Abstract
Patients with temporal lobe epilepsy (TLE) often display cognitive deficits. However, current epilepsy therapeutic interventions mainly aim at how to reduce the frequency and degree of epileptic seizures. Recovery of cognitive impairment is not attended enough, resulting in the lack of effective approaches in this respect. In the pilocarpine-induced temporal lobe epilepsy rat model, memory impairment has been classically reported. Here we evaluated spatial cognition changes at different epileptogenesis stages in rats of this model and explored the effects of long-term Mozart music exposure on the recovery of cognitive ability. Our results showed that pilocarpine rats suffered persisting cognitive impairment during epileptogenesis. Interestingly, we found that Mozart music exposure can significantly enhance cognitive ability in epileptic rats, and music intervention may be more effective for improving cognitive function during the early stages after Status epilepticus. These findings strongly suggest that Mozart music may help to promote the recovery of cognitive damage due to seizure activities, which provides a novel intervention strategy to diminish cognitive deficits in TLE patients.
Keywords: Status epilepticus, Mozart music, Morris water maze, Cognitive damage
Introduction
Temporal lobe epilepsy (TLE), the most common type of epilepsy in adult humans, is characterized by spontaneous recurrent seizures and pathologically changes of hippocampal neuronal loss (Tellez-Zenteno and Hernandez-Ronquillo 2012). Because of the hippocampus is involved in processing of spatial–temporal information (Wagatsuma and Yamaguchi 2007; Howe and Levy 2007), in addition to typical seizure activities, TLE affects significantly learning and memory capabilities due to hippocampal damage, which can reduce life quality of epilepsy patients (Jokeit et al. 2000). So far, the origin of cognitive deficits of TLE still remains unknown. Theoretically, many factors may affect cognitive ability, including seizures themselves (Marques et al. 2007), interictal epileptiform discharges (Aldenkamp and Arends 2004), and the reorganization of the underlying neuronal circuitry (Morimoto et al. 2004; Seth 2008; Brown 2013). Although antiepileptic drugs can relieve epileptic seizures, some studies have revealed that these drugs also have some adverse effects on cognition (Meador 2006). It is thus of importance to identify novel intervention strategies to diminish cognitive deficits in TLE patients.
Previous studies suggested that certain types of music are able to improve mental function (Sack 2006) and alleviate brain diseases (Kuester et al. 2010). For instance, it have been confirmed that the exposure to Mozart Sonata can improve spatial cognitive ability inducing in both humans and animals (Rauscher 1993; Ivanov and Geake 2003; Rauscher et al. 1998). Listening to music during the early post-stroke period enhanced cognitive recovery and prevent negative moods (Särkämö et al. 2008). Music therapy had benefits for improved cognitive impairment in Parkinsonism’s disease with dementia (Zhang 2015). Quite interestingly, recent studies have also indicated that music exposure might play a positive role in the treatment of epilepsy. Specifically, it has been found that listening to the Mozart sonata K.448 decreased epileptiform discharges in children with various types of epilepsy (Lin et al. 2011a, b). Nevertheless, relevant studies were primarily focused on the suppression of seizure activities through music intervention. And music effect in epilepsy intervention was confined only to the period during which the music was playing (Hughes et al. 1998). But whether appropriate music exposure can improve cognitive function in epileptic patients, and the possibility of long-term music exposure to cognitive improvement, still remain unclear.
In rodents, pilocarpine-induced experimental epilepsy reproduces both the clinical and neuropathological features of human TLE. Such epileptic animal model thus has been widely used to explore the mechanisms of TLE (Turski et al. 1983). After 1–2 days of pilocarpine-induced status epilepticus (SE), the rats enter a seizure-free period, known as the latent period, and then progressively develop a chronic epileptic condition after weeks (Curia et al. 2008). Classical tests have demonstrated hippocampus-dependent spatial memory deficits during the latent period in pilocarpine-treated rats (Faure et al. 2013; Chauvière et al. 2009).
The Morris water maze (MWM) task testing was widely used to evaluate the hippocampus-dependent spatial memory ability. In the present study, using MWM, we employed the pilocarpine-induced rats to determine the time course of cognitive deficits during epileptogenesis and explore the effects of longer-term Mozart music exposure on cognitive recovery in SE rats. Our results showed a persistent deficit of spatial memory during epileptogenesis. In addition, appropriate music exposure indeed attenuated spatial cognitive deterioration correlated with hippocampus and, remarkably, music intervention was found to be more effective for improving cognitive function during the early stages after SE.
Materials and methods
Animals preparation
All experiments were performed according to the experimental guidelines of the University of Electronic Science and Technology of China, and the experimental protocol was approved by the ethics committee of the University of Electronic Science and Technology of China. Adult male Sprague–Dawley rats (250 ± 30 g) were obtained from the Animal Research Institute of Sichuan Province, maintained on a 12-h light/12-h dark cycle (light on at 08:00 h) at a controlled ambient temperature (22 ± 2 °C). Food and water were made available ad libitum.
Seventy rats were used in this experiment. The rats were injected intraperitoneally with pilocarpine (60 mg/kg, Sigma) 18–20 h after the injection of lithium chloride (127 mg/kg, Sigma). Scopolamine methyl-nitrate (1 mg/kg, Sigma) was injected to limit peripheral cholinergic effects 30 min before the injection of pilocarpine. SE was stopped or reduced by diazepam (10 mg/kg) after 120 min. Sixty of the rats underwent lithium–pilocarpine treatment and they, with seizure severity scores of 3, 4 or 5 according to Racine (1972), were used in the following experiments. For comparison, the other 10 rats received saline injections instead of pilocarpine as a control group. Among the 60 rats that underwent lithium–pilocarpine treatment, 20 rats induced successfully SE were randomly assigned to the epilepsy group (SE rats without any additional musical exposure, n = 10) and the Mozart-epilepsy group (SE rats exposure to Mozart music, n = 10). The rest of 60 rats, including 14 rats whose seizure severity did not reach the above criteria and 26 rats died during or shortly after the SE, were excluded from our study. Control group rats did not been operated both pilocarpine injection and additional musical exposure.
Music exposure
Mozart’s piano sonata K.448, often used in studies on the “Mozart effect,” was employed in this experiment (Aoun et al. 2005; Hughes 2001). The Mozart music stimulus intensity was 65–75 dB. The musical material was presented on the day after SE consecutively for 34 days (First day after SE is denoted as SED1), and played repeatedly for 2 h per day from 8.00 p.m. to 10.00 p.m. The control group and epilepsy group rats were not exposed to any additional music and kept approximately 70 dB ambient noise intensity of feeding condition.
Morris water maze task testing
All rats were tested in a standard version of the hidden platform Morris water maze (MWM), which is usually used to measure hippocampus-dependent spatial memory ability (Özdemir et al. 2014). Before the experiment, the exercise capacity of all rats in MWM task testing was assessed to ensure their equal status. The testing was carried out three times, each time for 5 days beginning 10, 20, 30 days after SE (denoted as SED10, SED20, and SED30).
The MWM (WMT-200, Taimeng, China) was placed in a sound-isolated testing room, illuminated by normal housing lights and surrounded by a number of fixed extramaze visual cues. The cues remained constant during testing. The MWM consists of a black circular pool (130 cm diameter, 50 cm high) filled with water (27 cm depth) at 26 ± 1 °C and virtually divided into four equivalent quadrants: target quadrant, left quadrant, right quadrant and opposite quadrant. The water was made opaque with a mixture of white, thick, non-toxic milk. A 2 cm circular submerged escape platform (10 cm diameter) was positioned in the centre of target quadrant. The rats behavior (including latency to escape, time spent in each quadrant, swimming speed and distance) was monitored by a video camera and quantified using WMT-200 software.
The rats were trained with the standard MWM learning protocol during the 5-day testing. One day before each testing period, each rat was placed in the pool for 2 min, and then allowed to climb onto the platform where it could rest for 20 s and the procedure was repeated three times in order to habituate the rat to the training environment. On days 1–4 of testing, each rat performed four trials respectively and each trial began with the rat being placed in the pool and released facing the sidewall at one of the four quadrants. The latency time from immersion into the pool to escape onto the platform was recorded by software to provide a measure of spatial reference memory for each trail. The rat was guided to the platform if it failed to escape within 60 s and was allowed to stay in the platform for 20 s. The testing consisted of four trails a day, so there were totally 16 trials for each rat. On day 5, a probe trial was conducted, in which the platform was removed and the rats were placed in the opposite side of the target quadrant and then allowed to explore the pool for 60 s and the time spent in each quadrant was recorded.
The acquisition learning phase lasting 4 days provided a measure of spatial learning and reference memory, and the probe trial on the 5th day measured spatial memory and retrieval capabilities. Spatial memory ability was measured as the duration of time spent in the target quadrant (% total time, chance level = 25 %). In addition, we evaluated the swimming speed and distance of all rats in the probe trial in order to evaluate the motor ability after SE. All the behavior variables were analyzed by the software.
Statistical analysis
Differences between the groups with respect to the escape latency of the hidden platform acquisition training were assessed using repeated measures analysis of variance (ANOVA) with the general linear model. Differences in the duration of time spent in each quadrant for the different groups were analyzed using one-way ANOVA. Statistical analyses were performed using SPSS 16.0. The post hoc comparison with the Least-significant difference (LSD) method was used to provide more details about the differences among groups. The level of statistical significance was set at P < 0.05.
Results
Spatial memory impairment in MWM task
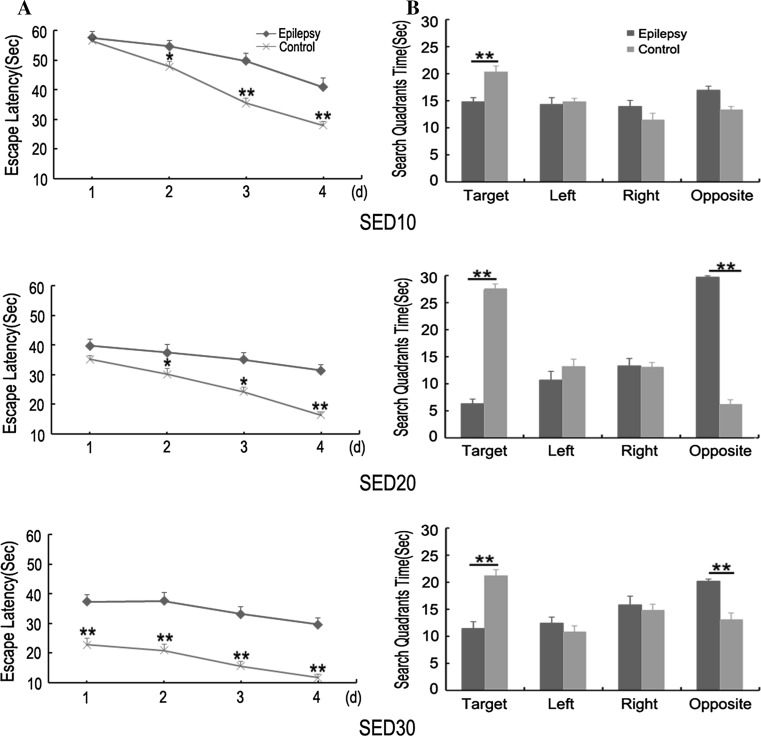
In the MWM task, the rats learn to locate a submerged platform in a pool by orienting themselves using distal visual cues. As shown in Fig. 1, there were significant differences in MWM performance between the control and epilepsy groups at three testing stages (P < 0.05). The control group rats showed better task acquisition and searched accurately for the platform, whereas the epilepsy group rats were less capable of finding the platform. Although both the control and epilepsy groups showed gradually decrease in escape latency with the acquisition trial training, the epilepsy group rats performed significantly worse than the control. In the probe trial, the epilepsy group rats spent less time in the target quadrant compared with the control during all three testing stages. These results demonstrated that SE rats had persistent spatial cognitive capability impairment following epileptogenesis.
Fig. 1.
Morris water maze performance in the control and epilepsy groups. a Escape latency for control rats compared with epilepsy group rats. b Time in quadrants for control rats compared with epilepsy group rats. Data is presented mean ± SEM. Single asterisk represents statistical differences at P < 0.05 and double asterisk represents statistical differences at P < 0.01. SED10: from 10 to 14 days after SE; SED20: from 20 to 24 days after SE; SED30: from 30 to 34 days after SE. Control group n = 10; Epilepsy group n = 10
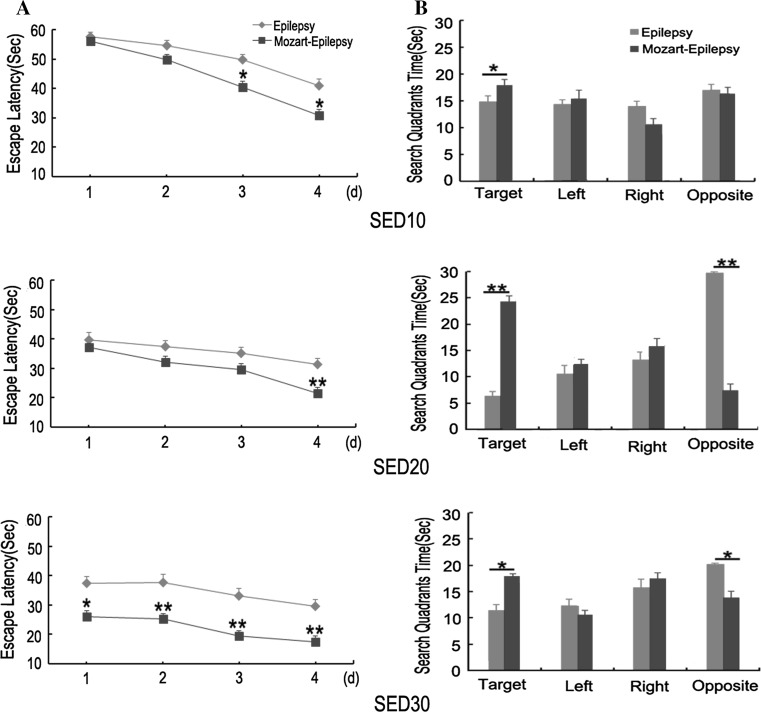
On the other hand, although the rats showed improvements in escape latency across the three testing periods (P < 0.01), there were marked differences between the Mozart-epilepsy and epilepsy groups (P < 0.01; Fig. 2). The Mozart-epilepsy group rats performed better than epilepsy group in water maze task (P < 0.01). During the probe trial, there was a substantial difference between the Mozart-epilepsy and epilepsy groups (P < 0.05). The epilepsy group rats spent less time in the target quadrant than the Mozart-epilepsy group rats. Taken together, these findings indicated that water maze performance was significantly enhanced in the Mozart-epilepsy as compared to epilepsy group.
Fig. 2.
Morris water maze performance in the Mozart-epilepsy and epilepsy groups. a Escape latency for Mozart-epilepsy group compared with epilepsy group. b Time in quadrants for Mozart-epilepsy group compared with epilepsy group. Data is presented mean ± SEM. Single asterisk represents statistical differences at P < 0.05 and double asterisk represents statistical differences at P < 0.01. SED10: from 10 to 14 days after SE; SED20: from 20 to 24 days after SE; SED30: from 30 to 34 days after SE. Epilepsy group n = 10; Mozart-epilepsy group n = 10
Mobility ability assessment after SE
Seizure activity can lead to abnormal mobility ability in rats, which may influence performance of the rats in the MWM. During the water maze testing, experimental rats did not show any seizure activity by video recording. In order to examine whether SE or seizure activity affected the mobility ability of the rats and then led to a decrease of their performance in the MWM, we further assessed their motion ability in terms of swimming speed and distance in the probe task. The results in Table 1 showed that there were no significant differences among the three groups across different testing times (P > 0.05). This finding implied that cognitive impairment of SE rats in MWM task was not due to abnormal movements in our experiment.
Table 1.
Swimming speed and distance in the water maze probe trial (Mean ± SEM)
| Stage | Group | Average swimming speed (cm/s) | Distance (cm) | P |
|---|---|---|---|---|
| SED10 | Epilepsy | 12.46 ± 0.32 | 745.45 ± 10.83 | |
| Mozart-epilepsy | 13.21 ± 0.18 | 791.19 ± 11.28 | >0.05 | |
| Control | 12.94 ± 0.52 | 774.72 ± 9.87 | ||
| SED20 | Epilepsy | 13.43 ± 0.42 | 802.52 ± 12.34 | |
| Mozart-epilepsy | 12.71 ± 0.19 | 760.82 ± 11.43 | >0.05 | |
| Control | 12.62 ± 0.36 | 755.43 ± 15.69 | ||
| SED30 | Epilepsy | 11.97 ± 0.20 | 716.88 ± 13.10 | |
| Mozart-epilepsy | 11.85 ± 0.72 | 709.34 ± 12.87 | >0.05 | |
| Control | 12.45 ± 0.61 | 745.25 ± 10.22 |
Assessment of learning rates after music exposure
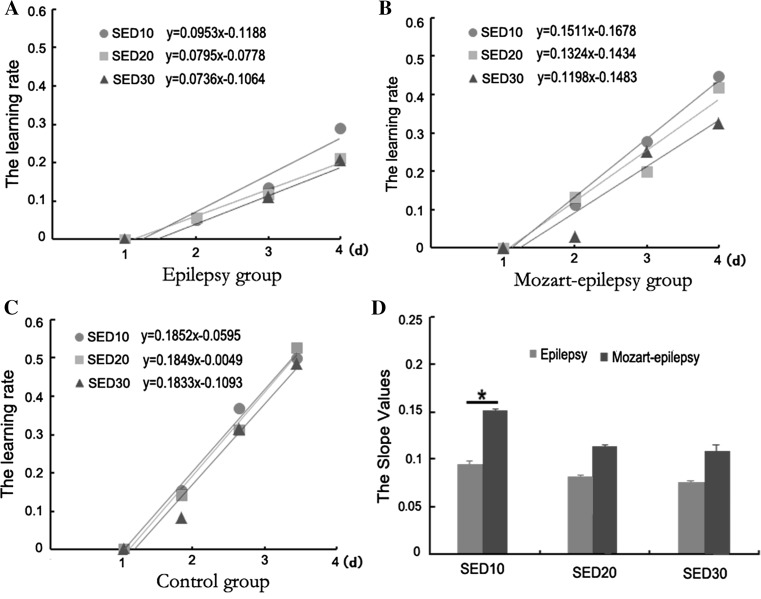
To analyze the influence of long-term music intervention on cognitive recovery, we further assessed the learning rates of the rats in the MWM. The learning rate was calculated as follows: (Mean latency of the first day—Mean latency of the second, third, or fourth day)/Mean latency of the first day.
As shown in Fig. 3, the linear slopes of the learning rates in the control group were nearly equal in three testing periods (Fig. 3c) and much higher than the epilepsy and Mozart-epilepsy groups (Fig. 3a, b). In contrast, the linear slopes of the learning rates in SE rats reduced gradually over the three periods following epileptogenesis. In addition, we also observed that the learning rates were different at the various testing periods between the epilepsy (Fig. 3a) and Mozart-epilepsy (Fig. 3b) groups. Compared with Mozart-epilepsy group, the epilepsy group showed relatively lower learning rates for all three testing periods. However, further statistical analysis revealed that there was only a significant difference at SED10 testing between these two groups (Fig. 3d; P < 0.05). Our above finding indicated that music intervention might be more effective for improving cognitive function during the early stages after SE.
Fig. 3.
Comparison of the learning rates among the different groups. Three groups are: Epilepsy group (a), Mozart-epilepsy group (b) and Control group (c). d The slope values for Mozart-epilepsy group compared with epilepsy group. Single asterisk represents P < 0.05. Control group n = 10; Epilepsy group n = 10; Mozart-epilepsy group n = 10
Discussion
Using a classical MWM task, we have demonstrated that pilocarpine experimental rats exhibit spatial memory deficits soon after the initial insult and such deficit persists during epileptogenesis. Music intervention is able to enhance the spatial memory capability and exerts a stronger effect on cognitive recovery during the early period after SE.
Cognitive deficits remain a major complaint in TLE. Although the majority of patients with TEL have normal intelligence, overall, they are more likely to suffer from impaired cognitive performance when compared with healthy controls (Meador 2002). Annemarieke Rutten et al. (2002) reported that impairments in visual-spatial memory were present shortly after SE in immature rats and persisted until adulthood. Impairment of a variety of memory types has been extensively described in TLE patients, including verbal, episodic and spatial working memory (Wieser 2004).
A wide range of variables may underlie cognitive impairment in epilepsy including biologic, psychosocial, and treatment-induced factors (Meador et al. 2001). There is convincing evidence showing that higher frequency and duration of TLE are associated with more severe hippocampal atrophy and cognitive deficiency (Eichenbaum 1999). However, few studies have addressed whether the degree of cognitive impairment is stable following the epileptogenesis process. In this study, our results showed that cognitive impairment was persistent and the degree was varied during the different epileptogenesis periods in the MWM testing. We didn’t find the presence of seizure activity or the change of motor ability indexed by both swimming speed and path distance at water maze task testing, which may suggest that cognitive impairment of SE rats in MWM task was not due to abnormal movements in our experiment. In future studies, further experiments and evaluations are required to investigate the detailed neural mechanisms of cognitive impairment in model rats.
Although there have been putative effects of music on learning and memory, accumulating evidence has demonstrated that spatial learning and working memory acquisition and retention in mouse, rat or human are improved by appropriately exposure to music. Rauscher (1993) observed an immediate enhancement in spatial–temporal reasoning in college students exposed to 10 min of the Mozart Sonata K.448. In addition to Mozart’s music, other musical pieces, including Schubert and Bach music, have been found to have the similar effect (Ivanov and Geake 2003; Caldwell and Riby 2006).
At present music has been used to ameliorate memory loss in Parkinson’s disease (Pacchetti et al. 2000) and demented patients (Foster and Valentine 2001), to assist language acquisition in learning-impaired children, to enhance student academic performance on examinations (Bridgett and Cuevas 2000) and attention-deficit/hyperactivity disorder (Maguire 2012). Particllarly, Mozart music is used to cure children with generalized seizures in clinical. Lin et al. (2010, 2011a, b) reported the effect of Mozart K.448 on epileptiform discharges and seizure frequencies in Taiwanese children with generalized seizures. By continuous EEG monitoring occurred before, during, and after 8 min of exposure to the Mozart sonata, in 81 % of the patients there was a reduction in interictal discharges. Similar reduction was also reported when patients with rolandic types of seizures were exposed to Mozart K.448 (Turner 2004). Hughes (2001) observed that exposure to a Mozart sonata dramatically reduced pathological ictal spiking activity in epilepsy subjects. Animal experiments also found long-term enhancement of maze learning in mice via a generalized Mozart effect (Aoun et al. 2005). Mozart K.448 attenuates spontaneous absence seizure and related high-voltage rhythmic spike discharges in Long Evans rats (Lin et al. 2013).
The therapeutic potential of music has largely been explored in cognitive science (Raglio et al. 2014). However, the effect of music on patients or animals with epileptic seizures is complicated and at present poorly understood. Previous studies paid more attention to effects of music therapy on epilepsy activity, such as the sustained time and frequency of seizures. In this study, we investigated mainly the effects of music on cognition improvement following epileptogenesis. Our results indicated that epilepsy rats had poorer learning and memory capability compared with normal rats, validating that there are dramatic and persistent impairments in spatial learning and memory following SE. Exposure to Mozart music significantly attenuated cognitive impairments in SE rats. Furthermore, we found that the learning rate was significantly different only at SED10 between the epilepsy rats exposed to music and the epilepsy rats without music exposure, indicating that early music intervention may be more helpful to the recovery of cognitive impairment.
Deficits in cognitive ability with TEL are often associated with cell loss in the hippocampus, and repeated seizures can aggravate neuronal loss and cell death, thereby exacerbating cognitive impairment (Eichenbaum 1999). Recent experimental evidence has shown that some specific music (such as Mozart music) can decrease spontaneous apoptotic cell death, increase neurotrophic factors, neurogenesis, synaptogenesis, dendritic spine number, dendritic arborization, and change the levels of neurotransmitters or receptors in rodents (Kim et al. 2006; Van Praag et al. 2000; Chikahisa et al. 2006). Exposure to music in the perinatal period enhances learning performance and alters BDNF/TrkB signaling in mice as adults (Castren et al. 1992). Lin et al. (2014) found that listening to Mozart K.448 significantly reduced the EEG oscillatory power and affected brain cortical function in healthy adults. Previous fMRI studies also revealed a correlation between cortical activity and increased spatial–temporal task performance during exposure to Mozart K.448 (Bodner et al. 2001).
However, it should be noted that in this study we didn’t estimate music effects on the number of spontaneous seizures and epileptiform discharges. Accordingly, our current results cannot rule out the possibility that cognitive improvement is associated with music decreased seizure activity or epileptiform discharges. It is of importance and deserves to be further examined the correlations between anticonvulsant effects and improved cognitive performance in our future studies.
Conclusions
In conclusion, our research revealed that the rats undergo progressive cognitive impairment following epileptogenesis. Music intervention significantly improves cognitive ability and plays a stronger effect on cognitive recovery during the early period after SE. Although it is still unclear how specifically music improves cognitive ability, it is likely that music can be used as an auxiliary tool for the treatment of clinical brain damage. Further experiments are needed to clarify both intervention strategies and the neural mechanisms underlying music therapy.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81371636, 81071222, and 61201278), the 111 Project (B12027) and the PCSIRT project. This study was also funded by the Scientific and Technological Research program of Chongqing Municipal Education Commission (No. KJ121309).
References
- Aldenkamp A, Arends J. The relative influence of epileptic EEG discharges, short nonconvulsive seizures, and type of epilepsy on cognitive function. Epilepsia. 2004;45:54–63. doi: 10.1111/j.0013-9580.2004.33403.x. [DOI] [PubMed] [Google Scholar]
- Aoun P, Jones T, Shaw GL, Bodner M. Long-term enhancement of maze learning in mice via a generalized Mozart effect. Neurol Res. 2005;27:791–796. doi: 10.1179/016164105X63647. [DOI] [PubMed] [Google Scholar]
- Bodner M, Muftuler LT, Nalcioglu O, et al. FMRI study relevant to the Mozart effect: brain areas involved in spatial-temporal reasoning. Neurol Res. 2001;23:683–690. doi: 10.1179/016164101101199108. [DOI] [PubMed] [Google Scholar]
- Bridgett DJ, Cuevas J. Effects of listening to Mozart and Bach on the performance of a mathematical test. Percept Mot Skills. 2000;90(3):1171–1175. doi: 10.2466/pms.2000.90.3c.1171. [DOI] [PubMed] [Google Scholar]
- Brown SR. Emergence in the central nervous system. Cogn Neurodyn. 2013;7(3):173–195. doi: 10.1007/s11571-012-9229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell GN, Riby LM. The effect of music exposure and own genre preference on conscious and unconscious cognitive processes: a pilot ERP study. Conscious Cognit. 2006;16:992–996. doi: 10.1016/j.concog.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Castren E, Zafra F, Thoenen H, et al. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci USA. 1992;89(20):9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvière L, Rafrafi N, Thinus-Blanc C, et al. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J Neurosci. 2009;29(17):5402–5410. doi: 10.1523/JNEUROSCI.4699-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikahisa S, Sei H, Morishima M, et al. Exposure to music in the perinatal period enhances learning performance and alters BDNF/TrkB signaling in mice as adults. Behav Brain Res. 2006;169(2):312–319. doi: 10.1016/j.bbr.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, et al. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Meador KJ. Cognitive and behavioral effects of antiepileptic drugs. Epilepsy Behav. 2002;3(5 Suppl):S49–S53. doi: 10.1016/S1525-5069(02)00502-9. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Conscious awareness, memory and the hippocampus. Nat Neurosci. 1999;2:775–776. doi: 10.1038/12137. [DOI] [PubMed] [Google Scholar]
- Faure JB, Akimana G, Carneiro JE, et al. A comprehensive behavioral evaluation in the lithium–pilocarpine model in rats: effects of carisbamate administration during status epilepticus. Epilepsia. 2013;54:1203–1213. doi: 10.1111/epi.12219. [DOI] [PubMed] [Google Scholar]
- Foster NA, Valentine ER. The effect of auditory stimulation on autobiographical recall in dementia. Exp Aging Res. 2001;27:215–228. doi: 10.1080/036107301300208664. [DOI] [PubMed] [Google Scholar]
- Howe AG, Levy WB. A hippocampal model predicts a fluctuating phase transition when learning certain trace conditioning paradigms. Cogn Neurodyn. 2007;1(2):143–155. doi: 10.1007/s11571-006-9012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. The Mozart effect. Epilepsy Behav. 2001;2(5):396–417. doi: 10.1006/ebeh.2001.0250. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Daaboul Y, Fino JJ, et al. The ‘Mozart effect’ on epileptiform activity. Clin Electroencephgr. 1998;29(3):109–119. doi: 10.1177/155005949802900301. [DOI] [PubMed] [Google Scholar]
- Ivanov VK, Geake JG. The Mozart effect and primary school children. Psychol Music. 2003;31:405–413. doi: 10.1177/03057356030314005. [DOI] [Google Scholar]
- Jokeit H, Luerding R, Ebner A. Cognitive impairment in temporal-lobe epilepsy. Lancet. 2000;355:1018–1019. doi: 10.1016/S0140-6736(05)74765-6. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee MH, Chang HK, et al. Influence of prenatal noise and music on the spatial memory and neurogenesis in the hippocampus of developing rats. Brain Dev. 2006;28(2):109–114. doi: 10.1016/j.braindev.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Kuester G, Rios L, Ortiz A, et al. Effect of music on the recovery of a patient with refractory nonconvulsive status epilepticus. Epilepsy Behav. 2010;18(4):491–493. doi: 10.1016/j.yebeh.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Lin LC, Lee WT, Wu HC. Mozart K.448 and epileptiform discharges: effect of ratio of lower to higher harmonics. Epilepsy Res. 2010;89:238–245. doi: 10.1016/j.eplepsyres.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Lin LC, Lee WT, Wang CH, et al. Mozart K.448 acts as a potential add-on therapy in children with refractory epilepsy. Epilepsy Behav. 2011;20:490–493. doi: 10.1016/j.yebeh.2010.12.044. [DOI] [PubMed] [Google Scholar]
- Lin LC, Lee WT, Wu HC, et al. The long-term effect of listening to Mozart K.448 decreases epileptiform discharges in children with epilepsy. Epilepsy Behav. 2011;21(4):420–424. doi: 10.1016/j.yebeh.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Lin LC, Juan CT, Chang HW, et al. Mozart K.448 attenuates spontaneous absence seizure and related high-voltage rhythmic spike discharges in Long Evans rats. Epilepsy Res. 2013;104(3):234–240. doi: 10.1016/j.eplepsyres.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Lin L-C, Ouyang C-S, Chiang C-T, et al. Listening to Mozart K.448 decreases electroencephalography oscillatory power associated with an increase in sympathetic tone in adults: a post-intervention study. J R Soc Med Open. 2014;5(10):1–7. doi: 10.1177/2054270414551657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J. Music and epilepsy: a critical review. Epilepsia. 2012;53(6):947–961. doi: 10.1111/j.1528-1167.2012.03523.x. [DOI] [PubMed] [Google Scholar]
- Marques CM, Caboclo LO, da Silva TI, et al. Cognitive decline in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsy Behav. 2007;10:477–485. doi: 10.1016/j.yebeh.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Meador KJ. Cognitive outcomes and predictive factors in epilepsy. Neurology. 2002;58(Suppl 5):S21–S26. doi: 10.1212/WNL.58.8_suppl_5.S21. [DOI] [PubMed] [Google Scholar]
- Meador KJ. Cognitive and memory effects of the new antiepileptic drugs. Epilepsy Res. 2006;68:63–67. doi: 10.1016/j.eplepsyres.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Gilliam FG, Kanner AM, et al. Cognitive and behavioral effects of antiepileptic drugs. Epilepsy Behav. 2001;2(4 Suppl):SS1–SS17. doi: 10.1006/ebeh.2001.0235. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Özdemir HH, Kara M, Yumrutas O, et al. Determination of the effects on learning and memory performance and related gene expressions of clothianidin in rat models. Cogn Neurodyn. 2014;8(5):411–416. doi: 10.1007/s11571-014-9293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacchetti C, Mancini F, Aglieri R. Active music therapy in Parkinson’s disease: an integrative method for motor and emotional rehabilitation. Psychosom Med. 2000;62:386–393. doi: 10.1097/00006842-200005000-00012. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–284. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raglio A, Farina E, Giovagnoli AR. Can music therapy alleviate psychological, cognitive, and behavioral impairment in epilepsy? Epilepsy Behav. 2014;31:7–8. doi: 10.1016/j.yebeh.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Rauscher FH. Music and spatial task performance. Nature. 1993;365:611. doi: 10.1038/365611a0. [DOI] [PubMed] [Google Scholar]
- Rauscher FH, Robinson KD, Jens JJ. Improved maze learning through early music exposure in rats. Neurol Res. 1998;20:427–432. doi: 10.1080/01616412.1998.11740543. [DOI] [PubMed] [Google Scholar]
- Rutten A, Van Albada M, Silveira DC, et al. Memory impairment followingstatus epilepticus in immature rats: time-course and environmental effects. Eur J Neurosci. 2002;16(3):501–513. doi: 10.1046/j.1460-9568.2002.02103.x. [DOI] [PubMed] [Google Scholar]
- Sack O. The power of music. Brain. 2006;129:2528–2832. doi: 10.1093/brain/awl234. [DOI] [PubMed] [Google Scholar]
- Särkämö T, Tervaniemi M, Laitinen S, et al. Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain. 2008;131(3):866–876. doi: 10.1093/brain/awn013. [DOI] [PubMed] [Google Scholar]
- Seth AK. Causal networks in simulated neural systems. Cogn Neurodyn. 2008;2(1):49–64. doi: 10.1007/s11571-007-9031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Zenteno JF, Hernandez-Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res Treat. 2012;12:1–5. doi: 10.1155/2012/630853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RP. The acute effect of music on interictal epileptiform discharges. Epilepsy Behav. 2004;5:662–668. doi: 10.1016/j.yebeh.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, et al. Limbic seizures produced by pilocarpine in rats: a behavioral, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Wagatsuma H, Yamaguchi Y. Neural dynamics of the cognitive map in the hippocampus. Cogn Neurodyn. 2007;1(2):119–141. doi: 10.1007/s11571-006-9013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser HG. ILAE Commission on Neurosurgery of Epilepsy. ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- Zhang S. Improvement of cognitive impairment and hallucination by pharmacological and non-pharmacological therapies for a parkinsonism’s disease with dementia. J Neurol Stroke. 2015;2(2):00049. doi: 10.15406/jnsk.2015.02.00049. [DOI] [Google Scholar]