Abstract
The broad availability of cheap three-dimensional (3D) printing equipment has raised the need for a thorough analysis on its effects on clinical accuracy. Our aim is to determine whether the accuracy of 3D printing process is affected by the use of a low-budget workflow based on open source software and consumer’s commercially available 3D printers. A group of test objects was scanned with a 64-slice computed tomography (CT) in order to build their 3D copies. CT datasets were elaborated using a software chain based on three free and open source software. Objects were printed out with a commercially available 3D printer. Both the 3D copies and the test objects were measured using a digital professional caliper. Overall, the objects’ mean absolute difference between test objects and 3D copies is 0.23 mm and the mean relative difference amounts to 0.55 %. Our results demonstrate that the accuracy of 3D printing process remains high despite the use of a low-budget workflow.
Electronic supplementary material
The online version of this article (doi:10.1007/s10278-015-9810-8) contains supplementary material, which is available to authorized users.
Keywords: Multidetector computed tomography, Computer-aided design, Printing, Imaging three-dimensional, Dimensional measurement accuracy
Background
Rapid manufacturing or three-dimensional (3D) printing technologies consist of a group of techniques—developed since the late 1980s—aimed at producing 3D objects. For a long time, these techniques have been used only in design and in industrial professional settings to build prototypes or mechanical parts. Nonetheless, the situation has deeply changed over the last years. 3D printing has been progressively made available to consumer users thanks to a reduction in the cost of 3D printers and print materials but also thanks to the development of simple, object-oriented, print software.
Several different manufacturing processes have been developed during the years: stereolitography (SLA), selective laser sintering (SLS), plaster-based 3D printing (PP), electron beam freeform fabrication (EBF), and laminated object manufacturing (LOM) [1]. Nowadays, the vast majority of consumer’s commercially available 3D printers uses a fused deposition modeling (FDM) process. This process produces 3D objects by adding layers of material one upon the other. Every layer is produced by deposing fused material in small drops released by the printer’s nozzle. Several materials can be used but acrylonitrile butadiene styrene (ABS) and polylactic acid (PLA) are the most frequently used. Nozzles function as glue guns melting the plastic material and placing the drops in the right order to build every layer. Once one layer is deposed and solidified, another one is added on the top of it. Layer after layer the entire object is manufactured.
The 3D printing spread drew the attention of medical professionals in order to convert imaging datasets into 3D objects. Both computed tomography (CT) and magnetic resonance imaging (MRI) datasets can be converted into 3D objects, the use of CT datasets being the most diffuse thanks to the Hounsfield units that simplifies the segmentation process. This allows an easy isolation of bone or contrast-filled vascular structures to produce 3D replicas of fractures [2] or aneurisms [3]. MRI datasets are instead more difficult to manipulate (since they lack of proper standardization and calibration concerning the presentation of measured voxel values) and their use is more limited [4]. Converting an image into a physical object has several advantages in the medical fields: it helps the surgeon simulating and planning the intervention [4]; it simplifies fracture classification [5] and prosthesis design [6]; it allows a preoperative selection of orthopedic metal hardware [2]; and it helps the physician communicating with the patients [7].
Nowadays, the use of 3D printing technology is still often limited to academic settings. Nonetheless, we believe it is going to rapidly spread to smaller hospitals thanks to printing solutions which can fit every budget. The broad availability of cheap 3D printing equipment has raised the need for thorough analysis on its effects on clinical accuracy.
Our aim is to determine whether the accuracy of 3D printing process is affected by the use of a low-budget workflow based on open source software and consumer’s commercially available 3D printers. We set aside about $2500 to buy the software and hardware needed to create 3D objects with a low-budget workflow; this cost reflects the one of a “state of the art” 3D printer for consumer use (not for professional use) plus the elaboration software. To our knowledge, no other previous study on this subject has been performed so far. Nonetheless, we believe that understanding whether these consumer segment solutions can fit the needs of general medical applications is of paramount importance to guide and regulate the spread of the 3D printing in smaller hospitals.
Methods
In order to test the accuracy of a low-budget workflow, a group of three test objects was designed using a computer-aided design software (AutoCAD for Mac 2014, Autodesk, San Rafael, USA) and printed out with a commercially available 3D printer (Strato 3D, Btek, Cornate d’Adda, Italy) (Fig. 1). Printing area is X 280 mm - Y 170 mm - Z 170 mm. Nozzle diameter is 0.40 mm, and repeatability reported on manufacturer technical specification amounted to 0.1 mm.
Fig. 1.

3D printer
A cube, a cylinder, and a low-height cylinder with holes were selected as test objects (Fig. 2).
Fig. 2.

Test object
Every test object was scanned with a 64-slice CT (Optima CT660, GE Healthcare, Little Chalfont, UK) in order to build the 3D copy of the test object. The scanning protocol was the one used routinely in the emergency setting (120 kV, collimation 0.625 mm, reconstruction gap 0.2 mm, matrix 512 × 512, convolution kernel for bone, pitch 0.52, rotation time 0.8 s).
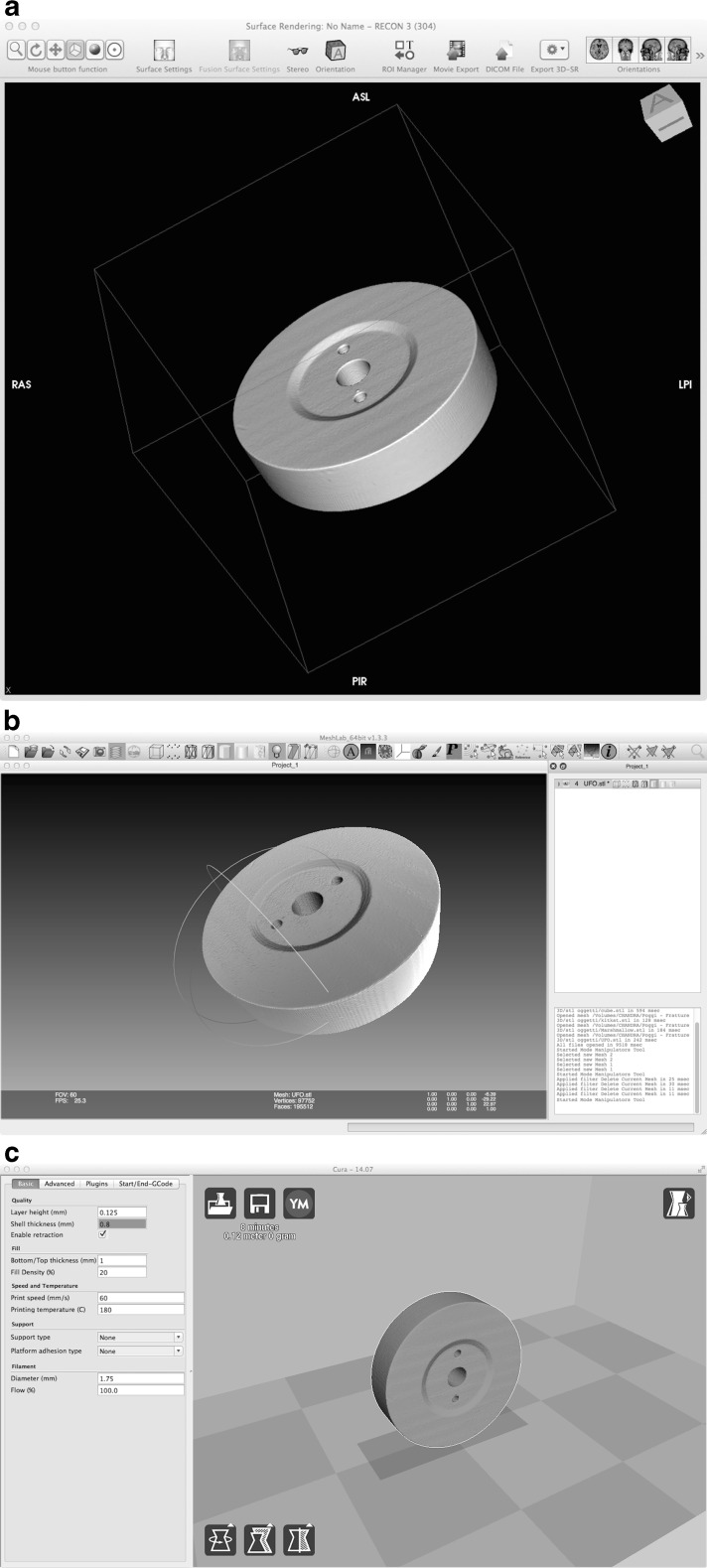
The Digital Imaging and Communications in Medicine (DICOM) dataset obtained was then elaborated by using the open source software OsiriX (OsiriX 32-bit v5.9, Pixmeo, Bernex, Switzerland; available at www.osirix-viewer.com/; accessed 6 April 2015). The dataset was loaded into the 2D/3D reconstruction tool (Fig. 3a, Vid. 1) using the 3D surface rendering option, and some surface settings were modified: resolution was set to the highest possible, smooth-iteration was set to zero (to avoid distortion of the borders), and pixel value of the first surface was set to −700. The other values were set to the default. Once obtained, the 3D surface render was exported using the export 3D-SR button and selecting export as STL (.stl). The extension .stl (STeroLitography) is used by the vast majority of 3D processing and print software.
Fig. 3.
Software chain. OsiriX (a), MeshLab (b), Cura (c)
The file .stl was loaded into another open source software MeshLab (Meshlab 64-bit v.1.3.3, Visual Computing Lab – ISTI – CNR, Italy; available at http://meshlab.sourceforge.net/; accessed 6 April 2015) (Fig. 3b, Vid. 2)—by using the function import mesh and then elaborated. The function select vertexes was used to select artifacts or structures that were present in the field of view and needed to be removed (e.g., the patient table). Once selected, the artifact or the structure was removed by using the delete the current set of selected vertices button. The program has several other selecting and cleaning options (both manual and automatic) that can fit specific issues.
In the last step the .stl file was loaded into an open source software (Cura 14.07, Ultimaker, Geldermalsen, Netherlands; available at https://software.ultimaker.com/; accessed 6 April 2015) which is generally called “slicer” because it converts .stl files into slices that printers can sequentially produce to build 3D objects (Fig. 3c, Vid. 3). Several options were modified, but in every case, changes were necessary only to fit the characteristics of the 3D printer (e.g., nozzle size or printing temperature) and not to meet specific purposes related to the present study. The options vary accordingly to the manufacturer’s suggested working temperature or to the building characteristics of the 3D printer. If needed, most of modern slicer—including Cura—can check whether the target object is successfully printable by means of a simulation process. After checking the position of the object on the production plane, the user needs to switch from view mode to layers in order to check the absence of gaps in the building procedure. After this semiautomatic elaboration, the object information was exported as a G-CODE file and loaded into the printer by using a Secure Digital (SD) card. The 3D copy (Fig. 4) was then printed with the same commercially available printer used to manufacture the test objects. Manufacturing time is extremely variable and related not only to the objects’ dimensions but also to their complexity. During our tests, manufacturing time ranged approximately from 4 to 8 h with a mean of 6 h.
Fig. 4.

3D copy
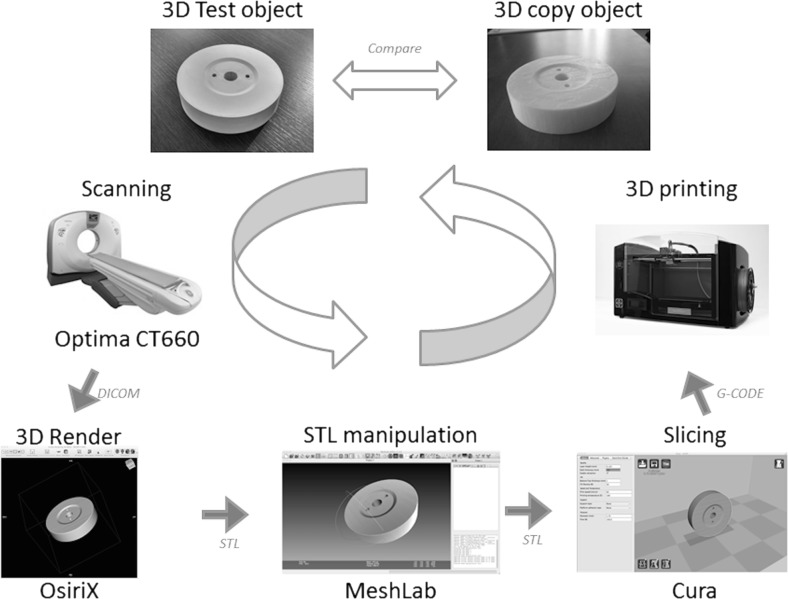
Both the 3D copies and the test objects were measured on all sides and radius using a digital professional caliper (Absolute, Mitutoyo, Takatsu-ku, Japan) with a resolution of 0.01 mm. The measurement was repeated only one time since the caliper is extremely precise and errors related to it are to be considered negligible. The entire workflow is summarized in Fig. 5.
Fig. 5.
Workflow
The mean absolute difference (mm) and the mean relative difference (%) were calculated according to the established practice which was followed in the accuracy study of rapid manufacturing techniques [8–10].
Results
The results of the measurement are summarized in Table 1.
Table 1.
Results
| Test object (mm) | 3D copy (mm) | Mean absolute difference (mm) | Mean relative difference (%) | ||
|---|---|---|---|---|---|
| Object no. 1, “cube” | Side A | 45.25 | 45.2 | −0,05 | 0.11 |
| Side B | 45.25 | 45.2 | −0.05 | 0.11 | |
| Side C | 46.7 | 46.8 | 0.1 | −0.21 | |
| Object no. 2, “cylinder” | Diameter | 25.9 | 26.12 | 0.22 | −0.84 |
| Height | 46.22 | 46.7 | 0.48 | −1.03 | |
| Object no. 3, “low height cylinder with holes” | Cylinder diameter | 121.1 | 120.5 | −0.6 | 0.50 |
| Cylinder height | 29.01 | 28.9 | −0.11 | 0.38 | |
| Hole diameter | 16.7 | 16.5 | −0.2 | 1.21 | |
| 0.23 | 0.55 |
For object no. 1 (“cube”), the three spatial axes (x, y, z) were measured and the mean relative differences were respectively −0.05, −0.05, and 0.1 %. For object no. 2 (“cylinder”), height and diameter were measured with mean relative differences respectively of 0.22 and 0.48 %. For object no. 3 (“low height cylinder with holes”), in addition to the same measure performed on object no. 2 (mean relative difference respectively of −0.6 and −0.11 %), the diameter of the hole was measured as well and it presented a mean relative difference between test object and 3D copy of −0.2 %.
Overall, the objects’ mean absolute difference between test objects and 3D copies is 0.23 mm and the mean relative difference amounts to 0.55 %.
Discussion
The overall mean relative difference in our study is 0.55 % and mean absolute difference between test objects and 3D copies is 0.23 mm. Asaumi et al. [8] suggest that dimensional changes may not affect the success of surgical applications if such changes are within a 2 % variation. Our results show that low-budget workflow’s overall accuracy is consistent and that it is likely that these types of low-budget workflows can deliver a degree of precision which is suitable to even the most demanding medical application (e.g., oral and maxillofacial interventions). Nevertheless, more focused studies are needed to actually demonstrate that these types of low-budget workflows can deliver such degree of precision in these specific settings.
However, our results indicate that technical and operator-independent factors (such as hardware and software) have reached nowadays a good level of accuracy even in the market consumer’s segment. For these reasons, the operator-dependent factors (such as the segmentation process accuracy) are rapidly becoming more and more important in influencing the overall workflow accuracy.
The importance of a precise segmentation in the daily use of a 3D print process to obtain an accurate end-model has been already extensively expressed in the recent literature [9].
Several studies on the accuracy of rapid manufacturing techniques have been published in the last 6 years. Silva and colleagues quantified the accuracy of several techniques (SLS, 3D printing™ and Polyjet™) both on mandibular anatomy [7] and on skull replicas [10]. More recently (2013), Salmi and colleagues [11] tested the accuracy of SLS, 3D printing™ and Polyjet™ on skull replicas as well. In 2012, Murugesan and colleagues [12] tested the accuracy of 3D printing™, Polyjet™, and FDM (preferred to SLS). These three techniques were applied on dental and mandibular anatomy. We would like to remember also the work by Fruhwald and colleagues [13] on skull replicas produced with SLA: maybe one of the first studies on the accuracy of a 3D printing technique applied to medical datasets. The methods and results of these studies are summarized in Table 2.
Table 2.
Accuracy studies present in literature
| Author | Printing technique | Test object | Mean absolute difference (%) |
|---|---|---|---|
| Salmi (2013) | SLS | 3D CT (original and model) | 0.79 ± 0.26 and 0.80 ± 0.32 |
| 3D printing (best quality) | 3D CT (original and measurement) | 0.67 ± 0.43 and 0.79 ± 0.44 | |
| 3D printing (moderate quality) | 3D CT | 0.38 ± 0.22 | |
| 3D printing (worse quality) | 3D CT | 0.55 ± 0.37 | |
| Polyjet | 3D CT (original and measurement) | 0.18 ± 0.12 and 0.18 ± 0.13 | |
| Murugesan (2012) | Polyjet | .stl file | 0.13 |
| 3D printing | .stl file | 1.67 | |
| FDM | .stl file | 1.73 | |
| El-Katatny (2010) | FDM | 3D CT skull | 0.24 ± 0.16 |
| FDM | 3D CT mandible | 0.22 ± 0.11 | |
| Ibrahim (2009) | SLS | Dry mandible | 1.79 |
| 3D printing | Dry mandible | 3.14 | |
| Silva (2008) | SLS | Dry skull | 2.10 |
| 3D printing | Dry skull | 2.67 | |
| Fruhwald (2008) | SLA | 2D/3D CT skull | 3 |
FDM fused deposition modeling, SLS selective laser sintering, SLA stereolitography
Our study walks the path set by the previous studies [7, 10–13], but it has several distinctive features. It tries not only to validate a workflow by testing its accuracy, but it also focuses on building a know-how so that every hospital’s department can start to actively produce accurate 3D objects with a low-budget workflow based entirely on open source software and consumer’s commercially available hardware.
In these part of the discussion, we will focus on some methodological differences between our study and the current literature. The first distinctive element is represented by our choice to use the FDM process. The world concerning 3D printing processes is very complex, as trademarks and patents often create confusion. A clear understanding of what is under the hood of a 3D printer can therefore be quite hard. Several studies have used Polyjet™ technology, but we must remember that Polyjet™ is a trademark which therefore narrows the universality of the results [7, 10, 11]. 3D printing™ is a trademark as well, but it generally refers to PP processes which are more widely used. Printers using these processes have several characteristic which make them less suitable than FDM printers for an everyday use. First of all, these kind of 3D printers are often extremely expensive (around $20.000) and their cost can be justified only for an intensive use in a 3D printing based business (e.g., graphics design). We must also remember that 3D printing is an ancillary activity in a hospital, even if it is extremely engaging. For these reasons, in many of the works cited above, the hospitals did not host the 3D printer and sent out to a research center the .stl files for printing [10]. Also, some kinds of 3D printers (e.g., the PP-based printer) may require a space which is especially designed or used to host the printer only.
On the other hand, FDM has more recently acquired new importance as leading technology for most of 3D consumer’s commercially available printers. 3D printers based on FDM are not only affordable (prices are lower than $2.000) but can also easily fit work spaces which are routinely used for other activities. Furthermore, FDM printers are low-maintenance: at every startup, the 3D printer performs a quality control on nozzle position in order to maintain stable accuracy, while some maintenance performed by a regular user is needed to ensure stable quality and repeatable results. After a few hours of printing, most of FDM printers generally need nozzle cleaning only (every printer presents precise indications on timing and mode of nozzle cleaning). This makes FDM-based 3D printer the best choice for a consumer savvy hospital which wants to be fully independent from external services. We must precise at this point that only few studies, besides ours, use FDM-based printers [12, 14].
With regard to the scanning process, we tried to maintain it as similar as possible to a routine scan. In several works, it is not specified whether the protocol is the one routinely used on patients. In some works [13], the scanning protocol seems not to be the one routinely used on patients because of its low pitch; in certain cases, this would lead to a severe increase in the dose delivered to patients. Our study’s protocol is not modified from the standard one we use everyday. This makes sure that CT images routinely obtained can be used to create accurate 3D objects.
After discussing the hardware features of the workflow, it is necessary to explore the software ones. The importance of the software in maintaining an high level of accuracy has been recently expressed [15]. Our 3D printing process is based on three different software: a medical image elaboration software used to convert DICOM into .stl files; an .stl elaboration software employed to clean small flaws and imperfections, and a slicer used to convert .stl files into a format which is suitable for the 3D printer. We managed to find a completely free and open source (FOSS) “software chain” with updated projects. The three software used (OsiriX, MeshLab, and Cura) are free and run smoothly on MacOSX and/or Windows machines. Other studies often underestimate the importance of software. Silva and colleagues [7–10] used InVesalius, an open source software able to produce .stl, but whose main function is 3D reconstruction. On the other hand, OsiriX is not only widespread but it is also a general medical image manipulation software with extensive picture archiving and communication system (PACS) features. Salmi and colleagues [11] used OsiriX and paid particular attention to the software of their study. In other works [12], proprietary software was used.
One last aspect concerning the software that needs to be underlined is the importance of the use of an .stl elaboration software. This kind of software is employed to clean small flaws and imperfections. Since some articles [16] are biased by the lack of use of this important tool, this step of the workflow should enter, in our opinion, in the medical imaging elaboration routine to create 3D objects.
In our study, geometrical surfaces only were used. Objects’ dimensions are comparable to those of the objects normally printed for medical purposes. In our study, dimensions ranged from a minimum of 17 mm to a maximum of 121 mm, while in other studies—such as the one of Silva’s and colleagues [10]—they ranged approximately from 20 to 180 mm. The majority of the other accuracy studies [7, 9–11, 16] start by directly manufacturing the real human anatomy (e.g., skulls or mandibular anatomy). This way of conceiving the study creates several distortions. First, some groups [7, 10] tend to “serialize” the studies: one article analyzes the accuracy of skull replicas, another one from the same group analyzes the accuracy for mandibular anatomy, and so on. Every bone, every organ, every structure, or part of a structure can be virtually suitable for an accuracy study. However, our intent is to validate the accuracy of the workflow in its more general meaning. Once we demonstrated that a determined chain of hardware and software has enough accuracy for medical use, then the workflow can be applied to every structure. Of course, the reproduction accuracy is not only determined by the hardware and software used, but it depends also on the ability of the health care professionals to manipulate images without distorting the structure he/she wants to reproduce. Health care professionals must choose wisely the Hounsfield unit interval and remove imperfections without affecting the original structure. Nonetheless, since these processes are not only operator-dependent but also purpose-dependent, they hardly can be standardized. Only the workflow can be fully standardized and tested for its accuracy in converting medical images into 3D objects. Reproducing objects like skulls is fascinating and challenging but, bearing our study’s aim in mind, we avoided testing the workflow on human anatomy. Self-designed test objects rather than anatomical structures allow us to perform the validation more accurately. For example, they remove several measurement bias since measuring the width of the mandible head can be difficult because of its shape. This leads therefore to the necessity of several measurements conducted by several operators in order to neutralize the arbitrary component of the measure. Geometrical forms, on the other hand, can be at the same time complex to print out—as a biological surface can be—but simple to measure. Since, in our article, precision is under evaluation, test objects are deliberately “complex” as they require a great degree of precision to be printed (e.g., a circular hole printed without shape distortion, a pyramid without asymmetric sides). Some objects are more “visually complex” than others, like, for example, a rough surface; however, it may be very difficult to compare the roughness and to establish precision in its reproduction. For all these reasons, we chose to test the accuracy in geometrical forms. We did not print out geometrical forms with overhangs. We think that overhangs are, nowadays, a secondary problem since most of the slicer software automatically add supports which are easily removable to sustain overhangs.
We can include—among the potential improvements of our study—the identification of other geometrical forms which are suitable to simulate biological surfaces without being complex to measure. As stated before, other limitations of this study are related to the necessity of simplifying operator-dependent factors (with a simplified and semiautomatic segmentation process) in order to take into account just technical and operator-independent factors (such as hardware and software) as the only factors defining accuracy. Different degrees of bias related to the segmentation process (generally speaking, a jaw is simpler to segment than a skull) are certainly present in other studies [7, 10–13]. If we consider that all these studies are performed by highly experienced professionals, we can assume that bias were reduced to the minimum but they are impossible to quantify.
Conclusions
In conclusion, our results demonstrate that the mean relative difference between test objects and 3D copies is 0.55 %. Therefore, we can state that accuracy of 3D printing process remains high despite the use of a low-budget workflow based on open source software and consumer’s commercially available hardware.
Electronic Supplementary Material
Video tutorial Osirix (MOV 6635 kb)
Video tutorial MeshLab (MOV 8493 kb)
Video tutorial Cura (MOV 9662 kb)
Acknowledgments
None
Conflicts of Interest
None for all authors
Ethics Statement
All human and animal studies have been approved and performed in accordance with ethical standards, and informed consent was obtained.
References
- 1.Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU, Giesel FL. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335–341. doi: 10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 2.Bizzotto N, Sandri A, Regis D, Romani D, Tami I, Magnan B. Three-dimensional printing of bone fractures: a new tangible realistic way for preoperative planning and education. Surg Innov. 2014 doi: 10.1177/1553350614547773. [DOI] [PubMed] [Google Scholar]
- 3.Tam MD, Laycock SD, Brown JR, Jakeways M. 3D printing of an aortic aneurysm to facilitate decision making and device selection for endovascular aneurysm repair in complex neck anatomy. J Endovasc Ther. 2013;20(6):863–867. doi: 10.1583/13-4450MR.1. [DOI] [PubMed] [Google Scholar]
- 4.Spottiswoode BS, van den Heever DJ, Chang Y, Engelhardt S, Du Plessis S, Nicolls F, Hartzenberg HB, Gretschel A. Preoperative three-dimensional model creation of magnetic resonance brain images as a tool to assist neurosurgical planning. Stereotact Funct Neurosurg. 2013;91(3):162–169. doi: 10.1159/000345264. [DOI] [PubMed] [Google Scholar]
- 5.Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR Am J Roentgenol. 2011;196(6):W683–W688. doi: 10.2214/AJR.10.5681. [DOI] [PubMed] [Google Scholar]
- 6.Smet MH, Marchal GJ, Baert AL, Van Hoe L, Van Cleynenbreugel J, Daniels H, Molenaers G, Moens P, Fabry G. Three-dimensional imaging of acetabular dysplasia: diagnostic value and impact on surgical type classification. Eur J Radiol. 2000;34(1):26–31. doi: 10.1016/S0720-048X(00)00156-X. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim D, Broilo TL, Heitz C, de Oliveira MG, de Oliveira HW, Nobre SM, Dos Santos Filho JH, Silva DN. Dimensional error of selective laser sintering, three-dimensional printing and PolyJet models in the reproduction of mandibular anatomy. J Craniomaxillofac Surg. 2009;37(3):167–173. doi: 10.1016/j.jcms.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Asaumi J, Kawai N, Honda Y, Shigehara H, Wakasa T, Kishi K. Comparison of three-dimensional computed tomography with rapid prototype models in the management of coronoid hyperplasia. Dentomaxillofac Radiol. 2001;30:330–335. doi: 10.1038/sj.dmfr.4600646. [DOI] [PubMed] [Google Scholar]
- 9.Fourie Z, Damstra J, Schepers RH, Gerrits PO, Ren Y. Segmentation process significantly influences the accuracy of 3D surface models derived from cone beam computed tomography. Eur J Radiol. 2012;81(4):e524–e530. doi: 10.1016/j.ejrad.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Silva DN, Gerhardt de Oliveira M, Meurer E, Meurer MI, Lopes da Silva JV, Santa-Bárbara A. Dimensional error in selective laser sintering and 3D-printing of models for craniomaxillary anatomy reconstruction. J Craniomaxillofac Surg. 2008;36(8):443–449. doi: 10.1016/j.jcms.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Salmi M, Paloheimo KS, Tuomi J, Wolff J, Mäkitie A. Accuracy of medical models made by additive manufacturing (rapid manufacturing) J Craniomaxillofac Surg. 2013;41(7):603–609. doi: 10.1016/j.jcms.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 12.Murugesan K, Anandapandian PA, Sharma SK, Vasantha Kumar M. Comparative evaluation of dimension and surface detail accuracy of models produced by three different rapid prototype techniques. J Indian Prosthodont Soc. 2012;12(1):16–20. doi: 10.1007/s13191-011-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frühwald J, Schicho KA, Figl M, Benesch T, Watzinger F, Kainberger F. Accuracy of craniofacial measurements: computed tomography and three-dimensional computed tomography compared with stereolithographic models. J Craniofac Surg. 2008;19(1):22–26. doi: 10.1097/scs.0b013e318052ff1a. [DOI] [PubMed] [Google Scholar]
- 14.El-Katatny I, Masood SH, Morsi YS. Error analysis of FDM fabricated medical replicas. Rapid Prototyp J. 2010;16:36e43. doi: 10.1108/13552541011011695. [DOI] [Google Scholar]
- 15.Huotilainen E, Jaanimets R, Valášek J, Marcián P, Salmi M, Tuomi J, Mäkitie A, Wolff J. Inaccuracies in additive manufactured medical skull models caused by the DICOM to STL conversion process. J Craniomaxillofac Surg. 2014;42(5):e259–e265. doi: 10.1016/j.jcms.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Fasel JH, Beinemann J, Schaller K, Gailloud P. A critical inventory of preoperative skull replicas. Ann R Coll Surg Engl. 2013;95(6):401–404. doi: 10.1308/003588413X13629960046994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video tutorial Osirix (MOV 6635 kb)
Video tutorial MeshLab (MOV 8493 kb)
Video tutorial Cura (MOV 9662 kb)