Abstract
Purpose
The aim of this study was to quantify the association between health information exchange (HIE) use and cost savings attributable to repeat imaging.
Methods
Imaging procedures associated with HIE were compared with concurrent controls on the basis of propensity score matching over the period from 2009 to 2010 in a longitudinal cohort study. The study sample (n = 12,620) included patients ages 18 years and older enrolled in the two largest commercial health plans in a 13-county region of western New York State served by the Rochester Regional Health Information Organization. The primary outcome was a continuous measure of costs associated with repeat imaging. The determinant of interest, HIE use, was defined as system access after the initial imaging procedure and before repeat imaging.
Results
HIE use was associated with an overall estimated annual savings of $32,460 in avoided repeat imaging, or $2.57 per patient. Basic imaging (radiography, ultrasound, and mammography) accounted for 85% of the estimated avoided cases of repeat imaging. Advanced imaging (CT and MRI) accounted for 13% of avoided procedures but constituted half of the estimated savings (50%).
Conclusions
HIE systems may reduce costs associated with repeat imaging. Although inexpensive imaging procedures constituted the largest proportion of avoided repeat imaging in our study, most of the estimated cost savings were due to small reductions in repeated advanced imaging procedures. HIE systems will need to be leveraged in ways that facilitate greater reductions in advanced imaging to achieve appreciable cost savings.
Keywords: Radiology, utilization, health care costs, electronic health records, health information exchange
INTRODUCTION
Imaging utilization increased rapidly during the first half of the past decade [1]. Although imaging utilization has stabilized [2–5], imaging procedures constitute a large portion of health care expenditures in the United States [6]. The federal Medicare program alone spends $10 billion annually on medical imaging [7]. Repeat imaging is a substantial contributor to imaging costs [8,9]. Patients frequently undergo repeat imaging procedures [10], particularly when prior images are difficult to obtain [11]. For these reasons, payers and policymakers have sought to reduce costs associated with repeat imaging [6,7].
Health information exchange (HIE), the electronic sharing of patient information, has the potential to improve the quality and efficiency of care by increasing provider access to patients' medical histories, including recent laboratory tests and imaging procedures [12–14]. Improved access to patients' medical histories may eliminate the need to repeat imaging procedures by making prior images more easily available [11,15,16] or by providing information indicating that a procedure is unwarranted [14].
Despite enthusiasm among policymakers and health policy experts, evidence supporting the ability of HIE to reduce imaging costs is inconsistent. For example, two studies found reductions in the use of neuroimaging and repeat imaging for lower back pain among adults presenting to emergency departments but did not identify cost savings [17,18]. Similarly, a recent investigation did not find an association between the implementation of HIE and imaging costs in ambulatory settings [19]. However, the results of two additional studies indicate potential reductions in imaging costs associated with HIE use in emergency departments. One of these studies projected cost savings through HIE on the basis of observed reductions in repeat imaging [20], whereas the other found that some imaging costs decreased with HIE use, but others increased [21]. Conversely, a nationwide analysis of exchange-capable electronic health records cited increased use of imaging, suggesting that these systems were not an effective mechanism for mitigating imaging costs [22]. The results of a more recent study of patients from a 13-county area of western New York State found potential reductions in repeat imaging associated with HIE that varied by imaging modality [23]. However, the study relied on a cross-sectional analysis to identify correlations between HIE use and counts of imaging procedures.
Understanding the relationship between HIE and imaging costs is critical given the nation's $30 billion investment in health IT [24–26]. Identifying cost savings attributable to specific imaging modalities will provide insights that allow payers and policymakers to better gauge potential savings from HIE use and develop policies that target specific imaging procedures that are more likely to drive efficiency gains from these systems. In this study, we examined the relationship between provider use of HIE and cost savings associated with repeat imaging, including changes in costs associated with specific imaging modalities, using a propensity score-matched cohort from the same population. The more rigorous study design mitigates confounding, and the analysis of specific imaging modalities potentially allows us to identify which procedures generate cost savings through provider use of HIE and which procedures should be targets of additional interventions to reduce costs.
METHODS
Study Setting
We conducted a cohort study of patients who underwent imaging procedures during 2009 and 2010 in western New York State. These patients consented to have their information made accessible to providers participating in the Rochester Regional Health Information Organization (RHIO). The Rochester RHIO is a nonprofit organization that facilitates HIE in a 13-county region [23,27]. The study was approved by the Human Research Protections Office of Weill Cornell Medical College.
HIE Intervention
Hospital systems, federally qualified health centers, private practices, reference laboratories, radiology groups, insurers, and county offices contribute data to the HIE. Authorized physicians, other clinicians, and nonclinical staff members can access patient information through a query-based web portal at the point of care. Data are fed from member sites continuously, giving users access to near real-time discharge summaries, prior diagnoses, radiology reports, medication history, and payer information [28]. More than two-thirds of the region's hospitals and physicians participate in the HIE system. At the time of the study, there were 1,318 authorized users of the HIE.
Data and Study Sample
Two commercial health insurance plans that cover more than 60% of the area's population supplied claims files for the individuals who had provided RHIO consent. Claims were limited to patients aged 18 years and older who were continuously enrolled in one of these plans. The data included six months of claims for each patient after their date of consent. These claims were merged with system logs that automatically track users' access of the HIE. A third-party data aggregation company managed the extraction of claims files and deidentification of patients.
Study participants were limited to patients who underwent imaging procedures within the first three months of their date of consent. This ensured a 90-day follow-up period after imaging. Procedures were identified using Current Procedural Terminology (CPT) codes. The third-party data aggregation company, which supplied the claims files, translated CPT codes to mutually exclusive imaging modality groups and body regions. We created a single indicator for each procedure regardless of the number of associated CPT codes used in billing. Each imaging procedure was defined as a unique combination of modality and body region on a calendar day for a given patient. For example, radiography of the chest and radiography of the abdomen on the same day would be counted as two different procedures. The earliest imaging procedure conducted after a patient's consent was defined as the index procedure, with each participant being observed only once during the study period.
Variables
The primary dependent variable was a continuous measure of costs associated with repeat imaging procedures. To derive our measure of costs, we first defined repeat imaging as a procedure within 90 days after the index procedure using the same modality for the same body location [9]. Next, the estimated cost associated with an imaging procedure was derived using the standard unit price per service, on the basis of CPT codes, as reported in the 2012 National Committee for Quality Assurance (NCQA) Relative Resource Use Standard Pricing Tables. Standardized pricing algorithms represent the average unit price for a service that includes both patient and payer liability. The NCQA tables provide standardized prices that allow estimates of changes in costs that can be generalized across providers regardless of geographic area, proprietary pricing, or fee schedules. Further documentation regarding the NCQA pricing tables can be found on the organization's website [29].
The primary independent variable for our analysis was any use of the HIE system after the initial imaging procedure and before a repeat procedure, if a second procedure was conducted. Other explanatory variables included demographic characteristics and utilization histories taken from the claims files. Patient characteristics included age, gender, and insurance status (private payer, Medicare managed care, or Medicaid managed care/state-subsidized insurance). Two measures of disease severity were used to describe patients, the number of major aggregated diagnostic groups in the year before consent and the total count of chronic conditions [30,31]. Both were generated using the Johns Hopkins ACG Case-Mix System. A variety of measures reflecting utilization in the six months before RHIO consent were also included: numbers of primary care visits, specialty care visits, emergency department or urgent care visits, inpatient admissions, laboratory tests, and imaging procedures. The number of encounters that occurred in the 90 days after the initial procedure, or until an imaging procedure was repeated (whichever was sooner), was also used as a utilization measure.
Statistical Analysis
Propensity Score Matching
Given that provider use of the HIE system is voluntary, we were concerned with the potential for selection bias. For example, patients whose records were accessed through the HIE may have had clinical indications that led to providers' viewing their information. Therefore, we used propensity score matching to select a group of patients without HIE accessed who were similar to the intervention group on the basis of their observable characteristics. Propensity score matching creates case and control groups with comparable conditional probabilities of HIE system access, which mitigates selection bias inherent in providers' decisions to view patient information [32].
First, we obtained the predicted probability of HIE system access using a logit model. Predictors of HIE use in the propensity score specification included patient demographic characteristics, prior health care utilization, imaging modality, and body locations. Next, to construct the matched pairs, we used 3:1 nearest neighbor matching with replacement. Each patient with HIE accessed was matched to three patients without HIE accessed within specified propensity score calipers (0.25 standard deviations of the logit of the propensity score) [33]. We tested other matching algorithms, including multiple matching ratios and calipers, to identify the one that yielded both the most balanced matches and adequate sample size to detect statistically significant effects. Last, the association between HIE system access and repeat imaging was estimated with our matched cohort using logistic regression models with heteroskedastic-robust standard errors.
Cost Analysis
Cost savings were estimated by determining the number of potentially avoided repeat imaging studies attributable to HIE system use [21]. First, the standard unit price per service was used to assign a cost to each CPT code [34], If claims for procedures included multiple CPT codes, we summed all associated standard pricing units to get a total cost. Second, for each modality, we determined the predicted probability of a repeated imaging study when the HIE system was accessed and when the system was not accessed. We multiplied the difference between these predicted probabilities and the number of procedures for each modality with HIE access to estimate the number of potentially avoided imaging studies. The product was then multiplied by the average cost for each modality. We summed the total costs across all modalities and annualized the dollar amounts.
RESULTS
Our final sample included 12,620 propensity score-matched patients. Baseline characteristics were well balanced between the intervention and control groups (Table 1). Before matching, the group without HIE accessed had lower average comorbidity scores, fewer chronic conditions, and lower utilization of health care services. The characteristics of the matched pairs show improved balance with no statistically significant differences between the intervention and control groups. Standardized differences in means (standardized bias) were well below 10% (0.1) for all variables [35,36]. The reduction in potential bias is illustrated in Figure 1.
Table 1.
Baseline characteristics of imaging procedures among patients in the Rochester Regional Health Information Organization, by HIE access status, with propensity score-matched comparison group
| Variable | HIE Access (n = 3,843) | Matched No HIE Access (n = 8,777) | Unmatched All No HIE Access (n = 31,473) | Standardized Bias Before Matching (%) | Standardized Bias After Matching (%) | P * |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Age, mean (y) | 58.1 | 58.1 | 56.2 | 11.2 | 0.1 | .959 |
| Men | 27.1% | 27.4% | 26.1% | 2.1 | −0.7 | .775 |
| No. of ADGs, mean | 1.21 | 1.21 | 1.04 | 13.2 | −0.1 | .975 |
| No. chronic conditions, mean | 3.1 | 3.2 | 2.8 | 13.0 | −0.6 | .816 |
| Commercial insurance | 60.0% | 59.9% | 62.0% | −3.8 | 0.5 | .843 |
| Medicaid managed care | 9.6% | 9.7% | 11.4% | −5.9 | −0.6 | .787 |
| Medicare managed care | 30.4% | 30.4% | 26.7% | 8.1 | −0.1 | .970 |
| Utilization in prior six mo | ||||||
| Laboratory tests, mean | 7.3 | 7.3 | 6.5 | 10.9 | −0.1 | .973 |
| Imaging procedures, mean | 1.6 | 1.6 | 1.4 | 8.1 | −0.6 | .790 |
| Primary care visits, mean | 1.9 | 1.9 | 1.8 | 7.1 | 0.7 | .766 |
| Specialty care visits, mean | 1.8 | 1.8 | 1.5 | 9.5 | 0.1 | .978 |
| ED encounters, mean | 0.3 | 0.3 | 0.3 | 2.6 | 1.1 | .549 |
| Inpatient admissions, mean | 0.1 | 0.1 | 0.1 | 2.6 | 0.7 | .755 |
| Utilization in 90 d after index imaging | ||||||
| Primary care visits, mean | 1.3 | 1.3 | 0.9 | 20.6 | 0.5 | .825 |
| Specialty care visits, mean | 1.8 | 1.8 | 1.0 | 42.2 | 0.2 | .947 |
| Inpatient admissions, mean | 0.2 | 0.2 | 0.1 | 15.4 | 0.7 | .800 |
| ED encounters, mean | 0.2 | 0.2 | 0.1 | 13.8 | 0.7 | .739 |
| Modality† | ||||||
| Radiography | 45.6% | 45.5% | 46.8% | −2.3 | 0.1 | .951 |
| CT | 15.3 | 15.7 | 11.2 | 12.0 | −1.3 | .592 |
| Mammography | 8.8 | 8.7 | 11.5 | −8.8 | 0.5 | .819 |
| MRI | 8.0 | 8.2 | 407 | 8.5 | −1.1 | .656 |
| Ultrasound | 15.7 | 15.1 | 19.2 | −9.3 | 1.5 | .504 |
Note: The standardized bias measures the differences in the mean of the HIE access group and no HIE access group in units of standard deviations. Standardized differences of less than 0.1 (10%) are considered adequate balance between treatment and comparison groups. ADG = aggregated diagnostic group; ED = emergency department; HIE = health information exchange.
P values comparing HIE access group with matched controls.
Propensity score matching included all modalities, but only the five most common are reported.
Fig 1.
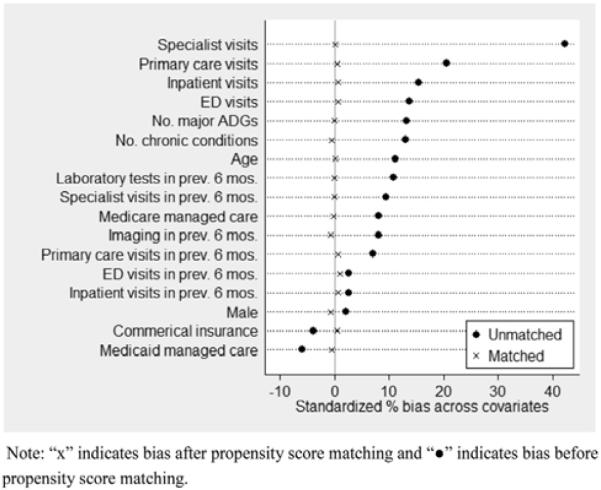
Standardized percentage bias across covariates before and after propensity score matching. Crosses indicate bias after propensity score matching and black circles indicate bias before propensity score matching. ADG = aggregated diagnostic group; ED = emergency department.
The majority of the patients in the sample underwent basic imaging procedures, with radiography (45.6%), mammography (9.5%), and ultrasound (15.7%) accounting for approximately 70% of all procedures. Among advanced imaging procedures, CT was the most common (14.8%), followed by MRI (7.9%). On average, repeated imaging procedures were conducted 40.8 days after the index imaging procedure.
Table 2 describes the associations between HIE system access and repeat imaging. In our matched sample, 6.4% of the imaging studies (n = 804) were repeated. Among those imaging studies for which the HIE system was accessed, 5.5% were repeated within 90 days. This was a statistically lower percentage of repeat imaging than was observed among the group with no HIE system use (6.7%). Overall, use of the HIE system after an index imaging procedure was associated with a 19% reduction in the odds of a procedure's being repeated within 90 days (odds ratio, 0.81; 95% confidence interval, 0.69–0.96).
Table 2.
Propensity score-adjusted associations between HIE system access and repeat imaging procedures
| Group | Repeat Imaging (n = 804) | No Repeat Imaging (n = 11,816) | P |
|---|---|---|---|
| HIE access | 213 (5.54%) | 3,630 (94.46%) | .012 |
| No HIE access | 591 (6.73%) | 8,186 (93.27%) |
Note: Pearson χ2 = 6.36; odds ratio, 0.81; 95% confidence interval, 0.69 to 0.96. HIE = health information exchange.
By applying the difference in predicted probabilities to observed imaging counts in our matched sample, we estimated that the use of the HIE system avoided 47 cases of repeated imaging procedures, generating $8,115 in cost savings (Table 3). Annualized, this translates into a savings of $32,460 in avoided imaging, or an annual per patient savings of $2.57.
Table 3.
Estimated savings associated with health information exchange system utilization
| Modality | Patients With Procedures | Patients With Repeated Imaging (%) | Estimated Avoided Cases | Estimated Savings | Annualized Estimated Savings |
|---|---|---|---|---|---|
| Aortography | 6 | 16.7 | 0 | 0 | 0 |
| CT | 1,863 | 3.1 | 4 (8.5%) | $2,009 | $8,038 |
| Fluoroscopy | 333 | 3 | 1 (2.1%) | $109 | $436 |
| Mammography | 1,202 | 3.6 | 2 (4.3%) | $151 | $602 |
| MRI | 1,001 | 3.2 | 2 (4.3%) | $2,060 | $8,240 |
| Ultrasound | 1,984 | 9.6 | 11 (23.4%) | $2,399 | $9,597 |
| Radiography | 5,760 | 8.2 | 27 (57.4%) | $1,387 | $5,548 |
| Other modalities without observed repeated imaging* | 471 | 0 | 0 (0.0%) | ($2) | † |
| Total | 12,620 | 6.4 | 47 | $8,115 | $32,460 |
| Average savings per patient (per 100,000 patients) | $2.57 ($257,211) |
Other imaging modalities included angioplasty, aortography, cystography, discography, echocardiography, myelography, urography, venography, and radiologic assist.
No expected savings.
The likelihood of repeat imaging varied by modality (Table 3). Aortography had the highest likelihood of being repeated, with 17% of these procedures repeated, but the procedure was rarely used. Among the more commonly used procedures, the highest rates were for radiography (8.2%) and ultrasound (9.6%). Basic imaging (radiography, ultrasound, and mammography) accounted for more than four-fifths (85.1%) of all the estimated avoided cases of repeat imaging and 46.7% of estimated cost savings. Although advanced imaging modalities (CT and MRI) constituted only 12.8% of the avoided utilization, these cases accounted for half of the estimated savings (50.1%).
DISCUSSION
Previous investigations have found that improving provider access to existing patient information through HIE has not been consistently associated with reductions in imaging utilization or cost savings. Our findings suggest that access to patient information through HIE was associated with an annual savings of $2.57 per patient from avoided repeat imaging in a community-based sample. The largest portion of estimated savings associated with HIE was attributed to small reductions in repeated advanced imaging procedures, such as CT and MRI. Inexpensive imaging procedures, such as radiography, constituted the largest proportion of avoided repeat imaging studies associated with HIE but accounted for only a small portion of estimated savings. Our results support the hypothesis that enabling provider access to existing patient information may lower costs through reductions in repeat imaging.
The identification of potential cost savings has important policy implications given the magnitude of public investments made in HIE and interoperable health IT. Since 2010, the State Health Information Exchange Cooperative Agreement Program has committed nearly $550 million to HIE [37], and funding through the Meaningful Use Program represents a $30 billion investment in health IT [24–26], Additionally, several states, including New York, have invested significant amounts of public dollars in HIE [38,39]. These investments have been made with expectations of gains in quality and improved efficiency in health care [40]. However, the lack of consistent evidence has led to skepticism surrounding the ability of HIE to reduce health care costs [41–43]. Our findings provide support for investments in HIE by demonstrating the potential for cost savings through reduced rates of repeat imaging.
The distribution of imaging modalities among the potentially avoided procedures identified in this study highlights the impact of advanced imaging procedures on health care costs. The majority of the estimated cost savings were realized through a relatively small number of potentially avoided cases of advanced imaging procedures, such as CT and MRI. However, the largest portion of avoided repeat imaging consisted of less expensive procedures, such as radiography. These findings are consistent with a Canadian study that found an association between the introduction of a multihospital PACS and reductions in repeat radiography, but not advanced imaging [44]. This suggests that reductions in repeat imaging associated with HIE may have only a modest impact on cost savings. Reducing imaging utilization likely requires multiple interventions [10]. To achieve more substantial savings through HIE, additional mechanisms that complement HIE will need to be developed to further reduce repeated advanced imaging procedures.
Our study extends the current literature in several ways. The participants reflect a more diverse population that received care in a broader range of settings than those evaluated in earlier investigations. The estimates of cost savings in our study were identified within a large community-based HIE consisting of commercially insured patients, including publicly insured, individuals in managed care plans, who underwent imaging procedures in conjunction with a variety of medical services. Previous studies of cost savings associated with reductions in repeat imaging through HIE focused on narrow populations treated in acute care settings [20,21]. Our study also provides important insights by identifying specific imaging modalities that are likely to drive cost savings through reductions in repeat imaging associated with HIE. Last, our analysis used a propensity score-matched approach that meets recent calls for stronger study designs in HIE research [45].
Despite these strengths, our study had several limitations to consider. First, our estimates of potentially avoided costs only consider repeat imaging using the same imaging modality. We did not consider potential savings or increases in costs from alternative imaging procedures. Other procedures may be appropriate given the course of treatment or diagnostic needs. Similarly, the measure of use in our study does not provide insight into the clinical decision-making process. We do not know what information was relevant to decisions to repeat images or to conduct alternative radiologic procedures. Some repeat imaging, using the same or an alternative modality, may be clinically appropriate. Our study period is not of sufficient length to follow all possible diagnostic choices over a course of illness. Importantly, avoided imaging may be attributable to mechanisms not explored in our study, such as decision support embedded in electronic health records [46]. Also, our measure of HIE system use was limited to the RHIO-supplied web portal. We did not account for other ways in which patient information was shared among providers, which could bias our estimates toward the null. Last, our study took place in one region of New York State. The providers in our sample may differ from those in other parts of the country, and the experiences of their patients may not generalize to broader populations.
Although our findings suggest that provider use of HIE leads to cost savings through reductions in repeat imaging, further study is warranted to see if greater savings are achieved as HIE is more widely implemented and providers gain experience with the systems.
TAKE-HOME POINTS.
-
■
Previous studies examining the relationship between HIE use and imaging costs have produced inconsistent results. Provider use of HIE was associated with modest, but significant, cost savings through reductions in repeat imaging.
-
■
Estimated savings found in our study support the hypothesis that the use of HIE may lead to cost savings through efficiency gains in the provision of medical services.
-
■
HIE systems will need to be leveraged in ways that facilitate greater reductions in advanced imaging studies to further achieve appreciable cost savings.
Footnotes
Dr. Unruh reports personal fees from naviHealth. No other authors have conflicts of interest related to the material discussed in this article.
REFERENCES
- 1.Iglehart JK. Health insurers and medical-imaging policy—a work in progress. N Engl J Med. 2009;360:1030–7. doi: 10.1056/NEJMhpr0808703. [DOI] [PubMed] [Google Scholar]
- 2.Lee DW, Duszak R, Jr, Hughes DR. Comparative analysis of Medicare spending for medical imaging: sustained dramatic slowdown compared with other services. AJR Am J Roentgenol. 2013;201:1277–82. doi: 10.2214/AJR.13.10999. [DOI] [PubMed] [Google Scholar]
- 3.Levin DC, Rao VM, Parker L. Physician orders contribute to high-tech imaging slowdown. Health Aff (Milwood) 2010;29:189–95. doi: 10.1377/hlthaff.2009.0528. [DOI] [PubMed] [Google Scholar]
- 4.Levin DC, Rao VM, Parker L. The recent downturn in utilization of CT: the start of a new trend? J Am Coll Radiol. 2012;9:795–8. doi: 10.1016/j.jacr.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Dodoo MS, Duszak R, Hughes DR. Trends in the utilization of medical imaging from 2003 to 2011: clinical encounters offer a complementary patient-centered focus. J Am Coll Radiol. 2013;10:507–12. doi: 10.1016/j.jacr.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Medicare Payment Advisory Commission Report to the Congress: Medicare payment policy. 2015 Mar; Available at: http://medpac.gov/documents/reports/march-2015-report-to-the-congress-medicare-payment-policy.pdf.
- 7.Medicare Payment Advisory Commission A data book: health care spending and the Medicare program. 2014 Jun; Available at: http://www.medpac.gov/documents/publications/jun14databookentirereport.pdf.
- 8.Haley T, Ghaemmaghami V, Loftus T, Gerkin RD, Sterrett R, Ferrara JJ. Trauma: the impact of repeat imaging. Am J Surg. 2009;198:858–62. doi: 10.1016/j.amjsurg.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Ip IK, Mortele KJ, Prevedello LM, Khorasani R. Repeat abdominal imaging examinations in a tertiary care hospital. Am J Med. 2012;125:155–61. doi: 10.1016/j.amjmed.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendee WR, Becker GJ, Borgstede JP, et al. Addressing overutilization in medical imaging. Radiology. 2010;257:240–5. doi: 10.1148/radiol.10100063. [DOI] [PubMed] [Google Scholar]
- 11.Sandberg JC, Ge Y, Nguyen HT, et al. Insight into the sharing of medical images: physician, other health care providers, and staff experience in a variety of medical settings. Appl Clin Inform. 2012;3:475–87. doi: 10.4338/ACI-2012-06-RA-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker J, Pan E, Johnston D, Adler-Milstein J, Bates DW, Middleton B. The value of health care information exchange and interoperability. Health Aff (Millwood) 2005;24:w10–8. doi: 10.1377/hlthaff.w5.10. [DOI] [PubMed] [Google Scholar]
- 13.Hripcsak G, Kaushal R, Johnson KB, et al. The United Hospital Fund meeting on evaluating health information exchange. J Biomed Inform. 2007;40:S3–10. doi: 10.1016/j.jbi.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao VM, Levin DC. The overuse of diagnostic imaging and the Choosing Wisely initiative. Ann Intern Med. 2012;157:574–6. doi: 10.7326/0003-4819-157-8-201210160-00535. [DOI] [PubMed] [Google Scholar]
- 15.Sung JC, Sodickson A, Ledbetter S. Outside CT imaging among emergency department transfer patients. J Am Coll Radiol. 2009;6:626–32. doi: 10.1016/j.jacr.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Swensen SJ. Patient-centered imaging. Am J Med. 2012;125:115–7. doi: 10.1016/j.amjmed.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Bailey JE, Wan JY, Mabry LM, et al. Does health information exchange reduce unnecessary neuroimaging and improve quality of headache care in the emergency department? J Gen Intern Med. 2013;28:176–83. doi: 10.1007/s11606-012-2092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey JE, Pope RA, Elliott EC, Wan JY, Waters TM, Frisse ME. Health information exchange reduces repeated diagnostic imaging for back pain. Ann Emerg Med. 2013;62:16–24. doi: 10.1016/j.annemergmed.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Ross SE, Radcliff TA, LeBlanc WG, Dickinson LM, Libby AM, Nease DE. Effects of health information exchange adoption on ambulatory testing rates. J Am Med Inform Assoc. 2013;20:1137–42. doi: 10.1136/amiajnl-2012-001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lammers EJ, Adler-Milstein J, Kocher KE. Does health information exchange reduce redundant imaging? Evidence from emergency departments. Med Care. 2014;52:227–34. doi: 10.1097/MLR.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 21.Frisse ME, Johnson KB, Nian H, et al. The financial impact of health information exchange on emergency department care. J Am Med Inform Assoc. 2012;19:328–33. doi: 10.1136/amiajnl-2011-000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick D, Bor DH, Woolhandler S, Himmelstein DU. Giving office-based physicians electronic access to patients' prior imaging and lab results did not deter ordering of tests. Health Aff (Millwood) 2012;31:488–96. doi: 10.1377/hlthaff.2011.0876. [DOI] [PubMed] [Google Scholar]
- 23.Vest J, Kaushal R, Silver M, Hentel K, Kern L. Health information exchange and the frequency of repeat medical imaging. Am J Manage Care. 2014;20:eSP16–24. [PubMed] [Google Scholar]
- 24.Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363:501–4. doi: 10.1056/NEJMp1006114. [DOI] [PubMed] [Google Scholar]
- 25.Steinbrook R. Health care and the American recovery and reinvestment act. N Engl J Med. 2009;360:1057–60. doi: 10.1056/NEJMp0900665. [DOI] [PubMed] [Google Scholar]
- 26.DesRoches CM, Campbell EG, Rao SR, et al. Electronic health records in ambulatory care—a national survey of physicians. N Engl J Med. 2008;359:50–60. doi: 10.1056/NEJMsa0802005. [DOI] [PubMed] [Google Scholar]
- 27.Greater Rochester Regional Health Information Organization About Rochester RHIO. Available at: http://www.grrhio.org/about/default.aspx.
- 28.Greater Rochester Regional Health Information Organization The Virtual Health Record links you to clinical reports, medication histories, radiology images and more from across the region. Available at: http://www.grrhio.org/providers/~/media/VHR%20Insert.ashx.
- 29.National Committee for Quality Assurance [Accessed September 3, 2013];RRU standard pricing tables. Available at: https://www.ncqa.org/tabid/l437/Default.aspx.
- 30.The Johns Hopkins ACG® System: technical reference guide version 10.0. The Johns Hopkins University; Baltimore, Maryland: 2011. The Health Services Research & Development Center at The Johns Hopkins University, Bloomberg School of Public Health. [Google Scholar]
- 31.Baldwin LM, Klabunde CN, Green P, Barlow W, Wright G. In search of the perfect comorbidity measure for use with administrative claims data: does it exist? Med Care. 2006;44:745–53. doi: 10.1097/01.mlr.0000223475.70440.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996;52:249–64. [PubMed] [Google Scholar]
- 33.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Committee for Quality Assurance RRU standard pricing tables. Available at: http://www.ncqa.org/RRUStandardPricingTables.aspx.
- 35.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–8. [Google Scholar]
- 36.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Office of the National Coordinator for Health Information Technology [Accessed September 13, 2012];State Health Information Exchange Cooperative Agreement Program. Available at: http://healthit.hhs.gov/portal/server.pt?open=512&objID=1488&mode=2.
- 38.Kern LM, Barron Y, Abramson EL, Patel V, Kaushal R. HEAL NY: promoting interoperable health information technology in New York State. Health Aff (Millwood) 2009;28:493–504. doi: 10.1377/hlthaff.28.2.493. [DOI] [PubMed] [Google Scholar]
- 39.Vest J. Health information exchange: national & international approaches. Adv Health Care Manage. 2012;12:3–24. doi: 10.1108/s1474-8231(2012)0000012005. [DOI] [PubMed] [Google Scholar]
- 40.Brailer DJ. Presidential leadership and health information technology. Health Aff (Millwood) 2009;28:w392–8. doi: 10.1377/hlthaff.28.2.w392. [DOI] [PubMed] [Google Scholar]
- 41.Waegemann CP. Health information technology's problems. Health Aff (Millwood) 2013;32:629. doi: 10.1377/hlthaff.2013.0091. [DOI] [PubMed] [Google Scholar]
- 42.Kellermann AL, Jones SS. What it will take to achieve the as-yet-unfulfilled promises of health information technology. Health Aff (Millwood) 2013;32:63–8. doi: 10.1377/hlthaff.2012.0693. [DOI] [PubMed] [Google Scholar]
- 43.Evidence on the costs and benefits of health information technology. 2008. The Congress of the United States, Congressional Budget Office. [Google Scholar]
- 44.You J, Yun L, Tu J. Impact of picture archiving communication systems on rates of duplicate imaging: a before-after study. BMC Health Serv Res. 2008;8:234. doi: 10.1186/1472-6963-8-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudin RS, Motala A, Goldzweig CL, Shekelle PG. Usage and effect of health information exchange: a systematic review. Ann Intern Med. 2014;161:803–11. doi: 10.7326/M14-0877. [DOI] [PubMed] [Google Scholar]
- 46.Wasser EJ, Prevedello LM, Sodickson A, Mar W, Khorasani R. Impact of a real-time computerized duplicate alert system on the utilization of computed tomography. JAMA Intern Med. 2013;173:1024–6. doi: 10.1001/jamainternmed.2013.543. [DOI] [PMC free article] [PubMed] [Google Scholar]


