregular to regular afferents after a contralateral vestibular lesion. Because of differences in sensitivity to galvanic stimulation between afferent types, such a change might significantly increase the overall sensitivity of the afferent population in a given ampulla to electrical stimulation delivered by a vestibular prosthesis. However, following bilateral gentamicin injections, vECAP amplitudes were not observed to increase, suggesting that adaptive processes did not produce peripheral changes to the afferents’ sensitivity to electrical stimulation. This result does not mean that efferent modulation of afferent responses is not an important mechanism for compensation for natural loss of vestibular function. We did not study that here. Rather, these results simply imply that such modulation does not produce the very significant changes seen with electrical stimulation with a vestibular prosthesis.
Our main conclusion, stated above, relies on the assumption that vECAPs reflect the state of the galvanically elicited vestibular afferent discharge. Indeed, this assumption underlies the use of such compound action potential recording in a variety of sensory systems. It is important to acknowledge that vECAPs may represent changes in afferent or other fiber discharge, or hair cell activity, which does not contribute to VOR. Such an interpretation seems somewhat unlikely to produce the observed results, however. Studies of the hair cell populations projecting to the VOR show that both regular and irregular afferents contribute to this reflex (Goldberg, 2000). One might expect that any increase in afferent input to the CNS would be reflected in an increase in vECAP amplitudes. Also, while other fibers of passage in the vicinity, such as facial nerve fibers, may theoretically contribute to vECAP amplitudes, there was no observable behavioral indication of such activation in the animals (i.e., no facial or lid movement) and transtympanic gentamicin is unlikely to affect such fibers.
A comparison of the slow phase eye velocity of elicited eye movements before and after injection of the contralateral ear with gentamicin suggested that central mechanisms of adaptation alone were likely involved in modifying the efficacy of electrical stimulation. If injection of the contralateral ear, which had little effect on the observed vECAP but had a dramatic effect on the natural VOR, drove an adaptive increase in the internal gain of the rotational VOR (i.e., the CNS became hypersensitive to electrically elicited vestibular input), then such a gain increase would be revealed by increases in the velocity of the electrically elicited slow phase eye movements. In three of four animals, contralateral injection of gentamicin not only decreased the natural rotational VOR, but it also increased the slow phase velocity of the electrically elicited eye movements. Therefore, experimental bilateral damage to the hair cells of the vestibular system not only reduced natural VOR and the galvanic sensitivity of the vestibular end organ but also increased the observed efficacy of electrical stimulation as measured by overt slow phase eye movement. These results demonstrate that central hypersensitivity is the likely mechanism of the improved observed efficacy of electrical stimulation. While it is not unexpected that such central changes would take place in response to a peripheral lesion, this study disambiguates such changes from the possible peripheral changes that also take place during the same time period.
Mechanism of Adaptation
The device under examination in this study employs trains of pulsed electrical stimuli to drive vestibular afferents. In this respect, it is similar to most vestibular prostheses under development. Under this scheme, all afferents that receive a supra-threshold electrical stimulus are driven to fire in synchrony with the stimulus train produced by a stimulating electrode. No matter what method is used to encode head velocity with the electrical stimulus, this type of afferent activity is inherently aphysiological. During natural activity, there is significant variability in firing properties among different groups of afferents innervating a given ampulla and afferent firing overall is stochastic (Goldberg et al. 1984). The activity of driven secondary vestibular neurons also exhibits a similar phasic relationship to the stimulation train. Because of this, it is not clear a priori how natural mechanisms of compensation interact with the aphysiologic input provided by a neurostimulator.
In this experiment, we examined the relationships between two stimulation parameters (pulse rate and pulse current amplitude) and the slow phase eye velocity of elicited eye movements before and after compensation for peripheral vestibular loss. In most cases, an increase in elicited slow phase eye velocity at a given current and pulse rate after adaptation was a product of an increase in the gain of the relationship between stimulation parameters and slow phase eye velocity, rather than a decrease in the threshold of the relationship without any change in gain. If we assume that the mechanism underlying the electrically elicited eye movements is based primarily on recruitment of neurons to the stimulation pulse rate, these results suggest that more or a greater proportion of secondary vestibular neurons are being recruited at a given current level or pulse rate after adaptation to vestibular loss. This could be the product of an overall increase in sensitivity to afferents driven by electrical stimulation by the entire population of secondary vestibular neurons. Conversely, a change in the threshold of the relationship alone, which was not observed in this experiment, might be suggestive of a change in sensitivity to electrical stimulation of only those secondary vestibular neurons that were already recruited prior to adaptation.
Implications for Human Studies
The present experiments can also be compared to longitudinal observations of electrically elicited eye movements in human Meniere’s subjects implanted with identical vestibular neurostimulators. In human patients, slow phase eye velocity resulting from electrical stimulation was initially robust following implantation, though it was far lower than those in rhesus monkeys using identical stimulation. Over time, the elicited slow phase velocity decremented significantly (Phillips et al. 2015b; Golub et al. 2014). We hypothesized that lesion of the vestibular end organ with gentamicin in monkeys with long-standing functional implants might replicate the loss of electrical stimulation efficacy observed in humans with preexisting pathology. For our study, we chose animals that had stable implants for long periods of time, so that the viability of the implant would not be an issue in the interpretation of the data. Following chemical lesion of the end organ, electrical stimulation remained effective even with greatly reduced electrically elicited afferent input, as indicated by vECAP. Indeed, the stimulation became far more effective following a loss of natural VOR. This suggests that central plasticity created a remarkably robust response even under conditions where the galvanic response of the end organ decreased significantly, at least under conditions of a progressive loss of vestibular function, and over the time periods studied in this experiment. However, in Meniere’s patients, this increase in sensitivity either does not occur or is overwhelmed by the reduced input that the prosthesis provides under these circumstances. It is possible that the specific pathology of Meniere’s disease has a unique and deleterious affect on the vestibular end organ and afferents following surgical implantation. It is also possible that the central plastic changes seen here are compromised in patients with Meniere’s disease.
In summary, we report peripheral and central changes in the response to electrical stimulation of the vestibular end organs with a chronically implanted neurostimulator following chemical lesion of the end organs with gentamicin. The results of the present study are encouraging for the overall development of vestibular prostheses. They suggest that even with some loss in the efficacy of the device over time, which is potentially an inevitable consequence of electrode implantation, CNS plasticity is capable of maintaining useful behavioral responses to electrical stimulation. At the same time, these same studies suggest that the efficacy of a vestibular implant in maintaining or producing electrically elicited VOR may not be a reliable indicator of the efficacy of the device in maintaining afferent activation over time.
Acknowledgments
This research was supported by the National Institute on Deafness and Other Communications Disorders (NIDCD) contract N01-DC-6-005 and the National Center for Research Resources ITHS ignition award RR00166. This study was also supported by the Coulter Foundation and Cochlear, Ltd. Publication of these results was supported by NIDCD grant 1 R01 DC014002.
Conflict of Interest
JR has been a paid consultant for Cochlear, Ltd., which manufactured and provided the UW/Cochlear vestibular implant. LL, KN, JP, JR, and the University of Washington hold intellectual property rights to the device used in this study.
References
- Angelaki DE, Newlands SD, Dickman JD (2000) Primate translational vestibuloocular reflexes. IV. Changes after unilateral labyrinthectomy. J Neurophysiol 83(5):3005–3018 [DOI] [PubMed]
- Aw ST, Todd MJ, Aw GE, Weber KP, Halmagyi GM. Gentamicin vestibulotoxicity impairs human electrically evoked vestibulo-ocular reflex. Neurology. 2008;71:1776–1782. doi: 10.1212/01.wnl.0000335971.43443.d9. [DOI] [PubMed] [Google Scholar]
- Black FO, Pesznecker S, Stallings V. Permanent gentamicin vestibulotoxicity. Otol Neurotol. 2004;25(4):559–569. doi: 10.1097/00129492-200407000-00025. [DOI] [PubMed] [Google Scholar]
- Cohen B, Suzuki JI. Eye movements induced by ampullary nerve stimulation. Am J Physiol. 1963;204:347–351. doi: 10.1152/ajplegacy.1963.204.2.347. [DOI] [PubMed] [Google Scholar]
- Cohen B, Suzuki J, Bender MB. Eye movements from semicircular canal nerve stimulation in cat. Ann Otol Rhinol Laryngol. 1964;73:153–169. doi: 10.1177/000348946407300116. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Minor LB, Beraneck M, Sadeghi SG (2009) Neural substrates underlying vestibular compensation: contribution of peripheral versus central processing. J Vestib Res 19(5–6):171–182 [DOI] [PMC free article] [PubMed]
- Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vestib Res. 1995;5:67–107. doi: 10.1016/0957-4271(94)00026-X. [DOI] [PubMed] [Google Scholar]
- Dai C, Fridman GY, Della Santina CC. Effects of vestibular prosthesis electrode implantation and stimulation on hearing in rhesus monkeys. Hear Res. 2011;277:204–210. doi: 10.1016/j.heares.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Fridman GY, Davidovics NS, Chiang B, Ahn JH, Della Santina CC. Restoration of 3D vestibular sensation in rhesus monkeys using a multichannel vestibular prosthesis. Hear Res. 2011;281:74–83. doi: 10.1016/j.heares.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Fridman GY, Chiang B, Rahman MA, Ahn JH, Davidovics NS, Della Santina CC. Directional plasticity rapidly improves 3D vestibulo-ocular reflex alignment in monkeys using a multichannel vestibular prosthesis. J Assoc Res Otolaryngol. 2013;14(6):863–77. doi: 10.1007/s10162-013-0413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovics NS, Fridman GY, Chiang B, Della Santina CC. Effects of biphasic current pulse frequency, amplitude, duration, and interphase gap on eye movement responses to prosthetic electrical stimulation of the vestibular nerve, neural systems and rehabilitation engineering. IEEE Trans. 2011;19:84–94. doi: 10.1109/TNSRE.2010.2065241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovics NS, Rahman MA, Dai C, Ahn J, Fridman GY, Della Santina CC. Multichannel vestibular prosthesis employing modulation of pulse rate and current with alignment precompensation elicits improved VOR performance in monkeys. J Assoc Res Otolaryngol. 2013;14(2):233–48. doi: 10.1007/s10162-013-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Santina C, Migliaccio A, Patel A. Electrical stimulation to restore vestibular function development of a 3-d vestibular prosthesis. Conf Proc IEEE Eng Med Biol Soc. 2005;7:7380–7385. doi: 10.1109/IEMBS.2005.1616217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Santina C, Migliaccio A, Patel A. A multichannel semicircular canal neural prosthesis using electrical stimulation to restore 3-D vestibular sensation. IEEE T Bio-Med Eng. 2007;54:1016–1030. doi: 10.1109/TBME.2007.894629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetter M, Zee DS. Recovery from unilateral labyrinthectomy in rhesus monkey. J Neurophysiol. 1988;59:370–393. doi: 10.1152/jn.1988.59.2.370. [DOI] [PubMed] [Google Scholar]
- Golub JS, Ling L, Nie K, Nowack A, Shepherd SJ, Bierer SM, Jameyson E, Kaneko CR, Phillips JO, Rubinstein JT. Prosthetic implantation of the human vestibular system. Otol Neurotol. 2014;35(1):136–47. doi: 10.1097/MAO.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res. 2000;130(3):277–97. doi: 10.1007/s002210050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Gong W, Haburcakova C, Merfeld DM. Vestibulo-ocular responses evoked via bilateral electrical stimulation of the lateral semicircular canals. IEEE T Bio-Med Eng. 2008;55(11):2608–19. doi: 10.1109/TBME.2008.2001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot JP, Gay A, Izabel Kos M, Pelizzone M. Ethical, anatomical and physiological issues in developing vestibular implants for human use. J Vestib Res. 2012;22:3–9. doi: 10.3233/VES-2012-0446. [DOI] [PubMed] [Google Scholar]
- Guyot JP, Sigrist A, Pelizzone M, Kos MI. Adaptation to steady-state electrical stimulation of the vestibular system in humans. Ann Otology, Rhinol Laryngology. 2011;120:143–149. doi: 10.1177/000348941112000301. [DOI] [PubMed] [Google Scholar]
- Guyot JP, Sigrist A, Pelizzone M, Feigl GC, Kos MI. Eye movements in response to electrical stimulation of the lateral and superior ampullary nerves. Ann Otol Rhinol Laryngol. 2011;120:81. doi: 10.1177/000348941112000202. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Fattore CM, Curthoys IS, Wade S. Gentamicin vestibulotoxicity. Otolaryngol Head Neck Surg. 1994;111(5):571–4. doi: 10.1016/S0194-5998(94)70523-2. [DOI] [PubMed] [Google Scholar]
- Hirvonen T, Minor L, Hullar T, Carey J. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J Neurophysiol. 2005;93:643–655. doi: 10.1152/jn.00160.2004. [DOI] [PubMed] [Google Scholar]
- Fridman GY, Della Santina CC. Progress toward development of a multichannel vestibular prosthesis for treatment of bilateral vestibular deficiency. Anat Rec (Hoboken) 2012;295(11):2010–29. doi: 10.1002/ar.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RF, Gong WS, Ramsey M, Minor L, Boyle R, Merfeld DM. Vestibular adaptation studied with a prosthetic semicircular canal. J Vestib Res. 2002;12:87–94. [PubMed] [Google Scholar]
- Lewis RF, Merfeld DM, Gong WS. Cross-axis vestibular adaptation produced by patterned electrical stimulation. Neurology. 2001;56:A18–A18. doi: 10.1212/WNL.56.12.18A. [DOI] [Google Scholar]
- Lewis RF, Haburcakova C, Gong W, Makary C, Merfeld DM. Vestibuloocular reflex adaptation investigated with chronic motion-modulated electrical stimulation of semicircular canal afferents. J Neurophysiol. 2010;103(2):1066–79. doi: 10.1152/jn.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RF, Haburcakova C, Gong W, Lee D, Merfeld D. Electrical stimulation of semicircular canal afferents affects the perception of head orientation. J Neurosci. 2013;33(22):9530–5. doi: 10.1523/JNEUROSCI.0112-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG (1994) Neural basis for motor learning in the vestibuloocular reflex of primates. III. Computational and behavioral analysis of the sites of learning. J Neurophysiol 72(2):974–998 [DOI] [PubMed]
- Lisberger SG, Pavelko TA, Broussard DM (1994) Neural basis for motor learning in the vestibuloocular reflex of primates. I. Changes in the responses of brain stem neurons. J Neurophysiol 72(2):928–953 [DOI] [PubMed]
- Merfeld DM, Haburcakova C, Gong W, Lewis RF. Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEE Trans on Biomed Eng. 2007;50(6):1005–15. doi: 10.1109/TBME.2007.891943. [DOI] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Robinson BK, Nourski KV, Zhang F, Jeng FC. Electrical excitation of the acoustically sensitive auditory nerve: single-fiber responses to electric pulse trains. J Assoc Res Otolaryngol. 2006;7(3):195–210. doi: 10.1007/s10162-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DE, Dai C, Rahman MA, Ahn JH, Della Santina CC, Cullen KE. (2013) Head movements evoked in alert rhesus monkey by vestibular prosthesis stimulation: implications for postural and gaze stabilization. PLoS One. 2013 Oct 17;8(10) [DOI] [PMC free article] [PubMed]
- Nakagawa T, Yamane H, Shibata S, Nakai Y. Gentamicin ototoxicity induced apoptosis of the vestibular cells of guinea pigs. Eur Arch Otorhinolyrngol. 1997;254:9–14. doi: 10.1007/BF02630749. [DOI] [PubMed] [Google Scholar]
- Newlands SD, Lin N, Wei M (2014) Responses of non-eye movement central vestibular neurons to sinusoidal horizontal translation in compensated macaques after unilateral labyrinthectomy. J Neurophysiol 112(1):9–21 [DOI] [PMC free article] [PubMed]
- Nie K, Bierer SM, Ling L, Oxford T, Rubinstein JT, Phillips JO. Characterization of the electrically evoked compound action potential of the vestibular nerve. Otol Neurotol. 2011;32(1):88–97. doi: 10.1097/MAO.0b013e3181f6ca45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie K, Ling L, Bierer SM, Kaneko CR, Fuchs AF, Oxford T, Rubinstein JT, Phillips JO. An experimental vestibular neural prosthesis: design and preliminary results with rhesus monkeys stimulated with modulated pulses. IEEE T Bio-Med Eng. 2013;60(6):1685–92. doi: 10.1109/TBME.2013.2241433. [DOI] [PubMed] [Google Scholar]
- Nourski KV, Abbas PJ, Miller CA, Robinson BK, Jeng FC. Acoustic-electric interactions in the guinea pig auditory nerve: simultaneous and forward masking of the electrically evoked compound action potential. Hear Res. 2007;232(1–2):87–103. doi: 10.1016/j.heares.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelizzone M, Fornos AP, Guinand N, van de Berge R, Kos I, Stokroos R, Kingma H, Huyot JP. First functional rehabilitation via vestibular implants. Cochlear Implants Int. 2014;15(1):S62–4. doi: 10.1179/1467010014Z.000000000165. [DOI] [PubMed] [Google Scholar]
- Peng GC, Baker JF, Peterson BW. Dynamics of directional plasticity in the human vertical vestibulo-ocular reflex. J Vestib Res. 1994;4(6):453–60. [PubMed] [Google Scholar]
- Perez Fornos A, Guiland N, van de Berg R, Stokroos R, Micera S, Kingma H, Pelizzone M, Guyot JP. Artificial balance: restoration of the vestibulo-ocular reflex in humans with a prototype vestibular neuroprosthesis. Front Neurol. 2014;29(5):66. doi: 10.3389/fneur.2014.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C, DeFrancisci C, Ling L, Nie K, Nowack A, Phillips JO, Rubinstein JT. Postural responses to electrical stimulation of the vestibular end organs in human subjects. Exp Brain Res. 2013;229(2):181–95. doi: 10.1007/s00221-013-3604-3. [DOI] [PubMed] [Google Scholar]
- Phillips C, Ling L, Oxford T, Nowack A, Nie K, Rubsintein JT, Phillips JO. Longitudinal performance of an implantable vestibular prosthesis. Hear Res. 2015;322:200–11. doi: 10.1016/j.heares.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JO, Bierer SM, Ling L, Nie K, Rubinstein JT (2011) Real-time communication of head velocity and acceleration for an externally mounted vestibular prosthesis. Conf Proc IEEE Eng Med Biol Soc. 2011:3537–3541 [DOI] [PubMed]
- Phillips JO, Shepherd SJ, Nowack AL, Ling L, Bierer SM, Kaneko CRS, Phillips CMT, Nie K, Rubinstein JT. Longitudinal performance of a vestibular prosthesis as assessed by electrically evoked compound action potential recording. IEEE Eng Med Biol Soc. 2012;2012:6128–31. doi: 10.1109/EMBC.2012.6347392. [DOI] [PubMed] [Google Scholar]
- Phillips JO, Ling L, Nie K, Jameyson E, Phillips CM, Nowack AL, Golub JS, Rubinstein JT (2015b) Vestibular implantation and longitudinal electrical stimulation of the semicircular canal afferents in human subjects. J Neurophysiol. Feb 4:jn.00171.2013. doi: 10.1152/jn.00171.2013. [DOI] [PMC free article] [PubMed]
- Rubinstein JT, Bierer S, Kaneko C, Ling L, Nie K, Oxford T, Newlands S, Santos F, Risi F, Abbas PJ. Implantation of the semicircular canals with preservation of hearing and rotational sensitivity: a vestibular neurostimulator suitable for clinical research. Otol Neurotol. 2012;33:789–796. doi: 10.1097/MAO.0b013e318254ec24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol. 2007;97(2):1503–14. doi: 10.1152/jn.00829.2006. [DOI] [PubMed] [Google Scholar]
- Schultheis LW, Robinson DA. Directional plasticity of the vestibule-ocular reflex in the cat. Ann Ny Acad Sci. 1981;374:504–512. doi: 10.1111/j.1749-6632.1981.tb30895.x. [DOI] [PubMed] [Google Scholar]
- Steyger PS, Karasawa T. Intra-cochlear trafficking of aminoglycosides. Commun Integr Biol. 2008;1(2):140–2. doi: 10.4161/cib.1.2.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki JI, Cohen B. Head eye body + limb movements from semicircular canal nerves. Exp Neurol. 1964;10:393–405. doi: 10.1016/0014-4886(64)90031-7. [DOI] [PubMed] [Google Scholar]
- Thompson LA, Haburcakova C, Gong W, Lee DJ, Wall C, Merfeld DM, Lewis RF. Responses evoked by a vestibular implant providing chronic stimulation. J Vestib Res. 2012;22(1):11–15. doi: 10.3233/VES-2012-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Honert C, Stypulkowski PH. Physiological properties of the electrically stimulated auditory nerve II. Single fiber recordings. Hear Res. 1984;14:225–243. doi: 10.1016/0378-5955(84)90052-2. [DOI] [PubMed] [Google Scholar]
- Wall C, Kos MI, Guyot JP. Eye movements in response to electric stimulation of the human posterior ampullary nerve. Ann Otol Rhinol Laryngol. 2007;116:369–374. doi: 10.1177/000348940711600509. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Thomassen JS, Dickman JD, Newlands SD, Angelaki DE (2014) Long-term deficits in motion detection thresholds and spike count variability after unilateral vestibular lesion. J Neurophysiol. 112(4):870–889 [DOI] [PMC free article] [PubMed]
- Zhang YB, Zhang R, Zhang WF, Steyger PS, Dai CF. Uptake of gentamicin by vestibular efferent neurons and superior olivary complex after transtympanic administration in guinea pigs. Hear res. 2012;283(1–2):169–79. doi: 10.1016/j.heares.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


