Abstract
OBJECTIVES:
The incidence of lung cancer in Koreans is increasing in women and in both men and women with a never-smoking history. Human papillomavirus (HPV) infection has been suggested as a modifiable risk factor of lung cancer in never-smokers and women (LCNSW). This systematic review (SR) aimed to evaluate an association between HPV infection and lung cancer risk in LCNSW.
METHODS:
Based on a prior SR and some expert reviews, we identified refereed, cited, or related articles using the PubMed and Scopus databases. All case-control studies that reported the odds ratio of HPV infection in LCNSW were selected. An estimate of the summary odds ratio (SOR) with 95% confidence intervals (CI) was calculated.
RESULTS:
A total of four case-control studies were included. The fixed-effect model was applied because of homogeneity (I-squared=0.0%). The SORs in women and in never-smokers were 5.32 (95% CI, 1.75 to 16.17) and 4.78 (2.25 to 10.15) respectively.
CONCLUSIONS:
These results showed a significant effect of HPV infection in LCNSW. It is evident that developing a preventive plan against LCNSW may be necessary.
Keywords: Lung neoplasms, Risk factor, Human papillomavirus, Meta-analysis
INTRODUCTION
Lung cancer ranks the first in cancer mortality in Korea and is the primary cancer with the heaviest disease burden [1]. According to the 2002-2012 statistics on lung cancer provided by Statistics Korea, the incidence rate in women increased and the rate of adenocarcinoma also increased during this time period [2]. These facts were corroborated by a study on lung cancer patients treated at a local cancer center [3], and particularly, the study authors reported that the majority of women with lung cancer were never-smokers (73.0%).
The increasing incidences of lung cancer among women never-smokers is a global trend [4,5], and it has been suggested that lung cancer in never-smokers should be considered separately, a disease different from lung cancer in smokers [6-8]. Among the hypotheses about the cause of lung cancer in women never-smokers, the one that has been given priority is second-hand smoke exposure [6,7,9-11]. However, a genome study has reported that the possibility of second-hand smoke involvement in lung cancer is low in Asian never-smokers [12]. In addition, second-hand smoke exposure also imposes a limit on cancer prevention efforts because control of second-hand smoke exposure cannot be achieved just by individuals making efforts, but requires efforts from society. Instead, a modifiable risk factor, human papillomavirus (HPV) infection, may be involved [6,7,9]. It is a risk factor for many cancers, such as cervical cancer [13], prostate cancer [14], and breast cancer [15], and currently, preventive vaccines are commercially available [16].
HPV deoxynucleic acid (DNA) is detected in approximately 20% of lung cancer tissues [17-20], and the detection rate is higher in lung cancer tissues of Asians [21-24]. The detection rate of HPV subtype 33 has been reported to be 31.3% among Koreans with lung cancer [25]. According to a systematic review reported in 2014 [26], the odds ratio (OR) of HPV infection was 5.67 (95% confidence interval [CI], 3.09 to 10.40), and among Asians it was 6.23 (95% CI, 2.78 to 13.97), showing a higher OR than for other races. However, this study did not present results from subgroup analysis on the OR of women and never-smokers. Thus, we have performed a systematic review that investigated the relationship between HPV infection and lung cancer in women and never-smokers.
MATERIALS AND METHODS
Search for and selection of relevant studies
In order to maximize the utility of previously conducted systematic reviews in the literature search, we used a manual search method rather than an automatic method [14,27,28]. Thus, we examined the references of five systematic review paper [17-20,26], and obtained, for each, the lists of “cited articles” and “similar (related) articles” provided by PubMed (www.ncbi.nlm.nih.gov/pubmed) and the Scopus (www.elsevier.com/solutions/scopus) database.
The finally selection criterion for the study objective was a case-control study in which HPV DNA was tested on never-smokers and women. Accordingly, for each study in the lists described above, the following exclusion criteria were applied to the abstract or the main body of the article: (1) a study dealing with different hypotheses, (2) an expert’s review or a systematic review study, and (3) a case report study. Of the remaining case-control studies after the first three exclusion criteria were applied, the final set of studies were selected using the following two exclusion criteria: (4) a study in which HPV DNA testing was not done on the pathological tissue, and (5) a study from which information on women or never-smokers cannot be obtained, even if it was a case-control study.
Statistical analysis
Two researchers applied the exclusion criteria and obtained HPV-related information from each study. ORs and 95% CIs were computed for the confirmed patient groups and positive HPV values. Heterogeneity was evaluated using the I-squared values (%), and when homogeneity was confirmed, a meta-analysis was performed to compute summary odds ratios (SORs) and 95% CIs using a fixed-effect model. Statistical significance was determined at the level of 5%, and the statistical program Stata version 14.0 (Stata Corp., College Station, TX, USA) was used to conduct the analyses.
RESULTS
Figure 1 shows the final study selection processes for the meta-analysis. We initially obtained 136 references from five systemic review papers and 1,219 cited and related studies through PubMed and Scopus. After the selection criteria were applied on a total of 1,355 studies, the number of studies excluded and the exclusion reasons were as follows: (1) 1,219 studies because they dealt with different hypotheses, (2) 19 studies because they were an expert’s review or a systematic review, (3) 96 case only studies, (4) six case-control studies that did not test for HPV DNA on the pathological tissue, and (5) 11 case-control studies in which information on women or never-smokers was not found. In summary, a total of 1,351 studies were excluded, and four studies were included [29-32].
Figure 1.
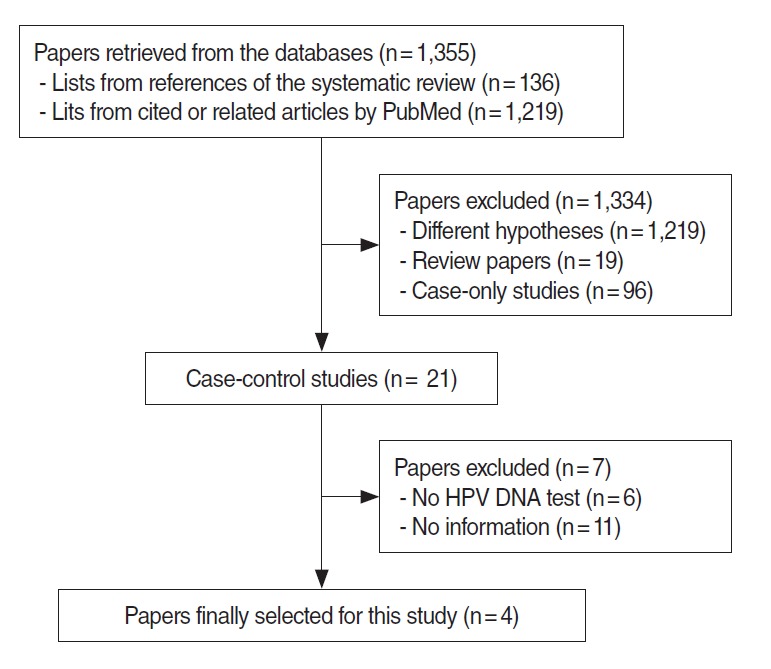
Flow chart of article selection. HPV, human papillomavirus; DNA, deoxynucleic acid.
Table 1 summarizes the four case-control studies, showing the nationality of the participants, test specimens, and distribution of participant groups of women and/or never-smokers, and the ORs and the 95% CIs computed depending on the presence or absence of positive HPV DNA. Information on a woman-only group and on a never-smoker group was presented in three studies each, and the I-squared value was 0% for both groups, suggesting homogeneity (Figure 2). Table 2 shows meta-analytic results from a fixed effect model on the HPV DNA subtypes 16/18. The SOR was 5.32 (95% CI, 1.75 to 16.17) in the women-only group and 4.78 (95% CI, 2.25 to 10.15) in the never-smoker group, showing a statistically significance.
Table 1.
Summary of four selected case-control studies for association of HPV 16/18 infection with lung cancer in women and never-smokers
| Author_year [Ref] | Nation | Specimen | Group | Case (n/N) | Control (n/N) | OR (95% CI) |
|---|---|---|---|---|---|---|
| Cheng_2001 [29] | Taiwan | PET | Women | 27/45 | 2/11 | 6.75 (1.30, 34.94) |
| Never-smokers | 38/78 | 7/48 | 5.56 (2.23, 13.91) | |||
| Nadji_2007 [30] | Iran | PET | Women | 6/25 | 2/23 | 3.32 (0.60, 18.45) |
| Never-smokers | 5/21 | 2/40 | 5.94 (1.04, 33.85) | |||
| Yu_2009 [31] | China | PET | Never-smoker | 4/11 | 2/8 | 1.71 (0.23, 12.89) |
| Sarchianaki_2014 [32] | Greece | PET | Women | 2/9 | 0/16 | 11.00 (0.47, 258.48) |
Author_year [Ref], name of first author_year of publication [reference number].
HPV, human papillomavirus; PET, paraffin-embedded tissue; OR, odds ratio; CI, confidence interval.
Figure 2.
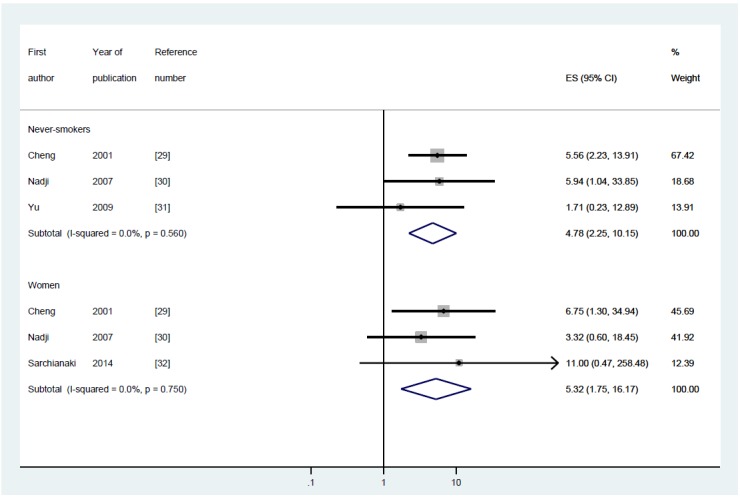
The forest plot of summary effect size (ES) with 95% confidence intervals (CI) using a fixed effect model by never-smokers and women.
Table 2.
Human papillomavirus detection and risk of lung cancer in women and never-smokers
| Type | Women SOR (95% CI) [I-squared, %] | Never-smokers SOR (95% CI) [I-squared, %] | Women & never-smokers AOR (95% CI)1 |
|---|---|---|---|
| 16/18 | 5.32 (1.75, 16.17) | 4.78 (2.25, 10.15) | |
| [0.0] | [0.0] | ||
| 16 | 3.98 (1.13, 13.98) | ||
| 18 | 11.66 (2.94, 46.27) |
SOR, summary odds ratio; CI, confidence interval; AOR, adjusted odds ratio.
Adjusted for age, tumor type, and tumor stage suggested by Cheng et al. [29].
DISCUSSION
The SOR for lung cancer associated with HPV infection was 5.32 for the women and 4.78 for the never-smokers. These are at a level similar to SOR 5.67, which is an odds ratio computed for men and women together [26]. Considering that the risk of lung cancer of subtype 18 infection was reported to be 11.66-fold (95% CI, 2.94 to 46.27) in women never-smokers (Table 2) [29], the actual risk level for women never-smokers with HPV infection is expected to be higher. The findings provide evidence for the argument that vaccines against HPV infection can prevent the occurrence of lung cancer in women as well as protect against other cancers. In addition, from the finding that the SOR was similar when men and women were not separated and when participants were limited to women, HPV infection can be inferred to have a greater impact on the occurrence of lung cancer in women never-smokers than in men smokers [33,34].
The four studies selected in this systematic review [29-32] were all case-control studies. This is because of the difficulty in conducting a cohort study involving HPV DNA. Of the four included studies, three studies [29,30,32] were also included in Zhai et al.’s analysis [26]. The fourth study, Yu et al. [31], was selected instead of the one by the same authors presented in 2013 [35], because the former showed analytic results on women. The reason why additional studies were not included in the current systematic review is because the study closed the literature search in September 2015 and it was closed in March 2014 for Zhai et al. [26]. Further study is needed in the future focusing on the occurrence of lung cancer in women never-smokers.
In contrast, via a manual search we were able to identify four studies that met the selection criteria of the systematic review by Zhai et al. [26], but were not included [36-39]. Of them, two studies [38,39] were published after their search was closed in March 2014, whereas two studies [36,37] were published before, which shows the importance of a hand search. Yu et al. [39] overlaps with two other studies presented by the same authors based on the same data sources [31,35], and thus, studies for a future meta-analysis should be selected carefully.
Lung tissues are known to have the highest sensitivity for HPV DNA detection [24], and six case-control studies were excluded from this meta-analysis because HPV infection was tested using DNA or antibody samples taken from bronchoalveolar lavage fluid or blood [40-45]. Using blood in lieu of tissue to test for HPV infection has the potential to be used as an early screening tool for lung cancer in women never-smokers [2]. In addition, there is some evidence that taking oral contraceptives is related to HPV proliferation [46], and thus, future studies are needed on the occurrence of lung cancer in women taking oral contraceptives [47,48]. As lung cancer in never-smokers is anticipated to become a serious problem soon [2,49], efforts should be made from different approaches to establish preventive policies.
In order to prove that a specific virus causes cancer, a case-control study must be conducted in order to meet necessary standards [50,51]. However, tumor-based case-control studies have the drawback of frequent measurement errors [52,53]. To overcome the problem, adaptive systematic reviews need to be continuously conducted.
Footnotes
The authors have no conflicts of interest to declare for this study.
SUPPLEMENTARY MATERIAL
Supplementary material (Korean version) is available at http://www.e-epih.org/.
REFERENCES
- 1.Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47:127–141. doi: 10.4143/crt.2015.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae JM. Modifiable risk factors of lung cancer in “never-smoker” women. Epidemiol Health. 2015;37:e2015052. doi: 10.4178/epih/e2015047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang H, Park CW, Kim W, Song SY, Jeong JU, et al. Never-smoker lung cancer is increasing. J Lung Cancer. 2012;11:89–93. (Korean) [Google Scholar]
- 4.Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003;123:21S–49S. doi: 10.1378/chest.123.1_suppl.21s. [DOI] [PubMed] [Google Scholar]
- 5.Zhou W, Christiani DC. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer. 2011;30:287–292. doi: 10.5732/cjc.011.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramanian J, Govindan R. Lung cancer in ‘never-smokers’: a unique entity. Oncology (Williston Park) 2010;24:29–35. [PubMed] [Google Scholar]
- 7.Alberg AJ, Wallace K, Silvestri GA, Brock MV. Invited commentary: the etiology of lung cancer in men compared with women. Am J Epidemiol. 2013;177:613–616. doi: 10.1093/aje/kws444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers: a different disease. Nat Rev Cancer. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 9.Planchard D, Besse B. Lung cancer in never-smokers. Eur Respir J. 2015;45:1214–1217. doi: 10.1183/09031936.00046915. [DOI] [PubMed] [Google Scholar]
- 10.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam WK. Lung cancer in Asian women-the environment and genes. Respirology. 2005;10:408–417. doi: 10.1111/j.1440-1843.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan VG, Ebert PJ, Ting JC, Lim E, Wong SS, Teo AS, et al. Whole-genome sequencing of asian lung cancers: second-hand smoke unlikely to be responsible for higher incidence of lung cancer among Asian never-smokers. Cancer Res. 2014;74:6071–6081. doi: 10.1158/0008-5472.CAN-13-3195. [DOI] [PubMed] [Google Scholar]
- 13.Wattleworth R. Human papillomavirus infection and the links to penile and cervical cancer. J Am Osteopath Assoc. 2011;111:S3–S10. [PubMed] [Google Scholar]
- 14.Bae JM. Human papillomavirus 16 infection as a potential risk factor for prostate cancer: an adaptive meta-analysis. Epidemiol Health. 2015;37:e2015052. doi: 10.4178/epih/e2015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bae JM. Two hypotheses of dense breasts and viral infection for explaining incidence of breast cancer by age group in Korean women. Epidemiol Health. 2014;36:e2015052. doi: 10.4178/epih/e2014020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila) 2012;5:18–23. doi: 10.1158/1940-6207.CAPR-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliani L, Favalli C, Syrjanen K, Ciotti M. Human papillomavirus infections in lung cancer. Detection of E6 and E7 transcripts and review of the literature. Anticancer Res. 2007;27:2697–2704. [PubMed] [Google Scholar]
- 18.Srinivasan M, Taioli E, Ragin CC. Human papillomavirus type 16 and 18 in primary lung cancers: a meta-analysis. Carcinogenesis. 2009;30:1722–1728. doi: 10.1093/carcin/bgp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa Y, Ando M, Kubo A, Isa S, Yamamoto S, Tsujino K, et al. Human papilloma virus in non-small cell lung cancer in never smokers: a systematic review of the literature. Lung Cancer. 2014;83:8–13. doi: 10.1016/j.lungcan.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Klein F, Amin Kotb WF, Petersen I. Incidence of human papilloma virus in lung cancer. Lung Cancer. 2009;65:13–18. doi: 10.1016/j.lungcan.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Syrjänen KJ. HPV infections and lung cancer. J Clin Pathol. 2002;55:885–891. doi: 10.1136/jcp.55.12.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YC, Chen JH, Richard K, Chen PY, Christiani DC. Lung adenocarcinoma and human papillomavirus infection. Cancer. 2004;101:1428–1436. doi: 10.1002/cncr.20538. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25:561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 24.Rezazadeh A, Laber DA, Ghim SJ, Jenson AB, Kloecker G. The role of human papilloma virus in lung cancer: a review of the evidence. Am J Med Sci. 2009;338:64–67. doi: 10.1097/MAJ.0b013e3181a393ba. [DOI] [PubMed] [Google Scholar]
- 25.Park MS, Chang YS, Shin JH, Kim DJ, Chung KY, Shin DH, et al. The prevalence of human papillomavirus infection in Korean non-small cell lung cancer patients. Yonsei Med J. 2007;48:69–77. doi: 10.3349/ymj.2007.48.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhai K, Ding J, Shi HZ. HPV and lung cancer risk: a meta-analysis. J Clin Virol. 2015;63:84–90. doi: 10.1016/j.jcv.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Bae JM. Narrative reviews. Epidemiol Health. 2014;36:e2015052. doi: 10.4178/epih/e2014018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bae JM. The necessity of an observational study on the interactions between allergic history and citrus fruit intake for the prevention of pancreatic cancer. Epidemiol Health. 2015;37:e2015052. doi: 10.4178/epih/e2015028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng YW, Chiou HL, Sheu GT, Hsieh LL, Chen JT, Chen CY, et al. The association of human papillomavirus 16/18 infection with lung cancer among nonsmoking Taiwanese women. Cancer Res. 2001;61:2799–2803. [PubMed] [Google Scholar]
- 30.Nadji SA, Mokhtari-Azad T, Mahmoodi M, Yahyapour Y, Naghshvar F, Torabizadeh J, et al. Relationship between lung cancer and human papillomavirus in north of Iran, Mazandaran province. Cancer Lett. 2007;248:41–46. doi: 10.1016/j.canlet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Yu Y, Yang A, Hu S, Yan H. Correlation of HPV-16/18 infection of human papillomavirus with lung squamous cell carcinomas in Western China. Oncol Rep. 2009;21:1627–1632. doi: 10.3892/or_00000397. [DOI] [PubMed] [Google Scholar]
- 32.Sarchianaki E, Derdas SP, Ntaoukakis M, Vakonaki E, Lagoudaki ED, Lasithiotaki I, et al. Detection and genotype analysis of human papillomavirus in non-small cell lung cancer patients. Tumour Biol. 2014;35:3203–3209. doi: 10.1007/s13277-013-1419-2. [DOI] [PubMed] [Google Scholar]
- 33.Gazdar AF, Thun MJ. Lung cancer, smoke exposure, and sex. J Clin Oncol. 2007;25:469–471. doi: 10.1200/JCO.2006.09.4623. [DOI] [PubMed] [Google Scholar]
- 34.Thun MJ, Hannan LM, Adams-Campbell LL, Boffetta P, Buring JE, Feskanich D, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5:e2015052. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Y, Yang A, Hu S, Zhang J, Yan H. Significance of human papillomavirus 16/18 infection in association with p53 mutation in lung carcinomas. Clin Respir J. 2013;7:27–33. doi: 10.1111/j.1752-699X.2011.00277.x. [DOI] [PubMed] [Google Scholar]
- 36.Krikelis D, Tzimagiorgis G, Georgiou E, Destouni C, Agorastos T, Haitoglou C, et al. Frequent presence of incomplete HPV16 E7 ORFs in lung carcinomas: memories of viral infection. J Clin Virol. 2010;49:169–174. doi: 10.1016/j.jcv.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Galvan A, Noci S, Taverna F, Lombardo C, Franceschi S, Pastorino U, et al. Testing of human papillomavirus in lung cancer and non-tumor lung tissue. BMC Cancer. 2012;12:512. doi: 10.1186/1471-2407-12-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anantharaman D, Gheit T, Waterboer T, Halec G, Carreira C, Abedi-Ardekani B, et al. No causal association identified for human papillomavirus infections in lung cancer. Cancer Res. 2014;74:3525–3534. doi: 10.1158/0008-5472.CAN-13-3548. [DOI] [PubMed] [Google Scholar]
- 39.Yu Y, Liu X, Yang Y, Zhao X, Xue J, Zhang W, et al. Effect of FHIT loss and p53 mutation on HPV-infected lung carcinoma development. Oncol Lett. 2015;10:392–398. doi: 10.3892/ol.2015.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papadakis ED, Soulitzis N, Spandidos DA. Association of p53 codon 72 polymorphism with advanced lung cancer: the Arg allele is preferentially retained in tumours arising in Arg/Pro germline heterozygotes. Br J Cancer. 2002;87:1013–1018. doi: 10.1038/sj.bjc.6600595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpagnano GE, Koutelou A, Natalicchio MI, Martinelli D, Ruggieri C, Di Taranto A, et al. HPV in exhaled breath condensate of lung cancer patients. Br J Cancer. 2011;105:1183–1190. doi: 10.1038/bjc.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiou HL, Wu MF, Liaw YC, Cheng YW, Wong RH, Chen CY, et al. The presence of human papillomavirus type 16/18 DNA in blood circulation may act as a risk marker of lung cancer in Taiwan. Cancer. 2003;97:1558–1563. doi: 10.1002/cncr.11191. [DOI] [PubMed] [Google Scholar]
- 43.Jain N, Singh V, Hedau S, Kumar S, Daga MK, Dewan R, et al. Infection of human papillomavirus type 18 and p53 codon 72 polymorphism in lung cancer patients from India. Chest. 2005;128:3999–4007. doi: 10.1378/chest.128.6.3999. [DOI] [PubMed] [Google Scholar]
- 44.Buyru N, Altinisik J, Isin M, Dalay N. p53 codon 72 polymorphism and HPV status in lung cancer. Med Sci Monit. 2008;14:CR493–CR497. [PubMed] [Google Scholar]
- 45.Simen-Kapeu A, Surcel HM, Koskela P, Pukkala E, Lehtinen M. Lack of association between human papillomavirus type 16 and 18 infections and female lung cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:1879–1881. doi: 10.1158/1055-9965.EPI-10-0356. [DOI] [PubMed] [Google Scholar]
- 46.Efird JT, Toland AE, Lea CS, Phillips CJ. The combined influence of oral contraceptives and human papillomavirus virus on cutaneous squamous cell carcinoma. Clin Med Insights Oncol. 2011;5:55–75. doi: 10.4137/CMO.S6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gasperino J, Rom WN. Gender and lung cancer. Clin Lung Cancer. 2004;5:353–359. doi: 10.3816/CLC.2004.n.013. [DOI] [PubMed] [Google Scholar]
- 48.Bae JM, Kim EH. Hormonal replacement therapy and the risk of lung cancer in women: an adaptive meta-analysis of cohort studies. J Prev Med Public Health. 2015;48:280–286. doi: 10.3961/jpmph.15.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25:472–478. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joshi D, Buehring GC. Are viruses associated with human breast cancer? Scrutinizing the molecular evidence. Breast Cancer Res Treat. 2012;135:1–15. doi: 10.1007/s10549-011-1921-4. [DOI] [PubMed] [Google Scholar]
- 51.Liang W, Tian H. Hypothetic association between human papillomavirus infection and breast carcinoma. Med Hypotheses. 2008;70:305–307. doi: 10.1016/j.mehy.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 52.Engels EA, Wacholder S, Katki HA, Chaturvedi AK. Tumor-based case-control studies of infection and cancer: muddling the when and where of molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2014;23:1959–1964. doi: 10.1158/1055-9965.EPI-14-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhai K, Ding J, Shi HZ. Author’s reply to “comments on HPV and lung cancer risk: a meta-analysis” [J. Clin. Virol. (in press)] J Clin Virol. 2015;63:92–93. doi: 10.1016/j.jcv.2014.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


