Abstract
During adolescence there is a normative increase in risk-taking behavior, which is reflected in, for example, increases in alcohol consumption. Prior research has demonstrated a link between testosterone and alcohol consumption, and between testosterone and neural responses to rewards. Yet, no study to date tested how testosterone levels and neural responses to rewards relate to and predict individual differences in alcohol use. The current study aimed to investigate this by assessing alcohol use, testosterone levels and neural responses to rewards in adolescents (12–17 years old) and young adults (18–26 years old). Participants were measured twice with a two-year interval between testing sessions. Cross-sectional analysis showed that at the second time point higher neural activity to rewards, but not testosterone levels, explained significant variance above age in reported alcohol use. Predictive analyses showed that, higher testosterone level at the first time point, but not neural activity to rewards at the first time point, was predictive of more alcohol use at the second time point. These results suggest that neural responses to rewards are correlated with current alcohol consumption, and that testosterone level is predictive of future alcohol consumption. These results are interpreted in the context of trajectory models of adolescent development.
Keywords: Adolescence, fMRI, Nucleus accumbens, Risk-taking, Alcohol
1. Introduction
Adolescence is an important developmental period characterized by increases in risk taking behavior, such as alcohol use, substance abuse and delinquency (Steinberg, 2004). Many of the changes in risk taking behavior are thought to be normative transitions in explorative behavior and sensation seeking, which help adolescents to explore their environment, gain independence and develop individual social and personal goals (Crone and Dahl, 2012, Steinberg, 2008). However, in some situations these risk taking tendencies can have detrimental consequences, such as in the case of excessive alcohol consumption. It was recently reported that 32% of all emergency treatments resulting from alcohol poisoning in the Netherlands were targeted at adolescents between 15 and 19 years of age (Valkenberg et al., 2012). Alcohol consumption is related to different types of risk taking behavior such as cigarette use and marijuana (Hill and Mrug, 2015). As such alcohol consumption can be used as a proxy for risk taking behavior and investigating alcohol consumption is therefore an important aspect of adolescent and young adult development. The neurobiological determinants and consequences of this behavior are not yet well understood.
Researchers have previously examined how hormonal changes related to pubertal development coincide with changes in alcohol consumption. Puberty onset starts approximately around age 9–10, although on average about 1.5 years earlier for girls than for boys, and it is marked by changes in both hormone levels and physical appearance (Susman and Rogol, 2004). Congruent with these changes, adolescents experience changes in the social, emotional and academic domains. Puberty specific change may instigate a transition not only in physical appearance, but also in social-affective domains such as risk-taking behavior, and specifically alcohol consumption. In a previous study we measured alcohol use in the last month and total lifetime use. We showed that in both boys and girls, more advanced puberty was associated with higher levels of alcohol consumption, while controlling for age. In addition, boys who had higher baseline levels of testosterone were also more likely to have consumed alcohol (de Water et al., 2013).
The psychological and hormonal changes in relation to puberty occur in conjunction with structural (Goddings et al., 2014, Peper et al., 2013) and functional changes in the brain (Blakemore et al., 2010), but few studies examined how affective brain reactivity and hormone levels relate to individual differences in alcohol consumption. One target region for affective brain reactivity in adolescence is the ventral striatum. Recent studies have shown that neural responses in the ventral striatum to rewards are heightened during adolescence, compared to childhood and adulthood (Braams et al., 2014, Galvan et al., 2006, Van Leijenhorst et al., 2010). Especially the nucleus accumbens (NAcc), a key region for processing rewards, shows elevated responses during adolescence. Intriguingly, neural activity in the NAcc corresponded with testosterone levels in early adolescence (Forbes et al., 2010, Op de Macks et al., 2011), and with self-reported risk taking tendencies (Galvan et al., 2007). In a recent longitudinal study including 299 participants, we examined NAcc activity across the whole range of childhood, adolescence and young adulthood using a simple gambling task that could result in gains or losses. We confirmed the adolescent peak in NAcc activity to rewards at two time points (Braams et al., 2015). The peak in neural activation in NAcc and the peak in risk taking behavior observed in adolescence are likely to be related. However, few studies have found a direct relationship between the two, possibly due to relatively small sample sizes of neuroimaging studies that assessed both neural reward sensitivity and alcohol consumption.
The current study aimed to shed light on the relationship between NAcc responses to rewards, testosterone levels and self-reported alcohol use in a longitudinal sample. We made use of the neural activity data that were previously reported in Braams et al. (2015). This longitudinal study examined neural activity to rewards during adolescence across two time points. In addition to the fMRI session, participants were asked to fill out an alcohol consumption questionnaire that tested three domains of alcohol use: average alcohol consumption per evening, alcohol consumption in the last month and total lifetime alcohol consumption. For a total of 169 participants between 12 and 26 years of age complete data on all measures was available. We hypothesized that age and alcohol use would be positively correlated, replicating prior studies (Spear, 2013). In addition, we hypothesized that the participants who showed elevated responses to rewards during a gambling task in the scanner, would also report higher use of alcohol, consistent with the hypothesis that adolescent specific changes in affective neural activity is related to higher risk taking (Galvan et al., 2007). Furthermore, we expect that higher levels of testosterone correspond with higher alcohol consumption (de Water et al., 2013). Given the longitudinal design, we were able to test whether neural activity and testosterone merely coincide with higher alcohol use, or also predict alcohol use at the second time point.
2. Materials and methods
2.1. Participants
Participants were part of a larger, longitudinal study in which participants were included between 8 and 27 years of age. Participants were recruited through high schools and advertisement in local newspapers. Participants who participated in the fMRI session and for whom testosterone data and self-report alcohol data at both time point were available were selected for the current study. In total, complete data from a total of 169 participants (87 males) was available. A description of participant data at both time points can be found in Table 1. Data from the full sample has previously been published in Braams et al., 2014, Braams et al., 2015, Peters et al. (2014) and Peper et al. (2013).
Table 1.
Participants were selected when data was available for testosterone, alcohol questionnaire and fMRI measure at both time points. Of this group, available data for per variable at time points 1 and 2 is described in the table.
| Time point 1 |
Time point 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | Minimum | Maximum | Mean (SE) | N | Minimum | Maximum | Mean (SE) | |
| Age | 169 | 9.95 | 24.55 | 15.30 (.25) | 169 | 12.18 | 26.62 | 17.30 (.25) |
| Testosterone | 169 | 0.60 | 2.96 | 1.72 (.05) | 169 | 0.95 | 2.93 | 1.93 (.05) |
| Left NAcc | 169 | −9.39 | 8.20 | 2.02 (.19) | 169 | −6.07 | 9.73 | 1.87 (20) |
| Right NAcc | 169 | −6.80 | 9.25 | 1.99 (.19) | 169 | −6.64 | 9.78 | 1.72 (.19) |
| Average number of glasses | 0 | n/a | n/a | n/a | 169 | 0.00 | 50.00 | 11.42 (1.19) |
| Alcohol use last month | 0 | n/a | n/a | n/a | 169 | 0.00 | 91.00 | 43.17 (3.07) |
| Alcohol use lifetime | 0 | n/a | n/a | n/a | 168 | 0.00 | 10.00 | 2.82 (.22) |
An approximation of IQ was determined by two subscales, vocabulary and picture completion, from the Wechsler Intelligence Scale for children (WISC-III) or Wechsler Intelligence Scale for adults (WAIS-III) (Wechsler, 1997). Estimated intelligence scores fell within the normal range (MIQ = 107.7, SEIQ = .73).
Participants received an endowment for participation in a larger scale study. Participants aged 17 and below received €30 and adults €60. Participants, or parents in the case of under aged participants, gave written informed consent prior to the start of the experiment. MRI scans for all participants were reviewed by a radiologist and checked for abnormalities. No abnormalities were reported.
2.2. Alcohol questionnaire
Participants filled out a questionnaire in which they indicated how much they drink on average when they go out, how much they drank last month and total lifetime alcohol use (Ames et al., 2007). This study made us of alcohol questionnaire that was filled out at the second time point, given that alcohol use was examined as an outcome variable. The average number of glasses of alcohol per night was measured with a free response question. Past month alcohol use in number of glasses was measured using a 10-point scale (0, 1–2, 3–4, 5–6, 7–10, 11–15, 16–20, 21–30, 31–50, >50). Lastly, for lifetime alcohol use participants indicated the amount of glasses of alcohol they consumed in total during their lifetime. Responses were collected on an 11-point scale (0, 1–10, 11–20, 21–30, 31–40, 41–50, 51–60, 61–70, 71–80, 81–90, and >90). Standard glass size was 250 ml or 8.4 oz, participants were asked to count bottles or cans of alcohol as 1.5 glasses. Self-report measures of substance use have been found to show high test–retest reliability with r's up to .86 (Brener et al., 2002).
2.3. Testosterone
Testosterone collection has been described previously (Braams et al., 2015, de Water et al., 2013, Peper et al., 2013, Peters et al., 2015). Testosterone levels were assessed in morning saliva samples. Samples were collected by passive drool, directly after waking up and before eating or brushing teeth. Females who had not yet reached menarche and males collected saliva on the day of fMRI testing. To control for menstrual fluctuations, post-menarcheal females and females who used contraceptives with a stopping period collected saliva on the 7th day of their menstrual cycle. At the 7th day of the menstrual cycle hormone levels are less influenced by fluctuations in the cycle (Mihm et al., 2011, Peper and Dahl, 2013). Females who used contraceptives without a stopping period such as hormonal intrauterine devices were excluded from testosterone assessment. Females who used the pill were included in the sample. In total 12 female participants reported to use the pill on the first time point and 22 female participants reported to use the pill on the second time point. Independent samples t-tests showed that testosterone levels (log transformed values are reported) did not differ (Time point 1: t = −.054, p = .124; Time point 2: t = .119, p = .539) between participants who did use the pill (Time point 1: N = 12; Mean = 1.28, SEmean = .047; Time point 2: N = 22; Mean = 1.38, SEmean = .054) and participants did not use the pill (Time point 1: N = 70; Mean = 1.28, SEmean = .047; Time point 2: N = 60; Mean = 1.38, SEmean = .054).
Testosterone levels for all saliva samples were assayed at the Department of Clinical Chemistry of the VU University Medical Centre. The lower limit of detection was 4 pmol/L. Salivary testosterone was determined by isotope dilution—online solid phase extraction liquid chromatography—tandem mass spectrometry (Peper et al., 2013). Intra-assay coefficients of variation were 11% and 4% at 10 and 140 pmol/L, respectively, and interassay coefficients of variation were 8% and 5% at 31 and 195 pmol/L, respectively (de Water et al., 2013). Testosterone levels were not normally distributed, therefore a log transformed measure for testosterone levels was used in all analyses. Testosterone samples were collected from 292 participants on T1 and 274 participants on T2. Testosterone levels from 25 participants on T1 and three participants on T2 fell below the detection limit of 4 pmol/L. These participants were excluded from further analyses. Seven participants on T1 and one participant on T2 did not collect sufficient amount of saliva for assessment. The final number of participants for whom testosterone data was available was 260 on T1 and 270 at T2. Note that only those participants for whom testosterone, alcohol and fMRI data was available at T2 were selected for this study. For a description of total participants see Table 1.
2.4. Experimental task
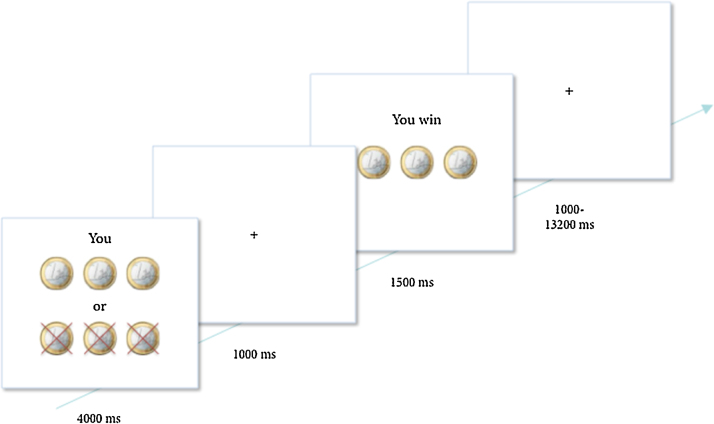
Participants played a heads or tails gambling game in which they could win or lose money (Braams et al., 2013, Braams et al., 2014). On each trial, participants guessed whether the computer would pick heads or tails and they won when the computer selected the chosen side of the coin. Each trial started with a trial onset screen (4000 ms) during which the participant indicated their choice to play for heads or tails. On the trial onset screen the participants also saw how much they could win or lose on that trial, explained in more detail below. The trial onset screen was followed by a fixation screen (1000 ms) and a feedback screen, which showed whether participants won or lost on that trial (1500 ms). Trials ended with a variable jitter (1000–13200 ms), see Fig. 1. Trial sequence and timing was optimized using OptSeq (Dale, 1999); see also (http://surfer.nmr.mgh.harvard.edu/optseq/). Probabilities for winning were 50%. Three different distributions of coins were included; trials on which 2 coins could be won and 5 lost, trials on which 3 coins could be won or 3 lost and finally trials on which 5 coins could be won or 2 could be lost. These different distributions of coins were included to keep participants engaged in the task, but were not analyzed separately (see also Braams et al., 2013, Braams et al., 2014). Participants were informed about the different distributions of coins and were familiarized with them during the practice task. Participants were explained that the coins won during the experiment translated to real money at the end of the experiment. Participants received 4, 5 or 6 euro's at the end of the task. Unbeknownst to the participants, the total earnings on the task did not relate to the amount won during the task but were chosen at random.
Fig. 1.
Example of a trial. On trial onset, participants were presented with a screen for 4000 ms indicating for whom they were playing (self, friend or antagonist) and how many coins could be won or lost. During this time, participants chose to play heads or tails by pressing the corresponding button. After a 1000 ms delay, trial outcome was presented for 1500 ms. Participants won when the computer randomly selected the same side of the coin as chosen by the participant.
Participants played 30 trials in the gambling game for themselves, 30 trials for their best friend and 30 trials for another person. The goal of the current study was to specifically assess neural responses to rewards for self, therefore for the current study only trials on which the participants played for themselves were included (see Braams et al., 2013, Braams et al., 2014 for a description of the data of the first time point for the full task).
2.5. Procedure
Participants received instructions regarding the testing session in a quiet laboratory room. Participants were familiarized with the MRI scanner with a mock scanner. Next the gambling game was explained and participants performed six practice trials. After the scanner session WISC-III or WAIS-III were administered. Participants filled out the alcohol questionnaire in a private setting at home, before the testing session. Participants were ensured that all information was collected anonymously and that results would not be shared with others. Testosterone was collected on the day of the scanning session for males and females who did not yet reached menarche, females who did reach menarche collected saliva on the 7th day of their menstrual cycle. Care was taken to schedule the scanning session as close as possible to the day of saliva collection.
2.6. MRI data acquisition
Scanning was performed on a 3 Tesla Philips scanner, with a standard whole-head coil. The functional scans were acquired using a T2*-weighted echo-planar imaging (EPI) (TR = 2.2 s, TE = 30 ms, sequential acquisition, 38 slices of 2.75 mm, field of view 220 mm, 80 × 80 matrix, in-plane resolution 2.75 mm). The first two volumes were discarded to allow for equilibration of T1 saturation effects. After the functional runs, a high-resolution 3D T1-weighted anatomical image was collected (TR = 9.751 ms, TE = 4.59 ms, flip angle = 8°, 140 slices, 0.875 mm × 0.875 mm × 1.2 mm, and FOV = 224 < .001 × 168 < .001 × 177.333). Visual stimuli were displayed on a screen in the magnet bore. A mirror attached to the head coil allowed participants to view the screen. Foam inserts inside the coil were used to limit head movement. MRI data acquisition was similar at the two time points (see also Braams et al., 2014).
2.7. fMRI preprocessing and statistical analyses
In total 299 participants were scanned at the first time point and 254 participants were scanned at the second time point. For fMRI-analyses, 36 participants at the first time point and 10 participants at the second time point were excluded for moving more than 1 voxel. An additional 14 participants at the first time point and six participants at the second time point were excluded for not finishing the task, technical problems and/or artifacts during data collection. For a total of 160 participants at T1 and 208 at T2 fMRI and data for the alcohol questionnaire was available. Whole brain fMRI analyses are reported for the group for whom data on the fMRI task and alcohol questionnaire was available.
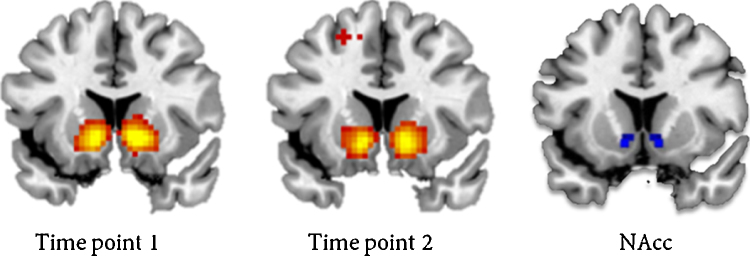
All data were analyzed with SPM8 (Wellcome Department of Cognitive Neurology, London). Images were corrected for slice timing acquisition and differences in rigid body motion. Structural and functional volumes were spatially normalized to T1 templates. Translational movement parameters never exceeded 1 voxel (<3 mm) in any direction for any participant or scan. The normalization algorithm used a 12-parameter affine transform together with a nonlinear transformation involving cosine basis functions and resampled the volumes to 3 mm cubic voxels. Templates were based on the MNI305 stereotaxic space (Cocosco et al., 1997). Functional volumes were spatially smoothed with a 6 mm FWHM isotropic Gaussian kernel. Statistical analyses were performed on individual subjects data using the general linear model in SPM8. The fMRI time series were modeled as a series of zero duration events convolved with the hemodynamic response function (HRF). On trial onset events were modeled separately for playing for self, friend and other. On feedback onset winning and losing for self, friend and antagonist were modeled. This resulted in three conditions at trial onset (self, friend, other) and six conditions at feedback onset (self win, self lose, friend win, friend lose, other win, other lose). Trials on which the participants failed to respond were modeled separately as covariate of no interest and were excluded from further analyses. The modeled events were used as regressors in a general linear model, along with a basic set of cosine functions that high-pass filtered the data, and a covariate for session effects. The least-squares parameter estimates of height of the best-fitting canonical HRF for each condition were used in pair-wise contrasts. The resulting contrast images, computed on a subject-by-subject basis, were submitted to random-effects group analyses. The contrast of interest was win > lose when playing for self, specified at the moment of feedback onset. Separate whole brain analyses were performed for time point 1 and time point 2. The whole brain contrast for winning > losing for self resulted in significant activation in the bilateral NAcc on both time points, see Fig. 2 and Table 2 for a whole brain table of all above threshold activation for this contrast.
Fig. 2.
Whole brain activation at the first and second time point for win > lose for self and the anatomical nucleus accumbens (NAcc) region used for analyses. A similar figure has been published before in Braams et al. (2015).
Table 2.
Whole brain table for neural activation for the contrast win > lose when playing for self at the first and second time point. Reported clusters survive family wise error correction, p < .05, at the voxel level. Only clusters comprised of 10 voxels or more are reported.
| Region | R/L | MNI |
T (168) | Voxels | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Time point 1 | ||||||
| Putamen | R | 15 | 11 | −5 | 12.57 | 574 |
| Caudate nucleus | L | −9 | 14 | −5 | 11.05 | |
| Anterior cingulate cortex | L | 0 | 50 | −2 | 6.67 | 112 |
| Inferior occipital gyrus | R | 45 | −79 | −14 | 6.15 | 50 |
| Hippocampus | L | −21 | −19 | −11 | 5.94 | 22 |
| Middle orbital gyrus | L | −30 | 41 | −5 | 5.89 | 16 |
| Middle cingulate cortex | R | 3 | −34 | 37 | 5.85 | 39 |
| Middle cingulate cortex | L | −3 | −7 | 34 | 5.47 | 16 |
| Hippocampus | R | 21 | −19 | −14 | 5.41 | 8 |
| Supplementary motor area | R | 6 | −22 | 55 | 5.33 | 12 |
| Time point 2 | ||||||
| Putamen | R | 15 | 11 | −8 | 10.76 | 225 |
| Caudate nucleus | L | −12 | 14 | −8 | 10.08 | 176 |
| Anterior cingulate cortex | R | 0 | 50 | −2 | 5.93 | 73 |
| Paracentral lobule | L | −12 | −22 | 58 | 5.73 | 41 |
| Middle cingulate cortex | R | 0 | −40 | 37 | 5.44 | 16 |
| Inferior parietal lobule | L | −51 | −61 | 46 | 5.30 | 9 |
| Middle frontal gyrus | L | −27 | 65 | 4 | 5.26 | 11 |
| Superior frontal gyrus | L | −21 | 17 | 58 | 5.21 | 18 |
| Right precentral gyrus | R | 42 | −1 | 25 | 5.13 | 3 |
| Right precentral gyrus | R | 24 | −16 | 58 | 5.12 | 6 |
| Anterior cingulate cortex | R | 6 | 5 | 28 | 5.08 | 4 |
| Middle cingulate cortex | R | 3 | −31 | 46 | 5.04 | 11 |
2.8. Region of interest analysis
We used the MarsBaR toolbox (Brett et al., 2002) (http://marsbar.sourceforge.net/) for SPM8 to perform region of interest (ROI) analyses to extract patterns of activation in an anatomically defined NAcc cluster. Average beta values, also known as parameter estimates, were used for ROI analyses. We used an anatomical mask of the left and right NAcc extracted from the Harvard-Oxford subcortical atlas, thresholded at 40%. We specifically focused on the NAcc, since previous studies have highlighted this part of the ventral striatum as a key region in reward-based processing (Braams et al., 2014, Delgado, 2007).
2.9. Hierarchical regression models
To investigate the relationship between testosterone levels, NAcc activation during reward processing and (1) average alcohol use per night, (2) alcohol use last month and (3) lifetime alcohol use, we performed hierarchical regressions for each measure.
In the first step we included linear, quadratic and cubic regressors for age. These regressors were included to account for the quadratic effects of age on NAcc activation and the cubic effects of age on testosterone levels (see Braams et al., 2015).
In the second step we included testosterone levels at time point 2 and NAcc activation at time point 2 to investigate cross-sectional relationships between alcohol use, testosterone and NAcc activation.
In the third step we included testosterone at time point 1 and NAcc activation at time point 1 to investigate whether testosterone and NAcc activation predict future alcohol use.
NAcc activation in the right and left NAcc are highly correlated at time point 1 (r = .802 p = < .001) and time point 2 (r = .745, p < .001), including regressors for highly correlated measures might result in erratic changes in coefficient estimates when these measures are included in the same model. Therefore separate models were fitted for left and right NAcc.
3. Results
3.1. The relationship between the alcohol questionnaire and age
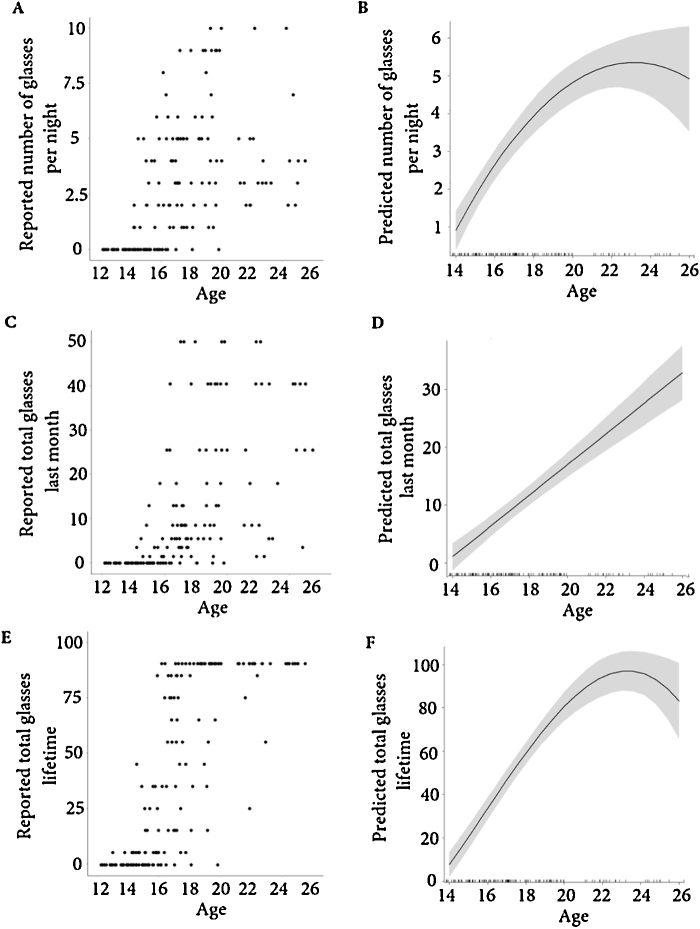
We tested whether a linear, quadratic of cubic regressor for age best described the relationship between the alcohol questionnaire and age. We used hierarchical regression analysis in which we included only a linear regressor for age in the first step, we added a quadratic regressor for age in the second step and a cubic regressor for age in the third step. We assessed improved model fit for each step by evaluating whether the F change was significant. Average amount of glasses was best described by a quadratic regressor of age, last month was best described by a linear regressor of age and lifetime alcohol use were best described by a cubic regressor of age, see Table 3 for all fitted models and Fig. 3 for a visual display of best fitting regression lines.
Table 3.
Hierarchical regressions for linear (age1), quadratic (age2) and cubic (age3) regressors of age for average amount of glasses consumed per night, total last month alcohol use and total lifetime alcohol use, see also Fig. 3.
| df | R2Δ | FΔ | pFΔ | B | SE | β | t | p | |
|---|---|---|---|---|---|---|---|---|---|
| Average amount of glasses | |||||||||
| Step 1 | 1166 | .31 | 75.79 | <.001 | |||||
| Constant | −5.66 | 0.99 | −5.71 | <.001 | |||||
| Age1 | 0.49 | 0.06 | 0.56 | 8.71 | <.001 | ||||
| Step 2 | 2165 | .07 | 17.44 | <.001 | |||||
| Constant | −8.26 | 1.13 | −7.30 | <.001 | |||||
| Age1 | 0.68 | 0.07 | 0.77 | 9.70 | <.001 | ||||
| Age2 | 0.06 | 0.01 | 0.33 | 4.18 | <.001 | ||||
| Step 3 | 3164 | .00 | .28 | .60 | |||||
| Constant | −0.48 | 14.89 | −0.03 | 0.98 | |||||
| Age1 | 0.28 | 0.76 | 0.32 | 0.37 | 0.72 | ||||
| Age2 | 0.02 | 0.07 | 0.12 | 0.31 | 0.76 | ||||
| Age3 | <.001 | <.001 | 0.36 | 0.52 | 0.60 | ||||
| Last month | |||||||||
| Step 1 | 1167 | 0.39 | 107.58 | <.001 | |||||
| Constant | −39.34 | 4.98 | −7.90 | <.001 | |||||
| Age1 | 2.94 | 0.28 | 0.63 | 10.37 | <.001 | ||||
| Step 2 | 2166 | 0.01 | 2.04 | .16 | |||||
| Constant | −44.05 | 5.96 | −7.39 | <.001 | |||||
| Age1 | 3.27 | 0.37 | 0.70 | 8.92 | <.001 | ||||
| Age2 | 0.10 | 0.07 | 0.11 | 1.43 | 0.16 | ||||
| Step 3 | 1165 | 0.01 | 2.05 | .15 | |||||
| Constant | −154.62 | 77.52 | −2.00 | 0.05 | |||||
| Age1 | 8.92 | 3.97 | 1.90 | 2.25 | 0.03 | ||||
| Age2 | 0.59 | 0.35 | 0.66 | 1.69 | 0.09 | ||||
| Age3 | −0.03 | 0.02 | −0.96 | −1.43 | 0.15 | ||||
| Lifetime | |||||||||
| Step 1 | 1167 | 0.57 | 219.95 | <.001 | |||||
| Constant | −113.98 | 10.79 | −10.57 | <.001 | |||||
| Age1 | 9.09 | 0.61 | 0.75 | 14.83 | <.001 | ||||
| Step 2 | 2166 | 0.06 | 27.58 | <.001 | |||||
| Constant | −148.84 | 12.02 | −12.38 | <.001 | |||||
| Age1 | 11.57 | 0.74 | 0.96 | 15.64 | <.001 | ||||
| Age2 | 0.74 | 0.14 | 0.32 | 5.25 | <.001 | ||||
| Step 3 | 1165 | 0.01 | 4.31 | .04 | |||||
| Constant | −470.21 | 155.32 | −3.03 | <.001 | |||||
| Age1 | 27.99 | 7.95 | 2.32 | 3.52 | <.001 | ||||
| Age2 | 2.16 | 0.70 | 0.95 | 3.09 | <.001 | ||||
| Age3 | −0.08 | 0.04 | −1.08 | −2.08 | 0.04 | ||||
Fig. 3.
Raw data and best fitting regression lines for age and the average amount of glasses that participant report to drink on average per night (panels A and B), the total amount of glasses that participant report to have consumed last month (panel C and D), the total amount of glasses that participant report to have consumed in their lifetime (panels E and F), also see Table 3.
3.2. The relationship between age, testosterone, NAcc activation and alcohol use
Hierarchical regression models were fitted to investigate the relationship between age, testosterone, NAcc activation and alcohol use. Separate models were fitted for each variable of alcohol use, i.e. average amount of glasses per night, total glasses consumed last month and total glasses consumed in the lifetime. The results of these analyses are described below.
3.2.1. Average amount of glasses
First we tested whether there was a cross-sectional relationship between average amount of glasses drunk per night, and age, testosterone and NAcc activation at the second time point. Results showed that, corrected for age, testosterone did not explain variance for the average amount of glasses at the second time point (all t < .47, p > .64), whereas right NAcc activation explained significant variance in the average amount of glasses (β = .13, t = 2.01, p = .046).
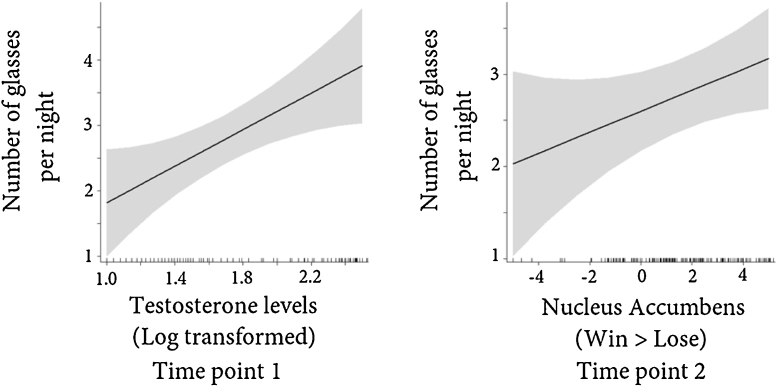
Second, we tested whether testosterone levels and NAcc activation at the first time point were predictive for the average amount of glasses drank per night at the second time point. Adding testosterone and NAcc at the first time point to the model showed that testosterone levels at the first time point significantly predicted the average amount of glasses at the second time point (β = .30, t = 2.66, p = .01), whereas NAcc activation at the first time point was not predictive of the average amount of glasses per night at the second time point (all t < .20, p > .84) (see Table 4 for a description of the regression models and Fig. 4 for a visual display of the significant effects).
Table 4.
Hierarchical regression testing the relationship between average amount of glasses of alcohol drank per night at the second time point (T2) and testosterone levels and left and right nucleus accumbens (NAcc) activation at the first time point (T1) and second time point (T2) for the left NAcc. Linear regressors of age are indicated by Age1, quadratic regressors of age by Age2 and cubic regressors of age by Age3. Asterisks indicate significant effects. See also Fig. 4.
| df | F | R2Δ | B | SE | β | t | p | |
|---|---|---|---|---|---|---|---|---|
| Right NAcc | ||||||||
| Step 1 | 3164 | 33.5 | .38 | |||||
| Constant | −0.48 | 14.89 | −0.03 | 0.98 | ||||
| Age1 | 0.28 | 0.76 | 0.32 | 0.37 | 0.72 | |||
| Age2 | 0.02 | 0.07 | 0.12 | 0.31 | 0.76 | |||
| Age3 | 0.00 | 0.00 | 0.36 | 0.52 | 0.60 | |||
| Step 2 | 5162 | 21.2 | .02 | |||||
| Constant | −3.70 | 15.35 | −0.24 | 0.81 | ||||
| Age1 | 0.41 | 0.78 | 0.47 | 0.53 | 0.60 | |||
| Age2 | 0.03 | 0.07 | 0.17 | 0.42 | 0.68 | |||
| Age3 | 0.00 | 0.00 | 0.23 | 0.32 | 0.75 | |||
| Testosterone T2 | 0.15 | 0.31 | 0.03 | 0.47 | 0.64 | |||
| Right NAcc win > lose T2 | 0.14 | 0.07 | 0.13 | 2.01 | 0.05* | |||
| Step 3 | 7160 | 16.6 | .03 | |||||
| Constant | −8.01 | 15.20 | −0.53 | 0.60 | ||||
| Age1 | 0.60 | 0.77 | 0.68 | 0.78 | 0.44 | |||
| Age2 | 0.04 | 0.07 | 0.26 | 0.64 | 0.52 | |||
| Age3 | 0.00 | 0.00 | −0.04 | −0.06 | 0.95 | |||
| Testosterone T2 | −0.90 | 0.50 | −0.18 | −1.79 | 0.08 | |||
| Right NAcc win > lose T2 | 0.11 | 0.07 | 0.10 | 1.65 | 0.10 | |||
| Testosterone T1 | 1.39 | 0.52 | 0.30 | 2.66 | 0.01** | |||
| Right NAcc win > lose T1 | −0.01 | 0.07 | −0.01 | −0.20 | 0.84 | |||
| Left NAcc | ||||||||
| Step 1 | 3164 | 33.5 | .38 | |||||
| Constant | −0.48 | 14.89 | −0.03 | 0.98 | ||||
| Age1 | 0.28 | 0.76 | 0.32 | 0.37 | 0.72 | |||
| Age2 | 0.02 | 0.07 | 0.12 | 0.31 | 0.76 | |||
| Age3 | 0.00 | 0.00 | 0.36 | 0.52 | 0.60 | |||
| Step 2 | 5162 | 20.5 | .01 | |||||
| Constant | −3.15 | 15.45 | −0.20 | 0.84 | ||||
| Age1 | 0.39 | 0.78 | 0.45 | 0.50 | 0.62 | |||
| Age2 | 0.03 | 0.07 | 0.17 | 0.41 | 0.69 | |||
| Age3 | 0.00 | 0.00 | 0.25 | 0.35 | 0.73 | |||
| Testosterone T2 | 0.12 | 0.32 | 0.03 | 0.38 | 0.70 | |||
| Left NAcc win > lose T2 | 0.10 | 0.08 | 0.09 | 1.39 | 0.17 | |||
| Step 3 | 7160 | 16.2 | .03 | |||||
| Constant | −7.67 | 15.27 | −0.50 | 0.62 | ||||
| Age1 | 0.58 | 0.77 | 0.67 | 0.76 | 0.45 | |||
| Age2 | 0.04 | 0.07 | 0.26 | 0.64 | 0.53 | |||
| Age3 | 0.00 | 0.00 | −0.03 | −0.05 | 0.96 | |||
| Testosterone T2 | −0.97 | 0.50 | −0.20 | −1.94 | 0.06 | |||
| Left NAcc win > lose T2 | 0.08 | 0.08 | 0.07 | 1.10 | 0.27 | |||
| Testosterone T1 | 1.45 | 0.52 | 0.32 | 2.77 | 0.01* | |||
| Left NAcc win > lose T1 | 0.00 | 0.07 | 0.00 | 0.01 | 0.99 | |||
Fig. 4.
Visual display of the predictive relationship between testosterone levels, values are log transformed, at the first time point and average number of glasses consumed per night at the second time point and the cross sectional relationship between nucleus accumbens activation, parameter estimates for win > lose when playing for self, and the average amount of glasses consumed per night at the second time point.
3.2.2. Alcohol use last month
We first tested whether there was a cross-sectional relationship between alcohol use in the last month, and age, testosterone and NAcc activation at the second time point. Testosterone and NAcc activation were not significantly related to alcohol use last month (all t < 1.18, p > .24).
We then tested whether testosterone and NAcc activation at the first time point were predictive of reported alcohol use in the last month at the second time point. Testosterone at the first time point was predictive of alcohol use at the second time point (β = .24, t(168) = 2.07, p = .04). NAcc activation at the first time point was not predictive of alcohol use on the second time point (all t < .62, p > .53) (see Table 5 for a description of the regression models).
Table 5.
Hierarchical regression testing the relationship between total amount of glasses of alcohol drank last month at the second time point (T2) and testosterone levels and left and right nucleus accumbens (NAcc) activation at the first time point (T1) and second time point (T2) for the left NAcc. Linear regressors of age are indicated by Age1, quadratic regressors of age by Age2 and cubic regressors of age by Age3. Asterisks indicate significant effects.
| df | F | R2Δ | B | SE | β | t | p | |
|---|---|---|---|---|---|---|---|---|
| Right NAcc | ||||||||
| Step 1 | 3165 | 37.68 | .41 | |||||
| Constant | −154.62 | 77.52 | −2.00 | 0.05* | ||||
| Age1 | 8.92 | 3.97 | 1.90 | 2.25 | 0.03* | |||
| Age2 | 0.59 | 0.35 | 0.66 | 1.69 | 0.09 | |||
| Age3 | −0.03 | 0.02 | −0.96 | −1.43 | 0.15 | |||
| Step 2 | 5163 | 22.93 | .01 | |||||
| Constant | −172.12 | 80.12 | −2.15 | 0.03* | ||||
| Age1 | 9.67 | 4.06 | 2.06 | 2.38 | 0.02* | |||
| Age2 | 0.67 | 0.36 | 0.75 | 1.86 | 0.06 | |||
| Age3 | −0.03 | 0.02 | −1.09 | −1.59 | 0.11 | |||
| Testosterone T2 | 1.74 | 1.64 | 0.07 | 1.06 | 0.29 | |||
| Right NAcc win > lose T2 | −0.26 | 0.36 | −0.05 | −0.73 | 0.47 | |||
| Step 3 | 7161 | 17.27 | .02 | |||||
| Constant | −189.04 | 79.95 | −2.36 | 0.02* | ||||
| Age1 | 10.38 | 4.04 | 2.21 | 2.57 | 0.01* | |||
| Age2 | 0.72 | 0.36 | 0.81 | 2.03 | 0.04* | |||
| Age3 | −0.04 | 0.02 | −1.29 | −1.88 | 0.06 | |||
| Testosterone T2 | −2.72 | 2.68 | −0.10 | −1.02 | 0.31 | |||
| Right NAcc win > lose T2 | −0.40 | 0.37 | −0.07 | −1.08 | 0.28 | |||
| Testosterone T1 | 5.79 | 2.79 | 0.24 | 2.07 | 0.04* | |||
| Right NAcc win > lose T1 | 0.15 | 0.39 | 0.02 | 0.39 | 0.70 | |||
| Left NAcc | ||||||||
| Step 1 | 3165 | 37.68 | .41 | |||||
| Constant | −154.62 | 77.52 | −2.00 | 0.05* | ||||
| Age1 | 8.92 | 3.97 | 1.90 | 2.25 | 0.03* | |||
| Age2 | 0.59 | 0.35 | 0.66 | 1.69 | 0.09 | |||
| Age3 | −0.03 | 0.02 | −0.96 | −1.43 | 0.15 | |||
| Step 2 | 5163 | 23.23 | .01 | |||||
| Constant | −169.80 | 79.90 | −2.13 | 0.04* | ||||
| Age1 | 9.57 | 4.05 | 2.04 | 2.36 | 0.02* | |||
| Age2 | 0.66 | 0.36 | 0.75 | 1.86 | 0.07 | |||
| Age3 | −0.03 | 0.02 | −1.07 | −1.56 | 0.12 | |||
| Testosterone T2 | 1.74 | 1.64 | 0.07 | 1.06 | 0.29 | |||
| Left NAcc win > lose T2 | −0.46 | 0.39 | −0.07 | −1.18 | 0.24 | |||
| Step 3 | 7161 | 17.55 | .02 | |||||
| Constant | −187.49 | 79.67 | −2.35 | 0.02* | ||||
| Age1 | 10.31 | 4.03 | 2.20 | 2.56 | 0.01* | |||
| Age2 | 0.72 | 0.36 | 0.81 | 2.03 | 0.05* | |||
| Age3 | −0.04 | 0.02 | −1.28 | −1.87 | 0.06* | |||
| Testosterone T2 | −2.78 | 2.66 | −0.11 | −1.05 | 0.30 | |||
| Left NAcc win > lose T2 | −0.60 | 0.40 | −0.09 | −1.49 | 0.14 | |||
| Testosterone T1 | 5.84 | 2.77 | 0.24 | 2.11 | 0.04* | |||
| Left NAcc win > lose T1 | 0.24 | 0.38 | 0.04 | 0.62 | 0.53 | |||
3.2.3. Lifetime alcohol use
For life time alcohol use, we first tested whether there was a cross-sectional relationship between lifetime alcohol use, and age, testosterone and NAcc activation at the second time point. Testosterone and NAcc activation were not significantly related to lifetime alcohol use (all t < .93, p > .35).
We then tested whether testosterone and NAcc activation at the first time point were predictive of lifetime alcohol use at the second time point. Testosterone at the first time point was predictive of alcohol use at the second time point (β = .25, t = 2.84, p = .005). NAcc activation at the first time point was not predictive of lifetime alcohol use on the second time point (all t's < .26, p > 79) (see Table 6 for a description of the regression models).
Table 6.
Hierarchical regression testing the relationship between total amount of glasses of alcohol drank in the lifetime at the second time point (T2) and testosterone levels and left and right nucleus accumbens (NAcc) activation at the first time point (T1) and second time point (T2) for the left NAcc. Linear regressors of age are indicated by Age1, quadratic regressors of age by Age2 and cubic regressors of age by Age3. Asterisks indicate significant effects.
| df | F | R2Δ | B | SE | β | t | p | |
|---|---|---|---|---|---|---|---|---|
| Right NAcc | ||||||||
| Step 1 | 3165 | 97.49 | .64 | |||||
| Constant | −470.21 | 155.32 | −3.03 | <.001** | ||||
| Age1 | 27.99 | 7.95 | 2.32 | 3.52 | <.001** | |||
| Age2 | 2.16 | 0.70 | 0.95 | 3.09 | <.001** | |||
| Age3 | −0.08 | 0.04 | −1.08 | −2.08 | 0.04* | |||
| Step 2 | 5163 | 58.29 | .00 | |||||
| Constant | −471.40 | 160.95 | −2.93 | <.001** | ||||
| Age1 | 28.02 | 8.15 | 2.33 | 3.44 | <.001** | |||
| Age2 | 2.14 | 0.72 | 0.94 | 2.98 | <.001** | |||
| Age3 | −0.08 | 0.04 | −1.09 | −2.02 | 0.05* | |||
| Testosterone T2 | −0.54 | 3.30 | −0.01 | −0.16 | 0.87 | |||
| Right NAcc win > lose T2 | 0.67 | 0.72 | 0.05 | 0.93 | 0.35 | |||
| Step 3 | 7161 | 44.36 | .02 | |||||
| Constant | −518.63 | 158.87 | −3.26 | <.001** | ||||
| Age1 | 30.05 | 8.03 | 2.49 | 3.74 | <.001** | |||
| Age2 | 2.30 | 0.71 | 1.01 | 3.26 | <.001** | |||
| Age3 | −0.09 | 0.04 | −1.30 | −2.45 | 0.02* | |||
| Testosterone T2 | −12.41 | 5.32 | −0.18 | −2.33 | 0.02* | |||
| Right NAcc win > lose T2 | 0.43 | 0.73 | 0.03 | 0.58 | 0.56 | |||
| Testosterone T1 | 15.78 | 5.55 | 0.25 | 2.84 | 0.01* | |||
| Right NAcc win > lose T1 | −0.20 | 0.77 | −0.01 | −0.26 | 0.79 | |||
| Left NAcc | ||||||||
| Step 1 | 3165 | 97.49 | .64 | |||||
| Constant | −470.21 | 155.32 | −3.03 | <.001** | ||||
| Age1 | 27.99 | 7.95 | 2.32 | 3.52 | <.001** | |||
| Age2 | 2.16 | 0.70 | 0.95 | 3.09 | <.001** | |||
| Age3 | −0.08 | 0.04 | −1.08 | −2.08 | 0.04* | |||
| Step 2 | 5163 | 57.82 | .00 | |||||
| Constant | −459.97 | 161.35 | −2.85 | 0.01* | ||||
| Age1 | 27.56 | 8.17 | 2.29 | 3.37 | <.001** | |||
| Age2 | 2.12 | 0.72 | 0.93 | 2.95 | <.001** | |||
| Age3 | −0.08 | 0.04 | −1.05 | −1.95 | 0.05* | |||
| Testosterone T2 | −0.79 | 3.31 | −0.01 | −0.24 | 0.81 | |||
| Left NAcc win > lose T2 | −0.08 | 0.79 | −0.01 | −0.10 | 0.92 | |||
| Step 3 | 7161 | 44.23 | .02 | |||||
| Constant | −510.00 | 158.98 | −3.21 | <.001** | ||||
| Age1 | 29.68 | 8.04 | 2.46 | 3.69 | <.001** | |||
| Age2 | 2.29 | 0.71 | 1.00 | 3.23 | <.001** | |||
| Age3 | −0.09 | 0.04 | −1.28 | −2.40 | 0.02* | |||
| Testosterone T2 | −13.20 | 5.30 | −0.20 | −2.49 | 0.01* | |||
| Left NAcc win > lose T2 | −0.33 | 0.80 | −0.02 | −0.41 | 0.68 | |||
| Testosterone T1 | 16.41 | 5.53 | 0.26 | 2.97 | <.001** | |||
| Left NAcc win > lose T1 | 0.03 | 0.75 | 0.00 | 0.04 | 0.97 | |||
4. Discussion
In this study we aimed to investigate the relationship between alcohol use, testosterone and neural responses to rewards. The current study is one of the first to combine neural and endocrinological measures with real life risk taking in a large longitudinal sample with continuous age range. We showed that neural responses to rewards were related to current alcohol use, whereas testosterone levels were predictive of future alcohol use. The discussion is organized along the lines of the cross-sectional versus predictive analyses.
4.1. Cross-sectional and predictive analyses
The results of the cross-sectional comparisons at the second time point showed that, after controlling for age, NAcc activation in response to receiving rewards explained significant variation in average amount of glasses consumed per night. These findings are consistent with prior research showing that activity in NAcc to rewards correlates with risk taking tendencies in adolescence and young adulthood (Galvan et al., 2007). In contrast, testosterone did not relate to reported alcohol use when analyzed cross-sectionally.
Next, we tested whether testosterone and NAcc activation predicted future alcohol use. This analysis showed that testosterone levels at the first time point, but not NAcc activation at the first time point, significantly predicted alcohol use on the second time point. This indicates a more protracted relationship than the cross-sectional relationship found between NAcc and alcohol use on the second time point.
Possibly this finding indicates a role for testosterone in (structural) NAcc development. Exactly how testosterone influences brain development is still unknown. To date, two studies have investigated this relationship using a longitudinal design. A study by Herting et al. (2014) found that testosterone levels influenced caudate development. In both boys and girls, low levels of testosterone were related to increases in caudate gray matter volume and high levels of testosterone were related to decreases in caudate gray matter volume. A study by Goddings et al. (2014) showed that pubertal development, measured with Tanner stages (Tanner and Whitehouse, 1976), explained variance in NAcc development above age for both sexes. Those individuals who were in a higher pubertal development stage compared to their age-matched peers had smaller NAcc volumes. NAcc development over time showed a linear decrease, which suggests that smaller NAcc volumes indicated a more adult like structure. These studies combined indicate that pubertal development has an influence on NAcc development above the influence of age. This fits with the protracted relationship between testosterone and alcohol use found in the current study. Testosterone effects on the brain can be slow, but also rapid (Goetz et al., 2014). In this case, the predictive association between testosterone and future alcohol use might reflect slow effects of testosterone on future behavioral outcomes.
5. Limitations
The current study used self-reported alcohol use as a proxy for risk-taking behavior. We only found a relationship between average amount of glasses and not alcohol use per month and lifetime alcohol use. Possibly there is large variation in circumstantial variables such as number of parties in a certain month, which is reflected in the score for past month alcohol use. Lifetime alcohol use was highly correlated with age and might be more reflective of risk taking behavior when assessed in a group with a smaller age range with preferably mostly young adolescents. Variation in the measure might reflect risk-taking behavior in this group more than in an older group. Furthermore, when alcohol use is assessed in a group with a large age range this might result in a non-normally distributed variable as many younger participants have never drank alcohol. Future studies could focus on alcohol use within a large sample of similar aged participants.
Risk-taking behavior entails a much greater spectrum of behaviors such as sexual risk-taking or traffic related risk-taking. Future studies could focus on a larger spectrum of risk-taking behaviors. Furthermore, the current study used baseline testosterone levels, measured at the beginning of the day. Testosterone levels are known to fluctuate throughout the day and can change rapidly due to environmental influences (Apicella et al., 2014). To assess the relationship between NAcc responses to the gambling task and testosterone levels, future studies could measure testosterone at several occasions around the moment of task administration.
6. Conclusion
In conclusion, NAcc activation to rewards is positively related to self-report alcohol use. This finding confirms the hypothesized relationship between neural activation to rewards and real-life risk-taking behavior. Even though there was no direct effect of testosterone on alcohol use, testosterone levels were found to be predictive of alcohol use two years later, indicating a more protracted relationship. A crucial question for the future is how testosterone levels influences brain development, and how the combination of both influences real life risk-taking (Crone and Dahl, 2012). Future studies assessing these relations across multiple times points will be important to unravel these dynamic relations.
Conflict of interest
The authors declare no competing financial interests.
Acknowledgements
This work was supported by a European Research Council (ERC) starting grant (ERC-2010-StG-263234) awarded to E.A.C. and by a VENI grant from the Dutch Science Foundation (NWO) awarded to J.S.P (VENI 451-10-007).
References
- Ames S.L., Grenard J.L., Thush C., Sussman S., Wiers R.W., Stacy A.W. Comparison of indirect assessments of association as predictors of marijuana use among at-risk adolescents. Exp. Clin. Psychopharmacol. 2007;15(2):204–218. doi: 10.1037/1064-1297.15.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella C.L., Carré J.M., Dreber A. Testosterone and economic risk taking: a review. Adapt. Hum. Behav. Physiol. 2014:1–28. [Google Scholar]
- Blakemore S.J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 2010;31(6):926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., Güroğlu B., de Water E., Meuwese R., Koolschijn P.C.M.P., Peper J.S., Crone E. Reward-related neural responses are dependent on the beneficiary. Soc. Cogn. Affect. Neurosci. 2013:1030–1037. doi: 10.1093/scan/nst077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., Peters S., Peper J.S., Güroğlu B., Crone E.A. Gambling for self, friends, and antagonists: differential contributions of affective and social brain regions on adolescent reward processing. NeuroImage. 2014;100:281–289. doi: 10.1016/j.neuroimage.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Braams B.R., Van Duijvenvoorde A.C.K., Peper J.S., Crone E.A. Longitudinal changes in adolescent risk-taking: a comprehensive study on neural responses to rewards, pubertal development and risk-taking behaviour. J. Neurosci. 2015;35(18):7226–7238. doi: 10.1523/JNEUROSCI.4764-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener N.D., Kann L., McManus T., Kinchen S.A., Sundberg E.C., Ross J.G. Reliability of the 1999 Youth Risk Behavior Survey questionnaire. J. Adolesc. Health. 2002;31(4):336–342. doi: 10.1016/s1054-139x(02)00339-7. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16(2):497. [Google Scholar]
- Cocosco R.A., Kollokian V., Kwan R.K.S., Evans A.C. Brain web: online interface to a 3D MRI simulated brain database. NeuroImage. 1997;5:S452. [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dale A.M. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 1999;8(2–3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Water E., Braams B.R., Crone E.A., Peper J.S. Pubertal maturation and sex steroids are related to alcohol use in adolescents. Horm. Behav. 2013;63(2):392–397. doi: 10.1016/j.yhbeh.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Delgado M.R. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Ryan N.D., Phillips M.L., Manuck S.B., Worthman C.M., Moyles D.L., Tarr J.A., Sciarrillo S.R., Dahl R.E. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(2) doi: 10.1097/00004583-201002000-00010. 162–172.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A., Hare T., Voss H., Glover G., Casey B.J. Risk-taking and the adolescent brain: who is at risk? Dev. Sci. 2007;10(2):F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G., Casey B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A.L., Mills K.L., Clasen L.S., Giedd J.N., Viner R.M., Blakemore S.J. The influence of puberty on subcortical brain development. NeuroImage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz S.M.M., Tang L.F., Thomason M.E., Diamond M.P., Hariri A.R., Carre J.M. Testosterone rapidly increases neural reactivity to threat in healthy men: a novel two-step pharmacological challenge paradigm. Biol. Psychiatry. 2014;76(4):324–331. doi: 10.1016/j.biopsych.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Gautam P., Spielberg J.M., Kan E., Dahl R.E., Sowell E.R. The role of testosterone and estradiol in brain volume changes across adolescence: a longitudinal structural MRI study. Hum. Brain Mapp. 2014;35(11):5633–5645. doi: 10.1002/hbm.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D., Mrug S. School-level correlates of adolescent tobacco, alcohol, and marijuana use. Subst. Use Misuse. 2015;50(12):1518–1528. doi: 10.3109/10826084.2015.1023449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihm M., Gangooly S., Muttukrishna S. The normal menstrual cycle in women. Anim. Reprod. Sci. 2011;124(3–4):229–236. doi: 10.1016/j.anireprosci.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Op de Macks Z.A., Gunther Moor B., Overgaauw S., Guroglu B., Dahl R.E., Crone E.A. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Dev. Cogn. Neurosci. 2011;1(4):506–516. doi: 10.1016/j.dcn.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper J.S., Dahl R.E. The teenage brain: surging hormones–brain–behavior interactions during puberty. Curr. Dir. Psychol. Sci. 2013;22(2):134–139. doi: 10.1177/0963721412473755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper J.S., Koolschijn P.C., Crone E.A. Development of risk taking: contributions from adolescent testosterone and the orbito-frontal cortex. J. Cogn. Neurosci. 2013;25(12):2141–2150. doi: 10.1162/jocn_a_00445. [DOI] [PubMed] [Google Scholar]
- Peters S., Braams B.R., Raijmakers M.E., Koolschijn P.C., Crone E.A. The neural coding of feedback learning across child and adolescent development. J. Cogn. Neurosci. 2014;26(8):1705–1720. doi: 10.1162/jocn_a_00594. [DOI] [PubMed] [Google Scholar]
- Peters S., Jolles D.J., Van Duijvenvoorde A.C., Crone E.A., Peper J.S. The link between testosterone and amygdala-orbitofrontal cortex connectivity in adolescent alcohol use. Psychoneuroendocrinology. 2015;53:117–126. doi: 10.1016/j.psyneuen.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Spear L. The teenage brain: adolescents and alcohol. Curr. Dir. Psychol. Sci. 2013;22(2):152–157. doi: 10.1177/0963721412472192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Ann. N. Y. Acad. Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman E.J., Rogol A. Puberty and psychological development. Handb. Adolesc. Psychol. 2004;2:15–44. [Google Scholar]
- Tanner J.M., Whitehouse R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976;51(3):170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkenberg H., Van Leeuwen L., Klein Wolt K., Goossens F. VeiligheidNL en Trimbos-instituut; 2012. Alcohol en jongeren: een vervolgonderzoek onder spoedeisende hulpbezoekers. [Google Scholar]
- Van Leijenhorst L., Zanolie K., Van Meel C.S., Westenberg P.M., Rombouts S.A., Crone E.A. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb. Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio: 1997. Wechsler Adult Intelligence Scale – Third Edition. Administration and Scoring Manual. [Google Scholar]