Abstract
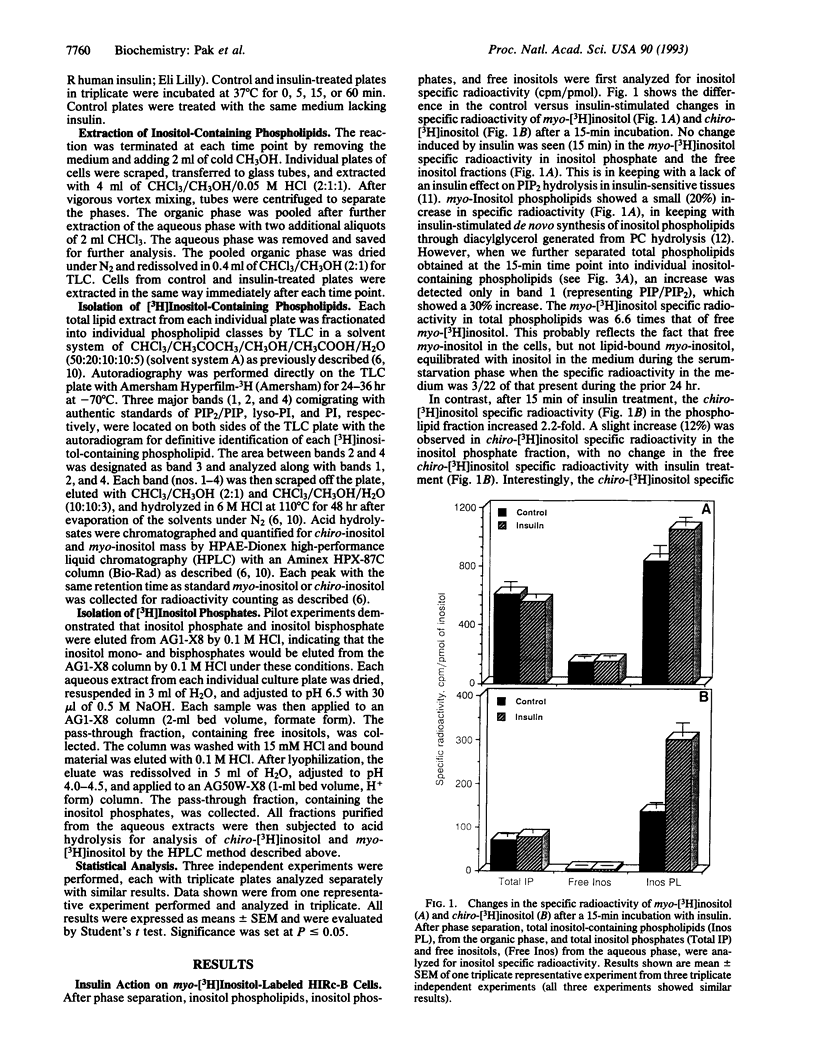
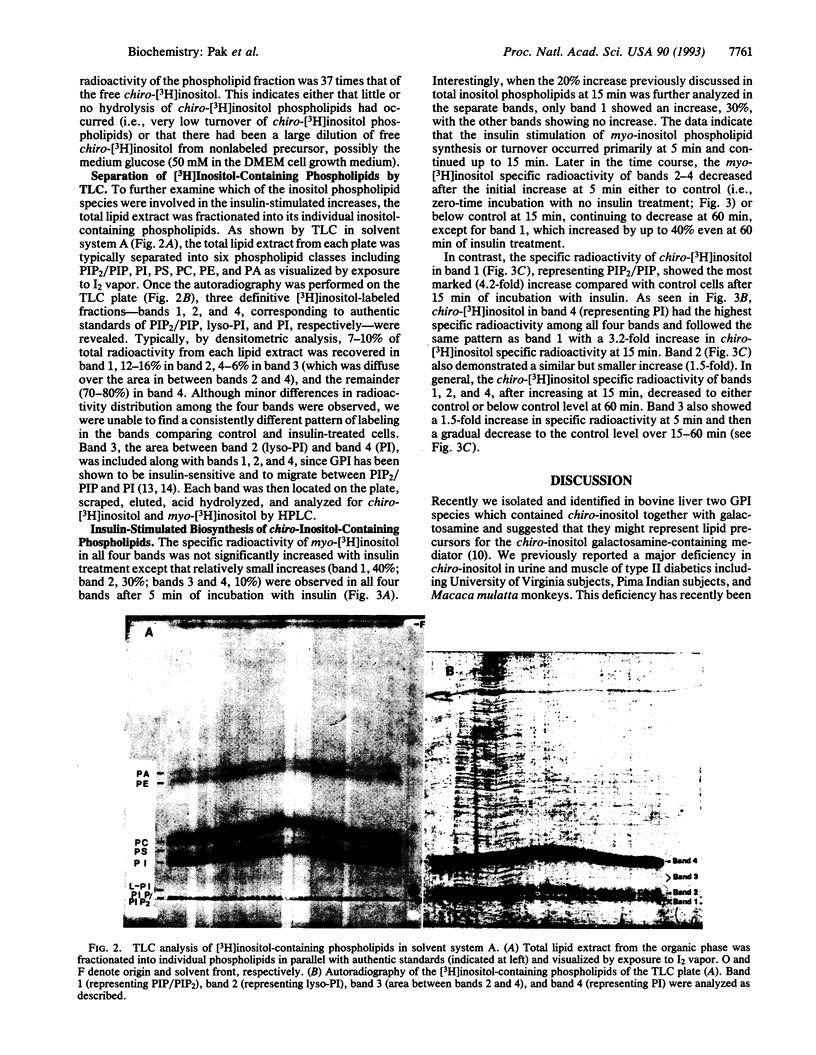
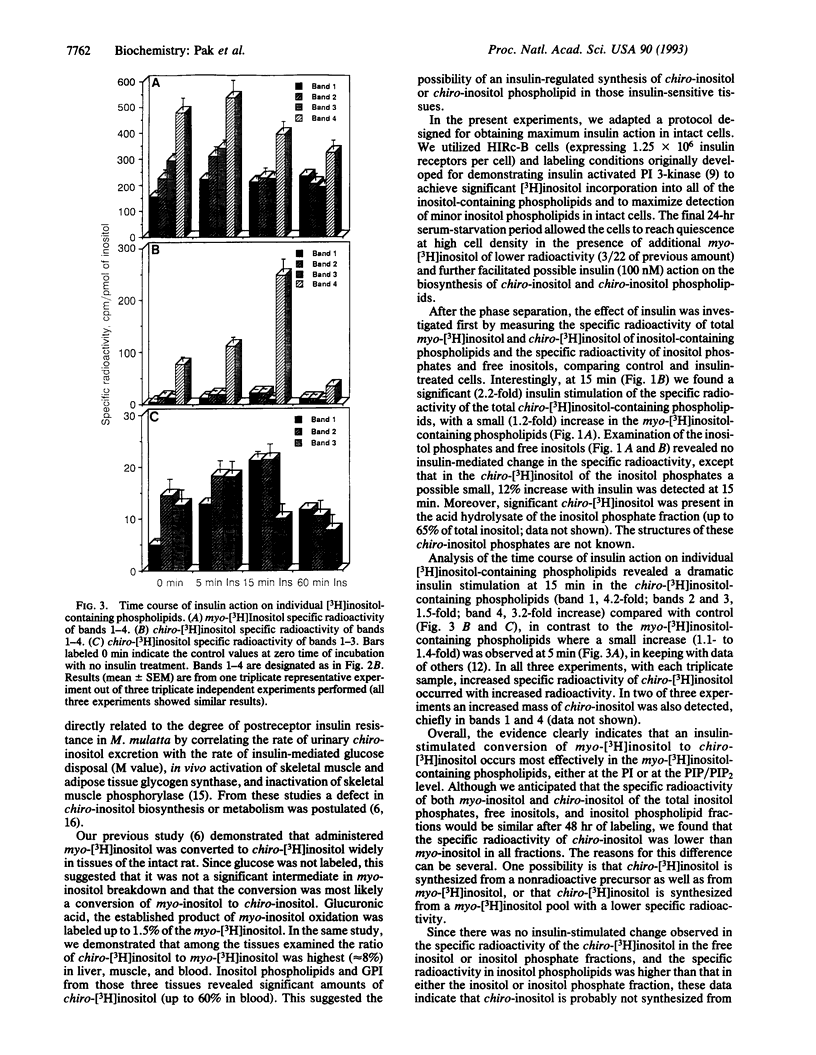
HIRc-B cells (rat fibroblasts expressing the human insulin receptor) were incubated with myo-[3H]inositol for 48 hr, and the biosynthesis of chiro-[3H]inositol was investigated in the absence and presence of insulin following a time course up to 60 min. After phase separation, treatment with insulin for 15 min caused a 2.2-fold increase in the specific radioactivity of chiro-[3H]inositol-containing phospholipids in contrast to a 1.2-fold increase in the specific radioactivity of myo-[3H]inositol-containing phospholipids. No insulin-mediated change in the specific radioactivity was observed in the inositol phosphates or free inositols. Further detailed analysis of individual [3H]inositol-containing phospholipids demonstrated marked increases in specific activity of the chiro-[3H]inositol phospholipids after 15 min of incubation with insulin: phosphatidylinositol 4-phosphate and 4,5-bisphosphate, 4.2-fold; lysophosphatidylinositol, 1.5-fold; phosphatidylinositol, 3.2-fold. In contrast, myo-[3H]inositol-containing phospholipids demonstrated relatively small increases (1.1- to 1.4-fold) after 5 min of incubation with insulin. These findings indicate that insulin stimulates de novo synthesis of chiro-inositol-containing phospholipids at the inositol phospholipid level.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farese R. V., Davis J. S., Barnes D. E., Standaert M. L., Babischkin J. S., Hock R., Rosic N. K., Pollet R. J. The de novo phospholipid effect of insulin is associated with increases in diacylglycerol, but not inositol phosphates or cytosolic Ca2+. Biochem J. 1985 Oct 15;231(2):269–278. doi: 10.1042/bj2310269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V. Lipid-derived mediators in insulin action. Proc Soc Exp Biol Med. 1990 Dec;195(3):312–324. doi: 10.3181/00379727-195-43150c. [DOI] [PubMed] [Google Scholar]
- Jarett L., Seals J. R. Pyruvate dehydrogenase activation in adipocyte mitochondria by an insulin-generated mediator from muscle. Science. 1979 Dec 21;206(4425):1407–1408. doi: 10.1126/science.505013. [DOI] [PubMed] [Google Scholar]
- Kelly K. L., Mato J. M., Merida I., Jarett L. Glucose transport and antilipolysis are differentially regulated by the polar head group of an insulin-sensitive glycophospholipid. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6404–6407. doi: 10.1073/pnas.84.18.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennington A. S., Hill C. R., Craig J., Bogardus C., Raz I., Ortmeyer H. K., Hansen B. C., Romero G., Larner J. Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. N Engl J Med. 1990 Aug 9;323(6):373–378. doi: 10.1056/NEJM199008093230603. [DOI] [PubMed] [Google Scholar]
- Larner J., Galasko G., Cheng K., DePaoli-Roach A. A., Huang L., Daggy P., Kellogg J. Generation by insulin of a chemical mediator that controls protein phosphorylation and dephosphorylation. Science. 1979 Dec 21;206(4425):1408–1410. doi: 10.1126/science.228395. [DOI] [PubMed] [Google Scholar]
- Larner J., Huang L. C., Schwartz C. F., Oswald A. S., Shen T. Y., Kinter M., Tang G. Z., Zeller K. Rat liver insulin mediator which stimulates pyruvate dehydrogenase phosphate contains galactosamine and D-chiroinositol. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1416–1426. doi: 10.1016/s0006-291x(88)80520-5. [DOI] [PubMed] [Google Scholar]
- Mato J. M., Kelly K. L., Abler A., Jarett L. Identification of a novel insulin-sensitive glycophospholipid from H35 hepatoma cells. J Biol Chem. 1987 Feb 15;262(5):2131–2137. [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- Mosthaf L., Grako K., Dull T. J., Coussens L., Ullrich A., McClain D. A. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. 1990 Aug;9(8):2409–2413. doi: 10.1002/j.1460-2075.1990.tb07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmeyer H. K., Bodkin N. L., Lilley K., Larner J., Hansen B. C. Chiroinositol deficiency and insulin resistance. I. Urinary excretion rate of chiroinositol is directly associated with insulin resistance in spontaneously diabetic rhesus monkeys. Endocrinology. 1993 Feb;132(2):640–645. doi: 10.1210/endo.132.2.8425483. [DOI] [PubMed] [Google Scholar]
- Pak Y., Huang L. C., Lilley K. J., Larner J. In vivo conversion of [3H]myoinositol to [3H]chiroinositol in rat tissues. J Biol Chem. 1992 Aug 25;267(24):16904–16910. [PubMed] [Google Scholar]
- Pak Y., Larner J. Identification and characterization of chiroinositol-containing phospholipids from bovine liver. Biochem Biophys Res Commun. 1992 Apr 30;184(2):1042–1047. doi: 10.1016/0006-291x(92)90696-i. [DOI] [PubMed] [Google Scholar]
- Romero G., Larner J. Insulin mediators and the mechanism of insulin action. Adv Pharmacol. 1993;24:21–50. doi: 10.1016/s1054-3589(08)60932-1. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Cuatrecasas P. Insulin stimulates the generation from hepatic plasma membranes of modulators derived from an inositol glycolipid. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5793–5797. doi: 10.1073/pnas.83.16.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serunian L. A., Auger K. R., Cantley L. C. Identification and quantification of polyphosphoinositides produced in response to platelet-derived growth factor stimulation. Methods Enzymol. 1991;198:78–87. doi: 10.1016/0076-6879(91)98010-4. [DOI] [PubMed] [Google Scholar]