Abstract
Although several effective therapies are available for the treatment of osteoporosis in postmenopausal women and older men, there remains a need for the development of even more effective and acceptable drugs. Several new drugs that are in late-stage clinical development will be discussed. Abaloparatide (recombinant parathyroid hormone related peptide [PTHrP] analogue) has anabolic activity like teriparatide. Recent data from the phase 3 fracture prevention trial demonstrate that this agent is effective in reducing fracture risk. Inhibiting cathepsin K reduces bone resorption without decreasing the numbers or activity of osteoclasts, thereby preserving or promoting osteoblast function. Progressive increases in bone mineral density (BMD) have been observed over 5 years. Early data suggest that odanacatib effectively reduces fracture risk. Lastly, inhibiting sclerostin with humanized antibodies promotes rapid, substantial but transient increases in bone formation while inhibiting bone resorption. Marked increases in BMD have been observed in phase 2 studies. Fracture prevention studies are underway. The new therapies with novel and unique mechanisms of action may, alone or in combination, provide more effective treatment options for our patients.
Keywords: Osteoporosis, Therapy, Parathyroid hormone-related protein, Cathepsin K, Sclerostin
INTRODUCTION
Osteoporosis is a disorder of low bone mass and damaged skeletal architecture resulting in impaired bone strength and an increased risk of fragility fracture. During the past 20 years, several classes of drugs with different mechanisms of action have been shown to protect patients with osteoporosis from fragility fractures. Most of our current treatment options are anti-remodeling agents that reduce both bone resorption and formation, bringing the balance of bone metabolism back toward or to normal. These drugs strengthen trabecular bone by reducing the number and depth of stress risers in thin trabeculae. They do not improve or rebuild the damaged trabecular architecture. They have less or even no effect on strengthening cortical bone. The most effective of these drugs, potent bisphosphonates and the receptor activator of nuclear factor kappa-B (RANK) ligand inhibitor denosumab, reduce the risk of vertebral fracture by about 70%, of hip fracture by 40% to 50% and of all non-vertebral fractures by 20% to 30% [1,2]. The only bone building or anabolic agents now available are parathyroid hormone (PTH) analogues, PTH 1-84 and teriparatide. These drugs stimulate both bone formation and bone resorption. In the early months of treatment, bone formation is activated more than is resorption, resulting in a positive bone balance, especially in the trabecular skeleton. While teriparatide therapy may thicken cortical bone, it also causes, at least temporarily, an increase in the porosity of cortical bone [3]. Both teriparatide and PTH 1-84 reduced the risk of vertebral fracture by 65% and 61%, respectively [4,5]. Teriparatide reduced the incidence of non-vertebral fracture by 35%. Neither of these drugs has been shown to reduce the risk of hip fracture.
There are several important limitations to our current treatments. Some drugs such as oral bisphosphonates require complex dosing regimens that are inconvenient and may result in poor compliance with the dosing rules. Patients sometimes object to the daily injections required with PTH drugs. Overall there is very poor adherence to recommended treatment regimens; more than half of patients discontinue their treatment within 12 months of beginning therapy [6]. Also, concerns about long-term safety with bisphosphonates and perhaps denosumab limit the acceptance of these drugs and cause concerns about the benefit: risk ratio of long-term treatment [7]. Thus, opportunities exist for new therapeutic agents to fill the unmet needs of having a drug that reduces the risk of non-vertebral fracture more effectively than current treatments, that has a good safety profile and that can be given conveniently.
This review will focus on the clinical development of three types of new drugs. One is a different form of PTH. The other two are drugs with unique mechanisms of action that have the potential to substantially strengthen cortical bone and to become important new treatment options to reduce fracture risk in patients with osteoporosis.
PARATHYROID HORMONE RELATED PEPTIDES
Parathyroid hormone related peptide (PTHrP) shares modest structural homology with PTH 1-84 and teriparatide. Both PTH and PTHrP bind to the same PTH receptor, but the kinetics of binding differ, and the duration of the cellular activation of cyclic AMP with PTHrP is shorter than with PTH [8,9]. Preclinical studies suggested that, compared to PTH, PTHrP could achieve an anabolic skeletal effect with less activation of bone resorption and less calcium mobilization causing hypercalcemia, thereby broadening the therapeutic window [10].
In a phase 2 clinical trial, 600 µg PTHrP 1-36 administered daily for 2 months resulted in similar gains in bone mineral density (BMD) as did teriparatide 20 µg daily [11]. The frequency of hypercalcemia and the tolerability between the two study drugs were similar.
A more promising candidate molecule is abaloparatide, a synthetic analog of PTHrP. In a phase 2 study, the stimulation of resorption and formation markers was less with abaloparatide 80 µg daily than with teriparatide 20 µg [12]. However, The BMD response to abaloparatide was greater in both the spine and especially in the hip than with teriparatide. Consistent with the preclinical studies, hypercalcemia appeared to occur less frequently with abaloparatide.
Results of the phase 3 pivotal fracture trial with abaloparatide were recently presented at the annual meeting of The Endocrine Society [13]. Over 18 months of treatment with abaloparatide and teriparatide, the incidence of vertebral fracture was decreased by 86% and 80%, respectively, compared to placebo. A significant 43% reduction in non-vertebral fracture risk was observed with abaloparatide. Teriparatide reduced non-vertebral fracture by 28%, a difference that was not statistically significant compared to placebo. The difference in non-vertebral risk reduction between abaloparatide and teriparatide was also not significant. The frequency of hypercalcemia was observed somewhat less with abaloparatide than with teriparatide.
The study is continuing for another 6 months during which time, all patients will take alendronate 70 mg once weekly. A more detailed analysis of the data from this study will be necessary to determine whether and how abaloparatide would provide meaningful clinical advantages over teriparatide, other than the convenience of not having to refrigerate the drug. A transdermal dosing system of abaloparatide is also under development.
CATHEPSIN K INHIBITORS
Cathepsin K (CatK) is the major osteoclast-derived proteolytic enzyme in bone [14,15]. It hydrolyzes type 1 collagen and other bone matrix proteins. The enzyme is highly expressed in osteoclasts with quite limited expression in other tissues. Genetic deficiency of CatK (pyknodysostosis) is characterized by an osteoclast-rich form of osteopetrosis with high bone mass, reduced bone resorption but with preservation of bone formation. Targeted disruption of the CatK gene in mice results in a phenotype with an osteosclerotic skeleton with increased trabecular and cortical bone mass of good quality [16]. Histomorphometry demonstrates a high rate of bone formation, normal bone mineralization and normal or increased osteoclast numbers, but a decrease in the osteoclasts' ability to resorb bone matrix. Compared to normal animals, CatK deficient mice have increased bone strength at the vertebral body and femoral mid-shaft.
Several small molecular weight inhibitors of human CatK have been evaluated in pre-clinical studies and clinical trials. The preclinical studies with these inhibitors have been restricted to monkeys and rabbits. Balicatib (Novartis, Basel, Switzerland) is a basic, highly selective nitrile-based CatK inhibitor in enzyme-based assays, but the selectivity is largely lost in whole cell assays due its accumulation in lysosomes (a property called lysosomotropism) where high concentrations exert effects on non-K cathepsins. Odanacatib (Merck, Kenilworth, NJ, USA) is a non-basic, non-lysosomotropic, nitrile based molecule that retains its high enzyme selectivity in cell-based assay systems [17]. ONO-5334 (ONO Pharmaceutical, Trenton, NJ, USA) is a potent hydrazine-based non-lysosomotropic inhibitor of CatK with high selectivity compared to other cathepsins.
In preclinical studies in ovariectomized rabbits and monkeys, CatK inhibitors caused dose-dependent decreases bone resorption at both trabecular and cortical sites and preservation of areal BMD [18,19,20,21,22]. Odanacatib treatment increased osteoclast numbers when assessed by histomorphometry, and increased serum levels of tartrate resistant acid phosphatase 5b (TRAP5b), a marker of osteoclast number. The effects of CatK inhibition on bone formation are more complex. Like other resorption inhibitors, balicatib and odanacatib inhibit trabecular bone formation in ovariectomized monkeys. In ovariectomized rabbits, both odanacatib and alendronate reduced bone resorption and prevented bone loss [21]. However, the reduction in bone formation rate was less with CatK inhibition than with alendronate. Unlike what occurs with other anti-resorptive agents, CatK inhibition is associated with maintenance of endocortical bone formation and increased periosteal bone formation in the femur, resulting in increased cortical bone thickness and volume in the hip and improved bending strength of long bones [19,23].
These effects in preclinical studies demonstrated the unique effects that CatK inhibition has on bone remodeling. Inhibiting CatK results in decreased capacity of viable osteoclasts to resorb bone. By inhibiting the extracellular activity of CatK in the resorption space, the depth of resorption cavities made by osteoclasts is markedly reduced, and the overall rate of bone resorption is decreased. However, the number and other functions of osteoclasts is preserved, unlike the effects of bisphosphonates or RANK ligand inhibitors which result in decreased numbers and function of osteoclasts. As a result, communication between these functioning osteoclasts and osteoblasts remains intact in the presence of CatK inhibition. Osteoclast-derived signaling molecules such as spingosphine-1-phosphate are present to regulate osteoblast activity [23,24]. Preservation of osteoblast function might also be due to reduced degradation by CatK of matrix-derived growth proteins such as transforming growth factor β and insulin-like growth factor 1.
The favorable preclinical studies led to clinical trials with the three CatK inhibitors. In a phase 2 study, treatment with balicatib led to reduced markers of bone resorption and increased BMD [25]. However, because several cases of morphea-like skin lesions were observed, further development of this compound was halted. The dermatologic toxicities of balicatib might be due to inhibition of CatK in skin or to the lysosomotropism described above, causing off-target inhibition of other cathepsins expressed in skin fibroblasts [26].
The effects of oral ONO-5334 (50 mg twice daily, 100 mg once daily, or 300 mg once daily) were compared with placebo and alendronate 70 mg weekly in postmenopausal women with osteoporosis in a phase 2 trial [27]. After 12 months of therapy, the 300 mg daily dose of ONO-5334 reduced bone resorption markers similarly to alendronate, but the effects on bone formation markers bone specific alkaline phosphatase (BSAP) and procollagen type I N-terminal peptide (P1NP) were less than that observed with alendronate. Levels of TRAP5b were reduced by alendronate but increased by the CatK inhibitor. The effects of ONO-5334 300 mg daily and alendronate on BMD of the lumbar spine were comparable. No clinically relevant safety concerns were noted, and no difference was detected in the rate of skin adverse events among the study groups.
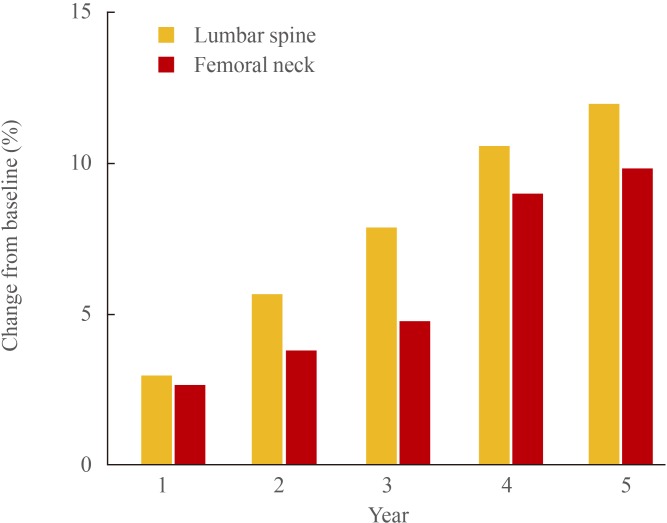
Odanacatib is the most extensively studied CatK inhibitor in clinical trials and is the only agent remaining in clinical development. In a placebo-controlled phase 2 trial, postmenopausal women with low BMD received odanacatib in once weekly doses ranging from 3 to 50 mg [28]. Over 24 months of treatment, a dose dependent increase in BMD at the lumbar spine and femoral neck was observed. At the highest dose of odanacatib (50 mg weekly), BMD at the lumbar spine and total hip increased by 5.7% and 4.1%, respectively, versus placebo. Urinary N-terminal telopeptide (NTX) was quickly reduced, with persistent decreases observed at 12 and 24 months (58% and 52% versus placebo, respectively). Markers of bone formation were initially reduced (20% for BSAP and 30% for P1NP), but levels then gradually returned toward baseline by 24 months. In the extension of this study, continued therapy with odanacatib 50 mg once weekly for 5 years resulted in almost linear increases in BMD from baseline at the lumbar spine (11.9%) and total hip (8.5%) (Fig. 1) [29]. Urinary NTX values remained well below baseline (50% at 3 years; 55.6% at 5 years), while after year 2, bone formation markers remained close to baseline levels.
Fig. 1. Bone mineral density change with odanacatib 50 mg once weekly.
The effects of odanacatib treatment on bone metabolism and BMD were rapidly reversible. After 2 years, therapy was withdrawn in a group of participants that had received odanacatib 50 mg weekly [30]. Markers of both bone resorption and formation quickly rose above baseline values, returning to baseline 12 to 24 months after therapy was discontinued. BMD values decreased to levels seen in the placebo group 12 months after withdrawing treatment. All doses of odanacatib were well tolerated. The incidence of skin and pulmonary events were similar in the treatment and placebo groups, and morphea-like skin lesions were not reported during the 5 years of therapy.
The effects of treatment with odanacatib 50 mg weekly on the incidence of hip and spine fracture is being evaluated in an international, placebo-controlled phase 3 event-driven study in more than 16,000 postmenopausal women with osteoporosis [31]. Based upon the results of a pre-planned interim analysis, this study was recently discontinued early because of "robust effectiveness." After a median duration of therapy of about 3 years, it was recently reported that odanacatib significantly reduced the incidence of vertebral, hip and non-vertebral fractures [32]. The overall safety profile of odanacatib did not differ from placebo. More detailed analyses of adverse events of special interest are being made. To collect more safety information, most patients will continue on their blinded treatment allocation for 5 years with additional follow-up on open label odanacatib therapy.
ANTI-SCLEROSTIN THERAPY
Sclerostin is an osteoblast inhibiting glycoprotein expressed primarily in osteocytes [33]. Secretion is increased by unloading or immobilization and is inhibited by PTH and by skeletal loading. Sclerostin inhibits osteoblast activity by interrupting the Wnt signaling pathway. Genetic deficiency of sclerostin results in the syndromes of sclerostiosis and Van Buchems disease. Both diseases are characterized by very high bone mass but, unlike the brittle bones of patients with osteopetrosis, the bone tissue is normal and of good quality. As a result, these patients with sclerostin deficiency are at very low risk for fracture. Homozygous patients experience cranial and facial distortion during growth and, as adults, bony overgrowth such as cranial basilar stenosis. Heterozygotes, who have intermediate levels of sclerostin, have a normal phenotype except for high bone mass, suggesting that inhibiting sclerostin has promise as a treatment strategy to activate bone formation and to improve bone mass as a treatment for osteoporosis and other bone disorders [34].
Sclerostin-deficient mice also exhibit high bone mass with increased rates of bone formation in both trabecular and cortical bone. These effects result in substantially stronger skeletons than in genetically normal mice [35].
Inhibiting sclerostin activity with an anti-sclerostin antibody in animals demonstrated the potential of sclerostin inhibition to improve bone mass and structure. In aged, ovariectomized rats given a sclerostin-inhibiting antibody, increased bone formation was observed on trabecular, endocortical, intracortical and periosteal bone surfaces [36]. Trabecular and cortical bone thickness was increased, and cortical porosity was reduced. Treatment for 5 weeks resulted in bone mass and bone strength that exceeded the sham-operated control animals and restored the skeletal abnormalities induced by ovariectomy.
In gonad-intact female cynomolgus monkeys, treatment with a humanized sclerostin-neutralizing antibody once monthly for 2 months transiently increased markers of bone formation, increased BMD in the lumbar spine, femoral neck, proximal tibia, and distal radius [37]. Increased modeling-based bone formation was observed on all skeletal surfaces. Substantial increase in bone strength occurred, with a strong correlation between bone mineral content (measured by quantitative computed tomography) and peak load observed in both the lumbar spine and femoral diaphysis.
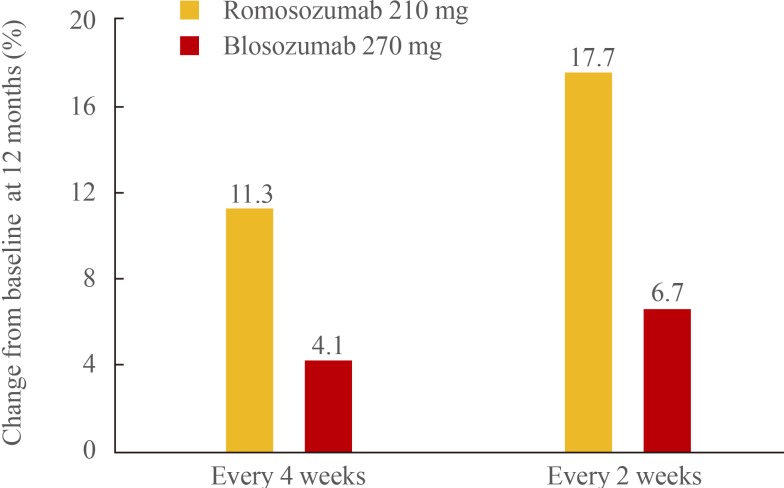
In clinical studies, single doses of humanized anti-sclerostin antibodies, administered subcutaneously, induced rapid and substantial increases in markers of bone formation accompanied by a moderate decrease in bone resorption markers, resulting in a very positive bone balance. In phase 2 studies in which patients received anti-sclerostin antibody injections every 2 to 4 weeks, does-dependent increases in BMD were observed (Fig. 2) [38,39]. The BMD responses to the larger doses of romosozumab were substantially greater than those observed in patients randomly assigned to receive open label alendronate or teriparatide therapy. Despite ongoing treatment, markers of bone formation returned to baseline levels within 6 to 12 months of beginning treatment. The mechanism(s) of this resistance to the anabolic effect of anti-sclerostin therapy is not known.
Fig. 2. Bone mineral density changes at 12 months in lumbar spine and femoral neck.
Anti-sclerostin therapy has been well tolerated, complicated only by injection site reactions that were generally mild and transient. Phase 3 studies with romosozumab, currently underway, will evaluate the efficacy of treatment to reduce fractures in women with postmenopausal osteoporosis and will provide much more information about the tolerability and safety of therapy with anti-sclerostin antibodies.
CONCLUSIONS
New forms of treatment, based on new or refined knowledge about the molecular mechanisms regulating bone metabolism, hold the promise of providing large gains in bone mass and reconstructing damaged skeletal architecture. The clinical challenge will be to most appropriately use these new medications to treat our patients most effectively.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 2.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 3.Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 4.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 5.Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, et al. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339. doi: 10.7326/0003-4819-146-5-200703060-00005. [DOI] [PubMed] [Google Scholar]
- 6.Lagari VS, McAninch E, Baim S. Considerations regarding adherence of anti-osteoporosis therapy. Postgrad Med. 2015;127:92–98. doi: 10.1080/00325481.2015.993278. [DOI] [PubMed] [Google Scholar]
- 7.McClung M, Harris ST, Miller PD, Bauer DC, Davison KS, Dian L, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med. 2013;126:13–20. doi: 10.1016/j.amjmed.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Dean T, Vilardaga JP, Potts JT, Jr, Gardella TJ. Altered selectivity of parathyroid hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol Endocrinol. 2008;22:156–166. doi: 10.1210/me.2007-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cupp ME, Nayak SK, Adem AS, Thomsen WJ. Parathyroid hormone (PTH) and PTH-related peptide domains contributing to activation of different PTH receptor-mediated signaling pathways. J Pharmacol Exp Ther. 2013;345:404–418. doi: 10.1124/jpet.112.199752. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-de la Rosa L, Lopez-Herradon A, Portal-Nunez S, Murillo-Cuesta S, Lozano D, Cediel R, et al. Treatment with N- and C-terminal peptides of parathyroid hormone-related protein partly compensate the skeletal abnormalities in IGF-I deficient mice. PLoS One. 2014;9:e87536. doi: 10.1371/journal.pone.0087536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz MJ, Augustine M, Khan L, Martin E, Oakley CC, Carneiro RM, et al. A comparison of parathyroid hormone-related protein (1-36) and parathyroid hormone (1-34) on markers of bone turnover and bone density in postmenopausal women: the PrOP study. J Bone Miner Res. 2013;28:2266–2276. doi: 10.1002/jbmr.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leder BZ, O'Dea LS, Zanchetta JR, Kumar P, Banks K, McKay K, et al. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2015;100:697–706. doi: 10.1210/jc.2014-3718. [DOI] [PubMed] [Google Scholar]
- 13.Miller PD, Leder BZ, Hattersley G, Lau E, Alexandersen P, Hala T, et al. LB-OR01-3 Effects of abaloparatide on vertebral and non-vertebral fracture incidence in postmenopausal women with osteoporosis: results of the phase 3 active trial; The Endocrine Society's 97th Annual Meeting & Expo; 2015 Mar 5-8; San Diego, CA. 2015. [Google Scholar]
- 14.Rodan SB, Duong LT. Cathepsin K: a new molecular target for osteoporosis. IBMS BoneKEy. 2008;5:16–24. [Google Scholar]
- 15.Costa AG, Cusano NE, Silva BC, Cremers S, Bilezikian JP. Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nat Rev Rheumatol. 2011;7:447–456. doi: 10.1038/nrrheum.2011.77. [DOI] [PubMed] [Google Scholar]
- 16.Pennypacker B, Shea M, Liu Q, Masarachia P, Saftig P, Rodan S, et al. Bone density, strength, and formation in adult cathepsin K (-/-) mice. Bone. 2009;44:199–207. doi: 10.1016/j.bone.2008.08.130. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier JY, Chauret N, Cromlish W, Desmarais S, Duong LT, Falgueyret JP, et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett. 2008;18:923–928. doi: 10.1016/j.bmcl.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 18.Stroup GB, Kumar S, Jerome CP. Treatment with a potent cathepsin K inhibitor preserves cortical and trabecular bone mass in ovariectomized monkeys. Calcif Tissue Int. 2009;85:344–355. doi: 10.1007/s00223-009-9279-x. [DOI] [PubMed] [Google Scholar]
- 19.Jerome C, Missbach M, Gamse R. Balicatib, a cathepsin K inhibitor, stimulates periosteal bone formation in monkeys. Osteoporos Int. 2011;22:3001–3011. doi: 10.1007/s00198-011-1529-x. [DOI] [PubMed] [Google Scholar]
- 20.Masarachia PJ, Pennypacker BL, Pickarski M, Scott KR, Wesolowski GA, Smith SY, et al. Odanacatib reduces bone turnover and increases bone mass in the lumbar spine of skeletally mature ovariectomized rhesus monkeys. J Bone Miner Res. 2012;27:509–523. doi: 10.1002/jbmr.1475. [DOI] [PubMed] [Google Scholar]
- 21.Pennypacker BL, Duong LT, Cusick TE, Masarachia PJ, Gentile MA, Gauthier JY, et al. Cathepsin K inhibitors prevent bone loss in estrogen-deficient rabbits. J Bone Miner Res. 2011;26:252–262. doi: 10.1002/jbmr.223. [DOI] [PubMed] [Google Scholar]
- 22.Cusick T, Chen CM, Pennypacker BL, Pickarski M, Kimmel DB, Scott BB, et al. Odanacatib treatment increases hip bone mass and cortical thickness by preserving endocortical bone formation and stimulating periosteal bone formation in the ovariectomized adult rhesus monkey. J Bone Miner Res. 2012;27:524–537. doi: 10.1002/jbmr.1477. [DOI] [PubMed] [Google Scholar]
- 23.Pennypacker BL, Chen CM, Zheng H, Shih MS, Belfast M, Samadfam R, et al. Inhibition of cathepsin K increases modeling-based bone formation, and improves cortical dimension and strength in adult ovariectomized monkeys. J Bone Miner Res. 2014;29:1847–1858. doi: 10.1002/jbmr.2211. [DOI] [PubMed] [Google Scholar]
- 24.Lotinun S, Kiviranta R, Matsubara T, Alzate JA, Neff L, Luth A, et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J Clin Invest. 2013;123:666–681. doi: 10.1172/JCI64840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Runger TM, Adami S, Benhamou CL, Czerwinski E, Farrerons J, Kendler DL, et al. Morphea-like skin reactions in patients treated with the cathepsin K inhibitor balicatib. J Am Acad Dermatol. 2012;66:e89–e96. doi: 10.1016/j.jaad.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 26.Falgueyret JP, Desmarais S, Oballa R, Black WC, Cromlish W, Khougaz K, et al. Lysosomotropism of basic cathepsin K inhibitors contributes to increased cellular potencies against off-target cathepsins and reduced functional selectivity. J Med Chem. 2005;48:7535–7543. doi: 10.1021/jm0504961. [DOI] [PubMed] [Google Scholar]
- 27.Eastell R, Nagase S, Ohyama M, Small M, Sawyer J, Boonen S, et al. Safety and efficacy of the cathepsin K inhibitor ONO-5334 in postmenopausal osteoporosis: the OCEAN study. J Bone Miner Res. 2011;26:1303–1312. doi: 10.1002/jbmr.341. [DOI] [PubMed] [Google Scholar]
- 28.Bone HG, McClung MR, Roux C, Recker RR, Eisman JA, Verbruggen N, et al. Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Miner Res. 2010;25:937–947. doi: 10.1359/jbmr.091035. [DOI] [PubMed] [Google Scholar]
- 29.Langdahl B, Binkley N, Bone H, Gilchrist N, Resch H, Rodriguez Portales J, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: five years of continued therapy in a phase 2 study. J Bone Miner Res. 2012;27:2251–2258. doi: 10.1002/jbmr.1695. [DOI] [PubMed] [Google Scholar]
- 30.Eisman JA, Bone HG, Hosking DJ, McClung MR, Reid IR, Rizzoli R, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res. 2011;26:242–251. doi: 10.1002/jbmr.212. [DOI] [PubMed] [Google Scholar]
- 31.Bone HG, Dempster DW, Eisman JA, Greenspan SL, McClung MR, Nakamura T, et al. Odanacatib for the treatment of postmenopausal osteoporosis: development history and design and participant characteristics of LOFT, the Long-Term Odanacatib Fracture Trial. Osteoporos Int. 2015;26:699–712. doi: 10.1007/s00198-014-2944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClung MR, Langdahl B, Papapoulos S, Saag KG, Adami S, Bone HG, et al. Odanacatib anti-fracture efficacy and safety in postmenopausal women with osteoporosis: results from the Phase III Long-term Odanacatib Fracture Trial (LOFT) Arthritis Rheumatol. 2014;66(Suppl 10):S987. [Google Scholar]
- 33.van Lierop AH, Hamdy NA, van Bezooijen RL, Lowik CW, Papapoulos SE. The role of sclerostin in the pathophysiology of sclerosing bone dysplasias. Clin Rev Bone Miner Metab. 2012;10:108–116. [Google Scholar]
- 34.Gardner JC, van Bezooijen RL, Mervis B, Hamdy NA, Lowik CW, Hamersma H, et al. Bone mineral density in sclerosteosis; affected individuals and gene carriers. J Clin Endocrinol Metab. 2005;90:6392–6395. doi: 10.1210/jc.2005-1235. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 37.Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, et al. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res. 2010;25:948–959. doi: 10.1002/jbmr.14. [DOI] [PubMed] [Google Scholar]
- 38.McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 39.Recker RR, Benson CT, Matsumoto T, Bolognese MA, Robins DA, Alam J, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res. 2015;30:216–224. doi: 10.1002/jbmr.2351. [DOI] [PubMed] [Google Scholar]