Abstract
To conduct a kinetic study of paraquat (PQ), we investigated 9 patients with acute PQ intoxication. All of them ingested more than 20 ml of undiluted PQ herbicide to commit suicide and arrived at our hospital early, not later than 7 h after PQ ingestion. The urine dithionite test for PQ in all of the nine patients was strongly positive at emergency room. Blood samples were obtained every 30 min for the first 2~3 h and then every 1 or 2 h, as long as the clinical progression was stable among the patients for 30 h after PQ ingestion. The area under the plasma concentration-time curve (AUCinf), which was extrapolated to infinity, was calculated using the trapezoidal rule. Toxicokinetic parameters, such as the terminal elimination half-life, apparent oral clearance, and apparent volume of distribution (Vd/F) were calculated. The maximum PQ concentration (Cmax) and the time to reach maximum PQ concentration (Tmax) were also obtained. Plasma PQ concentrations in nine patients were well described by a bi-exponential curve with a mean terminal elimination half-life of 13.1±6.8 h. Cmax and AUCinf were 20.8±25.7 mg/l and 172.5±160.3 h·mg/l, respectively. Apparent volume of distribution and apparent oral clearance were 50.9±61.3 l/kg and 173.4±111.2 l/h, respectively. There were a significant correlation (r =0.84; p<0.05) between the PQ amount ingested and Cmax. AUCinf also showed a significant correlation (r =0.83; p<0.05) with the PQ amount ingested. These correlations provide evidence that PQ has dose-linear toxicokinetic characteristics.
Keywords: Paraquat, Poisoning, Toxicokinetics
INTRODUCTION
Paraquat (1,1'-dimethyl-4,4'-bipyridinium dichloride, PQ) is an effective and widely used herbicide that has a proven safety record when appropriately applied to eliminate weeds [1]. However, it is notorious throughout the world as a potent human poison [2]. In humans, intentional or accidental ingestion of PQ is frequently fatal, as a result of multi-organ failure [3].
Over the past 30 years, several methods for modifying the toxicity of PQ have been examined, including prevention of absorption in the gastrointestinal tract [4], removal from the blood stream [5], prevention of accumulation in the lungs [6], scavenging oxygen free radicals [7,8] and prevention of lung fibrosis [9]. Unfortunately, most of these methods have proven ineffective, with the outcome already determined by the degree of exposure to PQ. Therefore, many toxicologists are trying to develop a new treatment modality for acute PQ intoxication.
Keeping in mind that toxicity is a function of exposure, and exposure is a function of dose and time, gaining an understanding of toxicokinetics and/or toxicodynamics is the fundamental step in understanding the pathophysiology and in developing an appropriate treatment modality. The plasma levels of PQ have an excellent prognostic value in acute PQ intoxication based on previous reports [10,11,12]. Therefore, any treatment modality should be developed based on the toxicokinetic information of PQ. A very elegant toxicokinetic study of PQ in 18 humans was reported by Houze et al., in 1990 [13]. Their report is the first and the last toxicokinetic study of PQ performed in humans until now. Most of the articles and text books of toxicology were used to reference the result in the report; mean distribution half-life of 5 h, a mean elimination half-life of 84 h, and volume of distribution ranging from 1.2 to 1.6 l/kg. In their report, among the 18 patients of acute PQ intoxication, only one patient was treated with hemodialysis. However, during the last two decades, hemoperfusion has been the early treatment modality for patients with acute PQ intoxication [14,15,16]. Therefore, it is uncertain whether the toxicokinetic data is accurate in the patients who were treated with hemoperfusion. The other issue with the previous study was a big variation in delay between poisoning and admission, with a mean time of 20±23 h (range, 3~96 h).
In the present study, we investigated toxicokinetics in patients with acute PQ intoxication. All of the patients arrived within 7 h after oral ingestion of PQ and hemoperfusion was initiated within 1 h.
METHODS
Patients
This study protocol was reviewed and approved by the Institutional Review Board of Soonchunhyang University Cheonan Hospital, and all procedures were performed after receiving informed consent from patients (IRB No. SCHCAIRB-2011-89). All patients attempted suicide by PQ ingestion and were admitted to the Institute of Pesticide Poisoning, Soonchunhyang University Cheonan Hospital. Standard medical emergency procedures were provided to all patients. Briefly, gastric lavage was performed within 2 h of PQ ingestion in all patients who immediately presented to the emergency room (ER). For those who had been intoxicated for up to 12 h before admission, 100 g fuller's earth in 200 ml 20% mannitol was given. Hemoperfusion was initiated within 1 h after arrival at ER. The amount ingested was estimated based on the number of swallows, where one mouthful was considered 20 ml. Survivors were defined as those patients who survived more than 3 months after PQ ingestion, maintained stable vital signs, had no evidence of progressive organ failure (especially the kidneys and lungs), and had no specific complaints. Eight out of the nine patients died and only one case survived.
Sampling and determination of plasma PQ concentration
Five ml of venous blood samples were collected when the patients arrived at the hospital and then sequential blood samples were collected every 30 min for the first 2 h followed by blood sampling at 1 or 2 h intervals. The mean duration of blood sampling was 14.3±6.8 h. Collected blood samples were centrifuged and the plasma was removed and stored at -70℃ until assayed. Ten ml of urine samples were also collected and assessed semi-quantitatively by the dithionite method, and the plasma PQ levels were determined by a well-validated HPLC method [17].
Toxicokinetic and statistical analysis
The PQ plasma concentration-time data were analyzed using a non-compartmental method with WinNonlin software version 6.1 (Pharsight Corporation, Mountain View, CA, USA). Time of ingestion was regarded as time zero. The area under the plasma concentration-time curve (AUCinf), which was extrapolated to infinity, was calculated using the trapezoidal rule. Toxicokinetic parameters, such as the terminal elimination half-life (t1/2), apparent oral clearance (CL/F: total body clearance divided by bioavailability, which is the extent of the ingested PQ that reaches the systemic circulation), and apparent volume of distribution (Vd/F), were calculated. The maximum PQ concentration (Cmax) and the time to reach maximum PQ concentration (Tmax) were also obtained. Because the patients had ingested a wide range of PQ amount (range, 9.8~36.8 g), Cmax and AUC were normalized by the PQ amount ingested and body weight or body mass index (BMI). The toxicokinetic parameters are reported as the mean±the standard deviation (SD). Descriptive analysis was carried out using medians±SD for non-normally distributed numerical data such as demographic data. The relationship between dose, BMI and toxicokinetic parameters was analyzed using linear regression (SigmaPlot software; Systat Software Inc., San Jose, CA, USA).
RESULTS
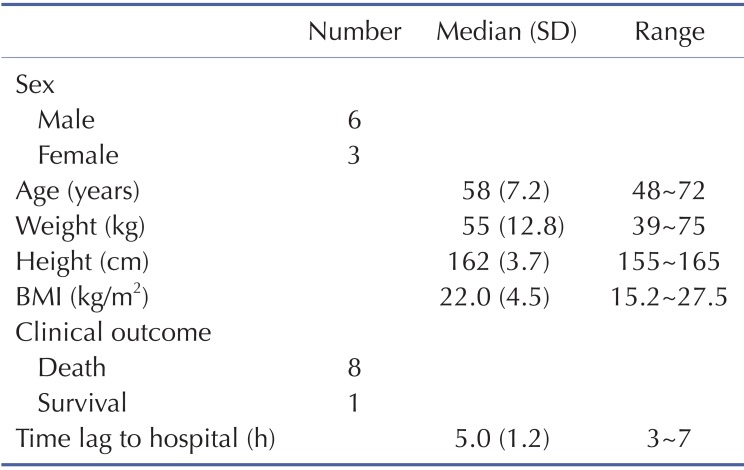
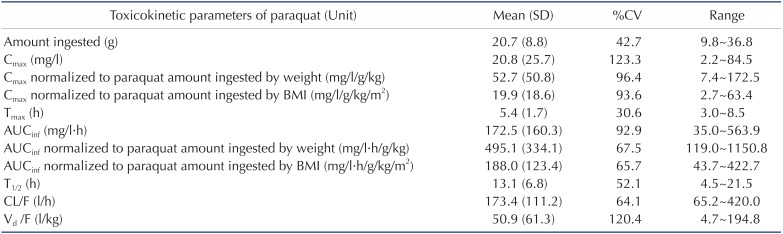
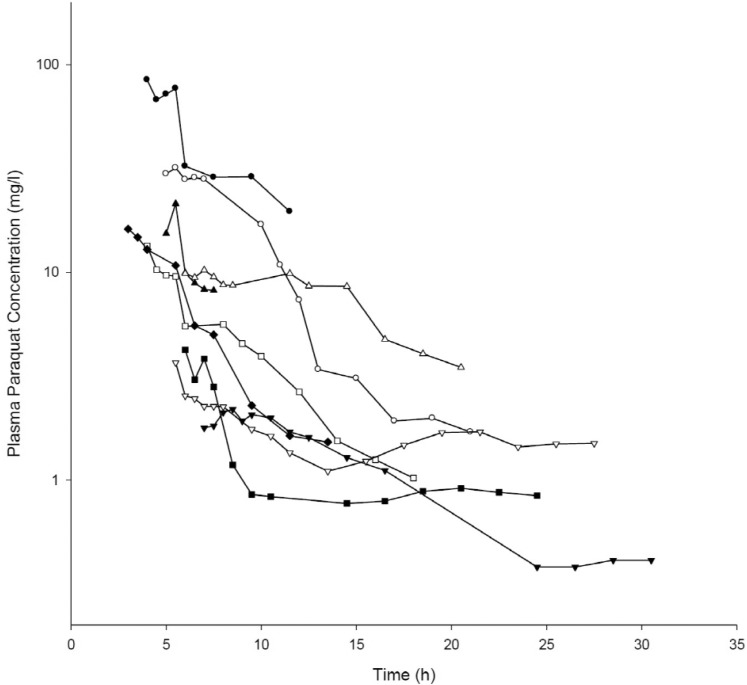
Demographic and clinical characteristics of included patients are summarized in Table 1. Two patients were females and the other patients were males. Age distribution ranged from 48 to 72 years. The mean ingested PQ amount was 20.7±8.8 g (range, 9.8~36.8 g). The urine dithionite test for PQ intoxication in nine patients showed strong positivity when they arrived at ER. The results of the toxicokinetic analysis for plasma PQ concentrations are shown in Table 2. Cmax and AUCinf were 20.8±25.7 mg/l and 172.5±160.3 h·mg/l, respectively. Plasma PQ concentration in nine patients was well described by a bi-exponential curve with a mean terminal elimination half-life of 13.1±6.8 h (Fig. 1). Apparent volume of distribution and apparent oral clearance were 50.9±61.3 l/kg and 173.4±111.2 l/h, respectively. There was a large interindividual variability in PQ toxicokinetic parameters despite efforts to adjust parameters for patient weight and weight-based PQ amount ingested. When Cmax was normalized with weightbased dose, coefficient of variation (%CV) was decreased from 123.6 to 96.4. Instead of using weight, BMI slightly improved %CV in Cmax normalization (from 96.4 to 93.6 %CV). Similar improvement was also observed in AUCinf.
Table 1. Demographic and clinical characteristics of patients with acute paraquat poisoning included in this study.
Table 2. Toxicokinetic parameters of paraquat in patients with acute paraquat poisoning included in this study.
Fig. 1. Plasma paraquat concentration-time curves of patients included in this study.
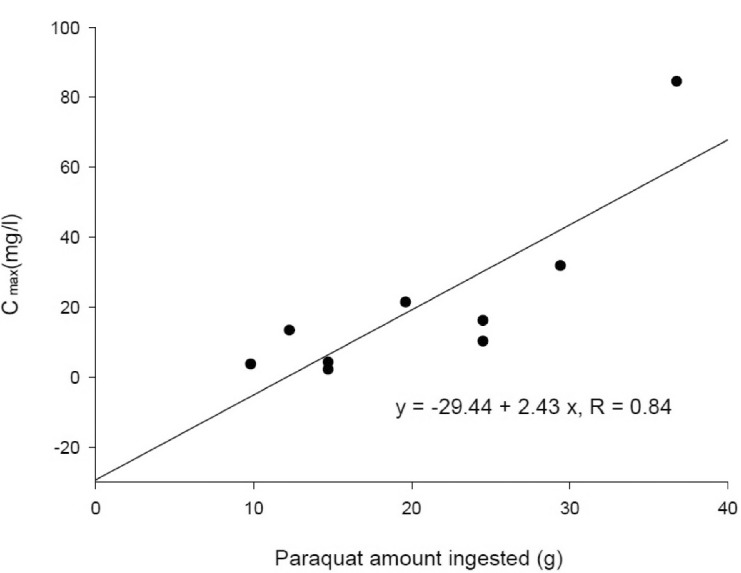
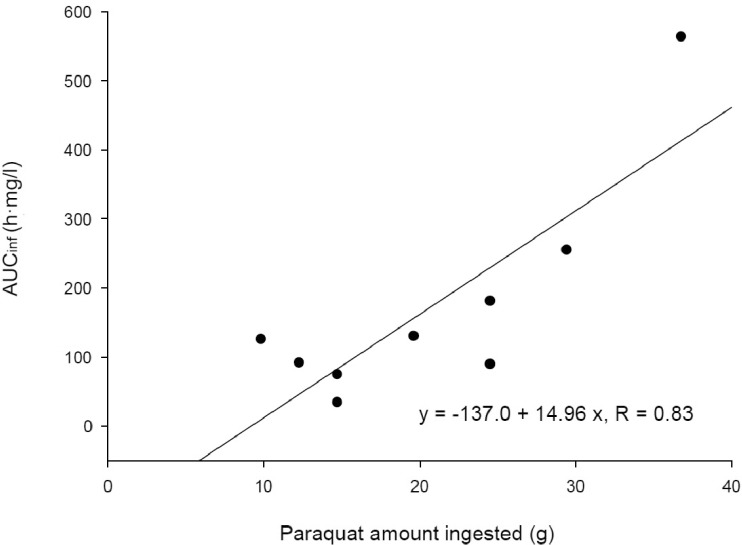
There was a significant correlation (r=0.84; p<0.05) between the PQ amount ingested and Cmax (Fig. 2). AUCinf also showed a significant correlation (r=0.83; p<0.05) with the PQ amount ingested (Fig. 3). These correlations provide evidence that PQ has dose-linear toxicokinetic characteristics.
Fig. 2. Correlation between the paraquat amount ingested and Cmax.
Fig. 3. Correlation between the paraquat amount ingested and AUCinf.
DISCUSSION
In the current study, the longest duration of sampling was 30.5 h. There are two reasons why we collected the blood samples for assessing PQ levels in a relatively short duration. First of all, the toxicity of PQ was too critical to collect blood samples for such a long duration for this study, in the majority of the cases. The other reason is that, in our clinical experience of thousands of patients with acute PQ intoxication, the PQ levels at 2 or 3 days after ingestion are far more complicated to interpret their clinical significance. The PQ level during this period is determined based on 3 factors including ingestion amount, degree of renal function deterioration, and the amount of PQ released from the other compartment into the circulation, so-called rebound.
Houzé et al. [13] reported that apparent volume of distribution was 1.2 to 1.6 l/kg in 3 patients and mean elimination half-life was 84 h. They calculated the renal clearance of PQ using urine toxicokinetic data, and they divided the patients according to admission time and time of death. In addition, they estimated the apparent volume of distribution in only three cases, in which renal clearance and plasma half-life were measured late during the course of poisoning. In their results, they selected 5 patients who died early from cardiovascular collapse and reported that the half-life was 7±2 h, which was similar to our results. Plasma PQ level at a given time has been regarded as the most reliable parameter for prediction of the clinical outcome. In general, the plasma concentration of a toxic substance at a given time is the end-result of the toxicokinetics of the substance. In the same way, unique toxicokinetics of a toxin help to determine the plasma concentration of the toxin at a given time. Based on this, the significance of plasma PQ level as a clinical marker can be maintained under the reproducible conditions of PQ toxicokinetics.
In the present study, toxicokinetic parameters showed dose-linearity, but there was an imperfect correlation between the PQ amount ingested and toxicokinetic parameters such as Cmax and AUCinf. The mean terminal elimination half-life was 13.1±6.8 h and the apparent volume of distribution was 50.9±61.3 l/kg. We cannot explain the precise mechanism of a large individual difference in these kinetic data. However, we believe that the following factors are responsible for the large individual difference in the kinetics: 1) Inaccuracy of ingestion amount: we determined the amount of PQ ingestion based on history taking. In general, the patient express the amount of PQ ingestion as "mouthful" or "cup" or "bowl". 2) Difference in vomiting intensity: most of the patients have vomiting after PQ ingestion, more or less, because the PQ herbicide includes some chemicals that induce vomiting. However, according to the pre-existing food material in the stomach, and the degree of alcohol drunken state, the amount of vomitus is different. 3) Difference in the aggressiveness of therapy such as gastric lavage, cathartics, extracorporeal elimination. 4) Heterogeneity in renal function deterioration: acute renal failure frequently develops in majority of cases of acute PQ intoxication. However, the degree of renal failure is very heterogeneous among the patients [18,19].
In order to minimize the heterogeneity in the above mentioned bias, we selected the patients who arrived early within 7 h after PQ ingestion; (It is extremely rare that the patients arrive early within 1 h or 52 h after PQ ingestion because all of the patients ingest PQ to commit suicide). All of the patients received the same treatment modality, including hemoperfusion which was applied for 3 h within 1 h after arrival to our hospital. In spite of these efforts, the kinetic data showed significant individual differences. The limitation of our study is that we have neither measured the PQ clearance by hemoperfusion, nor the renal clearance of PQ. In our previous study, the reduction rate of PQ, which was calculated based on the PQ levels in arterial and venous lines of hemoperfusion cartridge, was about 80% and the blood flow for hemoperfusion was about 250~300 ml/min [20]. Considering that the reduction rate of PQ is nearly 100% in the kidney, and renal blood flow is over 1200 ml/min, the efficacy of PQ elimination by hemoperfusion is far less than than that by the kidney. Perhaps this was the reason why there was no specific change in the declining pattern during hemoperfusion (between one and 4 h after hospital arrival).
In summary, we performed a kinetic study of PQ in nine patients with acute PQ intoxication. Plasma PQ concentration was well described by a bi-exponential curve and it showed dose-linear toxicokinetic characteristics. Despite large interindividual variation in toxicokinetic data, plasma PQ level and ingested PQ amount are useful clinical markers in patients with acute PQ intoxication.
ACKNOWLEDGEMENTS
This work was carried out with the support of the "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01083201)" of the Rural Development Administration, Republic of Korea.
Author contributions: H.J.K. and H.K.K. wrote the manuscript. H.L. and J.S.B. performed pharmacokinetic analysis. J.T.K. and H.W.G. performed sample collection and measurement of plasma concentration. H.K.K. and S.Y.H. supervised and coordinated the study.
CONFLICT OF INTEREST: The authors report no conflicts of interest.
References
- 1.Bromilow RH. Paraquat and sustainable agriculture. Pest Manag Sci. 2004;60:340–349. doi: 10.1002/ps.823. [DOI] [PubMed] [Google Scholar]
- 2.Onyon LJ, Volans GN. The epidemiology and prevention of paraquat poisoning. Hum Toxicol. 1987;6:19–29. doi: 10.1177/096032718700600104. [DOI] [PubMed] [Google Scholar]
- 3.Vale JA, Meredith TJ, Buckley BM. Paraquat poisoning: clinical features and immediate general management. Hum Toxicol. 1987;6:41–47. doi: 10.1177/096032718700600107. [DOI] [PubMed] [Google Scholar]
- 4.Meredith TJ, Vale JA. Treatment of paraquat poisoning in man: methods to prevent absorption. Hum Toxicol. 1987;6:49–55. doi: 10.1177/096032718700600108. [DOI] [PubMed] [Google Scholar]
- 5.Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72:745–757. doi: 10.1111/j.1365-2125.2011.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lock EA, Smith LL, Rose MS. Inhibition of paraquat accumulation in rat lung slices by a component of rat plasma and a variety of drugs and endogenous amines. Biochem Pharmacol. 1976;25:1769–1772. doi: 10.1016/0006-2952(76)90413-5. [DOI] [PubMed] [Google Scholar]
- 7.Hong SY, Hwang KY, Lee EY, Eun SW, Cho SR, Han CS, Park YH, Chang SK. Effect of vitamin C on plasma total antioxidant status in patients with paraquat intoxication. Toxicol Lett. 2002;126:51–59. doi: 10.1016/s0378-4274(01)00431-3. [DOI] [PubMed] [Google Scholar]
- 8.Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180:65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- 9.Ross JH, Krieger RI. Structure-activity correlations of amines inhibiting active uptake of paraquat (methyl viologen) into rat lung slices. Toxicol Appl Pharmacol. 1981;59:238–249. doi: 10.1016/0041-008x(81)90194-0. [DOI] [PubMed] [Google Scholar]
- 10.Proudfoot AT, Stewart MS, Levitt T, Widdop B. Paraquat poisoning: significance of plasma-paraquat concentrations. Lancet. 1979;2:330–332. doi: 10.1016/s0140-6736(79)90345-3. [DOI] [PubMed] [Google Scholar]
- 11.Hart TB, Nevitt A, Whitehead A. A new statistical approach to the prognostic significance of plasma paraquat concentrations. Lancet. 1984;2:1222–1223. doi: 10.1016/s0140-6736(84)92784-3. [DOI] [PubMed] [Google Scholar]
- 12.Scherrmann JM, Houze P, Bismuth C, Bourdon R. Prognostic value of plasma and urine paraquat concentration. Hum Toxicol. 1987;6:91–93. doi: 10.1177/096032718700600116. [DOI] [PubMed] [Google Scholar]
- 13.Houzé P, Baud FJ, Mouy R, Bismuth C, Bourdon R, Scherrmann JM. Toxicokinetics of paraquat in humans. Hum Exp Toxicol. 1990;9:5–12. doi: 10.1177/096032719000900103. [DOI] [PubMed] [Google Scholar]
- 14.Gil HW, Kim SJ, Yang JO, Lee EY, Hong SY. Clinical outcome of hemoperfusion in poisoned patients. Blood Purif. 2010;30:84–88. doi: 10.1159/000318585. [DOI] [PubMed] [Google Scholar]
- 15.Hong SY, Yang JO, Lee EY, Kim SH. Effect of haemoperfusion on plasma paraquat concentration in vitro and in vivo. Toxicol Ind Health. 2003;19:17–23. doi: 10.1191/0748233703th171oa. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki K, Takasu N, Okabe T, Ishimatsu S, Ueda A, Tanaka S, Fukuda A, Arita S, Kohama A. Effect of aggressive haemoperfusion on the clinical course of patients with paraquat poisoning. Hum Exp Toxicol. 1993;12:323–327. doi: 10.1177/096032719301200411. [DOI] [PubMed] [Google Scholar]
- 17.Blake DK, Gallagher RT, Woollen BH. Improved methods for the analysis of Paraquat in biological fluid. Chromatographia. 2002;55:S183–S185. [Google Scholar]
- 18.Gil HW, Yang JO, Lee EY, Hong SY. Clinical implication of urinary neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in patients with acute paraquat intoxication. Clin Toxicol (Phila) 2009;47:870–875. doi: 10.3109/15563650903306651. [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Gil HW, Yang JO, Lee EY, Hong SY. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol Dial Transplant. 2009;24:1226–1232. doi: 10.1093/ndt/gfn615. [DOI] [PubMed] [Google Scholar]
- 20.Kang MS, Gil HW, Yang JO, Lee EY, Hong SY. Comparison between Kidney and Hemoperfusion for Paraquat Elimination. J Korean Med Sci. 2009;24(Suppl 1):S156–S160. doi: 10.3346/jkms.2009.24.S1.S156. [DOI] [PMC free article] [PubMed] [Google Scholar]